Abstract
Lysosomal storage disorders are rare inborn errors of metabolism, with a combined incidence of 1 in 1500 to 7000 live births. These relatively rare disorders are seldom considered when evaluating a sick newborn. A significant number of the >50 different lysosomal storage disorders, however, do manifest in the neonatal period and should be part of the differential diagnosis of several perinatal phenotypes. We review the earliest clinical features, diagnostic tests, and treatment options for lysosomal storage disorders that can present in the newborn. Although many of the lysosomal storage disorders are characterized by a range in phenotypes, the focus of this review is on the specific symptoms and clinical findings that present in the perinatal period, including neurologic, respiratory, endocrine, and cardiovascular manifestations, dysmorphic features, hepatosplenomegaly, skin or ocular involvement, and hydrops fetalis/congenital ascites. A greater awareness of these features may help to reduce misdiagnosis and promote the early detection of lysosomal storage disorders. Implementing therapy at the earliest stage possible is crucial for several of the lysosomal storage disorders; hence, an early appreciation of these disorders by physicians who treat newborns is essential.
Keywords: lysosomal storage disorders, neonatal, hydrops, enzyme deficiency
The lysosomal storage disorders (LSDs) are rare diseases with a combined incidence of ~1 in 1500 to 7000 live births.1,2 LSDs result from the inherited deficiency of 1 or more of the many catabolic enzymes that are located within the lysosome. This group of inborn errors of metabolism encompasses >50 different diseases, each characterized by the accumulation of specific substrates.3–6 There are many steps necessary for the synthesis and processing of lysosomal enzymes, which makes this system prone to dysfunctions that can result from different mechanisms and at many different steps in the pathway.
LSDs classically have not been considered disorders of the newborn. Generally, clinicians are taught that newborns with LSDs appear normal at birth and that the symptoms develop progressively over the first few months of life or even after many years. However, a portion of these patients can be mildly symptomatic as early as the first few days of life or even before birth, or they may have transient symptoms in the newborn period. Often, these early symptoms evade recognition because they are not considered in the differential diagnosis of disorders of the neonate. Nonimmune hydrops fetalis (NIHF) can be the initial presentation, indicating prenatal involvement.4 Table 1 lists the different LSDs that have been reported in neonates and the specific enzymes and storage products involved.
TABLE 1.
LSDs With Neonatal Presentations
| Type/Disease | Enzyme | Accumulating Substrate | Inheritance and Gene | OMIM No. |
|---|---|---|---|---|
| Sphingolipid storage disease | ||||
| Fabry disease | α-Galactosidase A | Globotriaosyl-ceramide (ceramide trihexoside) | X-LR; Xq22; GLA; manifests in hemizygotes 1 in 40 000 | 301500 |
| Farber disease | Acid ceramidase | Ceramides | AR; 8p22-p21.3; ASAH1 | 228000 |
| Gaucher disease type 2 (infantile acute neuronopathic) | Glucocerebrosidase, acid β-glucosidase | Glucocerebroside | AR; 1q21; GBA | 230900, 608013 |
| GM1 gangliosidosis | β-Galactosidase | GM1 ganglioside, keratan sulfate | AR; 3p21.33; GLB1 | 230500 |
| Krabbe disease, globoid cell leukodystrophy | Galactocerebrosidase, β-galactosidase | Galactocerebroside, galactosylsphingosine (psychosine) | AR; 14q31; GALC | 245200 |
| Niemann-Pick disease, types A and B | Sphingomyelinase | sphingomyelin, cholesterol | AR; 11p15.1-p15.4 (SMPD1) | 257200, 607616 |
| Infantile-onset symptomatic epilepsy | Lactosylceramide α-2,3 sialyltransferase (GM3 synthase) | Lactosylceramide | AR; 2p11.2; ST3GAL5 | 609056 |
| Mucopolysaccharide storage disease | ||||
| MPS I, Hurler syndrome | α-l-iduronidase | Heparan sulfate, dermatan sulfate | AR; 4p16.3; IDUA | 607014 |
| MPS IVA, Morquio syndrome A | Galactosamine-6-sulphatase | Keratan sulfate, chondroitin sulfate | AR; 16q24.3; GALNS | 253000 |
| MPS VII, Sly syndrome | β-Glucuronidase | Dermatan sulfate, heparan sulfate, chondroitin sulfate | AR; 7q21.11; GUSB | 253220 |
| Glycogen storage disease | ||||
| Pompe disease | α-Glucosidase | Glycogen | AR; 17q25.2-q25.3; GAA | 232300 |
| Glycoprotein storage disease | ||||
| Sialidosis types I and II, mucolipidosis type 1 | Neuraminidase 1 | Sialic acid | AR; 6q21.3; NEU1 | 256550 |
| Schindler disease | N-acetyl-α-d-galactosaminidase | Glycosphingolipids | AR; 22q11; NAGA | 609241 |
| Complex lipid storage disease | ||||
| Wolman disease | Lysosomal acid lipase | Cholesterol esters, triglycerides | AR; 10q24-q25; LIPA | 278000 |
| Transport and trafficking disorder | ||||
| ISSD, sialuria, Salla disease | Sialin (membrane protein) | Sialic acid | AR; 6q14-q15; SLC17A5 | 269920, 604369 |
| Niemann-Pick disease type C | NPC1 and NPC2 | Lipids, cholesterol | AR; 18q11-q12 (NPC1), 14q24.3 (NPC2) | 257220, 607625 |
| Multienzyme defect | ||||
| Galactosialidosis | β-Galactosidase and neuraminidase | GM3, GM2, GM1, GD1a, lactose ceramide, GA2, and GA1 | AR; 20q13.1; PPCA | 256540 |
| I-cell disease, mucolipidosis type 2 | N-acetylglucosamine-l-phosphotransferase | Multiple substrate accumulation | AR; 12q23.3; GNPTAB | 252500 |
| Multiple sulfatase deficiency | Sulfatase-modifying factor 1; affects arylsulfatases A, B, C | Mucopolysaccharides and sulfatides | AR; 3p26; SUMF1 | 272200 |
| Prosaposin deficiency | Prosaposin, SAP A, B, C, D; activators for multiple lysosomal enzymes | ceramide, sulfatide, hexosylceramides, globotriaosyl-ceramide and lactosylceramide | AR; 10q22.1; PSAP | 176801 |
OMIM indicates Online Mendelian Inheritance in Man (see www.ncbi.nlm.nih.gov/sites/entrez?db=omim); X-LR, X-linked recessive; AR, autosomal recessive.
It is very likely that the incidence of perinatal manifestations of LSDs is vastly underestimated. Greater physician awareness of these early presentations has important clinical implications. The recent development and availability of enzyme-replacement therapy (ERT) for several of the LSDs makes diagnosis early in the clinical course particularly important. Early diagnosis and intervention is essential for maximizing the potential benefit from some of these therapies and may prevent irreversible organ damage. Early diagnosis can provide parents with realistic information about their child's prognosis and can enable appropriate genetic counseling for future pregnancies. It can also help families avoid the “diagnostic odyssey” that many patients undergo before a diagnosis is made.
The majority of the LSDs are inherited in an autosomal recessive manner, with 3 exceptions: the X-linked disorders Fabry disease, Hunter syndrome (mucopolysaccharidosis [MPS] II), and Danon disease. Ethnicity can also be an important factor when considering the diagnosis of LSDs. Certain ethnic groups have an increased carrier frequency for specific disorders. For example, Gaucher disease, which results from the deficiency of the enzyme glucocerebrosidase, is the most common genetic disorder in Ashkenazi Jews, with a frequency of ~1 in 855 live births.7 An increased incidence of galactosialidosis is found among individuals of Japanese ancestry.8 Pompe disease is reported to have an increased frequency in subjects of African or Chinese ancestry.9 Consanguinity also can be a factor to consider in diagnosis of neonates from isolated communities.
The intent of this review is to describe symptoms of LSDs that can manifest in the newborn period and should alert the clinician to the possibility of inherited lysosomal diseases. The discussion focuses on clinical manifestations that affect different organ systems and includes reference tables and an algorithm (Fig 1) to help in the differential diagnosis of these disorders in the neonatal period.
FIGURE 1.
Algorithm of the clinical evaluation recommended for an infant with a suspected LSD. GAGs indicates glycosaminoglycans.
FREQUENT CLINICAL MANIFESTATIONS IN THE NEONATAL PERIOD
A summary of the major clinical findings encountered among neonates with these inborn errors of metabolism is provided in Table 2. Most newborns with LSDs appear normal at birth, because many of the toxic metabolites cross the placenta during pregnancy and are cleared by the mother during gestation. The interval between birth and the onset of clinical symptoms can range from hours to months (Table 3).
TABLE 2.
Symptoms Encountered in Newborns With LSDs
| System | Manifestations |
|---|---|
| Neurologic | Hypotonia |
| Floppy-infant syndrome | |
| Trismus | |
| Strabismus | |
| Opisthotonus | |
| Spasticity | |
| Seizures | |
| Peripheral neuropathy | |
| Developmental delay | |
| Irritability | |
| Extrapyramidal movement disorder | |
| Hydrocephalus | |
| Respiratory | Congenital lobar emphysema |
| Impaired cough | |
| Recurrent respiratory infections | |
| Hoarseness | |
| Endocrine | Osteopenia |
| Metabolic bone disease | |
| Secondary hyperparathyroidism | |
| Congenital adrenal hyperplasia | |
| Cardiovascular | Cardiomegaly |
| Congenital heart failure | |
| Arrhythmias | |
| Wolff-Parkinson-White syndrome | |
| Cardiomyopathy | |
| Dysmorphology | |
| Head and neck | Microcephaly |
| Enlarged nuchal translucency | |
| Microstomia | |
| Micrognathia/microretrognathia | |
| Long philtrums | |
| Limbs | Bilateral broad thumbs and toes |
| Bilateral club feet | |
| Eversed lips | |
| Flattened nasal bridge | |
| Short nasal columella | |
| Oral | Macroglossia |
| Molar hypoplasia | |
| Hypertrophic gums | |
| Absent nasal septum | |
| Bilateral epicanthal inferior orbital creases | |
| Palpebral edema | |
| Hypertelorism | |
| Facial | Coarse facies |
| Low-set ears | |
| Gastrointestinal | Hepatosplenomegaly |
| Neonatal cholestasis | |
| Bones and joints | Lytic bone lesions |
| Joint contractures | |
| Dysostosis multiplex | |
| Hyperphosphatasemia | |
| Vertebral breaking | |
| Broadening of tubular bones | |
| Punctuate epiphysis | |
| Craniosynostosis | |
| Painful joint swelling | |
| Skin | Congenital ichthyosis |
| Collodion infant | |
| Hypopigmentation | |
| Telangiectasias | |
| Extended Mongolian spots | |
| Ocular | Corneal clouding |
| Megalocornea | |
| Glaucoma | |
| Cherry-red spots | |
| Fundi hypopigmentation | |
| Bilateral cataracts | |
| Hematologic | Anemia |
| Thrombocytopenia | |
| Hydrops fetalis | NIHF |
| Congenital ascites | |
| Recurrent fetal losses |
TABLE 3.
Clinical Manifestations Reported in Different LSDs in the Neonate
| Disease | Hypotonia | Irritability | Developmental Delay |
Seizures | Movement Disorder |
Hyperreflexia | Hydrocephalus | Corneal Clouding |
Cherry- Red Spot |
Hepatospleno- megaly |
Anemia/ Low Platelets |
CAH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pompe disease | X | |||||||||||
| Gaucher type 2 disease | X | X | X | X | ||||||||
| Krabbe disease | X | X | X | X | ||||||||
| I-cell disease | X | X | X | X | X | X | ||||||
| IOSE | X | X | ||||||||||
| Prosaposin deficiency | X | X | X | |||||||||
| Multiple sulfatase deficiency | X | X | ||||||||||
| Sialidosis | X | X | X | X | X | |||||||
| MPS VII | X | X | ||||||||||
| Niemann-Pick type B disease | X | |||||||||||
| Niemann-Pick type C2 disease | X | X | X | |||||||||
| Farber disease | ||||||||||||
| Galactosialidosis | X | X | X | X | ||||||||
| Fabry disease | ||||||||||||
| Sandhoff disease | ||||||||||||
| GM1 disease | X | |||||||||||
| ISSD | X | |||||||||||
| Schindler disease | ||||||||||||
| Wolman disease | X | |||||||||||
| Niemann-Pick type A disease |
| Cardiomegaly | Cardiomyopathy | Respiratory Distress |
Macroglossia | Hoarseness | Dysmorphic Features |
Osteopenia | Joint Contractures |
Dysostosis Multiplex |
Craniosynostosis | Ichthyosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pompe disease | X | X | X | X | X | ||||||
| Gaucher type 2 disease | X | X | X | X | |||||||
| Krabbe disease | |||||||||||
| I-cell disease | X | X | X | X | X | ||||||
| IOSE | |||||||||||
| Prosaposin deficiency | |||||||||||
| Multiple sulfatase deficiency | X | X | |||||||||
| Sialidosis | X | X | |||||||||
| MPS VII | X | ||||||||||
| Niemann-Pick type B disease | |||||||||||
| Niemann-Pick type C2 disease | X | ||||||||||
| Farber disease | X | ||||||||||
| Galactosialidosis | X | X | X | ||||||||
| Fabry disease | X | ||||||||||
| Sandhoff disease | X | ||||||||||
| GM1 disease | X | ||||||||||
| ISSD | X | X | |||||||||
| Schindler disease | X | ||||||||||
| Wolman disease | |||||||||||
| Niemann-Pick type A disease | X |
CAH indicates congenital adrenal hyperplasia; IOSE, infantile-onset symptomatic epilepsy.
Neurologic Manifestations
Some of the LSDs present with neuromuscular manifestations. Most often these manifestations include hypotonia or weakness caused by toxic effects of accumulating metabolites in the muscles.
Pompe disease, the inherited deficiency of α-glucosidase, also known as glycogen storage disorder type 2, is an uncommon cause of the floppy-infant syndrome and is often overlooked in the early differential diagnosis of such infants.10 There is a spectrum of clinical presentations of Pompe disease, with the infantile type being the most severe. These infants can have a very striking appearance and little to no spontaneous movements. There have also been descriptions of low “myopathic” carnitine levels in infants with Pompe disease. This can be a form of secondary deficiency that also manifests in the first few months with generalized hypotonia and absent reflexes.9,11
Gaucher disease also has a continuum of phenotypic presentations. The acute neuronopathic form, type 2, presents in infancy and has a devastating neurologic course. There is a subset of type 2 disease that manifests in utero or perinatally, with severe neurologic signs including strabismus, trismus, retroflexion of the head, and opisthotonos.12–14
Krabbe disease, the deficiency of galactocerebrosidase, is divided into 2 main clinical forms on the basis of the age of onset. The infantile form generally presents at the age of 3 to 6 months with irritability, spasticity, and arrested motor development. However, there are earlier disease manifestations that can be overlooked because of a low index of suspicion. Actually, accumulation of the toxic substrate galactosylsphingosine (psychosine) in tissues has been noted as early as 21 weeks’ gestation in a fetus diagnosed prenatally with Krabbe disease.15 Sahai et al16 described a newborn who presented at the age of 7 days with irritability that progressed to weakness and seizures at the age of 7 weeks. There was also a description of an asymptomatic infant who developed a peripheral neuropathy at the age of 7 weeks and was subsequently diagnosed with Krabbe disease.17 Clarke et al18 reported an extremely rare neonatal variant of Krabbe disease that presented in the newborn with both the typical neurologic symptoms and lung involvement.
Very early developmental arrest is also seen in some LSDs. I-cell disease (mucolipidosis type 2) is a rare lysosomal disorder that presents at birth or in the first few months of life with profound developmental delay and microcephaly.19 GM1 gangliosidosis type 1, infantile form, can also present with developmental delay. Denis et al20 described a female newborn who, by the third day of life, was hypotonic with a poor suck but had hyper-reflexia. Morrone et al21 described 5 Italian patients presenting at birth or within 3 months of age who had delayed psychomotor development, hypotonia, and, in 1 case, seizures.
Seizures are a neonatal neurologic presentation in other LSDs. A rare infantile-onset symptomatic epilepsy syndrome, caused by a premature termination in the GM3 synthase enzyme, presents between the ages of 2 weeks and 3 months, primarily with irritability and feeding problems. Infants with this syndrome later develop seizure activity and profound developmental regression.22 Prosaposin deficiency, a rarely diagnosed storage disease, manifests with rapidly progressive neurologic deterioration characterized by myoclonus and an extra-pyramidal movement disorder that proceeds to grand mal epilepsy.23
Another neurologic manifestation encountered rarely in the LSDs is hydrocephalus. Hydrocephalus has been described in multiple sulfatase deficiency, presenting at birth or later, and often precedes the actual diagnosis, usually made at ~8 years of age.24,25 Hydrocephalus can be a manifestation of an atypical variant of Gaucher disease associated with mutation D409H, which also has cardiac valve involvement; however, it usually presents at the age of a few months.26 Hydrocephalus has also been reported in early infancy in sialidosis and MPS VII.27,28
Respiratory Manifestations
Several different LSDs can present with respiratory distress. Respiratory problems can arise in conjunction with hydrops fetalis, which will be discussed later, or can develop in other circumstances.
Niemann-Pick disease type B is known to cause mild pulmonary involvement. Arda et al29 described the case of a female infant who had massive pulmonary involvement resembling congenital lobar emphysema. She was ultimately diagnosed by a lung biopsy that showed foamy cells, and the diagnosis was confirmed by enzyme analysis. In addition, at least 5 cases of Niemann-Pick disease type C2 have been described who presented shortly after birth with severe respiratory distress.30
In Pompe disease, the early respiratory manifestations are attributed mostly to progression of the disease, with muscular weakness leading to low lung volumes, an impaired cough, blood gas abnormalities, and sleep-apnea.9,31 These patients are also at increased risk for aspiration pneumonia. Although most patients with Pompe disease will develop cardiomegaly prior to the significant muscle weakness that leads to respiratory infections, there has been at least 1 description of recurrent neonatal respiratory infections as the presenting sign of Pompe disease. This was explained by hypotonicity of the respiratory muscles.32 Another important factor that contributes to the respiratory distress seen in Pompe disease is severe macroglossia, which can cause aspiration and uncoordinated deglutition.33
Farber disease is known to present at the first few months of life with hoarseness and progressive joint swelling. The hoarseness develops as a result of accumulation of ceramide and development of nodules on the vocal cords. Devi et al34 described a neonate who presented at the age of 1 month with hoarseness.
Endocrine Manifestations
Unger et al35 described 3 patients who presented at birth with generalized osteopenia. A review of the literature identified a subset of patients with I-cell disease who presented in the neonatal period with features of “metabolic bone disease” rather than with the common signs of dysostosis multiplex that usually develop later in life. This presentation was accompanied by increased serum parathyroid hormone and alkaline phosphatase activity but normal calcium concentrations.36–42
Secondary hyperparathyroidism is a rare diagnosis and is attributed mostly to maternal deficiencies. It is usually transient in I-cell disease,43 and patients are not diagnosed until later in life, when they develop the typical clinical and radiologic features of the disease. The severe secondary hyperparathyroidism is probably a result of impaired transplacental calcium transport due to the underlying lysosomal disorder. Treatment with vitamin D resulted in a more rapid resolution.39
Congenital adrenal hyperplasia (CAH) is occasionally a manifestation of the LSDs. Oohira et al44 described a patient who presented with CAH as a result of a 21-hydroxylase deficiency associated with sialidosis type 2 (mucolipidosis type 1) that was thought to be due to linkage between HLA and the neuroaminidase gene. Later, Kyllerman et al45 described another patient with suprarenal masses on computed tomography scans that were likely to represent adrenal changes. At birth the patient had an enlarged clitoris with moderately elevated levels of 17-hydroxyprogesterone and low levels of plasma cortisol, indicating a transient intrauterine dysfunction of the adrenal glands, although inappropriate steroid metabolites could not be demonstrated at 3 weeks of age. This adrenal hyperplasia was most likely secondary to the child's storage disorder, which was diagnosed as galactosialidosis.
Cardiovascular Manifestations
In classical infantile Pompe disease, cardiomegaly, congenital heart failure, arrhythmias such as supraventricular tachycardia, and cardiac arrest during surgery are examples of neonatal cardiovascular manifestations. This disorder is the only glycogen storage disease that is also an LSD, and the effects of glycogen accumulation are very pronounced in the heart. Lysosomal glycogen accumulation results in significant cardiac hypertrophy that may begin in utero and is apparent perinatally or at 4 to 8 weeks of age.46
In addition, the insulator effects of glycogen in conduction tissue can result in disruptions to the conduction system of the heart, leading to arrhythmias. Wolff-Parkinson-White syndrome has been reported to occur in Pompe disease as well.47 These conduction abnormalities, in conjunction with the hypertrophic cardiomyopathy, place affected infants at high risk for sudden death.
Cardiomyopathy may be associated with several other LSDs including Fabry disease, Gaucher disease, Niemann-Pick type A, I-cell disease, GM1 gangliosidosis, Sandhoff disease, sialidosis, galactosialidosis, infantile sialic acid storage disease (ISSD), and Schindler disease, although these generally do not manifest in the newborn.48,49
Dysmorphic Features
Several of the LSDs have classic dysmorphic features that can be identified in the newborn (Fig 2). MPS VII, the deficiency of β-glucuronidase, has a spectrum of clinical findings. A review of the literature reveals that some patients present as early as at birth, and have severe involvement, including a characteristic appearance of coarse facies with a depressed nasal bridge and widely spaced eyes.50 den Hollander51 also described a patient with MPS VII that presented with an enlarged nuchal translucency in early pregnancy.
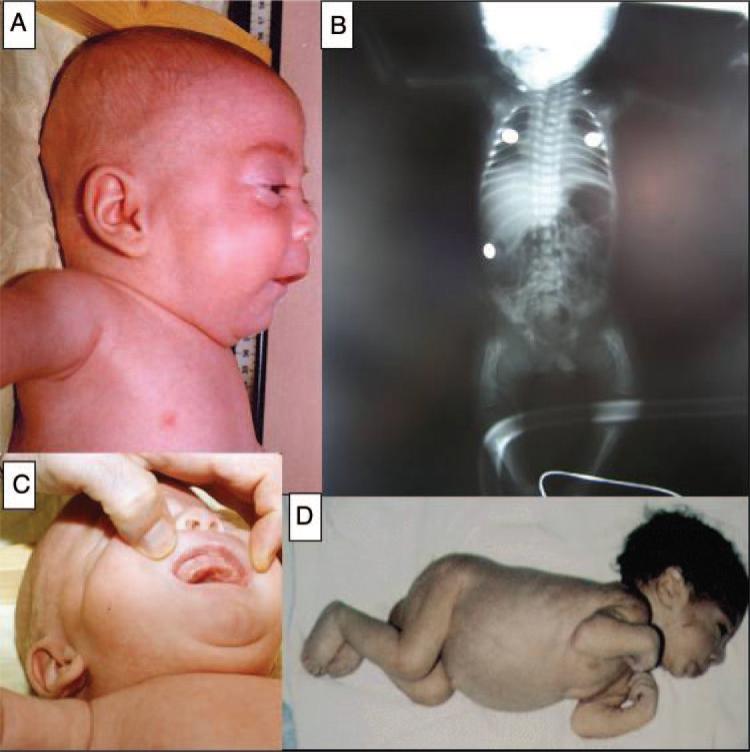
FIGURE 2.
Clinical photographs. A, Coarse facial features in a patient with I-cell disease. B, Skeletal survey showing lytic bony lesions in a patient with I-cell disease. C, Gum hyperplasia in a patient with I-cell disease. D, A newborn with perinatal-lethal type 2 Gaucher disease presenting with contractures and hepatosplenomegaly.
The early infantile form of galactosialidosis presents at birth with gargoyle-like features, including bilateral epicanthal inferior orbital creases, absent nasal septum, molar hypoplasia, high arched palate, and micrognathia.52 Infants with galactosialidosis have also been described with long philtrums and sparse temporal hair.53
One of the characteristic features of Pompe disease is severe macroglossia with a protruding tongue, which is also typical of several of the MPSs. The other accompanying features are flattened nasal bridge, facial hair, and coarse facial features.33
GM1 gangliosidosis type 1 has been described as presenting in the newborn with a slightly coarse facies and palpebral edema.20,54 I-cell disease is also characterized by coarse facial features and hypertrophic gums, which are unique to this disease at this age (Figs 2A and 2C).19
Several cases of perinatal-lethal Gaucher disease have been described with an unusual facial appearance, including low-set ears, a small nose with a flat nasal bridge, and, less frequently, hypertelorism, microstomia, eversed lips, microretrognathia, and microcephaly.14,55
Multiple sulfatase deficiency is not only rare, but also is not usually diagnosed until approximately the age of 8 years. There have been, however, a few descriptions of dysmorphic features seen at birth, including bilateral broad thumbs and great toes, both with angulation deformities, bilateral club feet, and a short nasal columella.24,25
Hepatosplenomegaly
The differential diagnosis of hepatosplenomegaly at birth is vast. When a newborn presents with hepatosplenomegaly, the first step is to rule out more common diagnoses such as viral infections, sepsis, and anatomic obstructions. LSDs should be considered in the differential diagnosis, particularly if there are additional manifestations such as coarse facies.
MPS VII commonly presents at birth (or even before birth with hydrops) and is associated with chronic hepatosplenomegaly.50,56,57 Sialidosis and galactosialidosis have also been described as a cause of hepatosplenomegaly in the newborn.54,58 Hepatosplenomegaly in the neonate is commonly seen with perinatal-lethal type 2 Gaucher disease14 (Fig 2D). Pueschel et al59 described the case of a patient with ISSD who had fetal ascites and chronic hepatosplenomegaly. Likewise, prosaposin deficiency, a very rare progressive LSD, presents with hepatosplenomegaly at birth, with no evidence of ascites.60,61
Niemann-Pick type C has been described in at least 3 patients who exhibited hepatosplenomegaly and jaundice in the first 2 months of life, which resolved later in life.62,63 The disease has also been described as manifesting with fetal ascites.64 Wolman disease is characterized by hepatosplenomegaly at birth or shortly thereafter.65,66 Multiple sulfatase deficiency and I-cell disease can present with neonatal hepatosplenomegaly.24,67–69
The biliary tract can also be involved in LSDs. Hochman et al70 described a patient who presented at the age of 9 days with mild jaundice who developed hepatosplenomegaly by the age of 1 month. This was later found to be a result of a bile duct injury that was diagnosed as part of I-cell disease. This case demonstrates that I-cell disease should be in the differential diagnosis of neonatal cholestasis. Neonatal cholestasis has also been described in Gaucher disease.71,72
Bones and Joints
Bone and joint involvement has been described occasionally in infants with LSDs. Lytic bone lesions have been observed in type 2 Gaucher disease.73 Joint contractures have been described in association with Gaucher disease in neonates and fetuses with hydrops fetalis (Fig 2D).74
Dysostosis multiplex, which results from abnormal bone formation, is a feature of several LSDs, but it usually presents later in life. However, it has been described in neonates and fetuses diagnosed with galactosialidosis, GM1 gangliosidosis, and I-cell disease in association with NIHF.19,75
Denis et al20 described a significant hyperphosphatasemia in the first few months of life in neonates with GM1 gangliosidosis who had radiographic evidence of lytic bone lesions. Severe bony changes, including vertebral breaking and broadening of tubular bones, have been described in a few infants with MPS VII.56 Galactosialidosis has been described in association with precocious calcification, which is also known as punctuate epiphysis.53
I-cell disease has been described together with craniosynostosis in the neonate in a few cases. Prenatal development of short femurs has also been seen (Fig 2B) in I-cell disease and must be differentiated from neonatal osteogenesis imperfecta.76 I-cell disease should be part of the differential diagnosis of significant craniosynostosis in the neonate or prenatal diagnosis of short femurs, especially if coarse facies is seen postnatally.77
Farber disease generally manifests after the first few months of life. There was a report of 1 patient, however, who presented with painful joint swelling at the age of 1 month.34
Skin Manifestations
Type 2 Gaucher disease can manifest with congenital ichthyosis. Ultrastructural and biochemical alterations are present in the epidermis of infants with type 2 Gaucher disease, even when clinical signs of skin involvement are not. Glucocerebrosidase is abundant in normal epidermis and results in the conversion of glucosylceramide into ceramide, both components of the lipid bi-layer that forms the epidermal barrier. In type 2 Gaucher disease, the ratio of glucosylceramide to ceramide is reversed.78 The clinical spectrum of skin involvement in type 2 Gaucher disease ranges from mild skin peeling and scaling that quickly resolves to a full-fledged “collodion baby” phenotype.79 Recognition of these skin manifestations is particularly important, because they often precede severe neurologic manifestations in these children.80–82
Multiple sulfatase deficiency can present in the neonatal period with variable skin involvement ranging from dry skin to ichthyosis.24,67,68 Early infantile galactosialidosis has been reported in association with diffuse skin hypopigmentation.53 There also has been a description of skin telangiectasia associated with this diagnosis.58
Another skin manifestation recorded in the literature is extended Mongolian spots. These have been reported in Hurler disease and type 1 gangliosidosis.83
Ocular Manifestations
Most of the common ocular manifestations of the LSDs do not present in the neonatal period. However, there can be early presentations. For example, Sergi et al84 described a Syrian newborn with neonatal sialidosis who was noted at birth to have corneal clouding.
Early eye involvement has also been described with I-cell disease. Of 35 patients with I-cell disease, the most frequent ocular finding was corneal clouding (14 cases), glaucoma (2 cases), or megalocornea (2 cases).85 There were no descriptions of macular cherry-red spots, a frequent later finding in several of the LSDs. Ultrastructural study of the eyes in 7 affected patients revealed changes in the conjunctival, corneal, scleral, and uveal fibroblasts, whereas other cells were not damaged.
Several ocular manifestations have been described in early-infantile galactosialidosis. These manifestations have included cherry-red spots, cloudy cornea, generalized stroma, and fundi hypopigmentation as well as bilateral cataracts with onset at 3 months of age.53
Hematologic Manifestations
Tekinalp et al86 described a patient diagnosed with galactosialidosis who presented with hydrops and coarse facial features, as well as anemia and thrombocytopenia from birth. There have been additional reports of galactosialidosis associated with anemia.
Gaucher disease and neonatal sialidosis type 2C can also present with anemia and thrombocytopenia in the newborn period.72,84 In general, thrombocytopenia is a well-recognized feature of Gaucher disease secondary to hypersplenism, due in part to the splenomegaly that develops. Roth et al72 described a newborn who presented at birth with persistent thrombocytopenia and was subsequently diagnosed with Gaucher disease.
Hydrops Fetalis/Congenital Ascites
Congenital ascites results from a wide range of etiologies including abnormalities of the genitourinary tract or gastrointestinal tract or cardiovascular anomalies, or it can arise secondary to a hematologic disorder. In recent years there has been increased awareness that ascites in the neonate may be a manifestation of 1 of several different LSDs. The event that triggers the accumulation of excessive fluid within the peritoneal cavity in infants with LSDs is a source of considerable controversy in the literature. The mechanism contributing to the development of hydrops in storage diseases may involve the obstruction of venous blood return resulting from organomegaly.87 Anemia, caused by either hypersplenism or the reduction of erythropoietic bone marrow stem cells caused by infiltrating storage cells, may be a trigger. Hydrops can also result from hypoproteinemia caused by liver dysfunction.88,89 Other conditions that may trigger ascites in metabolic disease are congestive heart failure and liver cirrhosis.
At present, 13 different LSDs are associated with NIHF or congenital ascites.1,90–92 These LSDs associated with NIHF include cases with type 2 Gaucher disease, sialidosis type II, galactosialidosis, ISSD, Salla disease, MPS types IV and VII, GM1 gangliosidosis, I-cell disease, Niemann-Pick types A and C, Wolman disease, and Farber disease. Often, the patients described had a previous affected sibling who was not diagnosed with such a disorder. For this reason, in cases of familial NIHF, one should consider the LSDs or other inborn errors of metabolism. When there is facial dysmorphism, irregularity of the epiphyses, and/or coarse trabeculations of the long bones in the presence of congenital ascites, the index of suspicion of a storage disease is even greater.
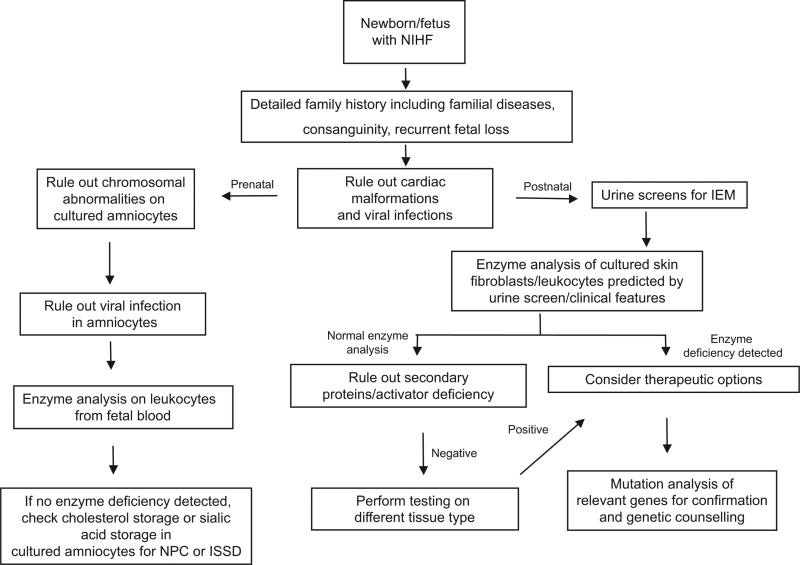
The relative frequency of the LSDs in the context of NIHF or ascites was 1.4% in a large retrospective series.93 In 2004, Burin et al91 searched for LSD in 33 cases of NIHF, including 28 cases in utero and 5 hydropic newborns. They detected 5 patients (15%) with LSD, including 4 diagnosed prenatally (I-cell disease, Niemann-Pick type A, galactosialidosis, and sialidosis) and 1 patient with MPS IVA diagnosed after birth. A recommended algorithm for the evaluation of cases of NIHF is shown in Fig 3.
FIGURE 3.
Algorithm for the clinical evaluation of a fetus or newborn with NIHF. IEM indicates inborn errors of metabolism; NPC, Niemann-Pick disease type C.
There are many descriptions of type 2 Gaucher disease presenting with hydrops fetalis and joint contractures. In the majority of cases with this severe phenotype, glucocerebrosidase activity is absent or severely deficient.13,14,74,78,88,94–99 In general, most patients with Gaucher disease do have some residual glucocerebrosidase activity, with no correlation between severity and the level of residual enzyme activity. Type 2 Gaucher disease is the rarest and most severe type, and untreated patients uniformly die before 3 years of age.
Cases of neonatal sialidosis (sialidosis type 2) presenting as hydrops fetalis or with neonatal ascites have been reported.27,84,100–108 NIHF has been reported in galactosialidosis.103,109,110 Claeys et al111 described a patient who presented prenatally with massive ascites and only after birth was diagnosed as having galactosialidosis. ISSD and Salla disease have been described in patients with massive fetal ascites.59,101,112–114
Although MPS IVA is a rare disease, prenatal manifestations of the disease include hydrops fetalis, with confirmation of the diagnosis by chorionic villus sampling or amniocentesis.115–117 MPS type VII also has been recognized as a cause of NIHF, which is actually the most common presentation of the disease.1,118,119 However, there is great variability in the associated clinical and biochemical manifestations.120
There have been at least 5 descriptions of cases with NIHF or congenital ascites, either transient or persistent, as the presenting symptom of GM1 gangliosidosis 1.20,75,121–123 Burin et al91 reported hydrops with I-cell disease and Niemann-Pick type A. Maconochie et al64 described a patient with severe congenital ascites diagnosed with Niemann-Pick type C, and Meizner et al124 reported a case of NIHF in which Niemann-Pick type C was diagnosed by electron microscopy. Although Wolman disease is usually accompanied with mild ascites, Uno et al described a case with massive milky ascetic fluid.65,125 Ben-Haroush et al125 described another case of isolated ascites that was later diagnosed as Wolman disease. Kattner et al126 reported a preterm infant with Farber disease and severe hydrops fetalis.
It is very important to examine the placenta carefully in cases where hydrops or ascites are present at birth or detected by ultrasound, even transiently. Placental histology can serve as an early diagnostic clue for a number of storage diseases, including GM1 gangliosidosis,20,127 MPS VII,128,129 ISSD,130,131 Gaucher disease,132 galactosialidosis,133 and Fabry disease.134 The presence of highly vacuolated cells or cells demonstrating storage should be followed up with enzymatic testing.
Recurrent Fetal Losses
Because most LSDs are autosomal recessive, consanguinity can be an important contributing factor. Many of the case descriptions of the LSDs found in the literature were identified in families with consanguinity, especially cases with NIHF. When taking a medical history, questions regarding previous gestations are very important, because in most families, the diagnosis is made only after previous pregnancies ended as stillbirths or with NIHF. For example, Nelson et al described a case of MPS type VII in consanguineous parents who had 3 previous unsuccessful pregnancies because of recurrent stillbirths.128 Venkat-Raman et al135 described a case of MPS Type VII in consanguineous parents who had a previous hydropic pregnancy. Landau et al109 described a family in which fetal hydrops occurred in 4 pregnancies, and the diagnosis of galactosialidosis was only made after the birth of the fourth affected child. Manning et al136 described the case of a twelfth pregnancy of a woman who had 4 first-trimester miscarriages and a previous child who died at 4 weeks of age. Ultimately, the diagnosis of Niemann-Pick type C was made.
DIAGNOSTIC METHODS
Accurate diagnosis is imperative for genetic counseling for future pregnancies, because most of the LSDs are autosomal recessively inherited. There are many ways to diagnose LSDs (Fig 1). With the development of new treatments for several of the LSDs, the diagnostic requirements are also changing. The efficacy of many of the proposed treatments relies heavily on early detection and initiation of treatment before the onset of irreversible pathology.137,138
Although many of the clinical presentations of different LSDs primarily result from substrate storage, these presentations vary greatly depending on the type, quantity, and site of the accumulated storage material. Because there is an overlap of clinical features in many of the LSDs, it is difficult to establish a diagnosis solely on the basis of clinical presentation. Fortunately, different accurate laboratory assays based on detection of the storage product, enzymatic assays, and DNA diagnostics have been developed. There are also biomarkers such as chitotriosidase that, although not optimally specific, can help monitor disease load. For example, in Gaucher disease, chitotriosidase levels decrease after ERT.
Urine screens that test for elevated levels of secreted substrate material are used routinely to examine the pattern of glycosaminoglycans and oligosaccharides in patients suspected of having MPS or disorders that present with oligosacchariduria, such as I-cell disease, mucolipidosis type 3, GM1 gangliosidosis, GM2 gangliosidosis type 2, fucosidosis, α-mannosidosis, sialidosis, galactosialidosis, and ISSD. After determining that the level of glycosaminoglycans is elevated, electrophoresis can further support the diagnosis of the MPSs, although the definitive diagnosis is made by enzyme analysis of either leukocytes or cultured skin fibroblasts. Although urine screens are very sensitive, there have been reports of affected individuals with normal urine screens; thus, when there is a strong index of suspicion, normal urine screen results should still be followed by enzyme analysis.139–141
Generally, panels of enzyme activity assays are performed on a combination of leukocytes and plasma and predominantly include enzymes involved in the digestion of glycosphingolipids and oligosaccharides. Diseases tested for in these panels include Gaucher disease, Niemann-Pick disease types A and B, acid lipase deficiency, GM1 and GM2 gangliosidosis, Krabbe disease, metachromatic leukodystrophy, mucolipidosis type 2 and 3, fucosidosis, α-mannosidosis, MPS type VII, and Schindler disease. Although measurement of enzyme activity in leukocytes and plasma enables the diagnosis of most LSDs in affected patients, a proportion may not be detected by this method. For example, in sialidosis and Pompe disease, the distinction between the normal and affected range in leukocytes can be very narrow, and the diagnostic analysis should be performed on other tissues or cultured fibroblasts. When leukocyte assays are not reliable, another method of enzyme analysis is to assay individual hair roots that develop from progenitor cells.138
A number of the LSDs result from deficiencies in secondary proteins or enzymes where the defect is in the activator protein. In these cases, the diagnosis can be achieved by examining the level of substrate secreted in the urine, or the rate of radiolabeled substrate turnover can be determined in cultured cells by substrate-loading tests. Ultimately, the relevant gene can be evaluated by mutation analysis. One of the examples for secondary protein deficiency is Niemann-Pick type C, which results from defective cholesterol transport in the lysosomal pathway and is diagnosed by analysis of cholesterol processing and accumulation in cultured fibroblasts.
Molecular analysis is rarely used as the primary screening tool for the diagnosis of LSDs. However, molecular analysis plays an important role with respect to carrier and prenatal testing for a variety of LSDs. Mutation data can also enable a rapid and accurate prenatal diagnosis. Because the demand for preimplantation diagnosis is rapidly increasing, it is very important that the causative LSD mutations be identified to enable the implantation of nonaffected fetuses. In a gene with several common mutations, this can be achieved more readily. In other disorders, however, the mutations identified may be novel or very rare. In cases when there is a strong diagnostic suspicion, sequencing of the relevant genes can be used to detect mutations. However, establishing that the nucleotide change identified is pathologic, rather than a mere polymorphism, can be challenging.138
Population screening for the LSDs is not performed routinely except for high-risk ethnic groups, for which screening for specific disorders may be appropriate, such as Tay-Sachs disease in the Ashkenazi Jewish population. Once a proband is identified in a given family, definitive carrier testing can be performed if the causative mutations are known.
Because of the early presentation of many of the LSDs, and the relative severity of these disorders, prenatal diagnosis is important for future pregnancies. Diseases that can be detected by enzymatic or biochemical changes in cultured cells can be detected using cultured chorionic villous cells or amniocytes for prenatal diagnosis. In many cases, direct analysis of chorionic villous tissue is also diagnostic, providing rapid and accurate results early in gestation. However, because some disorders can have pseudodeficiencies, it is often advisable to perform biochemical analysis on parental cultured cells, as well, to prevent a false-positive diagnosis.138
Whenever the causative gene is known, prenatal diagnosis can be made by mutation analysis. It is important that parental DNA is analyzed before prenatal testing to ensure that each parent carries 1 of the known causative mutations and that a corresponding normal allele can be detected also.
There are a few methods for the determination of enzymatic activities that have been developed recently on the basis of elution of the enzyme from a dried blood spot, followed by an assay of the enzyme activity by using fluorescent or radiolabeled substrates. Another alternative is the immune capture of the enzyme before the determination of enzyme activity. In addition, monoclonal and polyclonal antibodies can be used for the immune quantification of specific lysosomal proteins from biological samples, including dried blood spots. These assays provide a convenient and economical means for diagnosis and may have increasing importance as newborn screening for these disorders is considered.142,143 Electrospray ionization tandem mass spectrometry has been used effectively to investigate stored substrates in a number of the LSDs. This method has great potential and may enable the monitoring of responses to therapy.144,145 It is important to consider more than 1 type of assay as confirmation in any investigation of a patient suspected of having an LSD.
The decision regarding the advisability of newborn screening for the LSDs is complex. In addition to the requirements for a sensitive and specific assay, there are important ethical, economic, and counseling implications. Because the different LSDs have differing prognoses and therapeutic options, each disorder should be considered individually when deciding whether to implement newborn screening. Currently, screening for Krabbe and Pompe diseases are under discussion because of the therapeutic advances in these disorders, and it is likely that in the near future screening for other LSDs will be explored.
THERAPEUTIC MODALITIES
In the past, the only therapy available for patients affected by LSDs consisted of supportive care and treatment for disease complications. Significant progress has been made during the past few years, and therapies for type 1 Gaucher, Fabry, MPS types I, II, and VI, and Pompe diseases have been approved by the US Food and Drug Administration. Several new treatment modalities are under development for these and other LSDs that are, as yet, untreatable, with the goals of alleviating neurologic manifestations, improving visceral response, and reducing the cost of therapy. However, today the majority of newborns diagnosed with LSDs have an ominous prognosis.
On the basis of the premise that only 1% to 5% of enzyme activity is required to correct many of these metabolic defects, initial efforts were focused on the development of ERT. The prototype treatment was the now widely available ERT for Gaucher disease type 1 (the most common and nonneuronopathic type of Gaucher disease). ERT was approved recently for Fabry disease, MPS types I, II, and VI, and Pompe disease, and clinical trials of ERT in Niemann-Pick disease types A and B are in progress. However, there are limitations to this form of treatment: it does not affect all aspects of the disorder to the same degree, and clinical studies have shown that even after prolonged treatment, many symptoms of LSDs are not reversible. In addition, the extent of response seems to vary from individual to individual.146
The efficacy of treatments can be evaluated by close monitoring of clinical manifestations or by using diagnostic or surrogate biomarkers. Other parameters relevant to specific disease entities include tests such as forced expiratory capacity or walking distance in a specified amount of time.
Additional limitations of ERT relate to our incomplete knowledge of the natural history of these rare diseases. Because some of the clinical symptoms are results of secondary processes, ERT might not be expected to be beneficial. Another limitation is the expense of the replacement enzyme. These therapies often cost more than $250 000 per patient per year, and because of the length and chronicity of treatment, the cost can continue indefinitely. In addition, optimal dosing and maintenance doses have not been established definitively for all treated diseases. Because the replacement enzymes do not cross the blood-brain barrier, ERT does not correct central nervous system (CNS) manifestations. Safety studies have indicated that ERT is generally well tolerated. Even in patients who show seroconversion, a decrease in antibody titers occurs over time, and most continue to tolerate the drug. However, antigenicity does seem to impact therapeutic success of ERT for Pompe disease.146
Hematopoietic stem cell transplantation (HCT) or bone marrow transplantation, which provides a population of cells with the capacity to produce the missing enzyme, is also used to treat patients with LSD (reviewed in refs 147–150). Initial attempts at treatment of LSDs with marrow transplantation began in the 1980s. The success of marrow transplantation depends on the specific enzyme deficiency and the stage of the disease. Generally, visceral symptoms can be improved, whereas skeletal lesions remain relatively unaffected. HCT has been most successful in Hurler syndrome (MPS I), with improvement in visceral, corneal, and cardiopulmonary symptoms, growth, and developmental delay, especially when attempted before the age of 2 years, but not in some skeletal or cardiac valve manifestations. Although some improvements have been observed in MPS types VI and VII, HCT has had no effect on the other forms of MPS.151 The effect on neurologic symptoms varies in different LSDs. Although improvements have been documented in MPS I and Krabbe disease, neurologic disease progression was faster after transplantation in metachromatic leukodystrophy. For LSDs with variable neurologic forms, transplantation early in the disease course is desirable before extensive CNS injury becomes evident. Transplantation of nonallogeneic cord blood has also been performed when a matched bone marrow donor was not available. Encouraging results with this treatment modality have been reported for Hurler syndrome and Krabbe disease.152,153 The limitations of this treatment modality included nonresponsiveness, immune-related risks, and other complications associated with transplantation. Supplementing engrafted HCT subjects with additional mesenchymal stem cells has also shown promise for some LSDs.149,154,155
Another available treatment is substrate-reduction therapy, which aims to partially inhibit the biosynthetic cycle to reduce substrate influx in the compromised lysosome. A number of small molecule inhibitors of ceramide-specific glucosyltransferase, the first enzyme in the biosynthetic pathway that glycosylates ceramide, have been tested as therapeutics. Of these molecules, the one that has been most extensively developed for clinical use is miglustat (N-butyl-deoxynojirimycin). Miglustat has been approved for use in patients with Gaucher type 1 in Europe, the United States, and Israel. In Gaucher disease, substrate-reduction therapy is recommended only for adults when ERT is not a possibility or in combination with ERT. For the most part, this treatment modality is still experimental, and so far, results have been reported for only 209 patients enrolled in clinical trials using miglustat as a monotherapy. Of these patients, 108 had Gaucher type 1, 30 had Gaucher type 3, 41 had Niemann-Pick type C, and 30 had GM2 gangliosidosis.156 In these trials, clinical improvement was documented primarily in type 1 Gaucher disease. Although there have been some anecdotal stories of improvements in other LSDs, there are now ongoing clinical trials for late-onset Tay-Sachs and type 3 Gaucher disease.146
Pharmacologic chaperones are also under development as a potential therapy for some of the LSDs. Small-molecule chemical chaperones may be therapeutically useful for LSDs caused by specific mutant, yet catalytically active, enzymes. The hypothesized mode of action of these chaperones consists of reversible binding to the active site of a missense mutant enzyme, correcting protein misfolding and enhancing delivery to the lysosome. In the acidic lysosomal environment and in the presence of substrate, the chaperone would be released and the mutant enzyme would function better. This strategy is being tested in Fabry,157–159 type 1 Gaucher,160,161 Pompe,162,163 GM1 gangliosidosis,164 and Tay-Sachs and Sandhoff diseases.165 Large-scale screening of small-molecule libraries has been performed to identify compounds that might serve as improved chemical chaperones for Gaucher disease166 and Tay-Sachs disease, Sandhoff disease, and GM2 gangliosidosis.167
Because of the limited success of ERT in alleviating bone and CNS manifestations, and because of the risk of immune response after long-term treatment, another potential therapy under development is gene therapy. One of the major advantages of gene therapy is the potential for long-term expression of the therapeutic protein. The goal of gene therapy is to provide therapeutic levels of the deficient enzyme to all involved organ systems, which can be achieved by either ex vivo or direct in vivo gene-therapy strategies.168,169 The ex vivo strategy is to modify cells genetically by using a viral vector carrying the gene for the wild-type enzyme and then to transplant them into an affected patient, where they would express the enzyme and correct the deficiency. Hematopoietic progenitor cells are the primary therapeutic target, and retrovirus, adenovirus, adenovirus associated- and lentiviral vectors have been tested. Experiments using this method seemed promising in animals, but preliminary studies in patients with Gaucher disease demonstrated a poor response, probably because of the viral vector used.170 The in vivo approach is to inject a gene-transfer vector directly into a tissue or into the circulation. Studies have been performed in animal models of a number of LSDs, with injections administered in utero, neonatally, or in adult animals.169 Current research is focusing on resolving problems with transient expression of the therapeutic enzyme and severe immunoreaction to the foreign protein. Studies on direct injection into target tissues, including liver, muscle, and CNS, are also being conducted in animal models.168 Although proof-of-principal experiments in animal models have been promising for some LSDs, these therapies are not yet ready for clinical trials in patients.
CONCLUSIONS
The LSDs are rare diseases that are caused by deficient lysosomal enzyme activity or by a deficient lysosomal protein that interferes with enzyme activity. The accumulation of substrates within the lysosomes of cells is believed to contribute to the disease manifestations. Clinically, these disorders can have multiorgan presentations, resulting from different patterns of substrate accumulation. Because most patients appear normal at birth, it is likely that these disorders are underdiagnosed and their incidence is underestimated. This review highlights the different neonatal presentations of LSDs in order to enhance physician awareness of these disorders in the newborn. LSDs need to be considered in the differential diagnosis of many diverse neonatal symptoms. A high index of suspicion is essential, because treatment, when available, is most likely to be effective when begun early in the course of the disease. Prompt diagnosis may enable both early treatment to prevent irreversible clinical sequelae and timely genetic counseling.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health and National Human Genome Research Institute.
We acknowledge the clinical photographs of I-cell disease, which were graciously provided by H. Kayserili, MD, PhD, and A. Diliruba Aslander, MD (Medical Genetic Department, Istanbul Medical Faculty, Istanbul University, Turkey).
Abbreviations
- LSD
lysosomal storage disorder
- NIHF
nonimmune hydrops fetalis
- ERT
enzyme-replacement therapy
- MPS
mucopolysaccharidosis
- ISSD
infantile sialic acid storage disease
- CNS
central nervous system
- HCT
hematopoietic stem cell transplantation
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Stone DL, Sidransky E. Hydrops fetalis: lysosomal storage disorders in extremis. Adv Pediatr. 1999;46:409–440. [PubMed] [Google Scholar]
- 2.Fletcher JM. Screening for lysosomal storage disorders: a clinical perspective. J Inherit Metab Dis. 2006;29(2–3):405–408. doi: 10.1007/s10545-006-0246-7. [DOI] [PubMed] [Google Scholar]
- 3.Beaudet AL, Scriver CR, Sly WS, Valle D. Genetics, biochemistry and molecular basis of variant human phenotypes. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. McGraw-Hill; New York, NY: 1995. pp. 53–118. [Google Scholar]
- 4.Wraith JE. Lysosomal disorders. Semin Neonatol. 2002;7(1):75–83. doi: 10.1053/siny.2001.0088. [DOI] [PubMed] [Google Scholar]
- 5.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5(7):554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 6.Vellodi A. Lysosomal storage disorders. Br J Haematol. 2005;128(4):413–431. doi: 10.1111/j.1365-2141.2004.05293.x. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. McGraw-Hill; New York, NY: 1995. pp. 2641–2670. [Google Scholar]
- 8.Sakuraba H, Suzuki Y, Akagi M, Sakai M, Amano N. Beta-galactosidase-neuraminidase deficiency (galactosialidosis): clinical, pathological, and enzymatic studies in a postmortem case. Ann Neurol. 1983;13(5):497–503. doi: 10.1002/ana.410130505. [DOI] [PubMed] [Google Scholar]
- 9.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148(5):671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Howell RR, Byrne B, Darras BT, Kishnani P, Nicolino M, van der Ploeg A. Diagnostic challenges for Pompe disease: an under-recognized cause of floppy baby syndrome. Genet Med. 2006;8(5):289–296. doi: 10.1097/01.gim.0000204462.42910.b8. [DOI] [PubMed] [Google Scholar]
- 11.Verity MA. Infantile Pompe's disease, lipid storage, and partial carnitine deficiency. Muscle Nerve. 1991;14(5):435–440. doi: 10.1002/mus.880140509. [DOI] [PubMed] [Google Scholar]
- 12.Sarfati R, Hubert A, Dugue-Marechaud M, Biran-Mucignat V, Pierre F, Bonneau D. Prenatal diagnosis of Gaucher's disease type 2: ultrasonographic, biochemical and histological aspects. Prenat Diagn. 2000;20(4):340–343. doi: 10.1002/(sici)1097-0223(200004)20:4<340::aid-pd795>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Mignot C, Gelot A, Bessieres B, et al. Perinatal-lethal Gaucher disease. Am J Med Genet A. 2003;120A(3):338–344. doi: 10.1002/ajmg.a.20117. [DOI] [PubMed] [Google Scholar]
- 14.Eblan MJ, Goker-Alpan O, Sidransky E. Perinatal lethal Gaucher disease: a distinct phenotype along the neuronopathic continuum. Fetal Pediatr Pathol. 2005;24(4–5):205–222. doi: 10.1080/15227950500405296. [DOI] [PubMed] [Google Scholar]
- 15.Ida H, Rennert OM, Watabe K, Eto Y, Maekawa K. Pathological and biochemical studies of fetal Krabbe disease. Brain Dev. 1994;16(6):480–484. doi: 10.1016/0387-7604(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 16.Sahai I, Baris H, Kimonis V, Levy HL. Krabbe disease: severe neonatal presentation with a family history of multiple sclerosis. J Child Neurol. 2005;20(10):826–828. doi: 10.1177/08830738050200100901. [DOI] [PubMed] [Google Scholar]
- 17.Korn-Lubetzki I, Dor-Wollman T, Soffer D, Raas-Rothschild A, Hurvitz H, Nevo Y. Early peripheral nervous system manifestations of infantile Krabbe disease. Pediatr Neurol. 2003;28(2):115–118. doi: 10.1016/s0887-8994(02)00489-7. [DOI] [PubMed] [Google Scholar]
- 18.Clarke JT, Ozere RL, Krause VW. Early infantile variant of Krabbe globoid cell leucodystrophy with lung involvement. Arch Dis Child. 1981;56(8):640–642. doi: 10.1136/adc.56.8.640-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Güngör N, Coşkun T, Akçören Z, Cağlar M. I-cell disease: a case report and review of the literature. Turk J Pediatr. 1994;36(2):145–152. [PubMed] [Google Scholar]
- 20.Denis R, Wayenberg JL, Vermeulen M, et al. Hyperphosphatasemia in early diagnosed infantile GM1 gangliosidosis presenting as transient hydrops fetalis. Acta Clin Belg. 1996;51(5):320–327. doi: 10.1080/22953337.1996.11718526. [DOI] [PubMed] [Google Scholar]
- 21.Morrone A, Bardelli T, Donati MA, et al. Beta-galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum Mutat. 2000;15(4):354–366. doi: 10.1002/(SICI)1098-1004(200004)15:4<354::AID-HUMU8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Simpson MA, Cross H, Proukakis C, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36(11):1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- 23.Elleder M, Jerabkova M, Befekadu A, et al. Prosaposin deficiency: a rarely diagnosed, rapidly progressing, neonatal neurovisceral lipid storage disease. Report of a further patient. Neuropediatrics. 2005;36(3):171–180. doi: 10.1055/s-2005-865608. [DOI] [PubMed] [Google Scholar]
- 24.Burch M, Fensom AH, Jackson M, Pitts-Tucker T, Congdon PJ. Multiple sulphatase deficiency presenting at birth. Clin Genet. 1986;30(5):409–415. doi: 10.1111/j.1399-0004.1986.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 25.Santos RP, Hoo JJ. Difficulty in recognizing multiple sulfatase deficiency in an infant. Pediatrics. 2006;117(3):955–958. doi: 10.1542/peds.2005-1032. [DOI] [PubMed] [Google Scholar]
- 26.Inui K, Yanagihara K, Otani K, et al. A new variant neuropathic type of Gaucher's disease characterized by hydrocephalus, corneal opacities, deformed toes, and fibrous thickening of spleen and liver capsules. J Pediatr. 2001;138(1):137–139. doi: 10.1067/mpd.2001.109789. [DOI] [PubMed] [Google Scholar]
- 27.Tylki-Szymanska A, Lugowska A, Czartoryska B. Neuraminidase deficiency presenting as a nephrosialidosis: the first case detected in Poland. Acta Paediatr Jpn. 1996;38(5):529–532. doi: 10.1111/j.1442-200x.1996.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoyme HE, Jones KL, Higginbottom MC, O'Brien JS. Presentation of mucopolysaccharidosis VII (beta-glucuronidase deficiency) in infancy. J Med Genet. 1981;18(3):237–239. doi: 10.1136/jmg.18.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arda IS, Gençoğlu A, Coşkun M, Ozbek N, Demirhan B, Hiçsönmez A. A very unusual presentation of Niemann-Pick disease type B in an infant: similar findings to congenital lobar emphysema. Eur J Pediatr Surg. 2005;15(4):283–286. doi: 10.1055/s-2004-830362. [DOI] [PubMed] [Google Scholar]
- 30.Morisot C, Millat G, Coeslier A, et al. Fatal neonatal respiratory distress in Niemann-Pick C2 and prenatal diagnosis with mutations in gene HE1/NPC2 [in French]. Arch Pediatr. 2005;12(4):434–437. doi: 10.1016/j.arcped.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med. 2006;8(5):267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akcam M. Infantile Pompe's disease presenting with pulmonary infections during the newborn period. Saudi Med J. 2004;25(12):2022–2023. [PubMed] [Google Scholar]
- 33.Metzl JD, Elias ER, Berul CI. An interesting case of infant sudden death: severe hypertrophic cardiomyopathy in Pompe's disease. Pacing Clin Electrophysiol. 1999;22(5):821–822. doi: 10.1111/j.1540-8159.1999.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 34.Devi AR, Gopikrishna M, Ratheesh R, Savithri G, Swarnalata G, Bashyam M. Farber lipogranulomatosis: clinical and molecular genetic analysis reveals a novel mutation in an Indian family. J Hum Genet. 2006;51(9):811–814. doi: 10.1007/s10038-006-0019-z. [DOI] [PubMed] [Google Scholar]
- 35.Unger S, Paul DA, Nino MC, et al. Mucolipidosis II presenting as severe neonatal hyperparathyroidism. Eur J Pediatr. 2005;164(4):236–243. doi: 10.1007/s00431-004-1591-x. [DOI] [PubMed] [Google Scholar]
- 36.Patriquin HB, Kaplan P, Kind HP, Giedion A. Neonatal mucolipidosis II (I-cell disease): clinical and radiologic features in three cases. AJR Am J Roentgenol. 1977;129(1):37–43. doi: 10.2214/ajr.129.1.37. [DOI] [PubMed] [Google Scholar]
- 37.Cipolloni C, Boldrini A, Donti E, Maiorana A, Coppa GV. Neonatal mucolipidosis II (I-cell disease): clinical, radiological and biochemical studies in a case. Helv Paediatr Acta. 1980;35(1):85–95. [PubMed] [Google Scholar]
- 38.Babcock DS, Bove KE, Hug G, Dignan PS, Soukup S, Warren NS. Fetal mucolipidosis II (I-cell disease): radiologic and pathologic correlation. Pediatr Radiol. 1986;16(1):32–39. doi: 10.1007/BF02387502. [DOI] [PubMed] [Google Scholar]
- 39.Pazzaglia UE, Beluffi G, Danesino C, Frediani PV, Pagani G, Zatti G. Neonatal mucolipidosis 2: the spontaneous evolution of early bone lesions and the effect of vitamin D treatment—report of two cases. Pediatr Radiol. 1989;20(1–2):80–84. doi: 10.1007/BF02010640. [DOI] [PubMed] [Google Scholar]
- 40.Beck M, Barone R, Hoffmann R, et al. Inter- and intrafamilial variability in mucolipidosis II (I-cell disease). Clin Genet. 1995;47(4):191–199. doi: 10.1111/j.1399-0004.1995.tb03958.x. [DOI] [PubMed] [Google Scholar]
- 41.Herman TE, McAlister WH. Neonatal mucolipidosis II (I-cell disease) with dysharmonic epiphyseal ossification and butterfly vertebral body. J Perinatol. 1996;16(5):400–402. [PubMed] [Google Scholar]
- 42.Saul RA, Proud V, Taylor HA, Leroy JG, Spranger J. Prenatal mucolipidosis type II (I-cell disease) can present as Pacman dysplasia. Am J Med Genet A. 2005;135(3):328–332. doi: 10.1002/ajmg.a.30716. [DOI] [PubMed] [Google Scholar]
- 43.Sathasivam A, Garibaldi L, Murphy R, Ibrahim J. Transient neonatal hyperparathyroidism: a presenting feature of mucolipidosis type II. J Pediatr Endocrinol Metab. 2006;19(6):859–862. doi: 10.1515/jpem.2006.19.6.859. [DOI] [PubMed] [Google Scholar]
- 44.Oohira T, Nagata N, Akaboshi I, Matsuda I, Naito S. The infantile form of sialidosis type II associated with congenital adrenal hyperplasia: possible linkage between HLA and the neuraminidase deficiency gene. Hum Genet. 1985;70(4):341–343. doi: 10.1007/BF00295374. [DOI] [PubMed] [Google Scholar]
- 45.Kyllerman M, Mansson JE, Westphal O, Conradi N, Nellstrom H. Infantile galactosialidosis presenting with congenital adrenal hyperplasia and renal hypertension. Pediatr Neurol. 1993;9(4):318–322. doi: 10.1016/0887-8994(93)90073-l. [DOI] [PubMed] [Google Scholar]
- 46.Noori S, Acherman R, Siassi B, et al. A rare presentation of Pompe disease with massive hypertrophic cardiomyopathy at birth. J Perinat Med. 2002;30(6):517–521. doi: 10.1515/JPM.2002.081. [DOI] [PubMed] [Google Scholar]
- 47.Bulkley BH, Hutchins GM. Pompe's disease presenting as hypertrophic myocardiopathy with Wolff-Parkinson-White syndrome. Am Heart J. 1978;96(2):246–252. doi: 10.1016/0002-8703(78)90093-5. [DOI] [PubMed] [Google Scholar]
- 48.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81(6):349–372. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chabás A, Duque J, Gort L. A new infantile case of alpha-N-acetylgalactosaminidase deficiency: cardiomyopathy as a presenting symptom. J Inherit Metab Dis. 2007;30(1):108. doi: 10.1007/s10545-006-0470-1. [DOI] [PubMed] [Google Scholar]
- 50.Lee JE, Falk RE, Ng WG, Donnell GN. Beta-glucuronidase deficiency: a heterogeneous mucopolysaccharidosis. Am J Dis Child. 1985;139(1):57–59. doi: 10.1001/archpedi.1985.02140030059029. [DOI] [PubMed] [Google Scholar]
- 51.den Hollander NS, Kleijer WJ, Schoonderwaldt EM, Los FJ, Wladimiroff JW, Niermeijer MF. In-utero diagnosis of mucopolysaccharidosis type VII in a fetus with an enlarged nuchal translucency. Ultrasound Obstet Gynecol. 2000;16(1):87–90. doi: 10.1046/j.1469-0705.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 52.Arai Y, Edwards V, Takashima S, Becker LE. Vascular pathology in galactosialidosis. Ultrastruct Pathol. 1999;23(6):369–374. doi: 10.1080/019131299281338. [DOI] [PubMed] [Google Scholar]
- 53.Patel MS, Callahan JW, Zhang S, et al. Early-infantile galactosialidosis: prenatal presentation and postnatal followup. Am J Med Genet. 1999;85(1):38–47. [PubMed] [Google Scholar]
- 54.Gravel RA, Lowden JA, Callahan JW, Wolfe LS, Ng Yin Kin NM. Infantile sialidosis: a phenocopy of type 1 GM1 gangliosidosis distinguished by genetic complementation and uri-nary oligosaccharides. Am J Hum Genet. 1979;31(6):669–679. [PMC free article] [PubMed] [Google Scholar]
- 55.Finn LS, Zhang M, Chen SH, Scott CR. Severe type II Gaucher disease with ichthyosis, arthrogryposis and neuronal apoptosis: molecular and pathological analyses. Am J Med Genet. 2000;91(3):222–226. [PubMed] [Google Scholar]
- 56.Beaudet AL, DiFerrante NM, Ferry GD, Nichols BL, Jr, Mullins CE. Variation in the phenotypic expression of beta-glucuronidase deficiency. J Pediatr. 1975;86(3):388–394. doi: 10.1016/s0022-3476(75)80968-1. [DOI] [PubMed] [Google Scholar]
- 57.Gillett PM, Schreiber RA, Jevon GP, et al. Mucopolysaccharidosis type VII (Sly syndrome) presenting as neonatal cholestasis with hepatosplenomegaly. J Pediatr Gastroenterol Nutr. 2001;33(2):216–220. doi: 10.1097/00005176-200108000-00025. [DOI] [PubMed] [Google Scholar]
- 58.Nordborg C, Kyllerman M, Conradi N, Mansson JE. Early-infantile galactosialidosis with multiple brain infarctions: morphological, neuropathological and neurochemical findings. Acta Neuropathol (Berl) 1997;93(1):24–33. doi: 10.1007/s004010050579. [DOI] [PubMed] [Google Scholar]
- 59.Pueschel SM, O'Shea PA, Alroy J, et al. Infantile sialic acid storage disease associated with renal disease. Pediatr Neurol. 1988;4(4):207–212. doi: 10.1016/0887-8994(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 60.Harzer K, Paton BC, Poulos A, et al. Sphingolipid activator protein deficiency in a 16-week-old atypical Gaucher disease patient and his fetal sibling: biochemical signs of combined sphingolipidoses. Eur J Pediatr. 1989;149(1):31–39. doi: 10.1007/BF02024331. [DOI] [PubMed] [Google Scholar]
- 61.Hulková H, Cervenkova M, Ledvinova J, et al. A novel mutation in the coding region of the prosaposin gene leads to a complete deficiency of prosaposin and saposins, and is associated with a complex sphingolipidosis dominated by lactosylceramide accumulation. Hum Mol Genet. 2001;10(9):927–940. doi: 10.1093/hmg/10.9.927. [DOI] [PubMed] [Google Scholar]
- 62.Semeraro LA, Riely CA, Kolodny EH, Dickerson GR, Gryboski JD. Niemann-Pick variant lipidosis presenting as “neonatal hepatitis.”. J Pediatr Gastroenterol Nutr. 1986;5(3):492–500. doi: 10.1097/00005176-198605000-00030. [DOI] [PubMed] [Google Scholar]
- 63.Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD. Niemann-Pick disease type C: diagnosis and outcome in children, with particular reference to liver disease. J Pediatr. 1993;123(2):242–247. doi: 10.1016/s0022-3476(05)81695-6. [DOI] [PubMed] [Google Scholar]
- 64.Maconochie IK, Chong S, Mieli-Vergani G, Lake BD, Mowat AP. Fetal ascites: an unusual presentation of Niemann-Pick disease type C. Arch Dis Child. 1989;64(10 spec No):1391–1393. doi: 10.1136/adc.64.10_spec_no.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uno Y, Taniguchi A, Tanaka E. Histochemical studies in Wolman's disease: report of an autopsy case accompanied with a large amount of milky ascites. Acta Pathol Jpn. 1973;23(4):779–790. doi: 10.1111/j.1440-1827.1973.tb02775.x. [DOI] [PubMed] [Google Scholar]
- 66.Hoeg JM, Demosky SJ, Jr, Pescovitz OH, Brewer HB., Jr Cholesteryl ester storage disease and Wolman disease: phenotypic variants of lysosomal acid cholesteryl ester hydrolase deficiency. Am J Hum Genet. 1984;36(6):1190–1203. [PMC free article] [PubMed] [Google Scholar]
- 67.Perlmutter-Cremer N, Libert J, Vamos E, Spehl M, Liebaers I. Unusual early manifestation of multiple sulfatase deficiency [in English, French]. Ann Radiol (Paris) 1981;24(1):43–48. [PubMed] [Google Scholar]
- 68.Burk RD, Valle D, Thomas GH, et al. Early manifestations of multiple sulfatase deficiency. J Pediatr. 1984;104(4):574–578. doi: 10.1016/s0022-3476(84)80550-8. [DOI] [PubMed] [Google Scholar]
- 69.Lalwani SG, Kher A, Shridhar N, Bharucha BA, Naik GG. Mucolipidoses-II: a report of three cases. Indian J Pediatr. 1995;62(5):611–614. doi: 10.1007/BF02761891. [DOI] [PubMed] [Google Scholar]
- 70.Hochman JA, Treem WR, Dougherty F, Bentley RC. Mucolipidosis II (I-cell disease) presenting as neonatal cholestasis. J Inherit Metab Dis. 2001;24(5):603–604. doi: 10.1023/a:1012428113606. [DOI] [PubMed] [Google Scholar]
- 71.Barbier C, Devisme L, Dobbelaere D, Noizet O, Nelken B, Gottrand F. Neonatal cholestasis and infantile Gaucher disease: a case report. Acta Paediatr. 2002;91(12):1399–1401. doi: 10.1111/j.1651-2227.2002.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 72.Roth P, Sklower Brooks S, Potaznik D, Cooma R, Sahdev S. Neonatal Gaucher disease presenting as persistent thrombocytopenia. J Perinatol. 2005;25(5):356–358. doi: 10.1038/sj.jp.7211262. [DOI] [PubMed] [Google Scholar]
- 73.Adachi M, Wallace BJ, Schneck L, Volk BW. Fine structure of central nervous system in early infantile Gaucher's disease. Arch Pathol. 1967;83(6):513–526. [PubMed] [Google Scholar]
- 74.Sidransky E, Tayebi N, Stubblefield BK, et al. The clinical, molecular, and pathological characterisation of a family with two cases of lethal perinatal type 2 Gaucher disease. J Med Genet. 1996;33(2):132–136. doi: 10.1136/jmg.33.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tasso MJ, Martinez-Gutierrez A, Carrascosa C, Vazquez S, Tebar R. GM1-gangliosidosis presenting as nonimmune hydrops fetalis: a case report. J Perinat Med. 1996;24(5):445–449. doi: 10.1515/jpme.1996.24.5.445. [DOI] [PubMed] [Google Scholar]
- 76.Yuksel A, Kayserili H, Gungor F. Short femurs detected at 25 and 31 weeks of gestation diagnosed as Leroy I-cell disease in the postnatal period: a report of two cases. Fetal Diagn Ther. 2007;22(3):198–202. doi: 10.1159/000098717. [DOI] [PubMed] [Google Scholar]
- 77.Aynaci FM, Cakir E, Aynaci O. A case of I-cell disease (mucolipidosis II) presenting with craniosynostosis. Childs Nerv Syst. 2002;18(12):707–711. doi: 10.1007/s00381-002-0627-7. [DOI] [PubMed] [Google Scholar]
- 78.Sidransky E, Fartasch M, Lee RE, et al. Epidermal abnormalities may distinguish type 2 from type 1 and type 3 of Gaucher disease. Pediatr Res. 1996;39(1):134–141. doi: 10.1203/00006450-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 79.Lui K, Commens C, Choong R, Jaworski R. Collodion babies with Gaucher's disease. Arch Dis Child. 1988;63(7):854–856. doi: 10.1136/adc.63.7.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujimoto A, Tayebi N, Sidransky E. Congenital ichthyosis preceding neurologic symptoms in two sibs with type 2 Gaucher disease. Am J Med Genet. 1995;59(3):356–358. doi: 10.1002/ajmg.1320590315. [DOI] [PubMed] [Google Scholar]
- 81.Mau U, Kendziorra H, Kaiser P, Enders H. Restrictive dermopathy: report and review. Am J Med Genet. 1997;71(2):179–185. doi: 10.1002/(sici)1096-8628(19970808)71:2<179::aid-ajmg11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 82.Holleran WM, Ziegler SG, Goker-Alpan O, et al. Skin abnormalities as an early predictor of neurologic outcome in Gaucher disease. Clin Genet. 2006;69(4):355–357. doi: 10.1111/j.1399-0004.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 83.Rybojad M, Moraillon I, Ogier de Baulny H, Prigent F, Morel P. Extensive Mongolian spot related to Hurler disease [in French]. Ann Dermatol Venereol. 1999;126(1):35–37. [PubMed] [Google Scholar]
- 84.Sergi C, Beedgen B, Kopitz J, et al. Refractory congenital ascites as a manifestation of neonatal sialidosis: clinical, biochemical and morphological studies in a newborn Syrian male infant. Am J Perinatol. 1999;16(3):133–141. doi: 10.1055/s-2007-993847. [DOI] [PubMed] [Google Scholar]
- 85.Libert J, Van Hoof F, Farriaux JP, Toussaint D. Ocular findings in I-cell disease (mucolipidosis type II). Am J Ophthalmol. 1977;83(5):617–628. doi: 10.1016/0002-9394(77)90126-x. [DOI] [PubMed] [Google Scholar]
- 86.Tekinalp G, Aliefendioğlu D, Yüce A, Cağlar M, Beck M. A case with early infantile form of galactosialidosis with unusual haematological findings. J Inherit Metab Dis. 1999;22(5):668–669. doi: 10.1023/a:1005598517882. [DOI] [PubMed] [Google Scholar]
- 87.Hutchison AA, Drew JH, Yu VY, Williams ML, Fortune DW, Beischer NA. Nonimmunologic hydrops fetalis: a review of 61 cases. Obstet Gynecol. 1982;59(3):347–352. [PubMed] [Google Scholar]
- 88.Girgensohn H, Kellner H, Sudhof H. Congenital Gaucher's disease in erythroblastosis and vascular sclerosis [in German]. Klin Wochenschr. 1954;32(3–4):57–64. doi: 10.1007/BF01493524. [DOI] [PubMed] [Google Scholar]
- 89.Nicolaides KH, Warenski JC, Rodeck CH. The relationship of fetal plasma protein concentration and hemoglobin level to the development of hydrops in rhesus isoimmunization. Am J Obstet Gynecol. 1985;152(3):341–344. doi: 10.1016/s0002-9378(85)80224-6. [DOI] [PubMed] [Google Scholar]
- 90.Piraud M, Froissart R, Mandon G, Bernard A, Maire I. Amniotic fluid for screening of lysosomal storage diseases presenting in utero (mainly as non-immune hydrops fetalis). Clin Chim Acta. 1996;248(2):143–155. doi: 10.1016/0009-8981(95)06250-5. [DOI] [PubMed] [Google Scholar]
- 91.Burin MG, Scholz AP, Gus R, et al. Investigation of lysosomal storage diseases in nonimmune hydrops fetalis. Prenat Diagn. 2004;24(8):653–657. doi: 10.1002/pd.967. [DOI] [PubMed] [Google Scholar]
- 92.Kooper AJ, Janssens PM, de Groot AN, et al. Lysosomal storage diseases in non-immune hydrops fetalis pregnancies. Clin Chim Acta. 2006;371(1–2):176–182. doi: 10.1016/j.cca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Bouvier R, Maire I. Diagnosis of lysosomal storage diseases with fetal presentation [in French]. Ann Pathol. 1997;17(4):277–280. [PubMed] [Google Scholar]
- 94.Ginsburg SJ, Groll M. Hydrops fetalis due to infantile Gaucher's disease. J Pediatr. 1973;82(6):1046–1048. doi: 10.1016/s0022-3476(73)80441-x. [DOI] [PubMed] [Google Scholar]
- 95.Sun CC, Panny S, Combs J, Gutberlett R. Hydrops fetalis associated with Gaucher disease. Pathol Res Pract. 1984;179(1):101–104. doi: 10.1016/S0344-0338(84)80069-2. [DOI] [PubMed] [Google Scholar]
- 96.Sidransky E, Sherer DM, Ginns EI. Gaucher disease in the neonate: a distinct Gaucher phenotype is analogous to a mouse model created by targeted disruption of the glucocerebrosidase gene. Pediatr Res. 1992;32(4):494–498. doi: 10.1203/00006450-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 97.Sherer DM, Metlay LA, Sinkin RA, Mongeon C, Lee RE, Woods JR., Jr Congenital ichthyosis with restrictive dermopathy and Gaucher disease: a new syndrome with associated prenatal diagnostic and pathology findings. Obstet Gynecol. 1993;81(5 pt 2):842–844. [PubMed] [Google Scholar]
- 98.Strasberg PM, Skomorowski MA, Warren IB, Hilson WL, Callahan JW, Clarke JT. Homozygous presence of the crossover (fusion gene) mutation identified in a type II Gaucher disease fetus: is this analogous to the Gaucher knock-out mouse model? Biochem Med Metab Biol. 1994;53(1):16–21. doi: 10.1006/bmmb.1994.1052. [DOI] [PubMed] [Google Scholar]
- 99.Tayebi N, Cushner SR, Kleijer W, et al. Prenatal lethality of a homozygous null mutation in the human glucocerebrosidase gene. Am J Med Genet. 1997;73(1):41–47. doi: 10.1002/(sici)1096-8628(19971128)73:1<41::aid-ajmg9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 100.Beck M, Bender SW, Reiter HL, et al. Neuraminidase deficiency presenting as non-immune hydrops fetalis. Eur J Pediatr. 1984;143(2):135–139. doi: 10.1007/BF00445802. [DOI] [PubMed] [Google Scholar]
- 101.Gillan JE, Lowden JA, Gaskin K, Cutz E. Congenital ascites as a presenting sign of lysosomal storage disease. J Pediatr. 1984;104(2):225–231. doi: 10.1016/s0022-3476(84)80997-x. [DOI] [PubMed] [Google Scholar]
- 102.Guibaud P, Cottin X, Maire I, et al. Fetal ascites as a manifestation of infantile sialidosis. Significance of a study of oligosaccharides in amniotic fluid [in French]. J Genet Hum. 1985;33(3–4):317–324. [PubMed] [Google Scholar]
- 103.Schmidt M, Fahnenstich H, Haverkamp F, Platz H, Hansmann M, Bartmann P. Sialidosis and galactosialidosis as the cause of non-immunologic hydrops fetalis [in German]. Z Geburtshilfe Neonatol. 1997;201(5):177–180. [PubMed] [Google Scholar]
- 104.Ovali F, Samanci N, Guray A, et al. Congenital sialidosis. Turk J Pediatr. 1998;40(3):447–451. [PubMed] [Google Scholar]
- 105.Buchholz T, Molitor G, Lukong KE, et al. Clinical presentation of congenital sialidosis in a patient with a neuraminidase gene frameshift mutation. Eur J Pediatr. 2001;160(1):26–30. doi: 10.1007/pl00008412. [DOI] [PubMed] [Google Scholar]
- 106.Sergi C, Penzel R, Uhl J, et al. Prenatal diagnosis and fetal pathology in a Turkish family harboring a novel nonsense mutation in the lysosomal alpha-N-acetyl-neuraminidase (sialidase) gene. Hum Genet. 2001;109(4):421–428. doi: 10.1007/s004390100592. [DOI] [PubMed] [Google Scholar]
- 107.Pattison S, Pankarican M, Rupar CA, Graham FL, Igdoura SA. Five novel mutations in the lysosomal sialidase gene (NEU1) in type II sialidosis patients and assessment of their impact on enzyme activity and intracellular targeting using adenovirus-mediated expression. Hum Mutat. 2004;23(1):32–39. doi: 10.1002/humu.10278. [DOI] [PubMed] [Google Scholar]
- 108.Loren DJ, Campos Y, d'Azzo A, et al. Sialidosis presenting as severe nonimmune fetal hydrops is associated with two novel mutations in lysosomal alpha-neuraminidase. J Perinatol. 2005;25(7):491–494. doi: 10.1038/sj.jp.7211335. [DOI] [PubMed] [Google Scholar]
- 109.Landau D, Meisner I, Zeigler M, Bargal R, Shinwell ES. Hydrops fetalis in four siblings caused by galactosialidosis. Isr J Med Sci. 1995;31(5):321–322. [PubMed] [Google Scholar]
- 110.Haverkamp F, Jacobs D, Cantz M, Hansmann M, Fahnenstich H, Zerres K. Nonimmune hydrops fetalis with galactosialidosis: consequences for family planning. Fetal Diagn Ther. 1996;11(2):114–119. doi: 10.1159/000264289. [DOI] [PubMed] [Google Scholar]
- 111.Claeys M, Van der Hoeven M, de Die-Smulders C, et al. Early-infantile type of galactosialidosis as a cause of heart failure and neonatal ascites. J Inherit Metab Dis. 1999;22(5):666–667. doi: 10.1023/a:1005546501043. [DOI] [PubMed] [Google Scholar]
- 112.Lefebvre G, Wehbe G, Heron D, Vautjoer Brouzes D, Choukroun JB, Darbois Y. Recurrent nonimmune hydrops fetalis: a rare presentation of sialic acid storage disease. Genet Couns. 1999;10(3):277–284. [PubMed] [Google Scholar]
- 113.Lemyre E, Russo P, Melancon SB, Gagne R, Potier M, Lambert M. Clinical spectrum of infantile free sialic acid storage disease. Am J Med Genet. 1999;82(5):385–391. [PubMed] [Google Scholar]
- 114.Landau D, Cohen D, Shalev H, et al. A novel mutation in the SLC17A5 gene causing both severe and mild phenotypes of free sialic acid storage disease in one inbred Bedouin kindred. Mol Genet Metab. 2004;82(2):167–172. doi: 10.1016/j.ymgme.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Applegarth DA, Toone JR, Wilson RD, Yong SL, Baldwin VJ. Morquio disease presenting as hydrops fetalis and enzyme analysis of chorionic villus tissue in a subsequent pregnancy. Pediatr Pathol. 1987;7(5–6):593–599. doi: 10.3109/15513818709161423. [DOI] [PubMed] [Google Scholar]
- 116.Beck M, Braun S, Coerdt W, Merz E, Young E, Sewell AC. Fetal presentation of Morquio disease type A. Prenat Diagn. 1992;12(12):1019–1029. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- 117.Colmant C, Picone O, Froissart R, Labrune P, Senat MV. Second-trimester diagnosis of mucopolysaccharidosis type IV a presenting as hydrops fetalis. Prenat Diagn. 2006;26(8):750–752. doi: 10.1002/pd.1467. [DOI] [PubMed] [Google Scholar]
- 118.Nelson A, Peterson L, Frampton B, Sly WS. Mucopolysaccharidosis VII (beta-glucuronidase deficiency) presenting as nonimmune hydrops fetalis. J Pediatr. 1982;101(4):574–576. doi: 10.1016/s0022-3476(82)80707-5. [DOI] [PubMed] [Google Scholar]
- 119.Islam MR, Shah GN, Sly WS. PCR-based restriction fragment length polymorphism and haplotype of the most common mutation L176F in the beta-glucuronidase gene. Genet Test. 2007;11(1):72–74. doi: 10.1089/gte.2006.0505. [DOI] [PubMed] [Google Scholar]
- 120.Vervoort R, Islam MR, Sly WS, et al. Molecular analysis of patients with beta-glucuronidase deficiency presenting as hydrops fetalis or as early mucopolysaccharidosis VII. Am J Hum Genet. 1996;58(3):457–471. [PMC free article] [PubMed] [Google Scholar]
- 121.Abu-Dalu KI, Tamary H, Livni N, Rivkind AI, Yatziv S. GM1 gangliosidosis presenting as neonatal ascites. J Pediatr. 1982;100(6):940–943. doi: 10.1016/s0022-3476(82)80523-4. [DOI] [PubMed] [Google Scholar]
- 122.Vajro P, Strisciuglio P, Fontanella A, De Vincenzo A. Neonatal ascites disclosing GM1 gangliosidosis [in French]. Arch Fr Pediatr. 1990;47(7):544. [PubMed] [Google Scholar]
- 123.Sinelli MT, Motta M, Cattarelli D, Cardone ML, Chirico G. Fetal hydrops in GM(1) gangliosidosis: a case report. Acta Paediatr. 2005;94(12):1847–1849. doi: 10.1111/j.1651-2227.2005.tb01867.x. [DOI] [PubMed] [Google Scholar]
- 124.Meizner I, Levy A, Carmi R, Robinsin C. Niemann-Pick disease associated with nonimmune hydrops fetalis. Am J Obstet Gynecol. 1990;163(1 pt 1):128–129. doi: 10.1016/s0002-9378(11)90685-1. [DOI] [PubMed] [Google Scholar]
- 125.Ben-Haroush A, Yogev Y, Levit O, Hod M, Kaplan B. Isolated fetal ascites caused by Wolman disease. Ultrasound Obstet Gynecol. 2003;21(3):297–298. doi: 10.1002/uog.73. [DOI] [PubMed] [Google Scholar]
- 126.Kattner E, Schafer A, Harzer K. Hydrops fetalis: manifestation in lysosomal storage diseases including Farber disease. Eur J Pediatr. 1997;156(4):292–295. doi: 10.1007/s004310050603. [DOI] [PubMed] [Google Scholar]
- 127.Roberts DJ, Ampola MG, Lage JM. Diagnosis of unsuspected fetal metabolic storage disease by routine placental examination. Pediatr Pathol. 1991;11(4):647–656. doi: 10.3109/15513819109064796. [DOI] [PubMed] [Google Scholar]
- 128.Nelson J, Kenny B, O'Hara D, Harper A, Broadhead D. Foamy changes of placental cells in probable beta glucuronidase deficiency associated with hydrops fetalis. J Clin Pathol. 1993;46(4):370–371. doi: 10.1136/jcp.46.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Molyneux AJ, Blair E, Coleman N, Daish P. Mucopolysaccharidosis type VII associated with hydrops fetalis: histopathological and ultrastructural features with genetic implications. J Clin Pathol. 1997;50(3):252–254. doi: 10.1136/jcp.50.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hale LP, van de Ven CJ, Wenger DA, Bradford WD, Kahler SG. Infantile sialic acid storage disease: a rare cause of cytoplasmic vacuolation in pediatric patients. Pediatr Pathol Lab Med. 1995;15(3):443–453. doi: 10.3109/15513819509026980. [DOI] [PubMed] [Google Scholar]
- 131.Froissart R, Cheillan D, Bouvier R, et al. Clinical, morphological, and molecular aspects of sialic acid storage disease manifesting in utero. J Med Genet. 2005;42(11):829–836. doi: 10.1136/jmg.2004.029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soma H, Yamada K, Osawa H, Hata T, Oguro T, Kudo M. Identification of Gaucher cells in the chorionic villi associated with recurrent hydrops fetalis. Placenta. 2000;21(4):412–416. doi: 10.1053/plac.1999.0483. [DOI] [PubMed] [Google Scholar]
- 133.Groener J, Maaswinkel-Mooy P, Smit V, et al. New mutations in two Dutch patients with early infantile galactosialidosis. Mol Genet Metab. 2003;78(3):222–228. doi: 10.1016/s1096-7192(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 134.Vedder AC, Strijland A, vd Bergh Weerman MA, Florquin S, Aerts JM, Hollak CE. Manifestations of Fabry disease in placental tissue. J Inherit Metab Dis. 2006;29(1):106–111. doi: 10.1007/s10545-006-0196-0. [DOI] [PubMed] [Google Scholar]
- 135.Venkat-Raman N, Sebire NJ, Murphy KW. Recurrent fetal hydrops due to mucopolysaccharidoses type VII. Fetal Diagn Ther. 2006;21(3):250–254. doi: 10.1159/000091350. [DOI] [PubMed] [Google Scholar]
- 136.Manning DJ, Price WI, Pearse RG. Fetal ascites: an unusual presentation of Niemann-Pick disease type C. Arch Dis Child. 1990;65(3):335–336. doi: 10.1136/adc.65.3.335-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meikle PJ, Hopwood JJ. Lysosomal storage disorders: emerging therapeutic options require early diagnosis. Eur J Pediatr. 2003;162(suppl 1):S34–S37. doi: 10.1007/s00431-003-1348-y. [DOI] [PubMed] [Google Scholar]
- 138.Meikle PJ, Fietz MJ, Hopwood JJ. Diagnosis of lysosomal storage disorders: current techniques and future directions. Expert Rev Mol Diagn. 2004;4(5):677–691. doi: 10.1586/14737159.4.5.677. [DOI] [PubMed] [Google Scholar]
- 139.Harzer K, Cantz M, Sewell AC, et al. Normomorphic sialidosis in two female adults with severe neurologic disease and without sialyl oligosacchariduria. Hum Genet. 1986;74(3):209–214. doi: 10.1007/BF00282535. [DOI] [PubMed] [Google Scholar]
- 140.Peelen GO, de Jong JG, Wevers RA. HPLC analysis of oligosaccharides in urine from oligosaccharidosis patients. Clin Chem. 1994;40(6):914–921. [PubMed] [Google Scholar]
- 141.Harzer K, Rolfs A, Bauer P, et al. Niemann-Pick disease type A and B are clinically but also enzymatically heterogeneous: pitfall in the laboratory diagnosis of sphingomyelinase deficiency associated with the mutation Q292 K. Neuropediatrics. 2003;34(6):301–306. doi: 10.1055/s-2003-44668. [DOI] [PubMed] [Google Scholar]
- 142.Brooks DA, McCourt PA, Gibson GJ, Ashton LJ, Shutter M, Hopwood JJ. Analysis of N-acetylgalactosamine-4-sulfatase protein and kinetics in mucopolysaccharidosis type VI patients. Am J Hum Genet. 1991;48(4):710–719. [PMC free article] [PubMed] [Google Scholar]
- 143.Umapathysivam K, Hopwood JJ, Meikle PJ. Determination of acid alpha-glucosidase activity in blood spots as a diagnostic test for Pompe disease. Clin Chem. 2001;47(8):1378–1383. [PubMed] [Google Scholar]
- 144.Carpenter KH, Wiley V. Application of tandem mass spectrometry to biochemical genetics and newborn screening. Clin Chim Acta. 2002;322(1–2):1–10. doi: 10.1016/s0009-8981(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 145.Chace DH, Kalas TA, Naylor EW. The application of tandem mass spectrometry to neonatal screening for inherited disorders of intermediary metabolism. Annu Rev Genomics Hum Genet. 2002;3:17–45. doi: 10.1146/annurev.genom.3.022502.103213. [DOI] [PubMed] [Google Scholar]
- 146.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum Genet. 2007;121(1):1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 147.Malatack JJ, Consolini DM, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr Neurol. 2003;29(5):391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 148.Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immunopathol. 2004;26(1–2):119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- 149.Boelens JJ. Trends in haematopoietic cell transplantation for inborn errors of metabolism. J Inherit Metab Dis. 2006;29(2–3):413–420. doi: 10.1007/s10545-005-0258-8. [DOI] [PubMed] [Google Scholar]
- 150.Orchard PJ, Blazar BR, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151(4):340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 151.Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31(4):229–239. doi: 10.1038/sj.bmt.1703839. [DOI] [PubMed] [Google Scholar]
- 152.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350(19):1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 153.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352(20):2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 154.Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002;30(4):215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 155.Müller I, Kustermann-Kuhn B, Holzwarth C, et al. In vitro analysis of multipotent mesenchymal stromal cells as potential cellular therapeutics in neurometabolic diseases in pediatric patients. Exp Hematol. 2006;34(10):1413–1419. doi: 10.1016/j.exphem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 156.Butters TD. Pharmacotherapeutic strategies using small molecules for the treatment of glycolipid lysosomal storage disorders. Expert Opin Pharmacother. 2007;8(4):427–435. doi: 10.1517/14656566.8.4.427. [DOI] [PubMed] [Google Scholar]
- 157.Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290(4):C1076–C1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- 158.Fan JQ, Ishii S. Active-site-specific chaperone therapy for Fabry disease: yin and yang of enzyme inhibitors. FEBS J. 2007;274(19):4962–4971. doi: 10.1111/j.1742-4658.2007.06041.x. [DOI] [PubMed] [Google Scholar]
- 159.Hollak CE, Vedder AC, Linthorst GE, Aerts JM. Novel therapeutic targets for the treatment of Fabry disease. Expert Opin Ther Targets. 2007;11(6):821–833. doi: 10.1517/14728222.11.6.821. [DOI] [PubMed] [Google Scholar]
- 160.Butters TD. Gaucher disease. Curr Opin Chem Biol. 2007;11(4):412–418. doi: 10.1016/j.cbpa.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 161.Yu Z, Sawkar AR, Kelly JW. Pharmacologic chaperoning as a strategy to treat Gaucher disease. FEBS J. 2007;274(19):4944–4950. doi: 10.1111/j.1742-4658.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 162.Okumiya T, Kroos MA, Vliet LV, Takeuchi H, Van der Ploeg AT, Reuser AJ. Chemical chaperones improve transport and enhance stability of mutant alpha-glucosidases in glycogen storage disease type II. Mol Genet Metab. 2007;90(1):49–57. doi: 10.1016/j.ymgme.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 163.Parenti G, Zuppaldi A, Gabriela Pittis M, et al. Pharmacological enhancement of mutated alpha-glucosidase activity in fibroblasts from patients with Pompe disease. Mol Ther. 2007;15(3):508–514. doi: 10.1038/sj.mt.6300074. [DOI] [PubMed] [Google Scholar]
- 164.Iwasaki H, Watanabe H, Iida M, et al. Fibroblast screening for chaperone therapy in beta-galactosidosis. Brain Dev. 2006;28(8):482–486. doi: 10.1016/j.braindev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 165.Tropak MB, Reid SP, Guiral M, Withers SG, Mahuran D. Pharmacological enhancement of beta-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff patients. J Biol Chem. 2004;279(14):13478–13487. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zheng W, Padia J, Urban DJ, et al. Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A. 2007;104(32):13192–13197. doi: 10.1073/pnas.0705637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tropak MB, Blanchard JE, Withers SG, Brown ED, Mahuran D. High-throughput screening for human lysosomal beta-N-acetyl hexosaminidase inhibitors acting as pharmacological chaperones. Chem Biol. 2007;14(2):153–164. doi: 10.1016/j.chembiol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther. 2006;6(2):227–241. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- 169.Sands MS, Davidson BL. Gene therapy for lysosomal storage diseases. Mol Ther. 2006;13(5):839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 170.Dunbar CE, Kohn DB, Schiffmann R, et al. Retroviral transfer of the glucocerebrosidase gene into CD34+ cells from patients with Gaucher disease: in vivo detection of transduced cells without myeloablation. Hum Gene Ther. 1998;9(17):2629–2640. doi: 10.1089/hum.1998.9.17-2629. [DOI] [PubMed] [Google Scholar]