Abstract
Silver in various forms has long been recognized for antimicrobial properties, both in biomedical devices and in eyes. However, soluble drugs used on the ocular surface are rapidly cleared through tear ducts and eventually ingested, resulting in decreased efficacy of the drug on its target tissue and potential concern for systemic side effects. Silver nanoparticles were studied as a source of anti-microbial silver for possible controlled-release contact lens controlled delivery formulations. Silver ion release over a period of several weeks from nanoparticle sources of various sizes and doses in vitro was evaluated in vitro against Pseudomonas aeruginosa strain PA01. Mammalian cell viability and cytokine expression in response to silver nanoparticle exposure is evaluated using corneal epithelial cells and eye-associated macrophages cultured in vitro in serum-free media. Minimal microcidal and cell toxic effects were observed for several silver nanoparticle suspensions and aqueous extraction times for bulk total silver concentrations commensurate with comparative silver ion (e.g., Ag+(aq)) toxicity. This indicates that (1) silver particles themselves are not microcidal under conditions tested, and (2) insufficient silver ion is generated from these particles at these loadings to produce observable biological effects in these in vitro assays. If dosing allows substantially increased silver particle loading in the lens, the bactericidal efficacy of silver nanoparticles in vitro is one possible approach to limiting bacterial colonization problems associated with extended-wear contact lenses.
Keywords: silver, nanoparticle, cornea, bacteria, macrophage, antimicrobial, contact lens, infection
Introduction
Increased continuous and extended-wear contact lens use and on-eye lens wear duration have both exacerbated eye infection risk. Silicone hydrogel continuous wear (CW, defined as 30 days of continuous contact lens use) lenses have an incidence of microbial keratitis (MK) of 0.14% of eyes1 and MK incidence for extended wear (EW, defined as 6 to 7 days of continuous contact lens use) are 0.2% of patients and 0.27% of eyes for soft and disposable soft lens types worn as EW, respectively.1,2 Daily wear lenses (defined as lenses removed and replaced daily) certainly decrease MK risk substantially, with incidences ranging from 0.011% to 0.035% of patients for rigid gas permeable (RGP) and soft lenses, respectively.2 While not as serious, the incidence of Contact Lens-Induced Acute Red Eye (CLARE) infection is quite staggering at 1.4-6.2% of patients.3 With nearly a hundred million contact lenses used and consumed per year, this infection incidence from CLARE and MK produce considerable cost and morbidity, and limit further abilities to extend lens wear duration.
Strategies for controlling lens-based infection have focused on improved lens materials, designs and incorporating anti-microbial properties. Improved materials science in lens fabrication is limited by a combination of factors, including long-recognized tear film fouling of the lens surface, and resulting bacterial contamination.4-8 Bacterial contamination can also be controlled by using antimicrobial agents in contact lens materials, cleaning solutions, topical eye drops and storage components. In this regard, several antiseptics and antibiotics have been used for treating ocular infections, both with and without contact lens-associated infections, for many years.9-12 The recent advent of a new class of FDA-approvable biomedical devices -- combination devices – that incorporate drug releasing strategies into prosthetic devices13 now provides new opportunities to build more sophisticated anti-microbial approaches into contact lenses for extended wear use. This includes controlled release technology directly from the lens to mitigate bacterial contamination locally on-eye.
Silver is well known for its antimicrobial activity in a variety of physiological settings. Silver ions (Ag+(aq)) are generally recognized as the bioactive agent, supplied for clinical applications from numerous silver-containing formulations comprising silver salts, silver oxide, metallic silver, silver chelates, and silver particles.14-16 Silver ions form metal-organic complexes and insoluble compounds with sulfhydryl groups (e.g., cysteine residues) in cell walls of bacteria and fungi, generally inactivating essential enzymes responsible for energy metabolism and electron transport. Silver ions also block the electron transport chain functions most sensitively between cytochrome reductase and cytochrome oxidase, and less sensitively between NADH and succinate dehydrogenase.17 Silver may also exhibit many other less-specific biological effects, producing variable toxicity in both pathogens and mammalian cells.
Antimicrobial activity of silver ions is observed at concentrations ranging from nM to ∼ 5 μM, while mammalian macrophage/monocyte cellular toxicity is observed at concentrations approaching 12 μM in serum-containing solutions.18-20 Several biomedical devices releasing active silver antimicrobial agents are currently FDA-approved. The ARROWgard® catheter, a chlorhexidine-silver sulfadiazine-coated (CH-SS) catheter, has shown efficacy in reducing catheter-related blood stream infections (CRBSI) in high-risk patients21. Another catheter-related device is the silver-impregnated cuff (VITACUFF®, Integra Lifesciences, Inc.) placed around percutaneous catheters and producing significant reduction in colonization and incidence of CRBSI.22 Actisorb® Silver 220 (Ethicon, Inc.), an anti-microbial charcoal wound dressing containing 33μg of silver per square centimeter of wound closure fabric has shown good antibacterial activity against Staphylococcus aureus and vancomycin-resistant Enterococci versus wound dressings coated with antibiotics.23 Because of its relatively rich regulatory history as a device-released antibiotic (i.e., from combination devices13), broad therapeutic index, proven therapeutic efficacy, and minimal reports of resistance mechanisms24, silver is an attractive antimicrobial candidate to release from extended wear (EW) contact lens formulations. Possible adverse effects of locally released silver include argyria, the graying of tissue associated with silver ion exposure to light, increased incidence of bacterial resistance to silver via efflux pumps24, lack of efficacy due to dose formulation issues, and local cell toxicity. Development of a silver-based antibiotic contact lens formulation requires sustained release of silver from the contact lens material sufficient to inhibit bacterial proliferation, while having minimal adverse effects on ocular cells.25-29
This work investigates silver nanoparticles as a source of silver ion biocide and other killing mechanisms against a prominent contact lens-associated bacterium, P. aeruginosa, as well as effects on human corneal epithelial and murine macrophage cells in vitro. Silver antimicrobial effects in eye infections must effectively kill pathogens without adverse effects to endogenous host cells. Two operating mechanistic hypotheses for silver-induced bactericidal and ocular cell toxicity effects were investigated in these studies: (1) that these effects could be induced from production of silver ions from nanoparticle dissolution into the culture media, or (2) that silver nanoparticles themselves were intrinsically microcidal (as recently reported48,49), and also activating to mammalian cells. Results indicate that relatively high silver nanoparticle loading will be required to generate continuous silver ion fluxes on-eye sufficient to reliably kill P. aeruginosa while avoiding toxicity to both corneal epithelial cells and ocular macrophages.
Materials and Methods
Materials
Silver nanoparticles (Ted Pella, Redding, CA, 20 nm, 40 nm, 60 nm diameters supplied as aqueous suspensions at 7.0 × 1011, 9.0 × 1010 and 2.6 × 1010 particles/mL, respectively, diameter %CV = 8%) were used as received and diluted into media as described. Lipopolysaccharide (LPS) was purchased from Sigma Chemical (MO, USA).
Cell culture
Murine RAW264.7 monocyte/macrophage cell line (ATCC TIB-71, ATCC, Manassas, VA) and human HCE-T corneal epithelial cells (gift from CibaVision, Norcross, GA) were cultured in Ultraculture Serum-Free (UCSF) media supplemented with 200mM L-glutamine (Cambrex, North Brunswick, NJ). HCE-T cells do not contact inhibit in culture (unpublished personal communication, A. Wright, CibaVision, GA, USA). Cell cultures were maintained in T175 tissue culture polystyrene (TCPS) flasks (Nunc™, Rochester, NY) under standard conditions: incubation at 37°C, 98% humidity and 5% CO2. RAW264.7 cells were dissociated from culture flasks by incubation with Ca2+- and Mg2+- free cell culture grade Hank's balanced salt solution (HBSS, Life Technologies). All RAW264.7 subcultures were used prior to passage 15 as received from ATCC.53 HCE cells were used between five and fifteen passages, and routinely passaged using 0.25% trypsin/0.1% EDTA (Mediatech, Herndon, VA). Cell concentration and viability were assessed using trypan blue dye exclusion (BioWhittaker) and a hemacytometer. For silver toxicity assessments, both cell types were plated onto T25 TCPS flasks (Falcon®, Becton Dickinson) in Ultraculture Serum-Free (UCSF) media supplemented with 200mM L-glutamine (Cambrex, North Brunswick, NJ) to eliminate serum binding of silver ions and serum-induced aggregation of nanoparticles, and incubated at 37°C with 5% CO2.
Mammalian cell toxicity assays
Both RAW264.7 and HCE-T cell lines were seeded at 2.5×104 per T-25 cm2 flask (Nunc™) in UCSF media with 2μM, 4μM, or 6 μM of silver nitrate (stock 1 mg/mL ∼ 1000ppm, Sigma-Aldrich, St. Louis, MO) or 2μM, 4μM, or 6 μM silver nanoparticle solutions (40nm diameter, 9 ×1010 particles per mL, Ted Pella, Redding, CA) against control flasks containing only media. The molarity of silver nanoparticle solutions was determined from the manufacturer's specification that each colloid contained 0.001% silver or 10 ppm. This was then calculated into a molar silver amounts and this value served to compare the nanoparticle solutions to the soluble silver nitrate control solutions. Flasks were run in triplicate for each experimental group. Cell culture supernatant samples were collected at specified time points (1, 2, and 3 weeks) after seeding to determine the effects of the two forms of silver on cell viability. Supernatants were frozen at -70°C until used. After week 1, cells were trypsinized with 0.25% trypsin/0.1% EDTA (Mediatech, Herndon, VA) and counted using an Improved Neubauer Hemacytometer. Cells from each flask were then seeded into new culture flasks at original starting cell densities with new silver doses added. This was repeated both at weeks 2 and 3. Cell counts over time served as a method of quantifying cell viability and as reference for ToxiLight® bioluminescence assay (Cambrex, North Brunswick, NJ) that directly measures cell toxicity based on adenylate kinase release from damaged cell membranes.30-32
Silver bactericidal efficacy assays
Pseudomonas aeruginosa strain PA01 (gift of Prof. H. Schweizer, Colorado State University) was used as a model organism associated with bacterial eye infections.4 PA01 culture was initiated from frozen stocks and inoculated in UCSF media supplemented with 200 mM L-glutamine and incubated at 37°C while shaking. After 16-24 hours, liquid cultures were spread on Luria Bertani agar (Difco, Franklin Lakes, NJ) plates to obtain isolated colonies. Plates were incubated at 37° C for 16-24 hours. Individual PA01 colonies were picked using the BBL™ Prompt™ system (BD, Franklin Lakes, NJ) to rapidly inoculate 1 mL of 0.85% saline to a 0.5 McFarland standard (1.5×108 CFU/mL) – the accuracy of this inoculum was concurrently determined by serial dilution colony enumeration after 24 hours.
Media preparation for silver nanoparticle-bacterial interactions in vitro
Solutions of Ultraculture SF containing various concentrations (2, 4, 6, 10 μM based on nanoparticle concentrations and the % silver supplied as particles as explained above to produce a total silver concentration indicated) of 40nm silver nanoparticles were prepared at five-day intervals, twenty days prior to inoculation with P. aeruginosa. The aim of this experiment was to allow silver nanoparticle dissolution to soluble silver ions to achieve a 20-day dissolution range over time. These solutions were then used to represent different concentrations of silver ion derived from time-dependent particle dissolution in the cell culture media and added directly to bacterial cultures to assess ion killing efficacy. Silver ion concentrations in these samples were assessed using a Thermo Jarrell Ash IRIS Advantage Dual View High Resolution Inductively Coupled Plasma Optical Emission spectrometer (silver ion detection limit ∼ 0.01 mg/L ∼ 100ppb, variability ±0.005 mg/L).
On Day Twenty after media preparation, a BBL™ Prompt™ tube containing 1.5×108 CFU/mL P. aeruginosa was inoculated to approximately 1×105 CFU/mL in Erlenmeyer flasks containing 20 mL Ultraculture SF media containing silver nanoparticles. Also, on Day Twenty, solutions of 20 mL Ultraculture SF media containing various concentrations (2, 4, 6, 10 μM) of control silver nitrate (i.e., soluble salt) were inoculated to identical samples (1×105 CFU/mL) of bacteria, as were untreated control flasks containing only Ultraculture SF media. Flasks were incubated overnight at 37°C while shaking at 200 rpm.
Bacterial viability assays
After overnight incubation of bacteria with silver nanoparticles, silver nitrate doses or no silver treatment, all samples were serially diluted and plated on Luria Bertani agar for direct colony counting, while the remainder of the sample was tested for bacterial viability using the Live/Dead BacLight™ fluorescence kit (Molecular Probes-Invitrogen, Carlsbad, CA). Silver-containing samples (20 mL) were centrifuged at 10,000 × g, washed in 10 mL 0.85% (w/v, 8.5 mg/ml) saline (NaCl) and then centrifuged and resuspended again. These cultures were then adjusted to 1.5×108 CFU/mL. Likewise, untreated control flasks was centrifuged and resuspended in 2 mL 0.85% (w/v) saline. Equal volumes of the control culture were then transferred to either a 50 mL conical tube containing 20 mL 0.85% saline or to 20 mL 70% ethanol. These were both incubated at room temperature for 1 hour to ensure complete bacterial death in 70% ethanol. Both control samples were then centrifuged at 10,000 × g, and washed in 20 mL saline to remove traces of 70% ethanol in the ‘dead’ culture. The samples were then centrifuged again at 10,000 × g, and resuspended in a final volume of 10 mL saline. Both Live and Dead™ assay standards were diluted to a concentration of 1.5×108 CFU/mL. A standard curve for Live/Dead assay was prepared by mixing known proportions of these live and dead bacterial solutions.
The BacLight™ Live/Dead staining solution was prepared by mixing equal parts of Syto 9 and propidium iodide to 2 mL ASTM Type I purified water. Syto 9 stains all bacteria, live or dead, green. Bacteria showing signs of cell death (compromised membrane) allow propidium iodide to reduce Syto 9, labeling these bacteria red. Samples of all silver-containing bacterial cultures (100 μL) were added to the wells of a 96-well microtiter plate (Black F96 MicroWell™ Plates, Nunc, Rochester, NY) in duplicate, and standard curve samples were plated in triplicate. Samples (100 μL) of the 2× dye mixture were then added to these wells. After a 15-minute incubation at room temperature in dark conditions, samples were read using a fluorescent microplate reader (Tecan GENios) with excitation/emission filters set at 485/530nm and 485/630nm for Syto9 and propidium iodide dye, respectively. Spectral ratios of the observed fluorescence intensities in the green (shorter) and red (longer) wavelengths were used to assess bacterial viabilities and thereby compare the efficacy of silver ion as a biocide from sample to sample and to the colony enumeration assay.
Macrophage corneal cell co-culture activation assays
RAW264.7 cells (1×105 in Ultraculture SF media) (between five to fifteen passages) were seeded into the bottom of 6-well TCPS plates (Multidish 6, Nunc, Rochester, NY) and HCE-T cells (1×105 in Ultraculture SF media) (between five to fifteen passages) were seeded onto 0.2μm pore size Anopore membrane inserts (Nunc, Rochester, NY) set into the 6-well plates containing RAW264.7 cells. Co-cultures were allowed to incubate overnight at 37°C in 5% CO2. The media was refreshed the next day and either 1μg/mL lipopolysaccharide (LPS, positive cell activation control) or 1×1010 silver nanoparticles/mL of 20-, 40-, or 60-nm diameter silver nanoparticles were added to the wells. Nanoparticle concentration was delivered to the media in a common volume of water for all particle sizes. Untreated control, LPS-treated positive control, and nanoparticle-treated cells were lifted with 0.25% trypsin/0.1% EDTA (Mediatech, Herndon, VA) and counted at 4, 8, 12, and 24-hour culture time points. These experiments were run in triplicate and cell culture media supernatant samples collected at these time points and frozen at -70°C until their ELISA analysis.
Immunoassay (ELISA) analysis of cell culture media samples
Possible silver nanoparticle-induced activation of both cell types was studied by measuring the expression of specific cytokines using immunoassay (ELISA) analysis (OptEIA™, BD, Franklin Lakes, NJ) for human IL-1β, IL-4, IL-6, IL-8 in the human corneal epithelial cell line (HCE-T) and murine IL-1β, IL-4 and IL-6 in the murine macrophage line (RAW264.7). Microwell plate optical density was measured in a Tecan GENios instrument at 450 nm.
Endotoxin assay
Plasticware and ASTM Type I filtered water were tested for the presence of contaminating endotoxin using a Pyrogene™ Assay kit (Cambrex), and endotoxin levels were determined to be below the kit detection limit (0.02 EU/ml) (data not shown).
Statistical analyses
Determinations of statistical significance between various data relied on a Student's T test.
Results
Mammalian cell toxicity to silver in serum-free culture
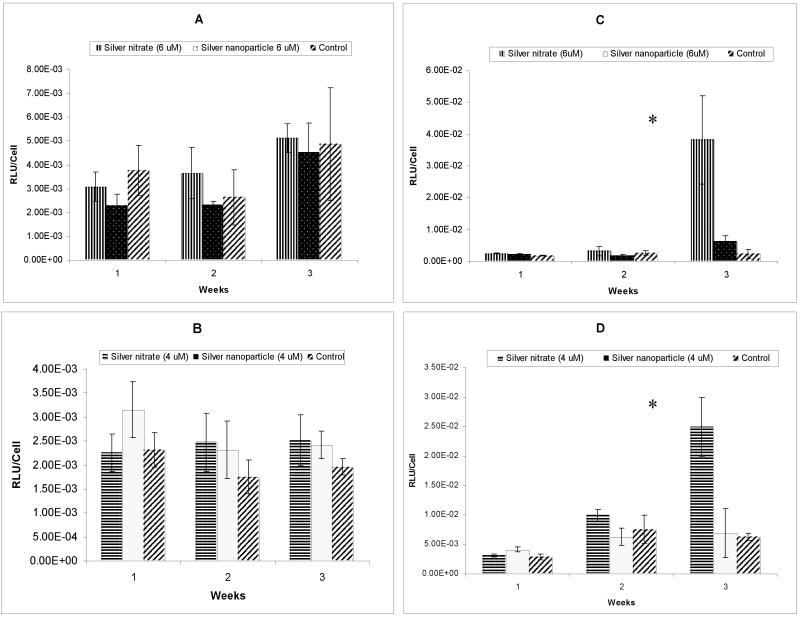
Cells cultured throughout three-week exposures to silver, in the form of either silver nitrate or silver nanoparticles, were assayed for the first sign of cell membrane damage by a non-destructive assay measuring adenylate kinase in the supernatant. Specifically, supernatant samples were frozen for future analysis, expanded cultured cells were lifted and reseeded at the original starting concentration (1×105 cells/well in Ultraculture SF media) in either fresh media containing (1) different concentrations of silver nitrate, (2) different concentrations of 40nm diameter silver nanoparticles or (3) no supplement addition (control). Silver nitrate (a water-soluble salt) convincingly killed both mammalian cell lines at concentrations of 8 μM and 10 μM in serum-free media, evidenced by cell monolayers lifting off of tissue culture surfaces (data not shown). This is consistent with previous reports of silver nitrate-induced cell toxicity20. Nanoparticle-induced cell toxicity data are shown in Figure 1: RAW264.7 macrophages consistently demonstrated less toxicity than the human corneal epithelial cell line. Notably, silver nanoparticles showed no significant impact on cell toxicity for either cell line, at any dosing, versus untreated controls.
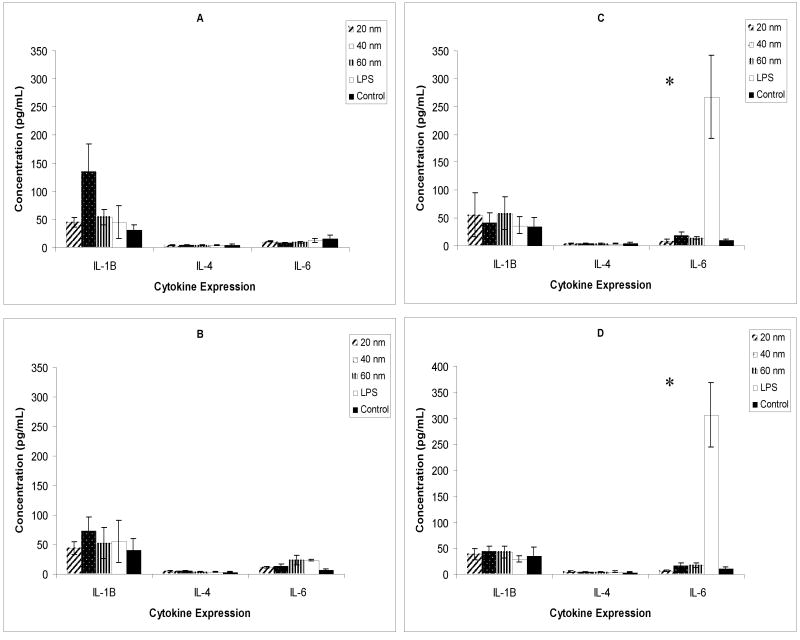
Figure 1.
Assay of adenylate kinase release from damaged mammalian cell membranes. Results from 6 μM silver treatment on murine RAW264.7 and human HCE-T cells (A & C) and 4 μM silver treatment on RAW264.7 and HCE-T cells (B & D) in serum-free culture. (*denotes p value < 0.05)
Bacterial toxicity by silver exposure
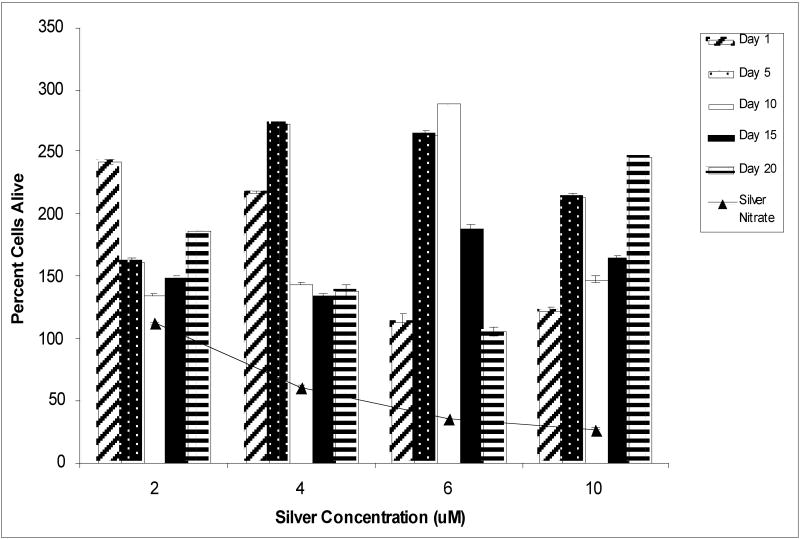
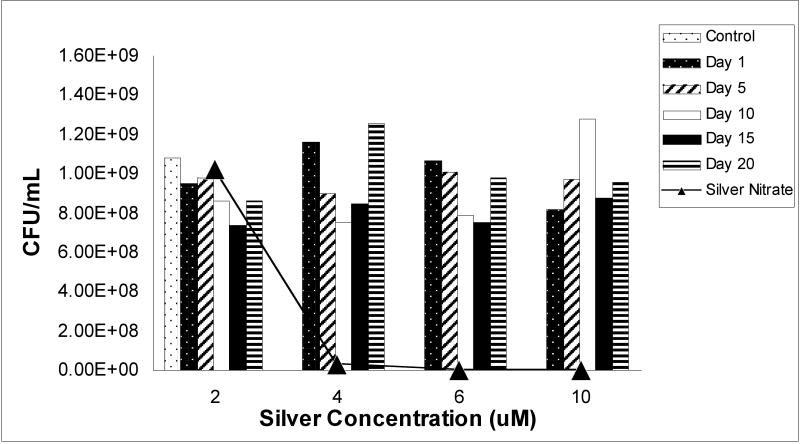
Time-dependent solubilization of silver ions from metallic silver nanoparticles, and the subsequent antimicrobial properties of these extracted particle suspensions on P. aeruginosa were studied. Significantly, silver ions concentrations detected at any time points in any silver nanoparticle concentrations tested using ICP-OES (assay detection limits of 100ppb silver ion under these media conditions) range from 0.1 to 0.28 μg/ml (maximum of 2.6μM silver ion concentration), well-below the known silver ion microcidal threshold shown in solution. Figure 2 shows bacterial viability results from 20-day nanoparticle solubilization experiment using the BacLight™ Live/Dead assay. Silver nitrate control solutions at 6μM and 10μM effectively halted bacterial growth with a 27% and 35% survival rate, respectively, compared to a 100% “live” cell assay fluorescence. However, the BacLight™ staining kit proved to be affected by the cell-attached or intracellular accumulation of silver nanoparticles in P. aeruginosa assays. This is an empirical conclusion because as samples are washed and centrifuged at 10,000 × g to remove all culture solution, remaining particles should accumulate either in or on the cell wall or even intracellularly. Importantly, media sample controls containing only silver nanoparticles intrinsically fluoresced 2-fold greater over that for the 100% “live” bacterial control samples, while silver nitrate-treated cultures showed the expected, consistent dose-response fluorescence signal correlation. Our method for determining anti-microbial activities of silver nanoparticle suspensions then shifted to using direct-colony enumeration of live bacteria (e.g., agar culture) for comparison. These results for bacterial viability are shown in Figure 3 and indicate that, consistent with Figure 2 data, silver nitrate effectively kills bacteria in a concentration-dependent manner while exposure to silver nanoparticle media has no apparent effects on viability. Again, this is consistent with the low levels of free silver ion detected in these cultures from nanoparticles (values ranging from 0.1-0.28 μg/mL, ∼2.6 μM), an order of magnitude below that expected for soluble ion-induced toxicity.
Figure 2.
Relative ratio of live to dead bacteria observed using the BacLight™ Live/Dead fluorescence assay within the culture population compared to initial bacteria inoculating density (100%) in vitro for silver nitrate (triangle symbol) and silver nanoparticles (bars shown at various nanoparticle aqueous extraction times). All nanoparticle aqueous extracts allowed bacterial growth beyond the 100% “LIVE” Pseudomonas aeruginosa control sample, possibly due to the confounding intrinsic fluorescence from the silver nanoparticles remaining within the bacterial cell or cell wall. All silver nitrate-treated (positive control) cultures (line connecting triangles) decreased with increasing silver ion concentration in the media.
Figure 3.
Bacterial live colony direct enumeration counts on agar after overnight incubation with either silver nitrate or silver nanoparticles. High toxicity is observed for silver nitrate-treated bacterial cultures (positive controls). No difference is observed between silver nanoparticle-treated cultures and untreated (negative) control cultures.
Cell cytokine immunoassays
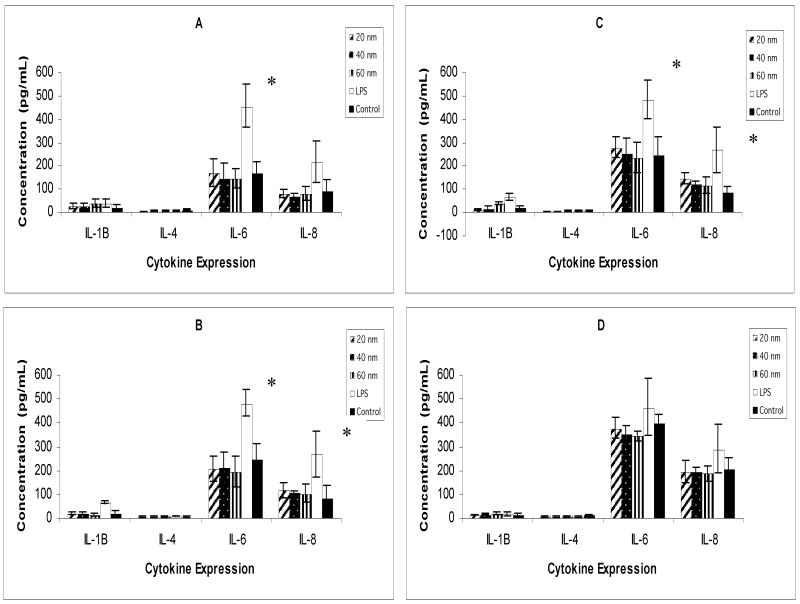
Mammalian cell activation by different sized silver nanoparticles in culture media was investigated in a co-culture system comprising corneal epithelial cells and macrophages. Analysis of murine RAW264.7 macrophages and human corneal epithelial cells required two different ELISA platforms (i.e., both murine and human, respectively) for each cytokine assayed. Levels of cytokines IL-1β, IL-4, IL-6, IL-8 from HCE-T cells are shown in Figure 4, and IL-1β, IL-4, and IL-6 assays from murine RAW264.7 cells are shown in Figure 5. Levels of IL-4 in both the human and murine kits from each respective cell type approached the limit of detection (< 4 pg/mL) and were indistinguishable from baseline controls throughout the study. Human HCE-generated IL-6 levels of LPS-activated HCE-T culture positive controls were significantly different from both nanoparticle-treated and untreated control samples until the 24-hour exposure time point. Human IL-8 levels of LPS-activated HCE-T culture controls were also significantly different, but only at 8 and 12 hours of exposure in culture. At all time points for human HCE cell IL-1β, IL-6 and IL-8 assay, nanoparticle samples were not significantly different from control samples. Murine IL-1β generated from nanoparticle and LPS-treated RAW264.7 macrophage cells were also indistinguishable from control samples. Murine IL-6 LPS-treated RAW264.7 cell samples peaked at 12 and 24 hours, while nanoparticle-treated samples were indistinguishable from RAW264.7 cell controls at each time point.
Figure 4.
Human HCE-T cell cytokine expression at 4, 8, 12 and 24 hours in serum-free culture (A, B, C, D, respectively). Silver nanoparticle treatments failed to produce cytokine expression significantly different than untreated cell culture controls. LPS-treated samples (positive controls) were significantly different from both untreated (negative) controls and silver nanoparticle treatments at 4, 8, and 12 hours for human IL-6, and at 8 and 12 hours for human IL-8. (*denotes p value < 0.05)
Figure 5.
Murine RAW264.7 cell cytokine expression assay results at 4, 8, 12 and 24 hours (A, B, C, D, respectively) in serum-free culture. Silver nanoparticle treatments failed to produce cytokine activation significantly different than untreated cell controls. LPS-treated samples were significantly different from both untreated cell controls and silver nanoparticle treatments at 12 and 24 hours for murine IL-6. (*denotes p value <0.05)
Discussion
Two operating mechanistic hypotheses for silver-induced bactericidal and ocular cell toxicity effects were investigated in these studies: (1) that these effects could be induced from production of silver ion from nanoparticle dissolution into the culture media, or (2) that silver nanoparticles themselves were intrinsically microcidal (as recently reported48,49), and also activating to mammalian cells. Results show that the nanoparticle loading studied here was insufficient to validate either of these hypotheses. Substantially higher silver nanoparticle dosing might possible produce effects, but whether these conditions might be commensurate with possible contact lens loading and lens optical quality, eye wash formulations, relevant silver chloride solubility concerns in physiological fluids, and overt ocular cell toxicity remain to be proven.
Biocidal activity of silver-containing media
Given the solubility limit for AgCl(s) in aqueous media (1.33×10-5M), and the chloride ion concentration in the USFM media of 0.6 g/L (0.06%, or 1.69 ×10-2M, provided in the product information), then silver ion would need to exceed 1.04 ×10-8M (>10nM) to precipitate in USFM as silver chloride. Silver ion detection using ICP-OES (2.6 μM) certainly exceeds this value. While this is media-specific and could precipitate as numerous silver salts with differing solubilities in vivo, this silver ion value exceeds known minimal toxicity onsets for bacteria (>nM18-20). Hence, silver ions in the media in experiments shown here should begin to be microcidal if reaching this lethal threshold. Absence of any detectable microcidal effects for silver nanoparticles asserts that such thresholds were not attained for nanoparticle-containing media. Recent silver particle surface modification resulting in positively charged particles has been recently invoked as an important determinant of silver nanoparticle biocidal activity, relying on electrostatic interactions between negatively charged bacteria and positively charges particles to produce toxicity in particle form.48,49 The commercial nanoparticle suspension used in this experiment comprises negatively charged particles bearing an ionized silver oxide outer layer (unpublished personal communication, Ted Pella, Inc. Redding, CA).
The commercial LIVE/DEAD BacLight™ kit was used to assess bacterial cell damage and viability in response to silver particle and ion exposure in vitro. Based on an untreated control culture, silver nitrate-treated bacterial cultures responded in a linear silver dosing-killing relationship for the four different silver doses. Use of this fluorescent dye assay to compare the efficacy of silver nanoparticle treatment was not possible: all nanoparticle-treated P. aeruginosa cultures were as optically dense as untreated control cultures (i.e., same cell count), yet most of them produced green (LIVE) fluorescent signal double that of the untreated control, indicating some optical anomaly confounding signal from the green dye (Syto 9) and possible optical activity for silver nanoparticles on or remaining within bacteria. Alone in media, silver nanoparticles at the dose used for bacteria do not autofluoresce at this green wavelength (data not shown). Combined with the LIVE/DEAD dye, silver nanoparticle suspensions do not fluoresce more than water samples containing the dye mixture alone (data not shown). Various contributions to this confounding effect in these milieus were difficult to distinguish. Hence, all cultures were serially plated and viable colonies enumerated the following day (Fig. 3) to confirm bacterial viability. While this is still a validated, accepted method of determining bacterial viability, it cannot demonstrate some initial, early kinetic effects on bacterial viability as the BacLight™ assay does. Nonetheless, all evidence is consistent in showing that silver nanoparticle preparations at 2, 4, 6, and 10 μM silver content exhibited no discernible effect on bacterial viability.
Cytokine assays on mammalian cell cultures
Cytokines IL-1β, IL-4, IL-6, and IL-8 production in cultured cells were profiled to represent different possible host immune responses to a Pseudomonal infection of the cornea33-34. Pro-inflammatory cytokines, IL-1β and IL-6, are commonly produced by macrophages and resident corneal cells in response to bacterial endotoxin.35 IL-4 (a Th2-type response cytokine) was chosen to include an immunosuppressive36 modulator and because of its down-regulation of human IL-8 in human corneal epithelial cells,37 a pro-inflammatory cytokine known to promote neutrophil mobilization to the site of infection.38-39 Accordingly, in this study design, IL-4 levels were nearly undetectable at high levels of IL-8 expression. While human IL-6 and IL-8 (from HCE-T cells) responded rapidly to positive control LPS activation at 4 hours post-treatment, continuing until 24 hours, murine IL-6 (from RAW264.7 cells) was not detected until 12 hours post-treatment. Though IL-1β is a pro-inflammatory cytokine, its expression in human corneal cells in these experiments was minimal. This result is supported by previous work in which an immortalized human corneal epithelial cell line expressed low levels of IL-1β and elevated levels of IL-6 and IL-8 during infection with P. aeruginosa.40 A recent study detected elevated expression of IL-1β in murine macrophage cells, which along with TNF-α, IL-12, and IL-6, are commonly produced by these cells41 and very much a determining factor in the pathogenesis of bacterial corneal infections.35,42-44
More accurate recapitulation of the in vivo eye environment was attempted in vitro by co-culturing methods with epithelial and macrophage cells. As the positive control for infection with Pseudomonas aeruginosa, LPS-treated cell cultures responded as anticipated: with elevated expression of IL-6 and IL-8, and to a lesser extent IL-1β. A drawback to the in vitro model for infection is the inability to accurately duplicate the effects of cytokine expression on the pathology of the infected tissue. While the two cell types are able to signal and communicate with each other through the shared in vitro culture environment, specific effects of concentrations of IL-1β or IL-6 expressed cannot be ascertained for their intrinsic abilities to recruit natural PMN infiltration or other tissue-based host reactions to infection.
Results obtained with silver nanoparticle-treated cell cultures were not as informative as the LPS positive controls. In the murine ELISA sets for RAW264.7 macrophages, nanoparticle-treated cell IL-1β and IL-6 expression was often at or above that of non-treated control cells and equivalent to LPS treatment in RAW264.7 cells, except for IL-6 at 12 and 24 hours. However, these results were not statistically significant. Even so, macrophage-activated cytokine expression might be expected at higher levels than epithelial cell cytokine expression because of the immuno-modulating phenotype of the macrophage, intrinsic reactivity to particles45-47 and expected reaction to nanoparticle exposure in this case. In support of this, nanoparticle-treated HCE-T cell IL-1β, IL-6 and IL-8 levels were either at or below that of controls. This result could be a dose-dependent effect, and that higher concentrations of nanoparticles would produce more potent cytokine reactions. Taken with the nanoparticle results showing general lack of biocidal activity with P. aeruginosa (vida infra), if a higher silver particle dose is required for bacterial kill, this dose might also induce adverse reactions with these two ocular cell types.
The normal, complex ocular environment contains many interacting cell types involved in the maintenance of the cornea. Macrophages coexist on the ocular surface with epithelial cells, and aid in keeping the eye environment free of foreign pathogens and debris, actively phagocytosing particulate matter and neutralizing it within the cell using enzymes and oxygen radicals. They also initiate innate immune response by releasing pro-inflammatory cytokines like IL-1β.
Various antigens and antigen-presenting cells promote mobilization of polymorphonuclear leukocytes (PMNs), ocular macrophages and Langerhans cells during infection. Like macrophages, Langerhans cells can also ingest foreign particles. They can also present ingested antigens on their cell surface and travel to T-lymphocytes, that then respond to presented antigens by initiating a cytokine cascade or signal other leukocytes by either a Th1 or Th2 response. PMNs are most responsible for corneal defense during sleep. However, their continued presence and subsequent inflammation has been shown to increase corneal tissue damage.50-52 Cytokines that regulate this response must be under strict control to mediate normal PMN response.52 This study intended to replicate this dynamic situation of resident corneal cells and scavenging macrophages and provoking of possible cytokines that regulate the host ocular immune response. That this response might be aggravated by nanoparticles possibly applied for antimicrobial use was a motivating hypothesis.
Cytotoxic effects of silver nitrate observed on the RAW264.7 macrophage cell line for the 4 μM and 6 μM treatments are were not significantly elevated by the third week of exposure, however there was significant toxicity at these concentrations for the human epithelial cell line at the third week of exposure. This was surprising, given previous studies showing silver ions to be some of the most toxic heavy metals on macrophage proliferation.18-20 However, their studies were carried out to four weeks in culture and it was at this latest time point that significant drops in cell proliferation occurred. Prolonging our studies would be important to ascertaining the true impact of silver dose and time. Additionally, determining effects of serum in these studies is imperative: no cells exist in vivo in serum-free media. Previous work in cells in 10% serum showed that 8 μM silver ion was well-tolerated by monocytes but 12 μM silver ion was not.20 Using serum-free media in our studies, silver concentrations of 8μM and 10 μM completely inhibited cell viability in both cell types. That silver ion partitioning into serum proteins affects its bioavailability and hence therapeutic index is intuitive: silver-binding proteins are ubiquitous. Assays to determine these precise behavioral differences between serum and serum-free media are difficult to interpret.
Conclusions
Both hypotheses proposed as motivation for this study remain unproven under the conditions tested: neither silver ion release from nanoparticles nor nanoparticles themselves exhibit microcidal effects at dosing levels studied in vitro. Poor bactericidal action against P. aeruginosa was shown for silver nanoparticles or their extracted media after 20 days of solubility. Mammalian cell toxicity was observed at high level (8-12μM silver ion) silver levels in serum-free culture. Low cell pro-inflammatory cytokine activation was observed for these all silver nanoparticle loadings in both human corneal epithelial cells and a murine macrophage cell line in serum-free cultures. These results contrast existing reports on microcidal properties of silver nanoparticles treated with a cationic modification,48,49 but indicate a possible therapeutic window for higher nanoparticles doses where microcidal effects might be enhanced without adverse response to ocular cells such as epithelium or leukocytes.
In the context of developing extended-wear or continuous-wear contact lens formulations that better protect the eye from infectious bacteria, polymer lenses might be able to be fabricated with relatively high silver nanoparticle loads for anti-microbial effects. Silver nanoparticles formulated into such an anti-microbial lens would have to be loaded so to reliably kill common ocular bacteria, remain non-toxic to endogenous ocular cells, and retain critical lens optical properties for vision correction despite high particle loads. The sub-visible wavelength size of dispersed nanoparticles eliminates many optical problems: nano-scale metal particle optical properties and aggregation effects would be a concern in such formulations. Nanoparticle susceptibility to producing well-known silver photochemical reactivity contributing to argyria in tissues from many silver compounds14 remains a significant unknown in these ocular applications.
Acknowledgments
The authors are grateful for graduate student support, technical assistance and the gift of human corneal epithelial cells from Ciba-Vision Novartis (Norcross, GA), as well as the gift of P. aeruginosa strain PA01 from H. Schweitzer (Colorado State University). Technical advice from Dr. M. Gabriel and A. Wright (CibaVision Novartis), and R.J. Christie, L. Chamberlain, M.L. Godek, P. Wu and P. Hogrebe (Colorado State University) is very much appreciated. Partial support from NIH grant EB000894 is also gratefully acknowledged.
References
- 1.Sweeney DF, du Toit R, Keay L, Jalbert I, Sankaridurg PR, Stern J, Skotnitsky C, Stephenson A, Covey M, Holden BA, Rao GN. In: Silicone Hydrogels: Continuous-wear Contact Lenses. 2nd. Sweeney DF, editor. Butterworth-Heinemann; Oxford: 2004. pp. 164–216. [Google Scholar]
- 2.Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, Kijlstra A. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- 3.Willcox MDP, Harmis N, Cowell BA, Williams T, Holden BA. Biomaterials. 2001;22:3235–3247. doi: 10.1016/s0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- 4.Rediske AM, Koenig AL, Barekzi N, Ameen LC, Slunt JB, Grainger DW. Biomaterials. 2002;23:4565–4572. doi: 10.1016/s0142-9612(02)00202-8. [DOI] [PubMed] [Google Scholar]
- 5.Carnoy C, Scharfman A, Van Brussell E, Lamblin G, Ramphal R, Roussel P. Infect Immun. 1994;62:1896–1900. doi: 10.1128/iai.62.5.1896-1900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landa AS, van der Mei HC, van Rij G, Busscher HJ. Cornea. 1998;17:293–300. [PubMed] [Google Scholar]
- 7.Bruinsma GM, van der Mei HC, Busscher HJ. Biomaterials. 2001;22:3217–3224. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 8.Lord MS, Stenzel MH, Simmons A, Milthorpe BK. Biomaterials. 2006;27:567–575. doi: 10.1016/j.biomaterials.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Tian X, Iwatsu M, Sado K, Kanai A. CLAO. 2001;27(4):216–220. [PubMed] [Google Scholar]
- 10.Hehl EM, Beck R, Luthard K, Guthoff R, Drewelow B. Eur J Clin Pharmacol. 1999;55(4):317–23. doi: 10.1007/s002280050635. [DOI] [PubMed] [Google Scholar]
- 11.Sano K, Tokoro T, Imai Y. Acta Ophthalmol Scand. 1996;74(3):243–8. doi: 10.1111/j.1600-0420.1996.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 12.Phinney RB, Schwartz SD, Lee DA, Mondino BJ. Arch Ophthalmol. 1988;106(11):1599–1604. doi: 10.1001/archopht.1988.01060140767052. [DOI] [PubMed] [Google Scholar]
- 13.Wu P, Grainger DW. Biomaterials. 2006;27(11):2450–67. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Russell AD, Hugo WB. Prog Med Chem. 1994;31:351–366. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 15.Stickler DJ. Curr Opin Infect Dis. 2000;13:389–393. doi: 10.1097/00001432-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Williams RL, Doherty PJ, Vince DG, Grashoff GJ, Williams DF. Crit Rev Biocompat. 1989;5(3):221–243. [Google Scholar]
- 17.Bragg PD, Rainnie DJ. Can J Microbiol. 1973;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 18.Wataha JC, Hanks CT, Sun Z. Dent Mater. 1995;11:239–245. doi: 10.1016/0109-5641(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 19.Wataha JC, Lockwood PE, Schedle A. J Biomed Mater Res. 2000;52:360–364. doi: 10.1002/1097-4636(200011)52:2<360::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Wataha JC, Lockwood PE, Schedle A, Noda M, Bouillaguet S. J Oral Rehab. 2002;29:133–139. doi: 10.1046/j.1365-2842.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 21.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. J Am Med Assoc. 1999;281(2):261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 22.Maki DG, Cobb L, Garman JK, Shapiro JM, Ringer M, Helgerson RB. Am J Med. 1988;85(3):307–314. doi: 10.1016/0002-9343(88)90579-7. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph P, Werner HP, Kramer A. Hyg Med. 2000;25:184–186. [Google Scholar]
- 24.Silver S. FEMS Micro Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 25.Nissen S, Furkert FH. Opthalmologe. 2000;97(9):640–643. doi: 10.1007/s003470070054. [DOI] [PubMed] [Google Scholar]
- 26.Neely F, Alli A. WO2005/065731 A1 “Anti-microbial contact lenses and methods for their production”. 2005. [Google Scholar]
- 27.Meyers AW, Neely FL, Enns JB. US2004/0115242 A1 “Anti-microbial contact lenses and methods for their production”. 2004. [Google Scholar]
- 28.Willcox M, Williams T, Schneider R, Vanderlaan D. US6591814 B2 “Biomedical devices with anti-microbial coatings”. 2003. [Google Scholar]
- 29.Vanderlaan DG, Meyers A, Brown-Skrobot S. WO 02/062402 A1 “Antimicrobial contact lenses containing activated silver and methods for their production”. 2002. [Google Scholar]
- 30.Crouch S, Kozlowski R, Slater K, Fletcher J. J Immunol Meth. 1993;160(1):81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 31.Olsson T, Gulliksson H, Palmeborn M, Bergstrom K, Thore A. J Appl Biochem. 1983;5:347–445. [PubMed] [Google Scholar]
- 32.Squirrell D, Murphy J. In: A practical guide to industrial uses of ATP luminescence in rapid microbiology. Stanley PE, Smither R, Simpson WJ, editors. Cara Technology Ltd.; Lindfield: 1997. pp. 107–113. [Google Scholar]
- 33.Szliter EA, Barrett RP, Gabriel MM, Zhang Y, Hazlett LD. Eye Contact Lens. 2006;32(1):12–18. doi: 10.1097/01.icl.0000167611.03883.58. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Barrett RP, McClellan SA, Hazlett LD. Invest Ophthalmol Vis Sci. 2005;46(11):4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 35.Hazlett LD. Prog Ret Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Proc Natl Acad Sci, USA. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takano Y, Fukagawa K, Shimmura S, Tsubota K, Oguchi Y, Saito H. Br J Opthamol. 1999;83:1074–1076. doi: 10.1136/bjo.83.9.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Ann Rev Immunol. 1991;19:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 39.Thakur A, Willcox MDP. Exp Eye Res. 2000;70:255–259. doi: 10.1006/exer.1999.0767. [DOI] [PubMed] [Google Scholar]
- 40.Xue ML, Willcox MDP, Lloyd A, Wakefield D, Thakur A. Clin Exp Opthamol. 2001;29:171–174. doi: 10.1046/j.1442-9071.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 41.Laskin DL, Pendino KJ. Annu Rev Pharmacol Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- 42.Rudner XL, Kernacki KA, Barrett RP, Hazlett LD. J Opthamol. 2000;164:6576–6582. doi: 10.4049/jimmunol.164.12.6576. [DOI] [PubMed] [Google Scholar]
- 43.Thakur A, Xue M, Stapleton F, Lloyd AR, Wakefield D, Willcox MDP. Infect Immun. 2002;70(4):2187–2197. doi: 10.1128/IAI.70.4.2187-2197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakur A, Kyd J, Xue M, Willcox MDP, Cripps A. Infect Immun. 2001;69(5):3295–3304. doi: 10.1128/IAI.69.5.3295-3304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii H, Hayashi S, Hogg JC, Fujii T, Goto Y, Sakamoto N, Mukae H, Vincent R, van Eeden SF. Respir Res. 2005;6(1):87. doi: 10.1186/1465-9921-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. J Mater Sci Mater Med. 2001;12(3):249–58. doi: 10.1023/a:1008967200706. [DOI] [PubMed] [Google Scholar]
- 47.Balduzzi M, Diociaiuti M, De Berardis B, Paradisi S, Paoletti L. Environ Res. 2004;96(1):62–71. doi: 10.1016/j.envres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Langmuir. 2002;18:6679–6686. [Google Scholar]
- 49.Hamouda T, Hayes M, Cao Z, Tonda R, Johnson K, Craig W, Brisker J, Baker J. J Infect Dis. 1999;180:1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- 50.Dinarello CA, Wolff SM. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 51.Niederkorn JY, Peeler JS, Mellon J. Reg Immunol. 1989;2:83–90. [PubMed] [Google Scholar]
- 52.Chusid MJ, Davis SD. Infect Immun. 1979;24:948–952. doi: 10.1128/iai.24.3.948-952.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godek ML, Malkov GS, Fisher ER, Grainger DW. Plasma Proc Polym. 2006;3:485–497. doi: 10.1002/ppap.200600007. [DOI] [PMC free article] [PubMed] [Google Scholar]