Abstract
Cocaine abuse is a significant problem among pregnant women. The present study tested the hypothesis that prenatal cocaine exposure impairs myogenic reactivity of coronary arteries in adult offspring. Pregnant rats received cocaine (30 mg kg−1 day−1) or saline from days 15 to 21 of gestational age and experiments were conducted in 3-month-old offspring. In pressurized coronary septal arteries, the diameter and vessel wall intracellular Ca2+ concentrations were measured simultaneously in the same tissue as a function of intraluminal pressure. Cocaine did not affect KCl-induced contractions of coronary arteries in either males or females, but decreased the distensibility in male vessels. In male offspring, cocaine treatment resulted in a significant decease in pressure-dependent myogenic contractions. Inhibition of eNOS with NG-nitro-L-arginine did not alter the myogenic response in either saline control or cocaine-treated animals. In females, cocaine caused a significant increase in pressure-dependent myogenic contractions. NG-nitro-L-arginine did not affect the myogenic response in the control animals, but blocked the cocaine-mediated effect. In both males and females, the presure-induced increases in vessel wall Ca2+ concentrations were not significantly different between cocaine and saline groups. The ratio of changes in the diameter to Ca2+ concentrations in the presurized arteries was significantly less in male but greater in female offspring after cocaine treatment. The results suggest that prenatal cocaine exposure causes reprogramming of coronary myogenic tone via changes in the Ca2+ sensitivity in a sex-dependent manner, leading to an increased risk of dysfunction of coronary autoregulation in adult offspring.
Keywords: cocaine, fetal programming, gender, coronary artery, myogenic tone
Introduction
Maternal cocaine abuse during pregnancy has been associated with numerous adverse perinatal outcomes. In addition to neurobehavioral effects, in utero cocaine exposure predisposes the fetus and neonate to various cardiovascular dysfunctions. Offspring born to mothers with a history of cocaine abuse have a high incidence of congenital cardiovascular malformations.1 It has been demonstrated in a rat model that fetal exposure to cocaine during gestation causes an increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring.2 In addition, prenatal cocaine resulted in programming of enhanced vascular contractility via changes in eNOS-regulated Ca2+ sensitivity of myofilaments in sex- and tissue-dependent manners in resistance arteries, leading to an increased risk of hypertension in male offspring.3
Consistently, epidemiological studies have shown a clear association of adverse intrauterine environment and an increased risk of coronary heart disease in adult life.4,5 However, it is unknown whether or to what extent fetal cocaine exposure affects coronary vascular reactivity in adult offspring. Coronary perfusion is essential for cardiac function, and autoregulation is an intrinsic tendency of coronary vasculature to maintain constant blood flow despite changes in perfusion pressure. Given that myogenic response is a major contributor to the autoregulation of coronary blood flow, the present study was designed to test the hypothesis that maternal cocaine administration during pregnancy alters coronary myogenic reactivity in adult offspring, which may lead to an increased risk of coronary heart disease in later life. The studies were performed in both male and female rat offspring to investigate the potential sex effects in fetal programming of the coronary reactivity caused by cocaine.
Methods
Experimental animals
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI), and were randomly divided into two groups: 1) saline control; and 2) cocaine 15 mg/kg i.p. twice daily from day 15 to 21 of gestational age, as described previously.3 Offspring were studied at 3 months of age. All procedures and protocols were approved by the Institutional Animal Care and Use Committee guidelines.
Coronary artery isolation and cannulation
Hearts were isolated and the right ventricle was opened along the free wall. Septal coronary arteries were chosen to study intramural coronary arteries.6 The arteries (~100–150 µm in diameter and 2–3 mm in length) were isolated and cannulated in an organ chamber (Living Systems, Burlington, VT), followed by placement on the stage of an inverted microscope. The proximal cannula was connected to a pressure transducer and reservoir of physiological salt solution (PSS). The intraluminal pressure was controlled by a servo-system to set transmural pressures. The distal cannula was connected to a luer-lock valve that was open to flush the lumen during the initial cannulation. After cannulation, the valve was closed and measurements were conducted under no-flow conditions. Arterial diameter was recorded using the SoftEdge Acquisition Subsystem (IonOptix Milton, MA) as described previously.7
Measurement of myogenic tone
Arteries were equilibrated in PSS for 10 minutes at intraluminal pressure of 20 mmHg, after which pressure was increased from 20 to 70 mmHg and returned to 20 mmHg immediately. The vessels were then allowed to equilibrate at 20 mmHg for another 30 minutes. After equilibration, intraluminal pressure was reduced to 10 mmHg, and arteries were further stabilized for 10 minutes. The pressure was then increased in a stepwise manner from 10 to 100 mmHg in 20-mmHg increments, and each pressure level was maintained for 5–10 minutes to allow vessel diameter to stabilize before measurement. The passive pressure-diameter relationship was conducted in Ca2+-free PSS containing 3 mM EGTA. Percent myogenic tone at each pressure step was calculated as % myogenic tone = (D1−D2)/D1×100, in which D1 was the passive diameter in Ca2+-free PSS and D2 was the active diameter with normal PSS in the presence of extracellular Ca2+.
Measurement of vascular wall [Ca2+]i
Intracellular free Ca2+ concentrations ([Ca2+]i) was measured in vascular wall loaded with fura-2 as previously described.8 Arterial segments were cannulated and pressurized to 10 mmHg and equilibrated at 37°C for 30 minutes. The cannulated arteries were incubated with 2.5 µM fura-2 AM in PSS at room temperature for 1 hour, followed by a washout period of 30 minutes at 37°C. Fura-2 fluorescence was measured by a photomultiplier system (IonOptix) with two alternated excitation wavelengths of 340 and 380 nm and an emission of 510 nm. The fluorescence intensity at each excitation wavelength (F340 and F380, respectively) and the ratio of these two fluorescence values (Rf340/380) were recorded simultaneously with arterial diameter measured with the use of the SoftEdge Acquisition Subsystem. Percent changes in [Ca2+]i (measured as fura-2 Rf340/380) at each pressure step was determined as (Ca12+−Ca22+)/Ca12+ × 100, where Ca12+ was [Ca2+]i obtained with normal PSS at given pressure and Ca22+ was [Ca2+]i obtained with Ca2+-free PSS at the same pressure.
Data analysis
Data are presented as means ± SEM. Experimental number (n) represents offspring from different dams. The differences were evaluated for statistical significance (P < 0.05) by two-way ANOVA.
Results
Effect of cocaine on coronary arterial distensibility
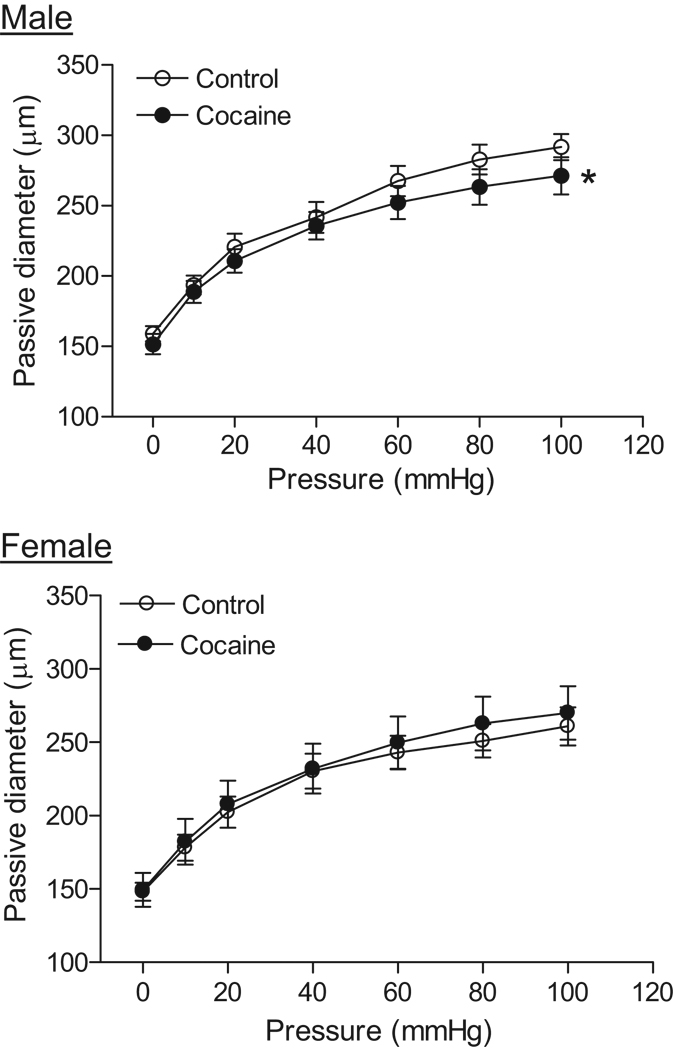
There were no significant differences in the resting coronary arterial diameters between the control male (135.8 ± 8.7 µm) and female (138.7 ± 5.7 µm) offspring. Cocaine treatment had no significant effect on the resting diameters in either males (147.1 ± 5.3 µm) or females (136.5 ± 9.4 µm). As shown in figure 1, in the absence of extracellular Ca2+, arterial passive diameters significantly increased in response to step increases of intraluminal pressure (0–100 mmHg). The pressure-mediated passive diameters were significantly greater in males than in females (P < 0.05). Cocaine had no effect on the passive diameters in females but significantly decreased them in males (P < 0.05) (Figure 1). In the cocaine-treated animals, there were no significant differences in the passive diameters between male and female offspring.
Figure 1. Effect of cocaine on passive arterial diameters of coronary arteries.
Pressure-induced passive arterial diameters were measured in Ca2+-free PSS in coronary arteries from 3 month old male and female offspring that had been exposed in utero to saline control or cocaine. Data are means ± SEM. * P < 0.05, cocaine versus control. n=6–8 rats.
Effect of cocaine on KCl-induced [Ca2+]i and contractions in pressurized coronary arteries
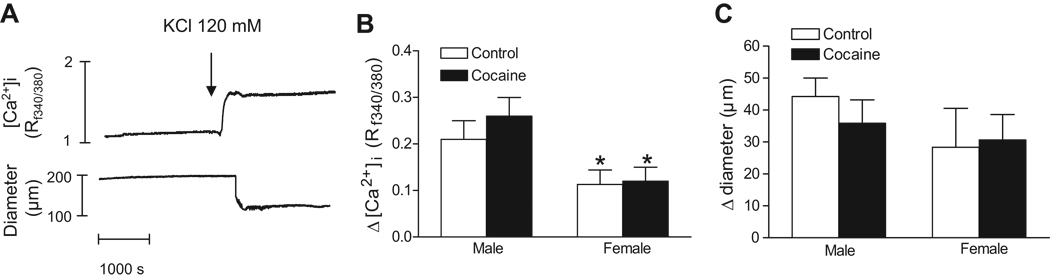
At intraluminal pressure of 20 mmHg, addition of 120 mM K-PSS caused a significant decrease in the vessel diameter, which was proceeded by an increase in [Ca2+]i (Figure 2A). Although there was no significant sex difference in KCl-mediated contractions, KCl-induced [Ca2+]i was significantly lower in female than in male arteries (Figure 2B and 2C). Cocaine had no significant effect on KCl-induced [Ca2+]i and contractions in either males or females (Figure 2B and 2C).
Figure 2. Effect of cocaine on KCl-induced constrictions and [Ca2+]i in pressurized coronary arteries.
KCl-induced changes in arterial diameters and [Ca2+]i were measured simultaneously at intraluminal pressure of 20 mmHg in coronary arteries from 3 month old male and female offspring that had been exposed in utero to saline control or cocaine. A, Typical recordings of KCl-induced increase of [Ca2+]i and decrease of the arterial diameter in the same tissue. B, KCl-induced increases of [Ca2+]i (Δ [Ca2+]i, Rf340/380). C, KCl-induced decreases of the arterial diameter (Δ diameter, µm). Data are means ± SEM. * P < 0.05, female versus male. n=5–7 rats.
Effect of cocaine on pressure-dependent myogenic response
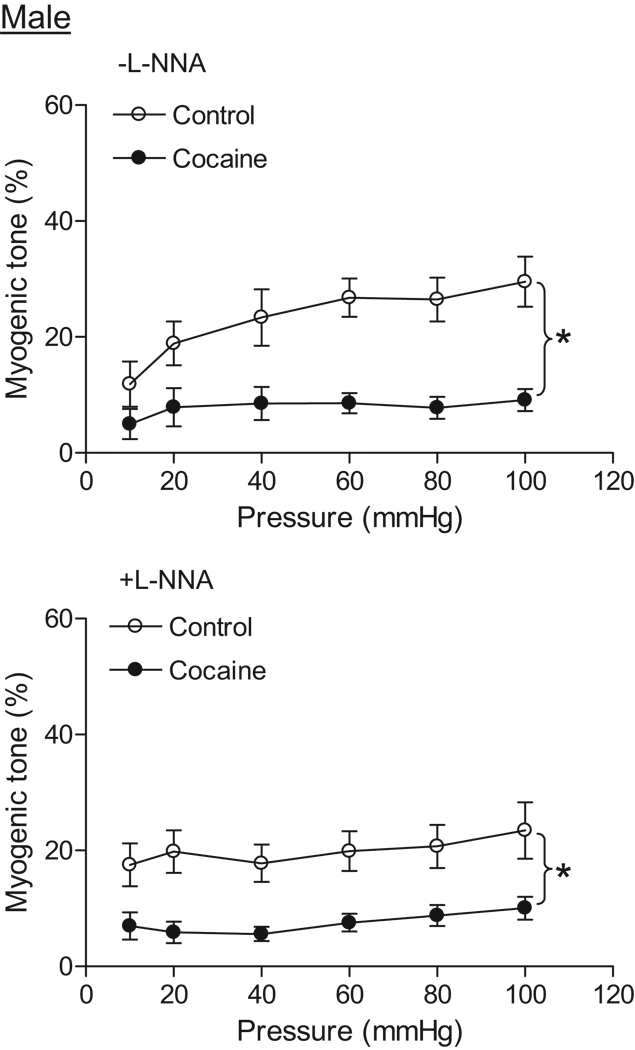
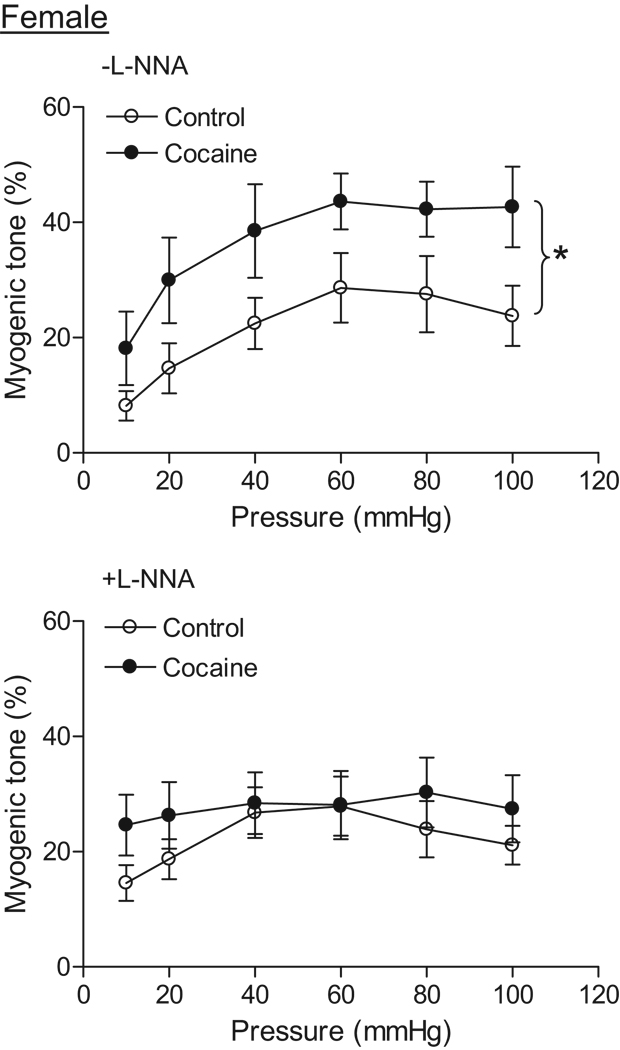
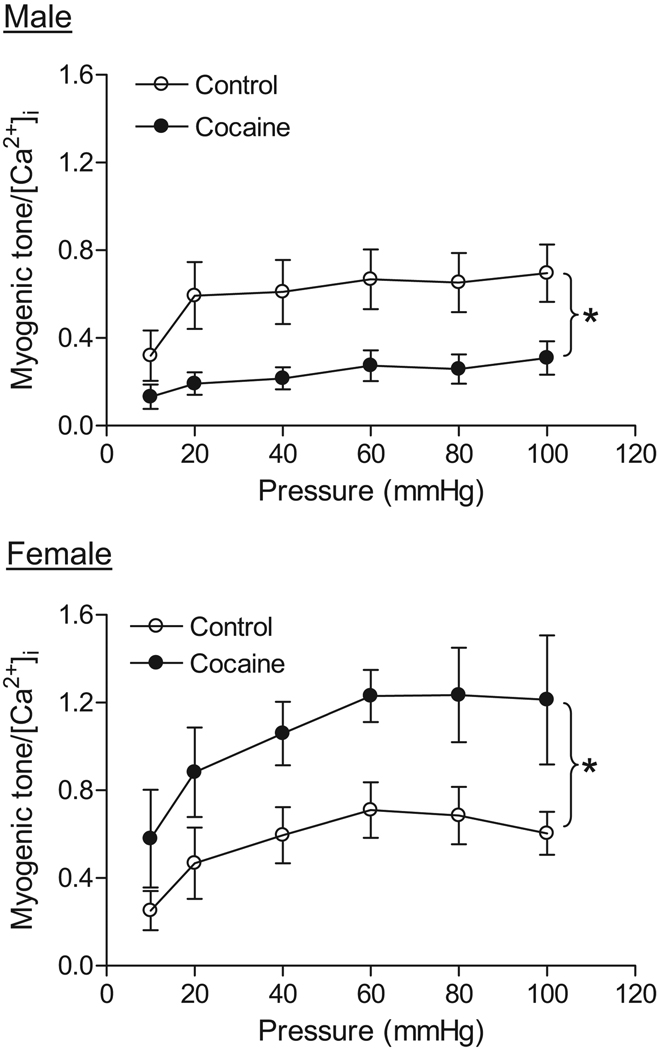
Coronary arteries of both male and female offspring developed myogenic tone in response to step increases of intraluminal pressure in the presence of extracellular Ca2+. In control animals, there was no significant difference in pressure-dependent myogenic tone between males and females in the absence of NG-nitro-L-arginine (L-NNA) (Figure 3 and Figure 4). In addition, L-NNA had no significant effect on the myogenic response in either control males (Figure 3) or females (Figure 4). In male offspring, prenatal cocaine caused a significant decrease in pressure-dependent myogenic tone in the absence or presence of L-NNA (Figure 3). In females, cocaine resulted in a significant increase in the myogenic tone of coronary arteries in the absence of L-NNA (Figure 4). In the presence of L-NNA, the myogenic tone was not significantly different between control and cocaine-treat female offspring (Figure 4). In cocaine-treated animals, pressure-dependent myogenic tone was significantly greater in female than in male offspring (Figure 3 and Figure 4).
Figure 3. Effect of cocaine on myogenic tone in male coronary arteries.
Pressure-dependent myogenic tone was determined in the absence or presence of L-NNA (100 µM, 20 min) in coronary arteries from 3 month old male offspring that had been exposed in utero to saline control or cocaine. Data are means ± SEM. * P < 0.05, cocaine versus control. n=6–8 rats.
Figure 4. Effect of cocaine on myogenic tone in female coronary arteries.
Pressure-dependent myogenic tone was determined in the absence or presence of L-NNA (100 µM, 20 min) in coronary arteries from 3 month old female offspring that had been exposed in utero to saline control or cocaine. Data are means ± SEM. * P < 0.05, cocaine versus control. n=6–8 rats.
Effect of cocaine on [Ca2+]i in pressurized coronary arteries
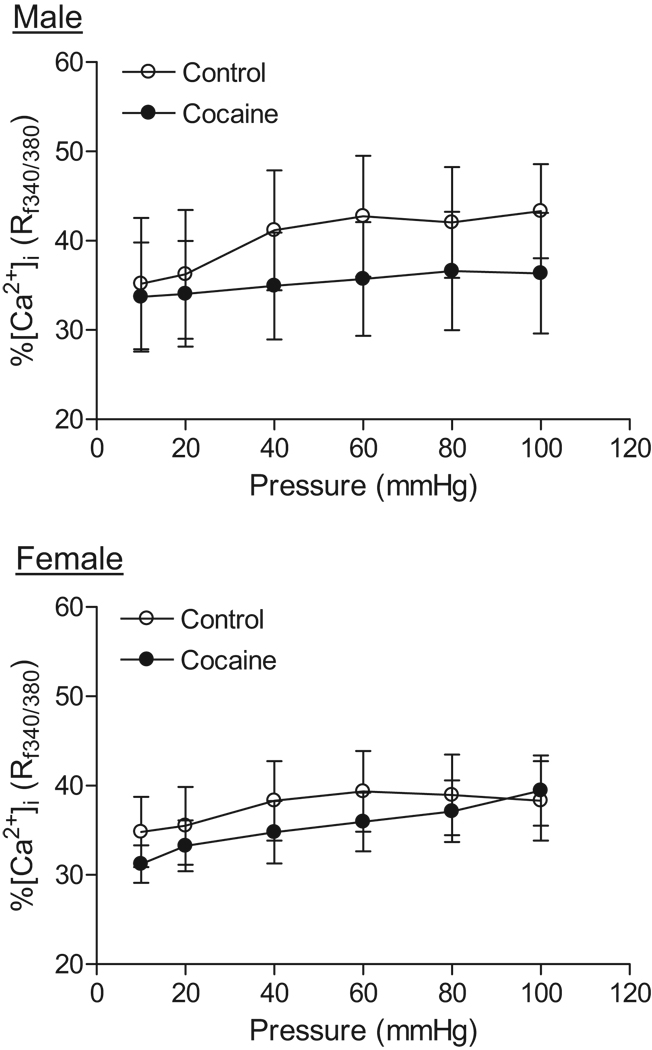
In the absence of extracellular Ca2+, increasing pressure (10–100 mmHg) did not affect [Ca2+]i in coronary arteries of either male or female offspring (data not shown). In the presence of extracellular Ca2+, step increases of intraluminal pressure significantly increased [Ca2+]i in both males and females (Figure 5). Prenatal cocaine treatment had no significant effects on [Ca2+]i of coronary arteries in either male or female offspring (Figure 5). To determine the effect of cocaine on the Ca2+ sensitivity in the regulation of myogenic response of coronary arteries, the ratio of myogenic tone to [Ca2+]i, measured simultaneously in the same tissue, in response to step increase of intraluminal pressure was determined. As shown in figure 6, prenatal cocaine significantly decreased the ratio of myogenic tone to [Ca2+]i in male offspring, but increased it in female offspring. In control animals, there was no significant difference in the ratio of myogenic tone to [Ca2+]i between males and females, but it became significantly greater in female than in male offspring in cocaine-treated animals (Figure 6).
Figure 5. Effect of cocaine on [Ca2+]i in pressurized coronary arteries.
Pressure-dependent [Ca2+]i were determined in coronary arteries from 3 month old male and female offspring that had been exposed in utero to saline control or cocaine. Data are means ± SEM. n=6–8 rats.
Figure 6. Effect of cocaine on the Ca2+ sensitivity of myogenic tone in pressurized coronary arteries.
Pressure-dependent myogenic tone and [Ca2+]i were determined simultaneously in coronary arteries from 3 month old male and female offspring that had been exposed in utero to saline control or cocaine. The ratio of myogenic tone to [Ca2+]i was determined as function of pressure. Data are means ± SEM. * P < 0.05, cocaine versus control. n=6–8 rats.
Discussion
The present study has demonstrated that prenatal cocaine exposure results in sex-dependent impairments in coronary myogenic response in adult offspring. Many previous studies have shown that adverse prenatal stimuli result in dysfunction of systemic vessels and an increased risk of hypertension in adult offspring.3,8–10 Nonetheless, only few studies examined fetal programming of vascular myogenic reactivity in offspring, despite its critical role in development of vascular tone and regulation of peripheral vascular resistance. It has been shown that fetal hypoxia causes an increase in pressure-dependent myogenic contractions of mesenteric arteries in male offspring.11 In addition, maternal undernutrition resulted in an increased mygenic response in the uterine artery.12 To our knowledge, the present study is the first to demonstrate in a rat model that adverse intrauterine environment causes an epigenetic modification of coronary vascular myogenic response in adult offspring. This finding is consistent with epidemiological studies showing an association of adverse intrauterine environment and an increased risk of coronary heart disease in adult life.4,5
The finding of increased pressure-mediated passive diameters in male, as compared with female, vessels suggests a greater distensibility in male coronary arteries. Although this sex-difference has not been previously examined in small coronary arteries, a study in rats showed reduced stiffness of small cerebral arteries in males compared with females.13 The physiological significance of the finding remains to be elucidated, but the less distensibility of female coronary arteries may suggest a lower coronary flow reserve in females.14,15 Cocaine decreased pressure-mediated passive diameters in males and abolished the sex difference in coronary distensibility. In agreement, it has been shown that cocaine reduces coronary flow reserve in male dogs.16
The lack of effect of cocaine on KCl-induced contractions of pressurized small coronary arteries in either females or males suggests that electromechanic-mediated contractile signal pathway in coronary arteries is not altered by fetal cocaine exposure. This is in agreement with the previous finding that prenatal cocaine had no effect on KCl-induced contractions of both aortas and resistance-sized mesenteric arteries in adult offspring.3 Similarly, another study showed that glucocorticoid exposure during early gestation had no significant effect on KCl-induced contractions in newborn lamb coronary arteries.17 In contrast, KCl-induced vascular contractility was enhanced in adult rats that exposed to nicotine before birth.18 These findings suggest a stimuli-specificity in fetal programming of electromechanic coupling in vascular smooth muscle in adult offspring.
The striking finding of opposite effects of fetal cocaine exposure on pressure-dependent myogenic response in male and female offspring is intriguing and suggests sex-specific fetal programming of coronary myogenic reactivity. The gender dichotomy in fetal programming of adult disease has been demonstrated in several animal models. Although the results are conflicting, it has been shown that female offspring are generally less sensitive in manifestation of cardiovascular disease caused by adverse prenatal stimuli.9 In the present study, we found that prenatal cocaine caused a significant decrease in coronary myogenic tone in male but an increase in female offspring. Pressure-induced myogenic tone is a major mechanism in coronary autoregulation.19–21 The heart is dependent on a continuous oxygen supply from the coronary circulation, as the myocardium has a very limited anaerobic capacity. Increased myocardial oxygen consumption in tachycardia and exercise is met principally by augmenting coronary blood flow. The striking decrease in coronary myogenic tone in cocaine-treated male offspring would predict a hemodynamic profile of “supranormal” coronary flow at rest and reduced coronary flow reserve, leading to an increased risk of ischemic heart disease when myocardial oxygen consumption increases several-fold in the transition from rest to exercise. In addition, a much dilated coronary circulation resulted from the decreased myogenic tone may cause abnormally high perfusion pressures to nutritive vessels and distort the balance of intravascular oncotic and hydrostatic pressure, increasing the risk of edema, ventricular stiffness and poor ventricular performance. This notion is supported by the findings in cardiac allografts in human and animal studies showing that the decreased coronary myogenic tone led to a reduced coronary flow reserve and myocardial edema and stiffness.6,22
In contrast to the finding in males, fetal cocaine exposure resulted in a significant increase in coronary myogenic tone in female offspring. Although the tightened myogenic regulation in females may suggest a greater capacity for coronary flow autoregulation and more effective buffering of capillary pressure from elevations in systemic pressure, it also impedes sufficient myocardial perfusion when oxygen demand increases. Indeed, the increased myogenic tone of coronary arteries is thought to play an important role in coronary heart disease in postmenopausal females.23
Myogenic tone is an intrinsic property of smooth muscle and occurs independent of neural, metabolic and endothelial influences. The role of nitric oxide (NO) in the regulation of vascular tone in the coronary resistance vessels is inconsistent, and numerous studies have shown that inhibition of NO synthesis with arginine analogs results in little or no change in coronary blood flow at rest or when myocardial oxygen consumption is elevated.24 In the present study, we found no effect of L-NNA on coronary myogenic tone in either male or female rats. The finding that cocaine-mediated reduction of myogenic tone was not affected by L-NNA in male offspring suggests a minimal role of NO in programming of coronary myogenic reactivity caused by cocaine in males. In contrast, in cocaine-treated female offspring, the increased coronary myogenic tone was blocked by L-NNA. This finding is surprising and suggests a stimulatory role of eNOS in the regulation of coronary myogenic tone in the cocaine-treated females. One possible explanation is that fetal cocaine exposure causes reprogramming of eNOS “uncoupling” in coronary arteries thereby generating reactive oxygen species (ROS) such as superoxide rather than NO, resulting in the increased myogenic tone. This notion is supported by the recent findings that cocaine increased NADPH oxidase and ROS production in the heart,25–27 and that sustained elevation of NADPH oxidase and ROS led to eNOS uncoupling and endothelial dysfunction.28–30
In the present study, we have demonstrated that cocaine-induced alterations in coronary myogenic tone are caused by changes in the Ca2+ sensitivity in both male and female offspring. This finding is consistent with growing evidence suggesting that mechanisms that regulate the Ca2+ sensitivity of the contractile apparatus in vascular smooth muscle form the backbone of pressure-induced myogenic vasoconstriction.31 Whereas the increased Ca2+ sensitivity in female offspring may be caused at least in part by aforementioned eNOS uncoupling and generation of ROS,31 distinct mechanisms are likely to be involved in the down-regulation of the Ca2+ sensitivity in male offspring. We have recently demonstrated that prenatal cocaine results in protein kinase C ε (PKCε) gene repression via promoter methylation in the heart of male, but not female, offspring.32 Given that PKC plays an important role in the regulation of the Ca2+ sensitivity and myogenic tone,31 it is plausible to suggest that the down-regulated PKC contributes to the decreased Ca2+ sensitivity in male coronary vessels.
PERSPECTIVES
The present finding of the long-term detrimental effect of prenatal cocaine exposure on coronary myogenic reactivity in adult offspring is in agreement with the recent findings that fetal cocaine results in the increased contractility of resistance vessels3 and heightened susceptibility of the heart to ischemia and reperfusion injury,2 and is consistent with other studies in humans and animal models,4,9,10 demonstrating that multiple in utero adverse factors during gestation cause fetal programming and increase the risk of hypertension and coronary heart disease in the adult. Given that cocaine abuse in pregnant women is a significant problem, these findings should have a clinical significance and suggest that intrauterine cocaine exposure is a risk factor for morbidity and mortality secondary to myocardial ischemia in later life in offspring. The striking sex difference in programming of coronary myogenic reactivity warrants further investigation of possible roles of sex steroid hormones in fetal origins of coronary heart disease.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants HL82779 (LZ), HL83966 (LZ), and S06GM073842 (SY).
Footnotes
Disclosures: None.
References
- 1.Lipshultz SE, Frassica JJ, Orav EJ. Cardiovascular abnormalities in infants prenatally exposed to cocaine. J Pediatr. 1991;118:44–51. doi: 10.1016/s0022-3476(05)81842-6. [DOI] [PubMed] [Google Scholar]
- 2.Bae S, Gilber RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia-reperfusion injury in adult male but not female rats. J Physiol. 2005;565:149–158. doi: 10.1113/jphysiol.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao D, Huang X, Xu Z, Yang S, Zhang L. Prenatal cocaine exposure differentially causes vascular dysfunction in adult offspring. Hypertension. 2009;53:937–943. doi: 10.1161/HYPERTENSIONAHA.108.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 6.Skarsgard PL, Wang X, McDonald P, Lui AH, Lam EK, McManus BM, van Breemen C, Laher I. Profound inhibition of myogenic tone in rat cardiac allografts is due to eNOS-and iNOS-based nitric oxide and an intrinsic defect in vascular smooth muscle contraction. Circulation. 2000;101:1303–1310. doi: 10.1161/01.cir.101.11.1303. [DOI] [PubMed] [Google Scholar]
- 7.Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of PKC/ERK1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do Carmo Pinho Franco M, Nigro D, Fortes ZB, Tostes RC, Carvalho MH, Lucas SR, Gomes GN, Coimbra TM, Gil FZ. Intrauterine undernutrition - renal and vascular origin of hypertension. Cardiovasc Res. 2003;60:228–234. doi: 10.1016/s0008-6363(03)00541-8. [DOI] [PubMed] [Google Scholar]
- 10.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–H682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hemmings DG, Veerareddy S, Baker PN, Davidge ST. Increased myogenic responses in uterine but not mesenteric arteries from pregnant offspring of diet-restricted rat dams. Biol Reprod. 2005;72:997–1003. doi: 10.1095/biolreprod.104.035675. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF. Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2006;290:H1081–H1089. doi: 10.1152/ajpheart.00752.2005. [DOI] [PubMed] [Google Scholar]
- 14.Nemes A, Forster T, Csanády M. Reduction of coronary flow reserve in patients with increased aortic stiffness. Can J Physiol Pharmacol. 2007;85:818–822. doi: 10.1139/y07-075. [DOI] [PubMed] [Google Scholar]
- 15.Kern MJ, Bach RG, Mechem CJ, Caracciolo EA, Aguirre FV, Miller LW, Donohue TJ. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28:1154–1160. doi: 10.1016/S0735-1097(96)00327-0. [DOI] [PubMed] [Google Scholar]
- 16.Mehta MC, Jain AC, Billie M. Effects of cocaine and caffeine alone and in combination on cardiovascular performance: an experimental hemodynamic and coronary flow reserve study in a canine model. Int J Cardiol. 2004;97:225–232. doi: 10.1016/j.ijcard.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Roghair RD, Segar JL, Sharma RV, Zimmerman MC, Jagadeesha DK, Segar EM, Scholz TD, Lamb FS. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1169–R1176. doi: 10.1152/ajpregu.00369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao D, Huang X, Lawrence J, Yang S, Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther. 2007;320:654–661. doi: 10.1124/jpet.106.113332. [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen AJM, Dankelman J, VanBavel E, Stassen HG, Spaan JAE. Myogenic reactivity and resistance distribution in the coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol. 2000;278:H1490–H1499. doi: 10.1152/ajpheart.2000.278.5.H1490. [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen AJM, Dankelman J, VanBavel E, Spaan JAE. Balance between myogenic, flow-dependent, and metabolic flow control in coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol. 2002;282:H2224–H2237. doi: 10.1152/ajpheart.00491.2001. [DOI] [PubMed] [Google Scholar]
- 21.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006;86:1263–1308. doi: 10.1152/physrev.00029.2005. [DOI] [PubMed] [Google Scholar]
- 22.Chan SY, Kobashigawa J, Stevenson LW, Brownfield E, Brunken RC, Schelbert HR. Myocardial blood flow at rest and during pharmacological vasodilation in cardiac transplants during and after successful treatment of rejection. Circulation. 1994;90:204–212. doi: 10.1161/01.cir.90.1.204. [DOI] [PubMed] [Google Scholar]
- 23.Mericli M, Nádasy GL, Szekeres M, Várbíró S, Vajo Z, Mátrai M, Acs N, Monos E, Székács B. Estrogen replacement therapy reverses changes in intramural coronary resistance arteries caused by female sex hormone depletion. Cardiovasc Res. 2004;61:317–324. doi: 10.1016/j.cardiores.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 25.Fan L, Sawbridge D, George V, Teng L, Bailey A, Kitchen I, Li JM. Chronic cocaine-induced cardiac oxidative stress and mitogen-activated protein kinase activation: the role of Nox2 oxidase. J Pharmacol Exp Ther. 2009;328:99–106. doi: 10.1124/jpet.108.145201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isabelle M, Vergeade A, Moritz F, Dautréaux B, Henry JP, Lallemand F, Richard V, Mulder P, Thuillez C, Monteil C. NADPH oxidase inhibition prevents cocaine-induced up-regulation of xanthine oxidoreductase and cardiac dysfunction. J Mol Cell Cardiol. 2007;42:326–332. doi: 10.1016/j.yjmcc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Moritz F, Monteil C, Isabelle M, Bauer F, Renet S, Mulder P, Richard V, Thuillez C. Role of reactive oxygen species in cocaine-induced cardiac dysfunction. Cardiovasc Res. 2003;59:834–843. doi: 10.1016/s0008-6363(03)00499-1. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 29.Münzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 30.Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 31.Schubert R, Lidington D, Bolz S. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Meyer K, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod. 2009;80:440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.