Abstract
Background
It is uncertain whether depression and antidepressant use are associated with decreased bone mineral density (BMD) and whether these relationships differ for men and women.
Methods
The study used a case-cohort design within the Baltimore Epidemiologic Catchment Area Study, a population-based sample of adults that recently completed its 23-year follow-up. Depression was measured at four time points during the follow-up period by the Diagnostic Interview Schedule. Lower spine BMD was measured at the fourth wave by dual-energy x-ray absorptiometry. The association of BMD with lifetime history of depression and antidepressant medication use was studied using linear regression with bootstrap standard errors.
Results
A history of depression was associated with lower spine BMD after controlling for age, sex, race, calcium intake, alcohol use, smoking status, level of physical activity, percent body fat, and antidepressant medication use (−0.140 g/cm2; p < .002). After controlling for depression, antidepressant medication use was associated with decreased BMD in women but not in men (−0.218 g/cm2; p < .016).
Conclusions
A history of depression predicted decreased lumbar spine BMD in men and women, and antidepressant use predicted decreased BMD in women even after controlling for depression. The magnitude of the effect of depression on BMD was approximately equivalent to 1 standard deviation in BMD and was therefore clinically significant. Providers should be aware of the physiologic consequences of depression as well as the possible risks to bone strength associated with antidepressant use in older patients.
Keywords: Depression, Antidepressants, Bone mineral density, Osteoporosis, Cohort study
Osteoporosis is a common disorder affecting both genders and all racial/ethnic groups. The National Osteoporosis Foundation has projected that by 2020 47.5 million U.S. adults older than 50 years will have osteopenia and another 13.9 million will have osteoporosis of the hip (1). Low bone mineral density (BMD) and risk of falling are primary mediators of fracture risk in elderly populations. Fractures, especially those of the hip and vertebrae, are associated with significant increases in functional disability and mortality (2). Major depressive disorder (MDD) is one of the most prevalent psychiatric conditions, affecting approximately 16% of the U.S. adult population (3). MDD and depressive symptoms commonly co-occur with physical ailments, and it has been suggested that depression may be an unrecognized risk factor for osteoporosis (4). Most studies have found an inverse association between depression and BMD; however, others have found no effect (5).
One potential confounder of the relationship between depression and BMD is the use of medications that have the potential to affect either bone strength or risk of fracture. There have been numerous studies indicating that antidepressant use increases risk of falls (6) and fracture (7) in geriatric populations, but the mechanism underlying this relationship is unknown. Animal studies have indicated that serotonin may influence bone mass (8), and Battaglino and colleagues (9) reported that daily injections of the selective serotonin reuptake inhibitor (SSRI) fluoxetine in mice increased bone formation, but that this effect was not observed in estrogen-deficient animals, which indicates that the effect of antidepressants on bone metabolism may depend on sex steroids (e.g., menopausal status in humans). Preliminary findings from human studies suggest that antidepressant use may adversely affect BMD. In cross-sectional analyses, current use of SSRIs, but not other classes of antidepressants, has been associated with lower lumbar spine (10,11) and hip (10,11) BMD in older men. In prospective analyses, both current (12) and baseline SSRI use (13), but not tricyclic antidepressant (TCA) use (13), have been associated with decreased hip BMD after 5 years. However, one cross-sectional study found no association between current use of any class of antidepressants and BMD (14). Notably, none of these studies controlled for MDD, although two reports adjusted for depressive symptoms as measured by the Short Form-36 and Geriatric Depression Scale (GDS) (12,13). In light of these results, it remains unresolved whether the association between antidepressant use and BMD is an example of confounding by indication.
This study investigates the relationships between MDD, antidepressant use, and BMD in a population-based sample of adults at least 60 years old. Two hypotheses are evaluated: (i) Are MDD and antidepressant use independently associated with BMD? If so, (ii) Do those associations vary by sex?
Methods
Sample
The Baltimore Epidemiologic Catchment Area (ECA) Study is a population-based sample of East Baltimore residents, and interviews were completed by 3481 participants older than 18 years in 1981 (15). The original study sample was 62% female and 63% white, with African Americans making up most of the remainder (33.9%). Follow-up interviews were conducted in 1982 (n = 2768), 1993–1996 (n = 1920), and 2004–2005 (n = 1071).
Recruitment for the Baltimore Health and Mental Health Study–Depression and Osteoporosis (BHMH-DO) was contingent on participation in the Baltimore Health and Mental Health Study: Mood and Memory Project (MMP) at Johns Hopkins Hospital (JHH), which targeted surviving ECA participants at least 60 years old interviewed in 2004–2005 (n = 398). In the MMP, eligible participants were recruited approximately 1 year after completion of the 2004–2005 follow-up and asked to come to JHH for a 4-hour neuropsychiatric evaluation. One hundred seven of the 398 ECA participants older than 60 years in 2004–2005 came to JHH in 2005–2006 to participate in the MMP. These participants were invited to have their BMD tested in the BHMH-DO. Efforts were made to have BMD measured on the same day as the MMP, and all dual-energy x-ray absorptiometry (DEXA) scans were completed within 6 months of the MMP visit.
The Johns Hopkins Medical Institutions Institutional Review Board (IRB) approved study procedures for the MMP. The Johns Hopkins Bloomberg School of Public Health IRB approved procedures for the BHMH-DO. All participants provided informed written consent.
Measures
Depression
Depression was assessed using the Diagnostic Interview Schedule (DIS) during all four ECA interviews. The DIS is a structured interview administered by laypersons and establishes diagnoses of MDD and depression syndromes using diagnostic algorithms, as well as the onset and recency of those conditions (16). The DIS has moderate concordance with clinical examinations such as the Schedules for Clinical Assessment in Neuropsychiatry (17). Measures summarizing lifetime MDD, number of depressive symptoms, and frequency and duration of depression episodes were generated by merging DIS responses from all four interviews. Current depressive symptoms were measured at the time of the MMP interview by the 30-item GDS (18).
BMD
BMD (g/cm2) and body composition (percent lean and fat) were assessed via whole-body DEXA scan (Hologic QDR 4500W, serial number 49694; Waltham, MA) and analyzed by a registered nurse using Hologic software version 12.3:5 at Johns Hopkins General Clinical Research Center Outpatient Clinic. The nurse was blinded as to the case status of the participants. Whole-body region BMD has been shown to have good concordance with site-specific DEXA measurements, with particularly good agreement between the lumbar spine subregion and the L2–L4 anteroposterior projection (r2 = 0.91–0.93) (19).
Medication use
Medication use was measured in two different modalities during the ECA fieldwork. During the 1981 and 1982 interviews, participants were asked explicitly about lifetime use of antidepressant medications by name while being shown color images of the medication pills. In the 1993–1996 and 2004–2005 interviews, participants were asked to list all medications taken in the past 7 days and to provide their medication bottles for inspection by the interviewer. Responses were evaluated using the Physicians Desk Reference (20) and consensus among the investigators. They were coded into eight categories: (i) statins, (ii) thiazide diuretics, (iii) antidepressants, (iv) antiepileptic agents, (v) antiosteoporotic agents, (vi) neuroleptic agents, (vii) estrogen/hormone replacement therapy, or (viii) medication not of interest. Antidepressants were further categorized according to class: SSRI or selective serotonin and neuroepinephrine reuptake inhibitor (SSNRI), mono-amine oxidase inhibitor (MAOI), or TCA. Indicators of lifetime, continuous, and recent use were generated by merging responses from all four interviews.
Other covariates
Demographic characteristics, years of education, smoking status (current [smoked within the past year] or former/never), alcohol use (number of days drank in past month [range 0–30]), and self-rated health were assessed at the 2004–2005 ECA interview. Level of physical activity and current calcium supplement use were ascertained by self-report on the same day as the DEXA scan. Level of physical activity was assessed by using a modified version of the Physical Activity Scale for the Elderly (PASE) (21). Adiposity was measured as total percent fat assessed by the DEXA scan. The indicator of total percent fat from DEXA scan has been shown to be a more accurate measure of body fat composition than more commonly used anthropomorphic measures (i.e., body mass index) (22,23).
Statistical Analysis
Linear regression models with lower spine (approximately lumbar regions L1–L4) BMD measurements as the outcome were fit using ordinary least squares (OLS). The BMD values were approximately normally distributed. Because standard OLS estimates of precision are dependent on the assumption of a large number of observations (n > 50), standard errors were obtained using the bootstrap method (replications: 1000). The bootstrap method builds a data set of replicated statistics through random sampling with replacement to estimate the average standard error of the regression coefficients, but does not change the estimation of the regression coefficient itself (24). Participants with medical devices that affected the lower spine BMD measurements were removed from analysis. Physical activity items from the PASE were evaluated individually and as a summed measure. The moderating influence of sex on the associations between depression and antidepressant medication use with BMD was investigated using both interaction terms and stratification. Because of the limited sample size, potential confounding variables (education, level of physical activity, percent fat, smoking status, alcohol intake, calcium use, other medications) were only included in the final regression models if (i) literature suggested that the covariate was an important predictor of bone strength, and (ii) the variable was associated (p < .20) with lower spine BMD when the primary exposures of interest, depression and antidepressant use, were included in the model. All analyses were conducted using STATA (version 9) software (STATA Corp., College Station, TX) and all p values refer to two-tailed tests.
Results
Ninety-eight of the 107 respondents (91.6%) who participated in the MMP at the JHH agreed to participate in the BHMH-DO Study, and whole-body BMD measurements were obtained for 97 of them. Five refused or were unable to make a return trip to JHH (4.7%), two (1.9%) could not be located, and two (1.9%) died before an appointment for the DEXA scan could be scheduled. A DEXA was unable to be performed on one participant because of the size restrictions of the apparatus. Persons who participated in the BHMH-DO Study were similar to the entire ECA cohort older than 60 years (Table 1). All women were postmenopausal, and 36 (54.5%) had used estrogen or hormone replacement therapy. Twenty-eight participants had used antidepressant medications, and 14 (50%) reported use at all four waves (continuous users). The average number of depressive symptoms at MMP visit as measured by the GDS was 5.2, and only two participants had moderate/severe (21–30) depressive symptomatology, indicating that the majority of the sample was not currently depressed at the time of the DEXA scan (data not shown).
Table 1.
Descriptive Characteristics of DEXA Participants and the 2004–2005 ECA Cohort
| Characteristic | Entire 2004–2005 ECA Sample Age 60+ | DEXA Participants | p Value* |
|---|---|---|---|
| N | 398 | 98 | |
| Age in years, mean (SD) | 72.8 (10.0) | 71.5 (6.6) | .22 |
| Women, n (%) | 257 (64.6) | 66 (67.4) | .60 |
| White, n (%) | 265 (66.6) | 65 (66.3) | .96 |
| Education in years, mean (SD) | 11.2 (3.0) | 11.4 (2.7) | .55 |
| Current smoker, n (%) | 70 (17.6) | 17 (17.4) | .96 |
| Number of days drank per month, mean (SD) | 3.2 (7.3) | 2.8 (6.7) | .62 |
| Lifetime MDD, n (%) | 33 (8.9) | 10 (10.3) | .67 |
| Lifetime antidepressant use, n (%) | 82 (20.6) | 28 (28.6) | .09 |
| Self-rated good/excellent health, n (%) | 198 (59.5) | 67 (69.8) | .06 |
Notes:
p value for t tests for continuous variables and likelihood chi-square tests for categorical variables.
DEXA = dual-energy x-ray absorptiometry; ECA = (Baltimore) Epidemiologic Catchment Area; SD = standard deviation; MDD = major depressive disorder.
Depression and Lower Spine BMD
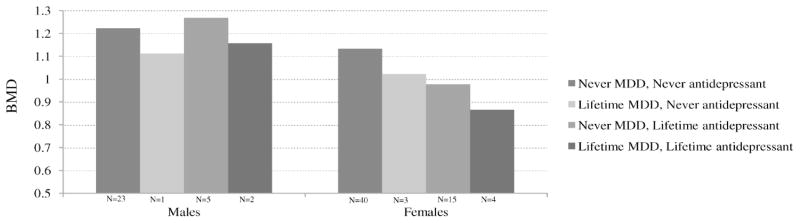
A history of depression was associated with decreased lumbar spine BMD (Figure 1), a finding that persisted after controlling for age, sex, race, total percent fat, current calcium use, smoking status, number of drinks in the past month, and lifetime antidepressant use (−0.140 g/cm2, p < .003), which together explained 32% of the variance in BMD (Table 2). The interaction of depression and sex was not statistically significant. In the sex-stratified analyses, depression was associated with significantly lower BMD in women (r2 = 25%), but was not associated with BMD in men (Table 3) (r2 = 57%). There was a marginally statistically significant (p < .1) association between number of depressive symptoms (−0.004 g/cm2) and duration of depression episodes (−0.086 g/cm2) and BMD, but their effects were attenuated and no longer statistically significant after adjusting for antidepressant use. There was no association between lifetime number of depressive episodes or number of depressive symptoms and BMD (data not shown). Use of glucocorticoids, statins, and thiazide diuretics did not substantially alter either the magnitude or statistical significance of the association between lifetime MDD and BMD (data not shown).
Figure 1.
Age-adjusted mean lower spine bone mineral density (g/cm2) by lifetime status of major depressive disorder (MDD) and antidepressant use. Never MDD, never antidepressant (N = 23 for men, N = 40 for women); lifetime MDD, never antidepressant (N = 1 for men, N = 3 for women); never MDD, lifetime antidepressant (N = 5 for men, N = 15 for women); lifetime MDD, lifetime antidepressant (N = 2 for men, N = 4 for women). Antidepressant = lifetime antidepressant use. BMD = bone mineral density (g/cm2). Excludes three participants with medical devices in the lower spine region.
Table 2.
Association of Depression and Antidepressant Use With Lower Spine Bone Mineral Density (g/cm2) for Entire Sample
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) |
p Value | Coefficient (95% CI) |
p Value | Coefficient (95% CI) |
p Value |
| Lifetime MDD (ref. Never) | −0.134 (−0.238, −0.029) |
.012 | −0.109 (−0.197, −0.022) |
.014 | −0.140 (−0.230, −0.049) |
.003 |
| Lifetime antidepressant use (ref. Never) | — | 0.052 (−0.121, 0.226) |
.554 | 0.067 (−0.073, 0.205) |
.354 | |
| Sex × Antidepressant use (ref. Male, Never use) | — | −0.204 (−0.402, −0.008) |
.042 | −0.218 (−0.398, −0.029) |
.017 | |
Notes: Model 1 is adjusted for age, race, and sex. Model 2 is adjusted for Model 1 plus lifetime antidepressant use and lifetime antidepressant use × sex. Model 3 is adjusted for Model 2 plus calcium use, smoking status, alcohol use, Physical Activity Scale for the Elderly score, and total percent fat. N = 94 for all models.
CI = confidence interval; MDD = major depressive disorder.
Table 3.
Association of Depression and Antidepressant Use With Lower Spine Bone Mineral Density (g/cm2) Stratified by Sex
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) |
p Value | Coefficient (95% CI) |
p Value | Coefficient (95% CI) |
p Value |
| Women | ||||||
| Lifetime MDD (ref. Never) | −0.180 (−0.299, −0.060) |
.003 | −0.132 (−0.244, −0.021) |
.020 | −0.162 (−0.285, −0.039) |
.010 |
| Lifetime antidepressant use (ref. Never use) | −0.156 (−0.266, −0.047) |
.005 | −0.141 (−0.263, −0.020) |
.023 | ||
| Men | ||||||
| Lifetime MDD (ref. Never) | 0.005 (−0.157, 0.166) |
.955 | −0.035 (−0.205, 0.136) |
.691 | −0.106 (−0.267, 0.056) |
.199 |
| Lifetime antidepressant use (ref. Never use) | 0.094 (−0.087, 0.274) |
.308 | 0.073 (−0.086, 0.232) |
.366 | ||
Notes: For women (N = 63): Model 1 is adjusted for age and race. Model 2 is adjusted for Model 1 plus lifetime antidepressant use. Model 3 is adjusted for Model 2 plus calcium use, total percent fat, and lifetime estrogen use. For men (N = 31): Model 1 is adjusted for age and race. Model 2 is adjusted for Model 1 plus lifetime antidepressant use. Model 3 is adjusted for Model 2 plus smoking status, alcohol use, Physical Activity Scale for the Elderly score, and total percent fat.
CI = confidence interval; MDD = major depressive disorder.
Antidepressant Use and BMD
The interaction between sex and lifetime antidepressant use was statistically significant (Table 2), indicating that antidepressant use was associated with lower BMD in women but not in men. In the sex-stratified models, anti-depressant use was associated with decreased BMD in women (Figure 1), a finding that persisted after controlling for depression, age, race, total percent fat, and calcium supplement use (Table 3). Use of other medications did not alter either the magnitude or statistical significance of the association between antidepressant use and BMD (data not shown). Continuous use of antidepressant medications (defined as use reported at all four survey waves) was associated with lower BMD (−0.124 g/cm2, p < .05), but this association was attenuated (−0.096 g/cm2) and no longer statistically significant after controlling for the effects of depression. There was no association between antidepressant use at the 2004–2005 interview as compared to former and never use (data not shown). In post hoc analyses, there was no association between antidepressant use and two conditions commonly treated with antidepressants, generalized anxiety disorder (GAD) and diabetic neuropathy (data not shown).
A sensitivity analysis was conducted to determine if the results reported above were caused primarily by a few influential data points. When outliers (as indicated by box plots) were excluded from the analysis, the results were similar and the interpretation of the effects of depression or antidepressants on BMD did not change (data not shown).
Discussion
In this nested case–cohort study of community-dwelling older adults, a lifetime history of major depression disorder, measured over a 23-year follow-up period, was associated with significantly reduced lower spine BMD after controlling for potential confounding influences. The magnitude of this difference (−0.125) is approximately equal to 1 standard deviation in lumbar spine BMD, which suggests that depression is a clinically significant risk factor for low BMD and osteoporosis. The World Health Organization estimates that for every 1 standard deviation decrease in BMD, the relative risk of fracture increases between 1.5- and 3.1-fold (25).
Characteristics of depression such as number of episodes and duration of symptoms were not associated with BMD in our analysis. Because of the relatively small sample size, this null finding may represent type II error as a result of limited power. Alternatively, this may suggest that the severity or duration of depression does not influence bone mass after the threshold of depressive disorder is reached.
In this population-based sample of older adults, nearly three times as many persons reported taking antidepressants as were identified as having a lifetime history of depression. Although the DIS is a conservative measure of clinically meaningful depression (17), this trend warrants concern given the finding that lifetime antidepressant use was associated with significantly decreased BMD even after controlling for depression status. Antidepressants may be prescribed for indications other than depression, but in post hoc analyses their use was not associated with either diabetic neuropathy or GAD. Antidepressant use is associated with risk of fracture in older adults (7), but the mechanism underlying this relationship is unclear. Therefore, although the results of this study are consistent with the hypothesis that these medications affect bone physiology, they do not preclude that antidepressants affect fracture risk through other pathways. It may be that antidepressant use is a marker of depression severity and that the effect on BMD associated with their use is partially attributable to depressive symptomatology, a type of confounding by indication. Animal studies had suggested that SSRIs may increase bone formation, but this effect was estrogen-dependent, and because the women in our study were postmenopausal we could not evaluate the impact of early-life SSRI use on BMD. Both depression and antidepressant use were associated with reduced BMD after controlling for the other’s effects, which is consistent with the hypothesis that they represent independent risk factors for low BMD rather than components of a common etiologic pathway.
These findings should be interpreted in light of the study limitations. Antidepressant medication use was measured at only four time points during the follow-up period, and subsequently it is possible that persons who only took antidepressant medications for a brief period of time were misclassified as never-users. BMD was measured at only one point in time—late in the life course—thus it cannot be determined if depression and antidepressant use affects bone accrual, bone loss, or both. Also, because of changes in patterns of prescribing antidepressants (26) the effects of recency of use from type of medication used could not be separated, which precludes a more nuanced examination of the physiologic mechanisms by which antidepressant use impacts bone physiology. As discussed above, the best concordance between region- and site-specific BMD measurements is for the lower spine/L2–L4 regions [i.e., concordance between pelvis region and total hip BMD is only r2 = 0.66–0.74 (19)], thus we only investigated lower spine BMD to improve the comparability of our findings with previous reports that used site-specific DEXA. However, a limitation of using this region is that it is an area that may be affected by osteoarthritis in older adults. Finally, the small sample size limited the statistical power to detect a significant effect or to completely exclude the effects of potential confounders, particularly among men in the stratified analyses and in the analyses of the influence of characteristics of depression and antidepressant use on BMD.
Despite these limitations, this study has the strengths of using a prospective, comprehensive diagnostic assessment of MDD and of evaluating the effects of antidepressant use while adjusting for depression, which previous studies of antidepressants and BMD had not done. Future research should examine which aspects of depression (e.g., physiologic changes such as hypercortisolism and/or behavioral changes such as reduced physical activity) mediate the relationship with bone physiology. Studies of biochemical mechanisms that link bone metabolism to other physiologic systems are needed to better inform how medications may impact these processes.
Acknowledgments
This work was supported by National Institute of Mental Health grants T32-MH14592, R01-MH47447, F31-MH78443, and K23-MH068793 and by Johns Hopkins General Clinical Research grant M01RR02719.
We thank Jessica Yokely for her assistance with data collection and study management.
Portions of the manuscript were presented at the American Psychopathological Association meeting, March 1–3, 2007, in New York, New York.
References
- 1.U.S. Department of Health and Services. Bone health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 2.Forsen L, Sogaard A, Meyer H, et al. Survival after hip fracture: short and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 3.Kessler R, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Cizza G. Depression: a major, unrecognized risk factor for osteoporosis? In: Eaton WW, editor. Medical and Psychiatric Comorbidity over the Course of Life. Arlington, VA: American Psychiatric Publishing, Inc.; 2006. pp. 129–153. [Google Scholar]
- 5.Mezuk B, Eaton WW, Golden S. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int. 2008;19:1–12. doi: 10.1007/s00198-007-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landi F, Onder G, Cesari M, et al. Psychotropic medications and risk for falls among community-dwelling frail older people: an observational study. J Gerontol A Biol Sci Med Sci. 2005;60:622–626. doi: 10.1093/gerona/60.5.622. [DOI] [PubMed] [Google Scholar]
- 7.Takkouche B, Montes-Martinez A, Gill S, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30:171–184. doi: 10.2165/00002018-200730020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Warden S, Robling A, Sanders M, et al. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 9.Battaglino R, Vokes M, Schulze-Spate U, et al. Fluoxetine treatment increases trabecular bone formation in mice. J Cell Biochem. 2007;100:1387–1394. doi: 10.1002/jcb.21131. [DOI] [PubMed] [Google Scholar]
- 10.Cauley J, Fullman R, Stone K, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 11.Haney E, Chan B, Diem S, et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–1251. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- 12.Richards J, Papaioannou A, Adachi J, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 13.Diem S, Blackwell T, Stone K, et al. Use of antidepressants and rates of hip bone loss in older women. Arch Intern Med. 2007;167:1240–1245. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo M, Setoguchi S, Schneeweiss S, et al. Bone mineral density in subjects using central nervous system-active medications. Am J Med. 2005;118:1414. doi: 10.1016/j.amjmed.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Regier D, Myers J, Kramer M, et al. The NIMH Epidemiologic Catchment Area program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41:934–941. doi: 10.1001/archpsyc.1984.01790210016003. [DOI] [PubMed] [Google Scholar]
- 16.Robins L, Helzer J, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 17.Eaton WW, Neufeld K, Chen L, et al. A comparison of self-report and clinical diagnostic interviews for depression: Diagnostic Interview Schedule and Schedules for Clinical Assessment in Neuropsychiatry in the Baltimore Epidemiologic Catchment Area followup. Arch Gen Psychiatry. 2000;57:217–222. doi: 10.1001/archpsyc.57.3.217. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage J, Brink T, Rose T, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ, 3rd, Looker A, Shepherd J, et al. Osteoporosis assessment by whole body region vs. site-specific DXA. Osteoporos Int. 2005;16:1558–1564. doi: 10.1007/s00198-005-1871-y. [DOI] [PubMed] [Google Scholar]
- 20.Physicians’ Desk Reference(PDR) Oradell, NJ: Medical Economics Co; 2006. [Google Scholar]
- 21.Washburn R, Smith K, Jette A, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:152–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 22.Ravaglia G, Forti P, Maioli F, et al. Measurement of body fat in healthy elderly men: a comparison of methods. J Gerontol A Biol Sci Med Sci. 1999;54A:M70–M76. doi: 10.1093/gerona/54.2.m70. [DOI] [PubMed] [Google Scholar]
- 23.Bolanowski M, Nilsson B. Assessment of human body composition using dual-energy x-ray absorptiometry and bioelectrical impedance analysis. Med Sci Monit. 2001;7:1029–1033. [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani R. Bootstrap measures for standard errors, confidence intervals and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 25.World Health Organization. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis: report of the WHO study group. World Health Org Tech Rep Ser. 1994;843:1–126. [PubMed] [Google Scholar]
- 26.Paulose-Ram R, Safran M, Jonas B, et al. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16:560–570. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]