Abstract
Aims
Leiomyosarcomas (LMS) are malignant neoplasms composed of cells that exhibit distinct smooth muscle differentiation. The molecular and cytogenetic features of LMS are complex and no consistent aberrations have been reported to date. Mitogen inducible gene-2 (Mig-2), kindlin and migfilin are recently identified cell–matrix adhesion proteins. The aim was to determine the expression and distribution of these proteins in human smooth muscle tumours of somatic soft tissue.
Methods and results
Immunohistochemistry was performed on a human LMS tissue microarray and on sections of human leiomyomas (LM) and normal smooth muscle. Migfilin was barely detectable in normal smooth muscle cells, whereas increased levels of migfilin were observed in the majority of LM and LMS. Furthermore, the cytoplasmic level of migfilin was strongly associated with higher tumour grades. Additionally, the cytoplasmic levels of migfilin and Mig-2 were correlated with each other, suggesting an association between the two in the cytoplasm. Kindlin was expressed in normal smooth muscle, LM and LMS, and its level did not correlate with tumour grade.
Conclusions
Our results suggest a role for cytoplasmic migfilin in the progression of LMS and identify cytoplasmic migfilin as a potentially important biological marker for human LMS progression.
Keywords: cell–matrix adhesion, kindlin, leiomyosarcoma, Mig-2, migfilin, tissue microarray
Introduction
Soft tissue leiomyosarcomas (LMS) are malignant neoplasms composed of cells that exhibit distinct smooth muscle differentiation.1,2 They usually occur in middle-aged or older people and represent 5–10% of all soft tissue sarcomas. Soft tissue LMS can be divided into four subgroups depending on their location: retroperitoneal LMS (including the pelvis), LMS arising from large blood vessels (commonly inferior vena cava), non-retroperitoneal soft tissue LMS (most frequently of the lower extremities) and LMS of dermis.2 Although LMS have been studied extensively, their pathogenesis is unclear. Their molecular and cytogenetic features are complex.3 Identification of biological markers and elucidation of the molecular mechanisms underlying the pathogenesis of LMS are therefore important.
Many fundamental cellular processes, such as cell proliferation, differentiation, survival and motility, are highly dependent on cell–cell and cell–extracellular matrix (ECM) adhesion.4,5 Integrins, the trans-membrane receptors that connect the ECM with the inner part of the cell, are associated with a number of proteins that can serve as docking sites for protein–protein interactions and/or as signal transducers facilitating communication.6-10 Thus, alterations in the expression or subcellular distribution of cell–ECM adhesion proteins are frequently associated with the pathogenesis and progression of human cancers.
Recent studies have identified mitogen inducible gene-2 (Mig-2) and kindlin (also known as kindlerin) as key regulators of cell–ECM adhesion, signalling and the actin cytoskeleton.11-14 They are encoded by different genes in humans but share significant sequence similarities. The functional importance of Mig-2 and kindlin in cell–ECM adhesion, cytoskeleton organization and signalling is manifested by recent findings from genetic, cell biological and clinical studies. First, genetic studies in Caenorhabditis elegans have shown that UNC-112, the C. elegans orthologue of human Mig-2/kindlin, is essential for integrin-mediated ECM adhesion.12 Second, in human cells, Mig-2 and kindlin are clustered at cell–ECM adhesions and loss of Mig-2 or kindlin reduces cell spreading.13,15 Third, human genetic analyses have shown that loss of kindlin results in the development of Kindler syndrome,16,17 which is characterized by skin blister formation, cytoskeleton alterations, atrophy and other defects in epidermis and mucosal membranes of the digestive, urinary tract and other tissues.18-20
Migfilin is a recently identified Mig-2- and filamin-binding focal adhesion protein.13,21-23 Interestingly, migfilin affects Mig-2 functions at cell–ECM adhesions, indicating that migfilin and Mig-2 work in concert to modulate cell–ECM adhesion and shape change.13 Notably, migfilin is also involved in the organization of cell–cell adhesion in epithelial and endothelial cells, since depletion of migfilin leads to disorganized adherens junctions and weakened cell–cell adhesion.23 In addition, migfilin can translocate into the nucleus and regulate gene expression.21 Although studies in cultured cells have suggested important roles of migfilin in cell adhesion, cytoskeleton organization and migration, the expression of migfilin in human tissues, either in normal or diseased conditions, is unknown.
In the present study, we analysed the expression and distribution of migfilin, Mig-2 and kindlin in normal human soft tissues (including smooth muscle from various locations, endothelium, fibroblasts and myofibroblasts), soft tissue leiomyomas (LM), as well as primary, recurrent and metastatic human LMS.
Materials and methods
SAMPLES
Forty well-characterized classical human LMS (31 high grade and nine low grade) and normal smooth muscle were used for the construction of the tissue microarray (TMA) following approval of the study by the Institutional Review Board (IRB). The TMA was constructed by a pathologist (U.N.M.R.). In addition, a set of 21 LM (12 were located in the soft tissue, nine were cutaneous) were examined. This was a blinded study, because the specimens were anonymized per IRB regulations and therefore the histopathological evaluations were objective. The clinical data were linked by an honest broker. Clinical information, including patient’s history, age and gender, tumour grade, size and location, was available only to the honest broker.
The tumours were classified as LMS based on morphological characteristics, as previously described.1,2 In addition, with immunohistochemistry they were positive for desmin, actin and calponin and negative for CD34, cytokeratin, S100 and C-KIT. Thirteen of the LMS patients were male (mean age 63 years, range 35–75 years) and 27 female (mean age 72 years, range 35–95 years). Twenty-five (62.5%) of the tumours were primary, whereas six (15.0%) were locally recurrent and nine (22.5%) metastatic. Fifteen (60%) of the primary LMS were located in the deep soft tissues, whereas 10 (40%) had a retroperitoneal location. Twenty-five LMS were ≥ 50 mm and 15 were < 50 mm in greater diameter. They were classified as grade 1, 2 and 3 according to their histological type, cellularity, pleomorphism and mitotic index.24,25 Grade 1 LMS were considered as low grade, grades 2 and 3 as high-grade neoplasms.24
GENERATION OF MONOCLONAL ANTI-KINDLIN ANTIBODY
Mouse monoclonal anti-migfilin and anti-Mig-2 antibodies have been previously described.13 To generate monoclonal anti-kindlin antibodies, a cDNA fragment was cloned encoding human kindlin residues 216–677 into the pGEX-5x-1 vector (Pharmacia, Sandwich, UK). The recombinant vector was used to transform Escherichia coli cells. The expression of the glutathione-S-transferase (GST)–kindlin fusion protein was induced with isopropyl β-D-1-thiogalactopyranoside. GST–kindlin fusion protein was purified by affinity chromatography using glutathione-Sepharose 4B. Purified GST–kindlin fusion protein was used as an antigen to immunize mice, as we have previously described.13,24 Hybridoma supernatants were initially screened for anti-kindlin activity by enzyme-linked immunosorbent assay (ELISA) using maltose-binding protein (MBP) fusion proteins containing kindlin residues 216–677. Antibodies that recognize MBP–kindlin in ELISA were selected and further tested by Western blotting using green fluorescent protein (GFP)-tagged kindlin expressed by mammalian cells. The specificity of the monoclonal antibodies was confirmed by Western blot of kindlin-expressing and knocking-down cells as described in Results.
IMMUNOHISTOCHEMISTRY
The study was conducted on formalin-fixed paraffin-embedded tissue samples from primary, locally recurrent and metastatic LMS that were retrieved from the Department of Pathology, University of Pittsburgh and the University of Pittsburgh Medical Center Presbyterian-Shadyside Hospitals. The most representative areas of each tumour were selected for the construction of the TMA cores. Following depa-raffinization, standard immunohistochemistry was performed using purified mouse monoclonal anti-migfilin (1 μg IgG/ml), anti-Mig2 (3 μg IgG/ml) and anti-kindlin (16 μg IgG/ml) antibodies. Formalin-fixed paraffin-embedded tissue samples from LM were analysed by immunohistochemistry following the same protocol.
The intensity of the immunopositivity was evaluated independently by two pathologists (D.J.P. and U.N.M.R.) and was scored on a scale of 0–3+ according to the following assessment: 0, no immunoreactivity; 1+, weak; 2+, moderate; 3+, strong immunopositivity. There was a high level of consistency of staining between the duplicate TMA cores.
SMALL INTERFERENCE RNA AGAINST KINDLIN
Human immortalized keratinocytes (HaCaT) were transfected with a kindlin-specific small interfering (si) RNA with target sequence 5′-AAGACACAUCCAUAG CAUACU-3′ or a non-specific control RNA, using the Oligofectamine transfection system (Invitrogen, Carlsbad, CA, USA). Kindlin siRNA transfectants and control transfectants were analysed by Western blotting with monoclonal anti-kindlin antibody 4A5. Equal loading was confirmed by re-probing the same membrane with an anti-actin antibody.
IMMUNOFLUORESCENT CELL STAINING
Immunofluorescent staining was performed as described previously.25 Briefly, HaCaT cells were plated on fibronectin-coated cover slips, fixed with 4% paraformaldehyde and stained with either the anti-kindlin mouse monoclonal antibody and rhodamine-red-conjugated goat antimouse secondary antibody, or just the secondary antibody (as a negative control).
STATISTICAL ANALYSIS
Statistical analysis (χ2 test, Mann–Whitney U-test, Kendall’s τ-test, binary logistic regression) was performed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was considered to be statistically significant. The Mann–Whitney U-test was employed in order to test whether there were significant differences in means of the examined variables between high- and low-grade tumours. Kendall’s τ-test was used in order to determine the strength of association between the immunohistochemical profiles of the different proteins.
Results
GENERATION AND CHARACTERIZATION OF MONOCLONAL ANTI-KINDLIN ANTIBODIES
Kindlin is a recently identified focal adhesion protein,15,16 loss of which has been associated with the Kindler syndrome. The level of kindlin protein in human tumours, however, has not previously been analysed. To facilitate studies of kindlin protein levels in human tumours, we generated a monoclonal antibody against human kindlin protein as described in Materials and methods. Hybridoma supernatants were initially screened for anti-kindlin activity by ELISA using MBP fusion proteins containing kindlin residues 216–677. Antibodies that recognize MBP–kindlin in ELISA were selected and further tested by Western blotting using GFP-tagged kindlin expressed by mammalian cells. A positive clone (clone 4A5.14) was selected and subsequently used to detect kindlin in normal human immortalized keratinocytes (HaCaT cells) transfected with either a control RNA or a siRNA against kindlin. As shown in Figure 1, clone 4A5.14 recognizes a protein band with predicted human kindlin molecular mass (77.4 kDa). The level of this protein was dramatically reduced in the kindlin–siRNA-transfected cells (Figure 1, compare lane 1 with lane 2), confirming that clone 4A5.14 specifically recognizes kindlin.
Figure 1.
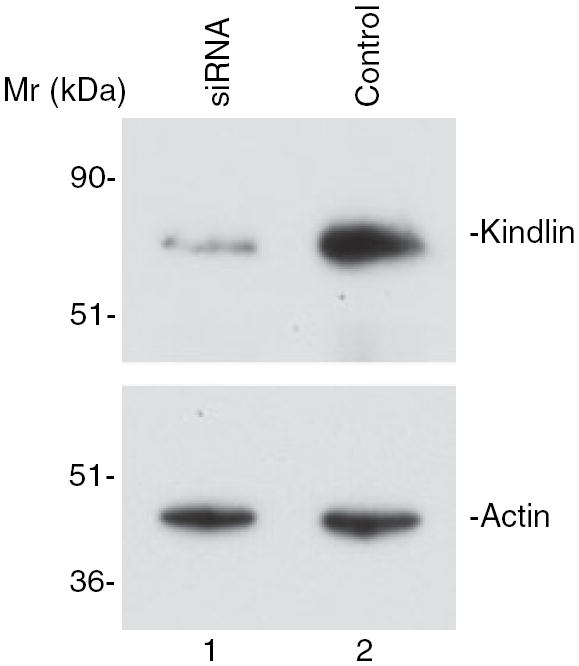
RNAi suppression of kindlin expression in human keratinocytes. Human HaCaT keratinocytes transfected with the kindlin small interfering (si) RNA (lane 1) or a control RNA (lane 2) were probed with monoclonal anti-kindlin antibody 4A5 (top panel) or an anti-actin antibody (bottom panel) as indicated. Note that the amount of kindlin-1 was substantially reduced in the kindlin siRNA transfectants (compare lanes 1 and 2, top panel).
To characterize further the 4A5.14 antikindlin antibody, immunofluorescence studies were performed using HaCaT cells. Clusters of kindlin were detected in focal adhesions consistent with previous findings18,19 (Figure 2B, arrows). No specific staining was observed when the cells were stained with just the secondary antibody (Figure 2A), confirming the specificity of the immunofluorescent staining.
Figure 2.
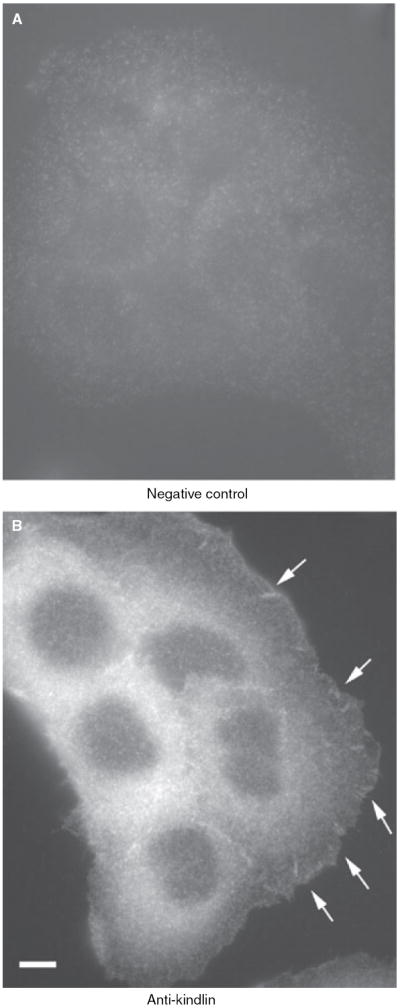
Localization of kindlin at cell–matrix adhesion sites in human keratinocytes. HaCat cells were stained with either the anti-kindlin monoclonal antibody (clone 4A5.14) and a Rhodamine Red™-conjugated goat antimouse IgG secondary antibody (B), or just the secondary antibody (A), which served as a negative control. (Leica DM R fluorescence microscope. Bar in panel B = 8 μm.)
EXPRESSION OF KINDLIN IN NORMAL HUMAN SMOOTH MUSCLE, LM AND LMS
To examine the level of kindlin in normal smooth muscle tissue, sections of normal smooth muscle were first stained using monoclonal anti-kindlin antibody 4A5.14. As shown in Figure 3A, kindlin was diffusely expressed in normal smooth muscle cells, its localization being exclusively cytoplasmic. Staining of LM with the monoclonal anti-kindlin antibody showed that kindlin was also diffusely expressed in the cytoplasm in the majority of the examined tumours (89%) (Figure 4A). Interestingly, strong kindlin immunoreactivity was detected in endothelial cells in LM tissues (Figure 4A).
Figure 3.
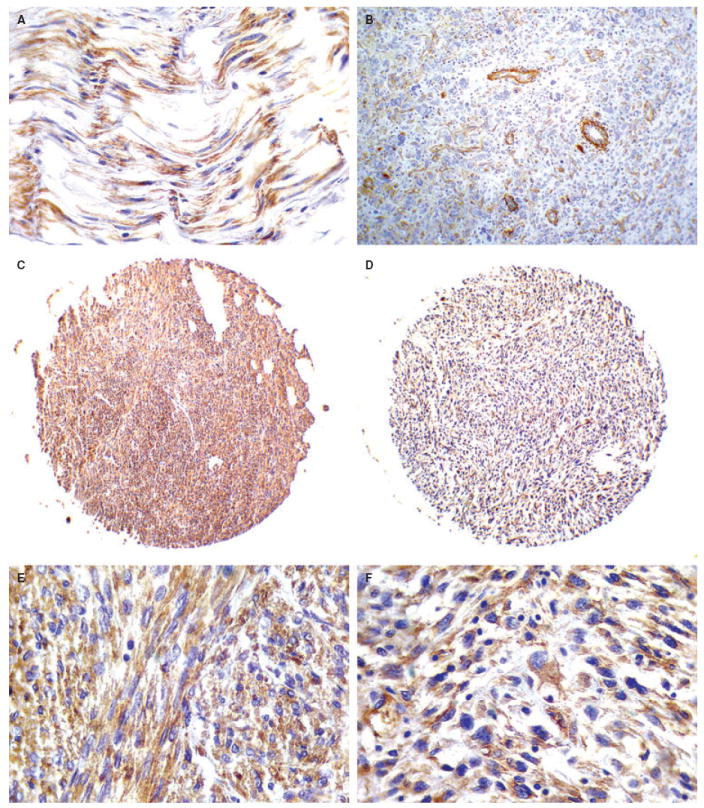
Expression of kindlin in normal smooth muscle, low- and high-grade leiomyosarcoma (LMS). A, Normal smooth muscle cells display immunopositivity for kindlin. B, Moderate immunopositivity for kindlin (2+) in a high-grade LMS. Endothelial cells of tumour vessels are strongly positive for kindlin. C,E, Strong kindlin immunopositivity (3+) in a low-grade LMS. D,F, High-grade LMS exhibiting strong immunoreactivity (3+) for kindlin.
Figure 4.
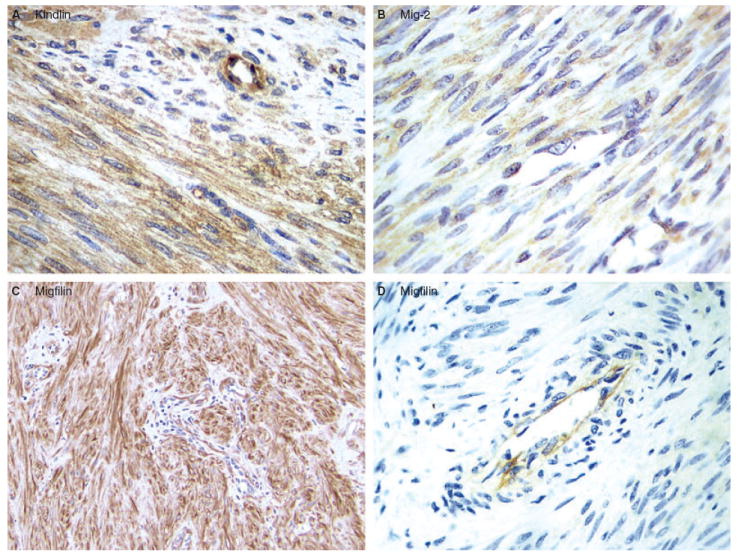
Expression of kindlin, Mig-2 and migfilin in leiomyoma (LM). A, Kindlin expression. Kindlin was detected in the majority (89%) of the LM cases. A representative diffuse, cytoplasmic kindlin staining of LM is shown. B, Mig-2 expression. Diffuse, cytoplasmic Mig-2 was detected in all of the LM cases. Representative Mig-2 reactivity is shown. C,D, Migfilin expression. Migfilin was detected in 14 out of 21 (66.7%) LM cases. C, Strongly positive, primarily cytoplasmic migfilin reactivity in a case of soft tissue LM. D, Negative migfilin reactivity in a case of soft tissue LM. Note that strong migfilin immunoreactivity was detected in endothelial cells (D).
Thirty-nine out of 40 (97.5%) LMS of all grades displayed diffuse, cytoplasmic immunoreactivity for kindlin (Figure 3C–F). There was no variation in the pattern of immunoreactivity in different grades of LMS. Kindlin immunoreactivity did not display significant differences between high- and low-grade neoplasms (Mann–Whitney U-test, P = 0.5). In addition, no significant differences between low-grade LMS and LM were observed (Mann–Whitney U-test, P = 0.45). Strong kindlin immunoreactivity was expressed in endothelial cells within the tumour parenchyma (Figure 3B).
EXPRESSION OF MIG-2 IN NORMAL HUMAN SMOOTH MUSCLE, LM AND LMS
Diffuse cytoplasmic and nuclear Mig-2 immunoreactivity was detected in normal smooth muscle (Figure 5A). Diffuse Mig-2 immunoreactivity was also detected in all of the LM cases that were analysed (Figure 4B). In LMS, 38/40 (95%) of LMS were Mig-2+ (Table 1) (Figure 5D,F). The staining pattern appeared similar in tumours of all grades. There was no significant correlation between the expression levels of Mig-2 among high- and low-grade LMS or between low-grade LMS and LM (Mann–Whitney U-test, P = 0.365 and 0.4, respectively).
Figure 5.
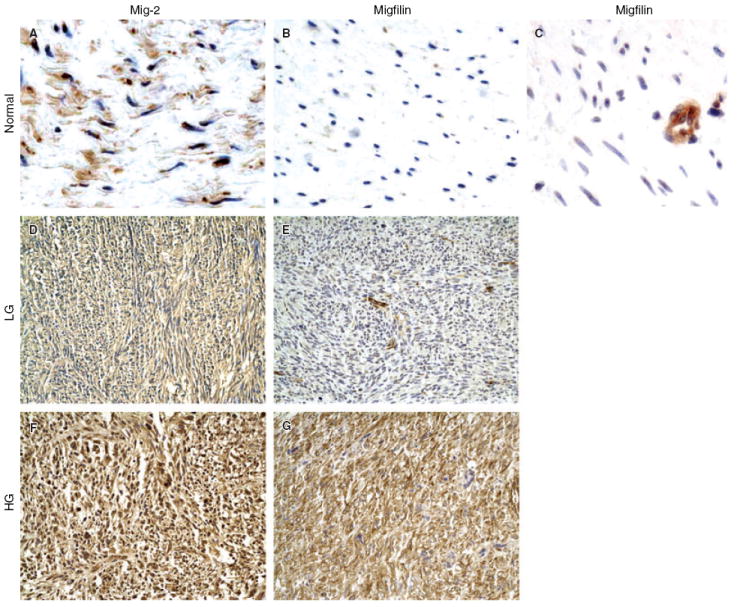
Expression of Mig-2 and migfilin in normal smooth muscle, low- and high-grade leiomyosarcoma (LMS). A, Normal smooth muscle cells exhibit positive nuclear and cytoplasmic immunoreactivity for Mig-2. B, Normal smooth muscle cells exhibit no immunoreactivity for migfilin. C, In normal soft tissue sections containing blood vessels, a high level of migfilin was detected in endothelial cells. D, Mig-2 exhibits moderate cytoplasmic immunoreactivity (2+) in a low-grade LMS. E, Low-grade LMS exhibiting weak immunopositivity for migfilin (1+). Endothelial cells show strong expression of migfilin. F, Strong nuclear and cytoplasmic immunoreactivity of Mig-2 (3+) in a high-grade LMS. G, High-grade LMS showing strong (3+), nuclear and cytoplasmic immunopositivity for migfilin. See Table 1 for a summary of tumour grade, clinical features and immunopositivity/localization of migfilin and Mig-2 in all 40 human LMS that were analysed.
Table 1.
Tumour grade, clinical features, immunohistochemistry and localization of migfilin and Mig-2 in human leiomyosarcoma
| Case | Sex | Tumour size (mm) | Migfilin expression | Mig-2 expression | Tumour grade |
|---|---|---|---|---|---|
| 1 | F | 40 | 2+ C | 2+ C | HG |
| 2 | F | 52 | – | 2+ C | HG |
| 3 | F | 120 | 2+ C/N | 2+ C | HG |
| 4 | F | 90 | 1+ C/N | 2+ C | HG |
| 5 | M | 170 | – | 1+ C | LG |
| 6 | M | 75 | 1+ C | 1+ C | LG |
| 7 | M | 70 | 2+ C | 2+ C | HG |
| 8 | F | 20 | 2+ C | 2+ C/N | LG |
| 9 | F | 45 | 2+ C | 2+ C/N | HG |
| 10 | F | 35 | – | 2+ C/N | HG |
| 11 | F | 52 | – | – | LG |
| 12 | F | 45 | 1+ C/N | 2+ C/N | HG |
| 13 | M | 240 | 1+ C | 1+ C/N | LG |
| 14 | F | 52 | 1+ C | 1+ C | HG |
| 15 | F | 90 | – | 2+ C/N | HG |
| 16 | F | 52 | 1+ C | 2+ C/N | LG |
| 17 | F | 100 | – | 2+ C | HG |
| 18 | F | 105 | 1+ C | 2+ C/N | LG |
| 19 | F | 175 | 3+ C | 2+ C | HG |
| 20 | F | 65 | 3+ C | 3+ C | HG |
| 21 | F | 70 | – | – | HG |
| 22 | F | 230 | 2+ C | 2+ C/N | HG |
| 23 | M | 100 | 2+ C | 2+ C | HG |
| 24 | F | 65 | – | 2+ C | HG |
| 25 | F | 85 | 2+ C | 2+ C | HG |
| 26 | F | 105 | 2+ C | 2+ C | HG |
| 27 | F | 150 | – | 1+ C/N | HG |
| 28 | F | 55 | 1+ C | 2+ C | LG |
| 29 | M | 235 | – | 3+ C | HG |
| 30 | M | 08 | 1+ C | 1+ C | HG |
| 31 | F | 130 | 1+ C | 2+ C | HG |
| 32 | M | 35 | – | 1+ C | HG |
| 33 | M | 18 | 1+ C | 2+ C/N | HG |
| 34 | F | 09 | 3+ C/N | 3+ C | HG |
| 35 | F | 10 | 3+ C | 3+ C | HG |
| 36 | M | 32 | 3+ C | 3+ C | HG |
| 37 | M | 250 | – | 2+ C/N | HG |
| 38 | M | 36 | 1+ C | 1+ C | HG |
| 39 | F | 80 | – | 3+ C | LG |
| 40 | M | 10 | 3+ C | 3+ C/N | HG |
F, Female; M, male; C, cytoplasmic immunolocalization; N, nuclear immunolocalization; HG, high-grade tumour; LG, low-grade tumour; 1+, weak immunopositivity; 2+, moderate immunopositivity; 3+, instense immunopositivity. See text for statistical analyses of associations between tumour grade and intensity of cytoplasmic and nuclear immunopositivity.
EXPRESSION OF MIGFILIN IN NORMAL HUMAN SMOOTH MUSCLE, LM AND LMS
Migfilin was barely detectable in normal smooth muscle cells (Figure 5B). Notably, however, intense migfilin immunopositivity was detected in endothelial cells (Figure 5C). Migfilin was diffusely expressed in 14 (10 conventional soft tissue LM and four cutaneous LM) out of 21 (66.7%) LM cases (Figure 4C,D). Migfilin immunoreactivity in LM appeared to be principally cytoplasmic. Strong migfilin reactivity was detected in endothelial cells in all samples analysed (see, for example, Figure 4D). Diffuse migfilin expression was observed in 67.5% (27/40) of the examined LMS. Twenty-one out of 31 (68%) high-grade and six of nine (67%) low-grade tumours were migfilin immunopositive (Table 1). Both cytoplasmic and nuclear immunoreactivity was observed, with more intense reactivity in the cytoplasm (Figure 5E,G). Migfilin was strongly expressed (2+/3+) in the cytoplasm of 14 out of 31 high-grade tumours, whereas only one out of nine low-grade tumours had strong cytoplasmic migfilin staining (2+) (Table 1).
CORRELATION OF THE EXPRESSION LEVEL OF MIGFILIN WITH TUMOUR GRADE IN HUMAN LMS
When both nuclear and cytoplasmic levels were taken into account, there was no apparent correlation between the level of migfilin and LMS grade. Nuclear levels of migfilin were not significantly associated with tumour grade (P = 0.602). However, when only cytoplasmic levels were considered for statistical analysis, a significantly stronger expression of migfilin in the high-grade compared with the low-grade LMS was demonstrated (Mann–Whitney U-test, P = 0.032). Statistical analysis did not reveal any difference between the protein levels of migfilin in LM and low-grade LMS (Mann–Whitney U-test, P = 0.37).
CORRELATION BETWEEN THE LEVELS OF MIG-2, MIGFILIN AND KINDLIN IN LMS
The nuclear level of migfilin was not significantly related to that of Mig-2 (Kendall’s τ = 0.274, P = 0.06). Interestingly, however, when the statistical analysis included only their cytoplasmic levels (Table 1), a significant correlation between migfilin and Mig-2 cytoplasmic levels was revealed (Kendall’s τ = 0.674, P = 0.014), indicating that the cytoplasmic levels of migfilin and Mig-2 are strongly associated in LMS. No significant association between kindlin and Mig-2 or between kindlin and migfilin in LMS was revealed (P > 0.05 for both associations).
CLINICOPATHOLOGICAL FINDINGS
Several clinicopathological parameters were analysed to determine the correlation of each adhesion molecule with the tumour grade, gender and age, histopathological grade and size, location and biological behaviour (recurrent or metastatic) of the tumour. The intensity and degree of immunoreactivity of Mig-2, migfilin and kindlin were not different within primary and recurrent/metastatic LMS, or between small (< 50 mm) and large (≥ 50 mm) neoplasms.
ROLE OF MIGFILIN AS A POTENT BIOLOGICAL MARKER
In order to investigate the importance of migfilin, Mig-2 and kindlin as potent biological predictors of tumour grade, their cytoplasmic levels were tested as categorical variables using binary logistic regression analysis. The most significant association among protein level and LMS grade was found for migfilin (P = 0.03). Evaluation of the levels of cytoplasmic expression of migfilin could predict a high-grade LMS tumour with a sensitivity of 70%, specificity of 84% and overall accuracy of 74%.
Discussion
Cell–cell and cell–ECM adhesions regulate cell morphology and participate in many fundamental cellular processes.7,8 Consequently, aberrations in the expression of cell adhesion molecules are often associated with tumour development.26,27 In the present study, we investigated the level and distribution of three cell–ECM adhesion proteins, namely kindlin, Mig-2 and migfilin, in normal human smooth muscle, LM and primary, recurrent and metastatic LMS.
Kindlin was expressed in normal smooth muscle, LM and LMS. Its level of expression did not display significant differences between high- and low-grade LMS, or between LM and low-grade LMS. No association was found between the immunohistochemical profiles of kindlin and Mig-2 or migfilin. This suggests that although kindlin and Mig-2 are structurally related, their levels of expression are not linked and up-regulation of one does not necessarily predict the expression level of the other.
With regards to migfilin, our results show that it is readily detectable in the majority (67.5%) of LMS and LM (66.7%), but not in normal smooth muscle cells. This suggests that migfilin is involved in the pathogenesis of smooth muscle neoplasms. Importantly, statistical analysis revealed that the cytoplasmic level of migfilin is significantly higher in high-grade compared with low-grade LMS. These findings, together with our recent findings that migfilin functions as an important component of cell–ECM and cell–cell adhesion networks and regulates cell migration,13,22,23,28 suggest a role for cytoplasmic migfilin in LMS progression.
Kato et al.29 have recently analysed Mig-2 expression in 13 cases of uterine LM and one case of LMS. The results showed that Mig-2 expression was elevated in 12 out of 13 cases of human uterine LM, but was reduced in the single case of uterine LMS that was analysed. The present study analysed the subcellular distribution and expression levels of Mig-2 in 40 cases of LMS (31 high grade and nine low grade), as well as in soft tissue LM and normal smooth muscle. Consistent with the study by Kato et al.,29 abundant Mig-2 was detected in all LM that were analysed. However, Mig-2 immunoreactivity was also detected in the majority (38 out of 40 LMS cases) of soft tissue LMS. This may reflect a different role for Mig-2 in the pathobiology of deep soft LMS analysed in the current study in comparison with uterine LMS analysed by Kato et al.29 Alternatively, this may stem from the difference in the numbers of LMS cases analysed in these studies (one single uterine LMS in the study by Kato et al.29 versus 40 soft tissue LMS cases in the current study).
Consistent with our recent results showing that migfilin and Mig-2 are co-localized at cell–ECM adhesions,13,22,23 statistical analysis of our immunohistochemical data reveal that the cytoplasmic levels of these proteins are positively and significantly correlated with each other. The parallel up-regulation of these proteins implies that the cytoplasmic migfilin–Mig-2 complex may be involved in the pathogenesis and progression of human LMS. Migfilin has been implicated in transcriptional regulation in mouse cardiomyocytes.21 Nuclear migfilin was also detected in the current study. It will be interesting to determine the role of nuclear migfilin in normal and transformed smooth muscle cells.
In conclusion, in the present study we have investigated the potential of migfilin and Mig-2 as molecular markers that might contribute to the distinction between high- and low-grade LMS. The results have revealed a significant association between cytoplasmic migfilin levels and LMS grade, suggesting that the migfilin cytoplasmic level may be used to aid the determination of different histological grades of LMS, especially in small biopsy specimens of deep soft tissue and retroperitoneal tumours.
Acknowledgments
This work was supported by NIH grants GM65188 and DK54639 to C.W. and DE016099 to H.L.
Abbreviations
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- IRB
Institutional Review Board
- LM
leiomyoma
- LMS
leiomyosarcoma
- MBP
maltose-binding protein
- Mig-2
mitogen inducible gene-2
- siRNA
small interfering RNA
- TMA
tissue microarray
References
- 1.Enzinger F, Weis SW. Leiomyosarcoma. In: Weis SW, Goldblum JR, editors. Soft tissue tumors. 4. Mosby; St Louis: 2001. pp. 724–748. [Google Scholar]
- 2.Evans H, Shipley J. Leiomyosarcoma. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002. pp. 131–134. [Google Scholar]
- 3.Ren B, Yu YP, Jing L, et al. Gene expression analysis of human soft tissue leiomyosarcomas. Hum Pathol. 2003;34:549–558. doi: 10.1016/s0046-8177(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 4.Sepulveda J, Gkretsi V, Wu C. Assembly and signaling of the cell–extracellular matrix adhesion complexes. Curr Top Dev Biol. 2005;68:183–225. doi: 10.1016/S0070-2153(05)68007-6. [DOI] [PubMed] [Google Scholar]
- 5.Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 8.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 9.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Schaller MD. UNC112. A new regulator of cell–extracellular matrix adhesions? J Cell Biol. 2000;150:F9–F11. doi: 10.1083/jcb.150.1.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG. The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell–matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol. 2000;150:253–264. doi: 10.1083/jcb.150.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein EJ, Bourner M, Head R, Zakeri H, Bauer C, Mazzarella R. URP1: a member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Biochim Biophys Acta. 2003;1637:207–216. doi: 10.1016/s0925-4439(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 15.Kloeker S, Major MB, Calderwood DA, Ginsberg MH, Jones DA, Beckerle MC. The Kindler syndrome protein is regulated by transforming growth factor-beta and involved in integrin-mediated adhesion. J Biol Chem. 2004;279:6824–6833. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- 16.Siegel DH, Ashton GH, Penagos HG, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin–extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobard F, Bouadjar B, Caux F, et al. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003;12:925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- 18.Hovnanian A, Blanchet-Bardon C, de Prost Y. Poikiloderma of Theresa Kindler: report of a case with ultrastructural study, and review of the literature. Pediatr Dermatol. 1989;6:82–90. doi: 10.1111/j.1525-1470.1989.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 19.Senturk N, Usubutun A, Sahin S, Bukulmez G, Erkek E, Topaloglu R. Kindler syndrome: absence of definite ultrastructural feature. J Am Acad Dermatol. 1999;40:335–337. doi: 10.1016/s0190-9622(99)70480-9. [DOI] [PubMed] [Google Scholar]
- 20.Haber RM, Hanna WM. Kindler syndrome. Clinical and ultrastructural findings. Arch Dermatol. 1996;132:1487–1490. doi: 10.1001/archderm.132.12.1487. [DOI] [PubMed] [Google Scholar]
- 21.Akazawa H, Kudoh S, Mochizuki N, et al. A novel LIM protein Cal promotes cardiac differentiation by association with CSX/NKX2-5. J Cell Biol. 2004;164:195–405. doi: 10.1083/jcb.200309159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C. Migfilin and its binding partners: from cell biology to human diseases. J Cell Sci. 2005;118:659–664. doi: 10.1242/jcs.01639. [DOI] [PubMed] [Google Scholar]
- 23.Gkretsi V, Zhang Y, Tu Y, et al. Physical and functional association of migfilin with cell–cell adhesions. J Cell Sci. 2005;118:697–710. doi: 10.1242/jcs.01638. [DOI] [PubMed] [Google Scholar]
- 24.Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol. 2001;153:585–598. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell–matrix focal adhesions but not cell–cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci. 1999;112:4589–4599. doi: 10.1242/jcs.112.24.4589. [DOI] [PubMed] [Google Scholar]
- 26.Christofori G. Changing neighbours, changing behaviour: cell adhesion molecule-mediated signalling during tumour progression. EMBO J. 2003;22:2318–2323. doi: 10.1093/emboj/cdg228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popov Z, Gil-Diez de Medina S, Lefrere-Belda M-A, et al. Low E-cadherin expression in bladder cancer at the transcriptional and protein level provides prognostic information. Br J Cancer. 2000;83:209–214. doi: 10.1054/bjoc.2000.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Tu Y, Gkretsi V, Wu C. Migfilin interacts with vasodilator-stimulated phosphoprotein (VASP) and regulates VASP localization to cell–matrix adhesions and migration. J Biol Chem. 2006;281:12397–12407. doi: 10.1074/jbc.M512107200. [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Shiozawa T, Mitsushita J, et al. Expression of the mitogen-inducible gene-2 (mig-2) is elevated in human uterine leiomyomas but not in leiomyosarcomas. Hum Pathol. 2004;35:55–60. doi: 10.1016/j.humpath.2003.08.019. [DOI] [PubMed] [Google Scholar]


