Abstract
The second to fourth (2D:4D) digit ratio, a sexually dimorphic, phenotypic characteristic putatively associated with perinatal androgen action, has been used to evaluate the hypothesized relation between prenatal hormonal factors and a variety of sexually dimorphic behaviors, including sex-linked psychopathology. Smaller digit ratios, suggestive of stronger perinatal androgen action, have been associated with male-linked disorders (e.g., autism), and larger digit ratios, suggestive of weaker perinatal androgen action, have been associated with female-linked disorders (e.g., depression and eating disorders). To evaluate the possible relation between digit ratio and another traditionally female-linked disorder, anxiety, 2D:4D ratios were measured in a non-clinical sample (58 men, 52 women). Participants also completed a battery of anxiety and gender role measures and performed two spatial/cognitive tasks typically showing a male advantage (mental rotation and targeting) and two tasks typically showing a female advantage (location memory and spatial working memory). Men with a more feminine pattern of sex-linked traits and behaviors (including digit ratios) reported greater anxiety. In contrast, greater anxiety in women was associated with both female-typical and male-typical traits and behaviors, and no significant association between digit ratio and anxiety was found. This pattern of results suggests that the development of anxiety is multiply determined, with contributing factors varying by sex.
Keywords: anxiety, sex-linked behavior, 2D:4D ratio, gonadal hormones
INTRODUCTION
Prenatal androgens are implicated in the development of behaviors showing sex differences, such that higher levels are associated with the expression of more male-typical behavior across a variety of species (Cohen-Bendahan, van de Beek, & Berenbaum, 2005; Collaer & Hines, 1995). Direct measurements of prenatal androgen levels are typically unavailable to researchers studying the early hormonal influences on adult behavior. For that reason, an alternative strategy adopted increasingly in this area of research is to correlate behaviors of interest with a putative measure of perinatal androgen action, such as the ratio of the index (2D) to ring (4D) finger.
The 2D:4D ratio is itself a sexually dimorphic trait found across species (Bailey, Wahlsten, & Hurd, 2005; McFadden & Bracht, 2005) and ethnic groups (Manning et al., 2000; Manning, Stewart, Bundred, & Trivers, 2004). It appears detectable in human fetuses as early as 10–40 weeks of gestation and is reportedly stable by two years of age (Malas, Dogan, Evcil, & Desdicioglu, 2006; Manning, 2002; Manning, Scutt, Wilson, & Lewis-Jones, 1998). Digit ratio also varies predictably between women with typical female prenatal development and women exposed to more “masculine” prenatal hormone levels because of an endocrine disorder (congenital adrenal hyperplasia or CAH) (Brown, Hines, Fane, & Breedlove, 2002; Okten, Kalyoncu, & Yarvis, 2002; but see Buck, Williams, Hughes, & Acerini, 2003). Some evidence suggests that the 2D:4D ratio is a direct correlate of prenatal sex steroid levels (Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer, & Manning, 2004; Manning et al., 1998). However, a recent proposal is that digit ratios may be better described as a measure of perinatal androgen action (McIntyre, 2006), consistent with findings that smaller digit ratios are associated with androgen receptor alleles showing fewer terminal domain CAG repeats (Manning, Bundred, Newton, & Flanagan, 2003), a marker of greater androgen sensitivity (Chamberlain, Driver, & Meisfeld, 1994; Kazemi-Esfarjani, Trifiro, & Pinski, 1995).
The 2D:4D ratio has been studied in the context of reproductive success (Manning et al., 2000), sex-typed behavior (Csathó et al., 2003a), spatial/cognitive abilities (Csathó et al., 2003b; Kempel et al., 2005; Manning, 2002; but see Coolican & Peters, 2003), adult personality characteristics (Austin, Manning, McInroy, & Matthews, 2002; Bailey & Hurd, 2005a; Fink, Manning, & Neave, 2004), and more recently in the context of psychopathology (Arato, Frecska, Beck, An, & Kiss, 2004; Bailey & Hurd, 2005b; Klump et al., 2006; Manning, Baron-Cohen, Wheelwright, & Sanders, 2001; McFadden, Westhafer, Pasanen, Carlson, & Tucker, 2005; Walder, Andersson, McMillan, Breedlove, & Walker, 2006). Generally, digit ratios show positive correlations with female-typical behaviors and negative correlations with male-typical behaviors (for review, see Putz, Gaulin, Sporter, & McBurney, 2004), and this pattern of results appears to generalize to sex-linked psychopathology. Smaller 2D:4D ratios have been associated with disorders that occur more frequently in males, such as autism, and other related finger-length ratios (2D:5D, 3D:5D, and 4D:5D) have been associated with attention-deficit/hyperactivity disorder (ADHD) (Manning et al., 2001; McFadden et al., 2005). In addition, girls with a more masculine 2D:4D ratio appear to express more male-typical characteristics, such as increased difficulties with social cognition, prosocial ability, and peer relationships (Williams, Greenhalgh, & Manning, 2003).
Recent research on eating disorders (Klump et al., 2006) and depression (Bailey & Hurd, 2005b, but see Martin, Manning, & Dowrick, 1999) suggests larger 2D:4D ratios may be associated with an increased risk for disorders that occur more frequently in females. Although anxiety disorders occur more often in women than in men (Pigott, 2003; Shear, Feske, & Greeno, 2000), to our knowledge no previous studies have reported the relation between digit ratios and adult levels of anxiety. Significantly, a potential role for prenatal androgens in the development of anxiety in women and men is supported by animal research showing that male rats deprived of androgens because of perinatal castration display female-typical patterns of anxious behavior (Lucion, Charchat, Pereira, & Rasia-Filho, 1996). Further, sensitivity to post-pubertal levels of hormones is a general feature of adult sex-linked behaviors influenced by prenatal hormones (Collaer & Hines, 1995). Incidentally, the onset of anxiety disorders tends to coincide with puberty (Yonkers & Kidner, 2002), a time of increased sex hormone production. Obsessive-compulsive disorder in women, for instance, increases following menarche and surpasses the rate for men (Pigott, 2003). In addition, anxiety symptoms appear sensitive to fluctuations in circulating hormone levels across reproductive life, including across the menstrual cycle (Cook et al., 1990; McLeod, Hoehn-Saric, Foster, & Hipsley, 1993; Williams & Koran, 1997), pregnancy, and the postpartum period (Altshuler, Hendrick, & Cohen, 1998; Hertzberg & Wahlbeck, 1999; Williams & Koran, 1997). Finally, previous findings of associations between a feminine 2D:4D and anxious behavior in childhood (Williams et al., 2003) and between a feminine 2D:4D and neuroticism (Fink et al., 2004), a personality feature considered a dimensional precursor to anxiety (Ehrler, Evans, & McGhee, 1999; Khan, Jacobson, Gardner, Prescott, & Kendler, 2005), suggest that adults with a more feminine digit ratio will also report greater levels of anxiety.
Therefore, the present investigation explored the relation between anxiety symptoms and the proposed measure of perinatal androgen action, the 2D:4D ratio. The association between anxiety scores and normative sex-linked behaviors was also examined because others have speculated that sex-linked disorders may represent an extreme expression of normative gender roles (Skodol, 2000), a general description of behaviors that also appear sensitive to levels of prenatal androgens (Berenbaum & Hines, 1992; Hampson, Rovet, & Altmann, 1998; Leveroni & Berenbaum, 1998; Meyer-Bahlburg et al., 2004; Servin, Nordenström, Larsson, & Bohlin, 2003; Udry, 2000; Udry, Morris, & Kovenock, 1995) and that have been correlated with 2D:4D (Csathó et al., 2003a, 2003b; Kempel et al., 2005; Manning, 2002; Peters, Manning, & Reimers, 2007). It was hypothesized that anxiety scores would differ between individuals with more masculine and more feminine digit ratios and that anxiety scores would be associated with other measures of sex-linked behavior.
As a first investigation of the relation between sex-linked behavior, 2D:4D ratio, and adult anxiety, this study included a variety of anxiety measures to determine whether specific components of anxiety (e.g., trait anxiety, state anxiety, cognitive symptoms, affective symptoms, and physical symptoms) and not others were related to digit ratio, spatial/cognitive measures, and gender role behavior. To remain true to this purpose and also to use measures of anxiety commonly found in the literature, we chose to include some measures for which clear sex differences have not been established (Foot & Koszycki, 2004; Novy, Nelson, Goodwin, & Rowzee, 1993; Morey, 1991; but see Battisti et al., 2004; Chambless & Mason, 1986). In addition, as an exploratory study, we chose to focus on a non-clinical population.
METHOD
Participants
As part of a larger study of hormones, personality, and sex-linked behavior, 58 men and 52 women were recruited from Introductory Psychology courses at Texas A&M University and from the community through an advertisement in the campus newspaper. Men and women were comparable in age (Men: M = 19.67, SD = 1.85; Women: M = 20.69, SD = 6.12), race (Men: 81% White, 5.2% Black, 3.4% Asian, 10.3% No Response; Women: 84.6% White, 3.8% Black, 9.6% Asian, 1.9% No Response), ethnicity (Men: 17.2% Hispanic, 81% Not Hispanic; Women: 11.5% Hispanic, 88.5% Not Hispanic), and in performance on a vocabulary test, a proxy measure of general intelligence (Men: M = 24.05, SD = 6.89; Women: M = 23.85, SD = 8.15). Participants were drawn from a non-clinical population to reflect the normal distribution of anxiety (i.e., no participants were excluded on the basis of anxiety scores). Those recruited through Introductory Psychology courses received credit applied towards a course requirement and those recruited from the community received $15 for their participation. All participants provided informed consent.
Measures
Beck Anxiety Inventory
The Beck Anxiety Inventory (BAI; Beck & Steer, 1993) consists of 21 items assessing somatic and affective symptoms of anxiety (e.g., “feeling hot,” “fear of dying,” “scared,” “dizzy or lightheaded”). Responses were on a scale from 0–3, with 0 representing the absence of a symptom and 1–3 representing increasing symptom levels. Scores on individual items were summed to provide an overall anxiety score. The BAI has shown high internal consistency (α = .85–.94) and test-retest reliability (r = .75). It also appears to be moderately correlated with other widely used measures of anxiety, such as the State-Trait Anxiety Inventory (Trait – r = .58, State – r = .51) and the Hamilton Anxiety Rating Scale-Revised (HARS-R – r = .51).
State-Trait Anxiety Inventory
The State-Trait Anxiety Inventory (STAI; Spielberger, 1983) is composed of 40 items and 2 scales (20 items per scale). On the State Scale, participants were asked to describe how they were feeling “right now, that is, at this moment.” Responses were on a 4-point scale ranging from 1 = “Not at all” to 4 = “Very Much So.” On the Trait Scale, participants were asked to describe how they “generally feel.” Responses were on a 4-point scale ranging from 1 = “Almost Never” to 4 = “Almost Always.” Half the items on each scale were scored in the positive direction and half in the reverse direction. Scores on the 20 items were summed to provide an overall scale score. In the standardization sample of college and high school students, military recruits, and working adults, the STAI demonstrated high internal consistency on both scales (α > .90) and high test-retest reliability (r = .65–.86) for the Trait Scale. As expected, low test-retest reliability (r = .33) was found for the State Scale. The STAI-Trait Scale also appears to be highly correlated (r = .70–.85) with other widely used measures of anxiety, such as the Manifest Anxiety Scale (MAS) and the Anxiety Scale Questionnaire (ASQ), and is moderately to highly correlated with the STAI-State Scale (r = .59–.75).
Personality Assessment Inventory-Anxiety Subscales
The Personality Assessment Inventory (PAI; Morey, 1991) Anxiety-Full Scale includes three subscales (24 items total) measuring cognitive (ANX-C), affective (ANX-A), and physiological (ANX-P) components of anxiety. Respondents chose whether symptoms were “Totally False,” “Slightly True,” “Mainly True,” or “Very True” of them. Scores on each item were weighted on a scale from 0–3. Items in the ANX-C scale focus on ruminative worry and impaired concentration, whereas items in the ANX-A scale measure tension and fatigue caused by perceived stress. Lastly, the ANX-P scale evaluates somatic symptoms of anxiety (e.g., “shortness of breath” and “trembling of hands”). The Anxiety-Full Scale of the PAI has shown high internal consistency (α = .89–.94) and test-retest reliability (r = .88). It also appears to be moderately to highly correlated with other widely used measures of anxiety, such as the BAI (r = .62), the Fear Survey Schedule (FSS) (r = .49), STAI-State (r = .62), and STAI-Trait (r = .73).
Personality Assessment Inventory-Anxiety-Related Disorders Subscales
The PAI Anxiety-Related Disorders-Full Scale (Morey, 1991) includes three subscales (24 items total) measuring obsessive-compulsiveness (ARD-O), phobias (ARD-P), and traumatic stress (ARD-T). Responses were on the same 4-point, weighted categories used in the Anxiety Subscales. The ARD-O scale contains items assessing inflexibility, perfectionism, and the presence of intrusive thoughts and behaviors, while the ARD-P scale evaluates fear of common objects and situations (e.g., “fear of heights” and “enclosed spaces”). Items on the ARD-T scale probe for a history of trauma and determine whether these events are presently causing distress. The Anxiety Related Disorders-Full Scale of the PAI has shown high internal consistency (α = .76–.86) and test-retest reliability (r = .83–.85). It also appears moderately correlated with other widely used measures of anxiety, such as the BAI (r = .48–.53), FSS (r = .66), Mississippi PTSD Scale (r = .81), Maudsley Obsessive-Compulsive Inventory (r = .62), STAI-State (r = .42) and STAI-Trait (r = .51).
Bem Sex-Role Inventory
The Bem Sex-Role Inventory (BSRI; Bem, 1981) consists of 60 items assessing masculinity and femininity as separate dimensions. Men typically score higher on the masculine scale and women typically score higher on the feminine scale, with effect sizes ranging from d = .44 to d = .96 (Murphy, 1994; Rammsayer & Troche, 2007).
Pre-School Activities Inventory
The Pre-School Activities Inventory (PSAI; Golombok & Rust, 1993) is a 24-item measure assessing childhood play preferences. Participants were instructed to respond according to their recollections of their preferences in early childhood (Hines et al., 2003). Individuals described how frequently they played with certain toys (e.g., “guns”), engaged in specific activities (e.g., “playing house”), and possessed several characteristics (e.g., “enjoys rough and tumble play”). Responses were on a 5-point Likert scale: N = “Never,” HE = “Hardly Ever,” S = “Sometimes,” O = “Often,” and VO = “Very Often.” Higher scores on this measure reflect male-typical play preferences while lower scores indicate female-typical play preferences. In previous research with adult populations, the sex difference in PSAI scores has generally shown a very large effect size (d = 2.65–3.25) (Alexander, 2006; Hines et al., 2003). The PSAI also shows moderate test-retest reliability for each sex (boys – r = .62, girls – r = .66) and moderate to high split-half reliability for each sex (boys – r = .66, girls – r = .80) (Golombok & Rust, 1993). In addition, PSAI scores correlate moderately with teacher ratings of gendered behavior (boys – r = .37, girls – r = .48) (Golombok & Rust, 1993).
Occupation, Activities, and Traits Attitudes and Personal Measures
The Occupation, Activities, and Traits-Attitudes Measure (short form) (OAT-AM; Liben & Bigler, 2002) is a 75-item questionnaire measuring gender attitudes towards others. It consists of three scales, each with 25 items, asking participants to describe whether men, women, or both sexes should do certain jobs (e.g., “secretary,” “plumber,” “florist”), activities (e.g., “fix a car,” “bake cookies,” “go to the beach”) or possess certain traits (e.g., “be emotional,” “be cruel,” “enjoy math”). Higher scores in this study indicate greater stereotyping of gender attitudes. In general, men tend to provide more stereotypic responses for each scale, whereas women provide more egalitarian responses (d = .39–.55). All three scales of the OAT-AM show high internal consistency (α = .75–.91) and test-retest reliability (r =.72–.77).
The Occupation, Activities, and Traits-Personal Measure (short form) (OAT-PM; Liben & Bigler, 2002), which measures gender typing of the self, also has 75 total items and three 25-item subscales. Participants were asked to describe their own occupational interests, involvement in activities, and personality characteristics. In general, women endorse more feminine items (d = .56–1.07) and men endorse more masculine items (d = .61–.93) on each scale. All three scales of the OAT-PM show moderate to high internal consistency (α = .65–.81) and high test-retest reliability (r = .72–.88). In addition, the feminine items on the OAT-PM are moderately correlated with the feminine items on other widely used measures of gender role behavior, such as the BSRI (r = .39–.56) and the PAQ (r = .19–.52), and the masculine items on the OAT-PM were slightly to moderately correlated to the masculine items on the BSRI (r = .05–.68) and the PAQ (r = −.07–.57).
Sex-Linked Spatial Tasks
Participants completed two tasks typically showing a female advantage (spatial location memory, spatial working memory) and two tasks typically showing a male advantage (mental rotation, targeting) in counterbalanced order. Memory for object locations was measured using the Silverman and Eals (1992) Location Memory Task, a task showing a small to moderate female advantage across various ethnic groups and 35 countries (Silverman, Choi, & Peters, 2007). As part of this task, a stimulus card with an array of 27 common objects (e.g., bird, flower, umbrella, iron, briefcase, teapot) was displayed for one minute followed by two response cards. The first response card, measuring object identity, displayed the 27 original objects plus 20 added objects. Participants were asked to indicate which objects were new or had been added. The second response card, measuring location memory, consisted of the 27 original objects. However, the positions of seven pairs of objects were exchanged. Participants were asked to indicate which objects had been moved. Response cards were displayed for a period of two minutes or until the participant was finished. Performance on both the identity and location tasks was measured using the following formula: 1 – [(omissions + commissions)/N], where N equals the total number of objects.
Spatial working memory was assessed with a game, similar to the card game “Memory” (Duff & Hampson, 2001). A 5 × 4 array made of beige-colored felt was mounted on the wall at the participant’s eye level. Ten pairs of colored dots (green, yellow, blue, orange, brown, red, black, gray, purple, and pink) were dispersed throughout the array in random order. Dots were hidden beneath cutout flaps and could only be seen when participants lifted the flap. To show participants the possible range of colors, sample dots of each color were displayed in a column to the right of the array. Participants were instructed to match the pairs of colored dots as quickly as possible, but only turning over two flaps at a time. Before starting the task, the experimenter removed all sample dots from the side display. Every time a participant matched a pair of dots, the experimenter placed the sample dot of that color back on the side display. This procedure was implemented to ensure that participants did not have to remember the color of the dots, but only the locations where the dots were matched or not matched. Performance on this task was recorded by a video camera and viewed at a later time for coding purposes. Two dependent variables were assessed: total time required to complete the task and total number of working memory errors (i.e., the number of times participants returned to already searched locations but did not produce a match plus the number of times they searched already matched locations).
Because a robust male advantage is typically found on mental rotation performance across age, ethnicity, and education level (Peters et al., 2007), spatial rotation ability was assessed via the re-drawn Mental Rotations Test (MRT-A; Peters et al., 1995; Vandenberg & Kuse, 1978). This test is composed of 24 items consisting of three-dimensional figures. For each item, a sample block design is given, along with four possible rotated representations of the design. Participants were asked to choose the two block patterns that matched the original figure. They were allowed three minutes to complete the first 12 items, followed by a two-minute break, and then another three minutes for the remaining 12 items. Performance on this task was defined as the total number of responses identifying both correct items (maximum score: 24).
To measure projectile throwing ability (Watson & Kimura, 1991), a target was constructed using a 36-inch-by-36-inch square of black felt. A bull’s eye, made of white Velcro, was placed at the center of the square, 18 inches across and 18 inches vertically. Five ping-pong balls were also covered in Velcro, allowing them to adhere to the target upon contact. Participants were given ten opportunities to hit the bull’s eye. Distance from the center was measured for each trial and averaged across the ten trials to yield a throw accuracy score. Higher scores indicate greater distances from the center, and thus worse performance on targeting.
Hormone Measures
Participants provided two saliva samples (< 15 mL) by passively drooling into a small vial. Prior to the test session, they were e-mailed instructions to avoid alcohol and dental work (for 24 hours pre-testing) and to not eat or brush their teeth (for 3 hours pre-testing), restrictions that were later verified by questionnaire. Saliva samples for each participant were not pooled and were stored separately at −80° C, a temperature that compared to −20° C increases the validity of the assay results (Granger, Shirtcliff, Booth, Kivlighan, & Schwartz, 2004). Frozen samples were shipped overnight on dry ice to Salimetrics Inc. (State College, Pennsylvania), where salivary levels of testosterone (in women and men) and estradiol (in women) were measured in duplicate using enzyme immunoassays. Because hormone measures did not differ between time 1 and time 2 for women or men, they were averaged across time.
The second to fourth digit ratio (2D:4D), the hypothesized proxy measure of perinatal androgen action (Manning et al., 1998, 2003; McIntyre, 2006), was calculated by obtaining a digital scan of the participant’s right hand using a Visioneer OneTouch 9220 scanner. Hand images were then printed in color using an HP deskjet 5550 printer, and printed copies were later used to measure the distance (in millimeters) from the basal crease to tip of the second and fourth fingers with digital vernier calipers. Two independent judges coded finger lengths for each hand copy and measurements were averaged across the two judges. Only right hand measurements were used in this study, as the sexual dimorphism in digit ratio appears strongest for this hand (Peters et al., 2007). Further, right hand digit ratio appears to be most consistently associated to sex-linked behaviors and hormone measures (Brown et al., 2002; Manning et al., 1998; McFadden & Shubel, 2002; Williams et al., 2000).
Procedure
After providing their first saliva sample, participants completed all anxiety and gender role measures, in standard order. They then completed the two female-linked, spatial/cognitive tasks (in counterbalanced order), followed by the two male-linked tasks (in counterbalanced order). After completing the spatial/cognitive testing, participants provided a second saliva sample, and a digital scan of their right hand was obtained.
RESULTS
Sex Differences in Behavior
Previously reported sex differences in behavior were evaluated using separate 2 × 2 multivariate analyses of variance (MANOVA), with sex (men, women) and digit ratio (high, low) as grouping factors and spatial tasks (working memory, mental rotation, targeting), gender role measures, and anxiety measures as three groups of dependent variables. Sex differences in location memory were assessed via an analysis of covariance (ANCOVA), controlling for performance on object identity. Sex differences in hormone measures were evaluated using separate analyses of variance (ANOVA) for testosterone and 2D:4D ratio.
Table I summarizes the sex differences in sex-linked behavior and hormone levels, with effect sizes reported for each. Note that a small number of individuals (4 men, 9 women) did not complete the entire battery of tests (some measures were unavailable). As a result, there was some variability in the number of participants available for the analyses of group differences. Briefly, men and women showed the expected differences in gender role behavior, as measured by the PSAI, F(1, 93) = 252.57, p < .001, OAT-PM masculine scale, F(1, 93) = 17.52, p < .001, OAT-PM feminine scale, F(1, 93) = 64.84, p < .001, OAT-AM, F(1, 93) = 13.02, p < .001, and the BSRI masculine, F(1, 93) = 5.51, p < .025, and feminine scales, F(1, 93) = 18.40, p < .001. Further, men compared to women showed significantly better performance on the paper and pencil measure of mental rotation, F(1, 101) = 29.32, p < .001, and significantly better targeting ability, F(1, 101) = 52.87, p < .001. In contrast, the female advantage in measures of spatial location memory and spatial working memory was small and non-significant, F(1, 74) < 1, ns, and F(1, 101) = 1.87, ns, respectively. As expected, salivary testosterone levels (average of time 1 and time 2) were significantly higher in men compared to women, F(1, 104) = 75.93, p < .001, and the 2D:4D ratio was lower in men (M = .96, SD = .03) compared to women (M = .97, SD = .03). However, the sex difference in digit ratio did not reach statistical significance, F(1, 108) = 2.10, ns, d = .28. Because digit ratios may vary by race/ethnicity (Manning et al., 2000, 2004), the sex difference in digit ratio was evaluated for only White, non-Hispanic participants. Results also did not reach statistical significance, F(1, 80) = 2.27, ns, d = .33.
Table I.
Mean Scores (SD) on Measures of Hormone Levels, Spatial Ability, and Gender Role Behavior Confirming Expected Sex Differences
| Task/Measure | Sex Difference? | Means | Effect Size | ||
|---|---|---|---|---|---|
| Men | Women | ||||
| Spatial Tasks | Mental Rotation | Yes*** | 13.78 (4.61) n = 55 |
9.06 (4.33) n = 50 |
d = 1.06 |
| Targeting | Yes*** | 6.23 (1.78) n = 55 |
9.53 (2.77) n = 50 |
d = 1.41 | |
| Spatial Memory – Errors | No | 7.82 (9.71) n = 55 |
5.60 (6.58) n = 50 |
d = .27 | |
| Location Memory | No | 0.74 (0.10) n = 41 |
0.76 (0.11) n = 38 |
d = .18 | |
| Gender Role Measures | PSAI | Yes*** | 76.26 (9.11) n = 54 |
36.74 (15.60) n = 43 |
d = 3.09 |
| OAT-PM – Masculine Score | Yes*** | 2.43 (0.32) n = 54 |
2.12 (0.40) n = 43 |
d = .87 | |
| OAT-PM Feminine Score | Yes*** | 2.00 (0.25) n = 54 |
2.46 (0.31) n = 43 |
d = 1.65 | |
| OAT-AM | Yes*** | 0.15 (0.15) n = 54 |
0.05 (0.09) n = 43 |
d = .78 | |
| BSRI – Feminine Scale | Yes*** | 4.71 (0.56) n = 54 |
5.27 (0.68) n = 43 |
d = .89 | |
| BSRI – Masculine Scale | Yes* | 5.53 (0.83) n = 54 |
5.16 (0.65) n = 43 |
d = .49 | |
| Digit Ratio | Right 2D:4D | No | 0.96 (0.03) n = 58 |
0.97 (0.03) n = 52 |
d =.28 |
| Left 2D:4D | No | 0.96 (0.04) n = 58 |
0.96 (0.04) n = 52 |
d =.17 | |
| Salivary Testosterone (pg/mL) | Average Testosterone | Yes*** | 230.99 (85.49) n = 58 |
110.70 (51.05) n = 50 |
d = 1.71 |
| Salivary Estradiol (pg/mL) | Average Estradiol | Not Applicable | Not measured | 11.30 (5.92) n = 47 |
Not Applicable |
Note. p < .05.
p < .001.
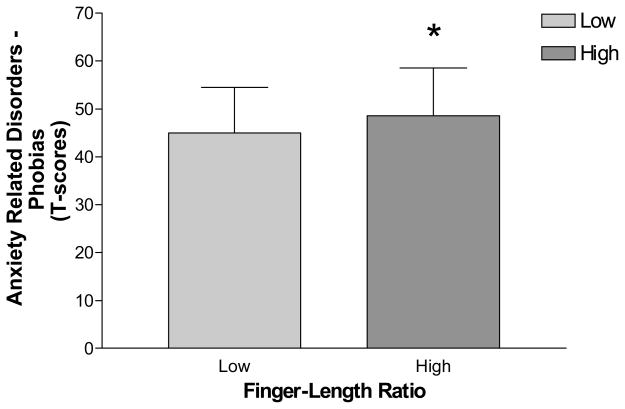
Table II summarizes the means and SDs for women and men on the anxiety measures, with effect sizes reported for each. Women generally reported higher levels of anxiety on the Anxiety-full scale (ANX) of the PAI, F(1, 93) = 7.83, p < .01, and all three of its component scales: cognitive (ANX-C), F(1, 93) = 5.69, p < .025, affective (ANX-A), F(1, 93) = 9.73, p < .01, and physical symptoms (ANX-P), F(1, 93) = 4.81, p < .05. They also reported greater phobia symptoms on the Anxiety-Related Disorders-Phobias Subscale (ARD-P), F(1, 93) = 7.01, p = .01. Similarly, a trend towards significance was found for the Beck Anxiety Inventory (BAI), F(1, 93) = 3.50, p < .10. In contrast, no sex differences were found on state, F(1, 93) < 1, ns, or trait anxiety, F(1, 93) = 1.31, ns, and the Anxiety-Related Disorders-Obsessions (ARD-O), F(1, 93) = 1.39, ns, and Trauma (ARD-T), F(1, 93) < 1, ns, Subscales of the PAI.
Table II.
Mean Scores (SD) on Measures of Anxiety in Women and Men
| Task/Measure | Sex Difference? | Means | Effect Size | ||
|---|---|---|---|---|---|
| Men (n = 54) | Women (n = 43) | ||||
| Anxiety Measures | ANX Scale | Yes** | 45.61 (10.00) | 51.22 (10.59) | d = .54 |
| ANX – Cognitions Subscale | Yes* | 46.81 (9.95) | 51.56 (10.99) | d = .45 | |
| ANX – Affect Subscale | Yes** | 43.96 (9.10) | 49.61 (8.58) | d = .64 | |
| ANX – Physical Sx Subscale | Yes* | 47.68 (9.57) | 52.15 (12.12) | d = .41 | |
| STAI – State | No | 31.11 (11.80) | 30.95 (9.62) | d = .01 | |
| STAI – Trait | No | 34.59 (11.58) | 36.86 (9.01) | d = .22 | |
| BAI | No | 7.63 (5.74) | 10.26 (8.26) | d = .37 | |
| ARD Scale | No | 47.96 (9.89) | 50.81 (10.06) | d = .29 | |
| ARD – Obsessions Subscale | No | 50.27 (9.42) | 52.44 (10.21) | d = .22 | |
| ARD – Phobias Subscale | Yes* | 44.52 (9.66) | 49.38 (9.47) | d = .51 | |
| ARD- Traumas Subscale | No | 49.92 (10.63) | 49.83 (10.43) | d = .008 | |
Note. ANX = Anxiety Scale of the Personality Assessment Inventory; STAI = State-Trait Anxiety Inventory; BAI = Beck Anxiety Inventory; ARD = Anxiety-Related Disorders Scale of the Personality Assessment Inventory.
p < .05.
p < .01.
Digit Ratio, Hormone Levels, and Anxiety
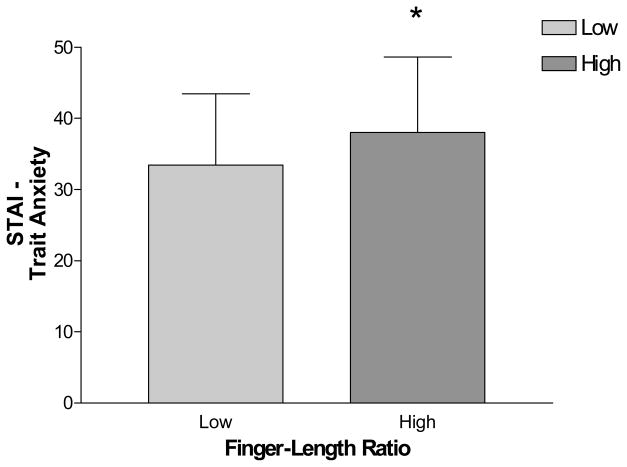
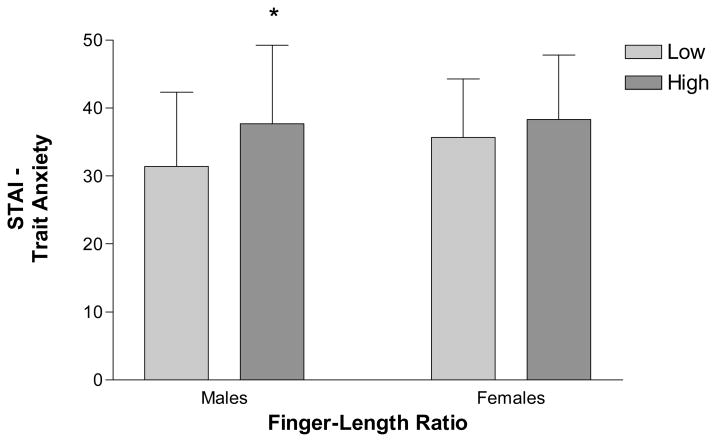
To examine the relation between the 2D:4D ratio and anxiety symptoms, bivariate correlations were conducted between the continuous measure of 2D:4D and scores on anxiety measures within each sex. Results of these analyses are reported in Table III. To summarize, only trait anxiety was positively correlated with digit ratio in men (r = .26, p < .05). Men with larger (i.e., more feminine) digit ratios reported greater trait anxiety than men with smaller (i.e., more masculine) digit ratios. No significant correlations were found between anxiety scales and digit ratio in women.
Table III.
Bivariate (Two-Tailed) Correlations Between Anxiety Measures and Digit Ratio
| Anxiety Measure | 2D:4D Ratio Correlation |
|||
|---|---|---|---|---|
| Men | Women | |||
| (N) | r | (N) | r | |
| ANX Scale | 54 | r = .105 | 43 | r = .110 |
| Cognitions Subscale | 54 | r = .181 | 43 | r = .110 |
| Affect Subscale | 54 | r = .074 | 43 | r = .019 |
| Physical Sx Subscale | 54 | r = .000 | 43 | r = .152 |
| STAI-State | 58 | r = .049 | 52 | r = .200 |
| STAI-Trait | 58 | r = .260* | 52 | r = .082 |
| BAI | 54 | r = −.065 | 43 | r = .030 |
| ARD Scale | 54 | r = .098 | 43 | r = .092 |
| Obsessions Subscale | 54 | r = .045 | 43 | r = .053 |
| Phobias Subscale | 54 | r = .063 | 43 | r = .138 |
| Traumas Subscale | 54 | r = .099 | 43 | r = .031 |
Note. ANX = Anxiety Scale of the Personality Assessment Inventory; STAI = State-Trait Anxiety Inventory; BAI = Beck Anxiety Inventory; ARD = Anxiety-Related Disorders Scale of the Personality Assessment Inventory.
p < .05.
Bivariate correlations were also conducted between hormone levels (average of time 1 and time 2) and anxiety measures for men and for women not taking oral contraceptives (n = 37). This analysis of hormones and behavior showed no significant associations between average estradiol or testosterone levels and anxiety scores in either men or women.
Sex-linked Behavior and Anxiety
Anxiety scores were hypothesized to covary with gender role and spatial abilities on the basis of previous research indicating that sex-linked behaviors share similar hormonal determinants. To test this hypothesis, within-sex, bivariate correlations were conducted between anxiety measures and the four sex-linked spatial tasks and between anxiety measures and all gender role questionnaires. Results of these correlational analyses are reported in Table IV and V. To summarize, of the 11 measures of anxiety, 7 (ANX-full scale, ANX-C, ANX-A, ANX-P, trait anxiety, ARD-full scale, and ARD-P) were significantly correlated with mental rotation performance and none were significantly correlated with targeting ability in men; for women, none were significantly correlated with mental rotation performance and 2 were significantly correlated with targeting ability (ARD-full scale and ARD-T). Men who performed poorly on mental rotation had higher anxiety scores on more than half of the anxiety measures, whereas women who performed better on targeting had higher scores on anxiety-related disorders symptoms, particularly related to trauma. No significant correlations were found between anxiety scores and the two female-linked tasks (spatial location memory and spatial working memory) either in men or women.
Table IV.
Bivariate (Two-Tailed) Correlations Between Anxiety Measures and Male-Linked Spatial Tasks
| Anxiety Mea sure | Mental Rotation | Targeting | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| N | r | N | r | N | r | N | r | |
| ANX Scale | 54 | −.360** | 43 | −.038 | 54 | .250 | 43 | −.044 |
| Cognitions Subscale | 54 | −.387** | 43 | −.021 | 54 | .240 | 43 | .053 |
| Affect Subscale | 54 | −.321* | 43 | −.178 | 54 | .236 | 43 | −.056 |
| Physical Sx Subscale | 54 | −.269* | 43 | .073 | 54 | .212 | 43 | −.147 |
| STAI-State | 58 | −.188 | 52 | .069 | 58 | .165 | 52 | .026 |
| STAI-Trait | 58 | −.262* | 52 | .027 | 58 | .199 | 52 | .152 |
| BAI | 54 | −.262 | 43 | .148 | 54 | .066 | 43 | .018 |
| ARD Scale | 54 | −.284* | 43 | .082 | 54 | .128 | 43 | −.357* |
| Obsessions Subscale | 54 | −.074 | 43 | .264 | 54 | .010 | 43 | −.297 |
| Phobias Subscale | 54 | −.303* | 43 | .001 | 54 | .137 | 43 | −.106 |
| Traumas Subscale | 54 | −.250 | 43 | −.081 | 54 | .131 | 43 | −.338* |
Note. ANX = Anxiety Scale of the Personality Assessment Inventory; STAI = State-Trait Anxiety Inventory; BAI = Beck Anxiety Inventory; ARD = Anxiety-Related Disorders Scale of the Personality Assessment Inventory.
p < .05.
p < .01.
Table V.
Bivariate (Two-Tailed) Correlations Between Anxiety Measures and Gender Role Behavior
| Anxiety Measure | PSAI | OAT-PM Masculine Score | OAT-PM Feminine Score | OAT-AM | BSRI- Feminine Scale | BSRI- Masculine Scale | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | |
| ANX Scale (54 men, 43 women) | −.286* | −.079 | .078 | −.086 | .170 | −.142 | .162 | −.150 | .101 | −.149 | −.207 | −.198 |
| Cognitions Subscale | −.334* | −.071 | .004 | −.078 | .221 | −.108 | .160 | −.161 | .144 | −.077 | −.179 | −.148 |
| Affect Subscale | −.277* | .003 | .077 | −.134 | .103 | −.127 | .091 | −.022 | −.003 | −.012 | −.242 | −.264 |
| Physical Sx Subscale | −.146 | −.133 | .170 | −.026 | .123 | −.153 | .202 | −.194 | .129 | −.311* | −.152 | −.140 |
| STAI-State (58 men, 52 women) | −.363** | .004 | −.003, n = 54 | −.161, n = 43 | .170, n = 54 | −.133, n = 43 | −.042, n = 54 | .016, n = 43 | .018, n = 54 | −.008, n = 43 | −.095, n = 54 | −.139, n = 43 |
| STAI-Trait (58 men, 52 women) | −.305* | .094 | −.009, n = 54 | −.119, n = 43 | .132, n = 54 | −.184, n = 43 | .050, n = 54 | −.026, n = 43 | .038, n = 54 | .005, n = 43 | −.293*, n = 54 | −.300, n = 43 |
| BAI (54 men, 43 women) | −.167 | −.059 | .005 | .123 | .172 | −.127 | −.014 | −.157 | .003 | −.175 | −.054 | −.116 |
| ARD Scale (54 men, 43 women) | −.208 | −.050 | −.005 | .008 | .261 | .062 | .133 | .005 | .191 | −.242 | −.062 | −.287 |
| Obsessions Subscale | −.064 | −.123 | .011 | −.018 | .251 | .381* | .149 | .196 | .127 | −.154 | .052 | −.214 |
| Phobias Subscale | −.294* | −.093 | .086 | −.237 | .139 | −.205 | .123 | −.159 | .174 | .151 | −.232 | −.366* |
| Traumas Subscale | −.126 | .079 | −.077 | .195 | .178 | −.081 | .038 | −.056 | .127 | −.426** | .005 | −.100 |
Note. ANX = Anxiety Scale of the Personality Assessment Inventory; STAI = State-Trait Anxiety Inventory; BAI = Beck Anxiety Inventory; ARD = Anxiety-Related Disorders Scale of the Personality Assessment Inventory.
p < .05.
p < .01.
Anxiety levels were also significantly correlated with reports of gender role orientation. Of the 11 measures of anxiety, 6 (ANX-full scale, ANX-C, ANX-A, state anxiety, trait anxiety, and ARD-P) were significantly correlated with PSAI scores and 1 (trait anxiety) was significantly correlated with BSRI masculine scores in men; for women, 1 (ARD-O) was significantly correlated with OAT-PM feminine scores, 1 (ARD-P) was significantly correlated with BSRI masculine scores, and 2 (ANX-P and ARD-T) were significantly correlated with BSRI feminine scores. As expected, men with more feminine play preferences reported greater affective and cognitive symptoms of anxiety, greater state and trait anxiety, and greater phobia symptoms. Men with less masculine traits on the BSRI masculine scale also reported greater trait anxiety. Similarly, women with a more feminine gender role on the OAT-PM feminine scale reported greater obsessive-compulsive symptoms, and women with less masculine traits on the BSRI masculine scale reported greater phobia symptoms. However, contrary to expectations, women reporting less feminine traits on the BSRI feminine scale reported greater physical and trauma-related symptoms of anxiety.
Hierarchical Regression Analyses
Because significant associations between trait anxiety and digit ratio and between trait anxiety and various sex-linked behaviors were found for men, we were interested in the relative contributions of gender role measures, spatial task performance, and hormone levels to scores on this measure. Therefore, separate hierarchical regression analyses were conducted for each sex using STAI-trait as the criterion variable. For each model, the BSRI masculine and feminine scales were entered at the first step, cognitive abilities showing the largest sex differences (i.e., mental rotation and targeting scores) were entered at the second step, and 2D:4D ratio and testosterone levels were entered at the third step. Hormone measures were entered last to determine if they added any significant contribution to scale scores beyond gender role behavior and spatial task performance.
For men, results of these analyses suggest that the variance in trait anxiety scores was influenced primarily by gender role behavior, particularly scores on the BSRI masculine scale. The first model using the BSRI masculine and feminine scales appeared to account for the greatest, though non-significant, portion of variance in anxiety, F(2, 51)= 2.75, R2 = .097, ns. Although adding the cognitive tasks at the second step produced a significant model, F(4, 49) = 2.59, R2 = .174, p < .05, this did not represent a significant change from the first model, Fchange = 2.29, ns. Examination of individual beta weights suggests that the BSRI masculine scale was the only significant predictor of trait anxiety in men, β = −.22, p < .025. No model significantly predicted trait anxiety scores in women.
DISCUSSION
Results of the present study confirmed the expected sex differences in average testosterone levels, all measures of social behavior, the two male-linked spatial tasks, and 5 of the 11 measures of anxiety (ANX-full scale, ANX-C, ANX-A, ANX-P, and ARD-P), with a 6th measure (BAI) showing near-significance. In contrast, no significant sex differences were found for the 2D:4D ratio, the two female-linked linked tasks, and 5 of the 11 measures of anxiety (trait or state anxiety, ARD-full scale, ARD-O, and ARD-T). As expected, reports of anxiety were related to various normative sex-linked behaviors in women and men. More specifically, men performing worse on mental rotation and those reporting more female-typical play preferences in childhood reported greater anxiety on more than half of the measures (7 and 6, respectively). Men reporting fewer masculine traits on the BSRI masculine scale also reported greater trait anxiety. In women, more feminine-typed behavior on the OAT-PM was associated with greater obsessive-compulsive symptoms, and less masculine-typed traits on the BSRI masculine scale were associated with greater phobia symptoms. However, contrary to predictions, women reporting less feminine-typed traits on the BSRI feminine scale reported greater physical and trauma-related symptoms of anxiety, and women with better (i.e., more masculine) performance on the targeting task reported greater anxiety-related disorders symptoms overall and greater trauma-related symptoms, in particular. Also contrary to hypotheses, reports of anxiety were unrelated to measures of estradiol in women, testosterone in women and men, and of the 11 measures of anxiety, only trait anxiety showed a significant, positive correlation with digit ratio in men. However, this relation was qualified by results from regression analyses suggesting that 2D:4D ratio did not predict any significant variance in trait anxiety beyond gender role behavior.
The present finding that sub-clinical levels of anxiety were unrelated to a measure of hormone levels at one test session does not necessarily contradict previous evidence suggesting that anxiety levels are sensitive to hormonal change across the menstrual cycle, pregnancy, and postpartum periods (Altshuler et al., 1998; Cook et al., 1990; Hertzberg & Wahlbeck, 1999; McLeod et al., 1993; Williams & Koran, 1997). However, the absence of a significant sex difference in 2D:4D ratio and a significant female-advantage on location memory (Silverman & Eals, 1992; Silverman et al., 2007) and spatial working memory (Duff & Hampson, 2001) in this research may be viewed as problematic for the general interpretation of the results for prenatal hormones, sex-linked behavior, and anxiety. Certainly, the hypothesized relation between digit ratio and perinatal androgen action (Lutchmaya et al., 2004; Manning et al., 1998; McIntyre, 2006) relies on the sexual dimorphism of this trait, and a significant difference is often found in the literature (Manning, 2002). However, it should be noted that mean digit ratios in this research were lower in men compared to women and that the magnitude of the sex difference (for entire sample – d =. 28, for White, non Hispanics – d = .33) is similar to several published studies (d = .19–.22), with those having a smaller sample (n = 29) not finding a sex difference and those with a larger sample (n = 196) reporting a significant difference (Lutchmaya et al., 2004; Williams et al., 2003). Similarly, the female advantage in location memory is small (d = .26) (Voyer et al., 2007), consistent with the general finding that sex differences in female-linked tasks compared to male-linked tasks generally yield smaller effects (Hyde & Linn, 1988). Thus, it is likely that our non-significant sex difference in 2D:4D ratio and object location memory is related to considerations external to the hypothesized effect (i.e., sample size; Thompson, 1999). On the other hand, the previously reported sex differences in working memory errors (d =.63) and completion time (d = .69) for the spatial working memory task (Duff & Hampson, 2001) are much larger than the effect size reported in this study (d = .27). Of interest, a female advantage has not been observed on other visuospatial working memory tasks (Robert & Savoie, 2006). It may be that the magnitude of the female advantage in the novel spatial working memory task is smaller than that documented in the earlier report.
Against this background of subject characteristics that, with the possible exception of spatial working memory, are consistent with the general findings from research on human sex differences, the sum of the results for men suggests that the development of gender roles contributes to the expression of anxiety. The relation between a more feminine pattern of behaviors and anxiety in men is consistent with previous research showing that the lack of instrumental traits (e.g., dominance and assertiveness) and the socialization of expressive traits (e.g., passiveness and dependence) associated with the female gender role may result in an increased susceptibility to anxiety symptoms (Arrindell, Kolk, Pickersgill, & Hageman, 1993; Chambless & Mason, 1986; Fodor, 1974; Ginsburg & Silverman, 2000; Peréz Blasco & Serra Desfilis, 1997). Adding to this perspective, results of this study suggest that beyond associations with sex-linked personality traits, anxiety in men appears related to other sex-linked behavior, such as cognitive processes and early social experiences (e.g., play preferences). In this context, it may be significant to note that although anxiety levels of men in these analyses were associated with mental rotation ability, they were unrelated to targeting ability. Previous findings that women who were exposed to higher levels of androgens showed enhanced targeting ability but not enhanced mental rotation ability (Hines et al., 2003) have suggested that the mechanisms supporting targeting accuracy may be organized by androgens in prenatal life whereas those supporting mental rotation may be more dependent on postnatal development. Therefore, the apparent sensitivity of anxiety levels in men to mental rotation ability further suggests that both are among a class of sex-linked variables that are more dependent on social experiences for their ultimate expression.
A greater role for socialization in the expression of sex-linked behavior, however, does not contradict a hormonal hypothesis (Wallen, 1996). For example, an association between men’s reports of their early play preferences and anxiety in this research may result because androgen effects on play styles (e.g., Berenbaum & Hines, 1992) promote gender-linked personality traits and social interaction patterns (Maccoby, 1998) that confer greater or lesser risk for anxiety. In this context, it may be informative that although trait anxiety was best predicted by scores on the BSRI masculine scale, this study also found a significant correlation between trait anxiety and 2D:4D ratio in men. Given the large number of analyses, this result may prove to be spurious. However, such an association between 2D:4D ratio and trait anxiety is consistent with other research showing significant associations between digit ratio and sex-linked psychopathology. Masculine 2D:4D ratios and other related digit ratios, hypothesized to reflect greater perinatal androgen action, have been associated with male-typical disorders, such as autism and ADHD, (Manning et al., 2001; McFadden et al., 2005), whereas feminine digit ratios, hypothesized to reflect lower perinatal androgen action, have been associated with female-typical psychopathology, such as trait depression (Bailey & Hurd, 2005b) and eating disorder symptoms (Klump et al., 2006). A relationship between digit ratios and anxiety in men is also supported by previous research showing a positive association between 2D:4D ratio and what are often thought as precursors to anxiety in adulthood: anxious behavior in childhood (Williams et al., 2003) and degree of neuroticism in adults (Fink et al., 2004). Taken together with animal research demonstrating a role for androgens in the expression of anxious behavior (Lucion et al., 1996), these findings in humans suggest that the association between measures of androgen action and the development and expression of anxiety in men merits further study.
Although our results in men suggest that lower anxiety levels are associated with the greater expression of male-typical social and cognitive behavior, results in women appear contradictory. Whereas some aspects of anxiety (obsessive-compulsive and phobia symptoms) in women were associated with more female-typical/less male-typical behavior, other aspects of anxiety (physical and trauma-related symptoms) were associated with less female-typical/more male-typical behavior. It may be that because anxiety is multi-determined (Fodor, 1974; Hettema, Neale, & Kendler, 2001), the specific risk factors that increase anxiety potential may differ between women and men due to sex differences in social development (Skodol & Bender, 2003). For example, risk factors that occur more frequently in women than in men (such as physical trauma or abuse) may be more important determinants of anxiety levels than other variables assessed in this research. It may also be relevant for theories of gender stereotypes and the expression of anxiety, that women’s reports of trauma-related symptoms were associated with less feminine traits and more masculine performance in targeting ability. More male-typical behavior in women (e.g., rough and tumble play and aggression) may lead to a greater risk for trauma, or perhaps adapting more male-typical behavior, such as targeting ability, is a coping resource developed by women following the experience of trauma. Our findings suggest that it may be informative in future research on anxiety disorders to measure the relationship between gender development and the different cognitive, affective and physical symptoms of anxiety.
Finally, the present investigation was exploratory in nature and a stronger test of the study hypothesis and conclusions will require replication using a more parsimonious set of analyses and repeated assessment on a larger sample, including individuals with different anxiety related disorders (e.g., phobia, generalized anxiety). It may also be useful in research on the relative contributions of social and biological factors to anxiety to consider other sexually dimorphic traits and other finger-length ratios that differ between males and females (McFadden et al., 2005). It may also be important to clarify whether other sex-linked psychopathology (e.g., borderline personality and antisocial personality) and disorders varying in their sensitivity to gender, like schizophrenia and schizotypal personality (Arato et al., 2004; Walder et al., 2006), are associated with 2D:4D ratio and gender role behavior, and subsequently, with the hypothesized androgen mechanisms believed to underlie these traits and behaviors.
Fig. 1.
Fig. 2.
Fig. 3.
Acknowledgments
This research was supported in part by the National Institute of Mental Health grant MH071414 (GMA). The authors thank Cecil Reynolds, Ph.D. and Leslie C. Morey, Ph.D. for their input on earlier versions of this manuscript and the various graduate and undergraduate assistants who collaborated in data collection for this project: Julia Makkaoui, Liz Wiley, Chris Strachan, Travis Smith, Ebun Akindile, and Troy Song.
References
- Alexander GM. Associations among gender-linked toy preferences, spatial ability, and digit ratio: Evidence from eye-tracking analysis. Archives of Sexual Behavior. 2006;35:699–709. doi: 10.1007/s10508-006-9038-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. Journal of Clinical Psychiatry. 1998;59:29–33. [PubMed] [Google Scholar]
- Arato M, Frecska E, Beck C, An M, Kiss H. Digit length pattern in schizophrenia suggests disturbed prenatal hemispheric lateralization. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2004;28:191–194. doi: 10.1016/j.pnpbp.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Armstrong KA, Khawaja NG. Gender differences in anxiety: An investigation of the symptoms, cognitions, and sensitivity towards anxiety in a nonclinical population. Behavioural and Cognitive Psychotherapy. 2002;30:227–231. [Google Scholar]
- Arrindell WA, Kolk AM, Pickersgill MJ, Hageman WJJM. Biological sex, sex role orientation, masculine sex role stress, dissimulation, and self-reported fears. Advances in Behaviour Research & Therapy. 1993;15:103–146. [Google Scholar]
- Austin EJ, Manning JT, McInroy K, Matthews E. A preliminary investigation of the associations between personality, cognitive ability and digit ratio. Personality and Individual Differences. 2002;33:1115–1124. [Google Scholar]
- Bailey AA, Hurd PL. Finger length ratio (2D:4D) correlates with physical aggression in men but not in women. Biological Psychology. 2005a;68:215–222. doi: 10.1016/j.biopsycho.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bailey AA, Hurd PL. Depression in men is associated with more feminine finger length ratios. Personality and Individual Differences. 2005b;39:829–836. [Google Scholar]
- Bailey AA, Wahlsten D, Hurd PL. Digit ratio (2D:4D) and behavioral differences between inbred mouse strains. Genes, Brain, & Behavior. 2005;4:318–323. doi: 10.1111/j.1601-183X.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Battisti F, De Francisis A, Tarsitani L, Di Clemente L, Di Stani F, Calabresi M, et al. Gender differences in anger, depression and anxiety dimensions and psychometric screening of psychopathology in a sample of students. Rivista di Psichiatria. 2004;39:184–188. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory manual. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Bem SL. Bem Sex Role Inventory professional manual. Palo Alto, CA: Consulting Psychologists Press; 1981. [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3:203–206. [Google Scholar]
- Brown W, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Hormones and Behavior. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Human Reproduction. 2003;18:976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Meisfeld RL. The length and location of the CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research. 1994;15:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambless DL, Mason J. Sex, sex-role stereotyping and agoraphobia. Behaviour Research & Therapy. 1986;24:231–235. doi: 10.1016/0005-7967(86)90098-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on childhood and adult sex-typed behavior: methods and findings. Neuroscience and Biobehavioral Reviews. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychological Bulletin. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Cook BL, Noyes RJ, Garvey MJ, Beach V, Sobotka J, Chaudhry D. Anxiety and the menstrual cycle in panic disorder. Journal of Affective Disorders. 1990;19:221–226. doi: 10.1016/0165-0327(90)90095-p. [DOI] [PubMed] [Google Scholar]
- Coolican J, Peters M. Sexual dimorphism in the 2D/4D ratio and its relation to mental rotation performance. Evolution and Human Behavior. 2003;24:179–183. [Google Scholar]
- Csathó Á, Osváth Á, Bicsák É, Karádi K, Manning J, Kállai J. Sex role identity related to the ratio of second to fourth digit length in women. Biological Psychology. 2003a;62:147–156. doi: 10.1016/s0301-0511(02)00127-8. [DOI] [PubMed] [Google Scholar]
- Csathó Á, Osvath A, Karádi K, Bicsák É, Manning J, Kállai J. Spatial navigation related to the second to fourth digit length in women. Learning and Individual Differences. 2003b;13:239–249. [Google Scholar]
- Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain & Cognition. 2001;47:470–493. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- Ehrler DJ, Evans JG, Mcghee RL. Extending big-five theory into childhood: A preliminary investigation into the relationship between big-five personality traits and behavior problems in children. Psychology in the Schools. 1999;36:451–458. [Google Scholar]
- Fink B, Manning JT, Neave N. Second to fourth digit ratio and the ‘big five’ personality factors. Personality and Individual Differences. 2004;37:495–503. [Google Scholar]
- Fodor IG. The phobic syndrome in women: Implications for treatment. In: Franks V, Burtle V, editors. Women in therapy. New York: Brunner/Mazel; 1974. pp. 132–168. [Google Scholar]
- Foot M, Koszycki D. Gender differences in anxiety-related traits in patients with panic disorder. 2005;20:123–130. doi: 10.1002/da.20031. [DOI] [PubMed] [Google Scholar]
- Ginsburg GS, Silverman WK. Gender role orientation and fearfulness in children with anxiety disorders. Journal of Anxiety Disorders. 2000;14:57–67. doi: 10.1016/s0887-6185(99)00033-x. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J. The pre-school activities inventory: a standardized assessment of gender role in children. Psychological Assessment. 1993;5:131–136. [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwatrz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet JF, Altmann D. Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Developmental Neuropsychology. 1998;14:299–320. [Google Scholar]
- Hertzberg T, Wahlbeck K. The impact of pregnancy and the puerperium on panic disorder: A review. Journal of Psychosomatics Obstetrics and Gynecology. 1999;20:59–64. doi: 10.3109/01674829909075578. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Kazemi-Esfarjani P, Trifiro MA, Pinski L. Evidence for a recessive function of the long polyglutamine tract in the human androgen receptor: Possible pathogenic relevance for the (CAG)n-expanded neuropathies. Human Molecular Genetics. 1995;4:523–527. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- Kempel P, Gohlke B, Klempau J, Zinsberger P, Reuter M, Hennig J. Second-to-fourth digit length, testosterone and spatial ability. Intelligence. 2005;33:215–230. [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. British Journal of Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Berenbaum SA. Early androgen effects on interest in infants: Evidence from children with congenital adrenal hyperplasia. Developmental Neuropsychology. 1998;14:321–340. [Google Scholar]
- Liben LS, Bigler RS. The developmental course of gender differentiation: Conceptualizing, measuring, and evaluating constructs and pathways. Monographs of the Society for Research in Child Development. 2002:67. [PubMed] [Google Scholar]
- Lucion AB, Charchat H, Pereira GAM, Rasia-Filho AA. Influence of early postnatal gonadal hormones on anxiety in adult male rats. Physiology & Behavior. 1996;60:1419–1423. doi: 10.1016/s0031-9384(96)00246-6. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone, and estradiol. Early Human Development. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Maccoby EE. The two sexes: Growing up apart, coming together. Cambridge, MA: Belknap Press/Harvard University Press; 1998. [Google Scholar]
- Malas MA, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hand, digits, and digit ratio. Early Human Development. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Manning JT. Digit ratio: A pointer to fertility, behavior, and health. New Brunswick, NJ: Rutgers University Press; 2002. [Google Scholar]
- Manning JT, Barley L, Walton J, Lewis-Jones DI, Trivers RL, Singh D, et al. The 2nd:4th digit ratio, sexual dimorphism, population differences, and reproductive success: Evidence for sexually antagonistic genes? Evolution and Human Behavior. 2000;21:163–183. doi: 10.1016/s1090-5138(00)00029-5. [DOI] [PubMed] [Google Scholar]
- Manning JT, Baron-Cohen S, Wheelwright S, Sanders G. The 2nd to 4th digit ratio and autism. Developmental Medicine & Child Neurology. 2001;43:160–164. [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Newton DJ, Flanagan BF. The second to fourth digit ratio and variation in the androgen receptor gene. Evolution and Human Behavior. 2003;24:399–405. [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of the 2nd and 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone, and oestrogen. Human Reproduction. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Human Development. 2004;80:161–168. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Martin SM, Manning JT, Dowrick CF. Fluctuating asymmetry, relative digit length, and depression in men. Evolution and Human Behavior. 1999;20:203–214. [Google Scholar]
- McFadden D, Bracht MS. Sex differences in the relative lengths of metacarpals and metatarsals in gorillas and chimpanzees. Hormones and Behavior. 2005;47:99–111. doi: 10.1016/j.yhbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Hormones and Behavior. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. [DOI] [PubMed] [Google Scholar]
- McFadden D, Westhafer JG, Pasanen EG, Carlson CL, Tucker DM. Physiological evidence of hypermasculinization in boys with the inattentive type of attention-deficit hyperactivity disorder (ADHD) Clinical Neuroscience Research. 2005;5:233–245. [Google Scholar]
- McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reproductive Biology and Endocrinology. 2006;4:10–18. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Hoehn-Saric R, Foster GV, Hipsley PA. The influence of premenstrual syndrome on ratings of anxiety in women with generalized anxiety disorder. Acta Psychiatrica Scandinavica. 1993;88:248–251. doi: 10.1111/j.1600-0447.1993.tb03451.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Archives of Sexual Behavior. 2004;33:97–104. doi: 10.1023/b:aseb.0000014324.25718.51. [DOI] [PubMed] [Google Scholar]
- Morey LC. The Personality Assessment Inventory professional manual (PAI) Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Murphy PL. Social interest and psychological androgyny: Conceptualized and tested. Individual Psychology: Journal of Adlerian Theory, Research & Practice. 1994;50:18–30. [Google Scholar]
- Novy DM, Nelson DV, Goodwin J, Rowzee RD. Psychometric comparability of the State-Trait Anxiety Inventory for Different Ethnic Subpopulations. Psychological Assessment. 1993;5:343–349. [Google Scholar]
- Okten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit ratio lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Human Development. 2002;70:47–54. doi: 10.1016/s0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Peréz Blasco J, Serra Desfilis E. Influence of the traditional feminine role on the anxiety symptoms in a sample of adult women. Anales de psicología. 1997;13:155–161. [Google Scholar]
- Peters M, Laeng B, Latham K, Jackson M, Zaiyouna R, Richardson C. A redrawn Vandenberg & Kuse mental rotations test: Different versions and factors that affect performance. Brain & Cognition. 1995;28:39–58. doi: 10.1006/brcg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Peters M, Manning JT, Reimers S. The effects of sex, sexual orientation, and digit ratio (2D:4D) on mental rotation performance. Archives of Sexual Behavior. 2007;36:251–260. doi: 10.1007/s10508-006-9166-8. [DOI] [PubMed] [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatric Clinics of North America. 2003;26:621–672. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Putz DA, Gaulin SJC, Sporter RJ, McBurney DH. Sex hormones and finger length: What does 2D:4D indicate? Evolution and Human Behavior. 2004;25:182–199. [Google Scholar]
- Rammsayer TH, Troche SJ. Sexual dimorphism in second-to-fourth digit ratio and its relation to gender-role orientation in males and females. Personality and Individual Differences. 2007;42:911–920. [Google Scholar]
- Robert M, Savoie N. Are there gender differences in verbal and visuospatial working-memory resources? European Journal of Cognitive Psychology. 2006;18:378–397. [Google Scholar]
- Servin A, Nordenström A, Larsson A, Bohlin G. Prenatal androgens and gender-typed behavior: A study of girls with mild and severe forms of congenital adrenal hyperplasia. Developmental Psychology. 2003;39:440–450. doi: 10.1037/0012-1649.39.3.440. [DOI] [PubMed] [Google Scholar]
- Shear MK, Feske U, Greeno C. Gender differences in anxiety disorders: Clinical implications. In: Frank E, editor. Gender and its effects on psychopathology. Washington, DC: American Psychiatric Press, Inc; 2000. pp. 151–165. [Google Scholar]
- Silverman I, Choi J, Peters M. The hunter-gatherer theory of sex differences in spatial abilities: Data from 40 countries. Archives of Sexual Behavior. 2007;36:261–268. doi: 10.1007/s10508-006-9168-6. [DOI] [PubMed] [Google Scholar]
- Silverman I, Eals M. Sex differences in spatial abilities: Evolutionary theory and data. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind. New York: Oxford; 1992. pp. 533–549. [Google Scholar]
- Skodol AE. Gender-specific etiologies for antisocial and borderline personality disorders? In: Frank E, editor. Gender and its effects on psychopathology. Washington, DC: American Psychiatric Press; 2000. pp. 37–60. [Google Scholar]
- Skodol AE, Bender DS. Why are women diagnosed borderline more than men? Psychiatric Quarterly. 2003;74:349–360. doi: 10.1023/a:1026087410516. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983 . [Google Scholar]
- Thompson B. Journal editorial policies regarding statistical significance tests: Heat is to fire as p is to importance. Educational Psychology Review. 1999;11:157–169. [Google Scholar]
- Udry JR. Biological limits of gender construction. American Sociological Review. 2000;65:443–457. [Google Scholar]
- Udry JR, Morris NM, Kovenock J. Androgen effects on women’s gendered behaviour. Journal of Biosocial Science. 1995;27:359–368. doi: 10.1017/s0021932000022884. [DOI] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations: A group test of three-dimensional spatial visualization. Perceptual and Motor Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D, Postma A, Brake B, Imperto-McGinley J. Gender differences in object location memory: a meta-analysis. Psychonomic Bulletin and Review. 2007;14:23–38. doi: 10.3758/bf03194024. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Andersson TLC, McMillan AL, Breedlove SM, Walker EF. Sex differences in digit ratio (2D:4D) are disrupted in adolescents with schizotypal personality disorder: Altered prenatal gonadal hormone levels as a risk factor. Schizophrenia Research. 2006;86:118–124. doi: 10.1016/j.schres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Wallen K. Nature needs nurture: The interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Hormones and Behavior. 1996;30:364–378. doi: 10.1006/hbeh.1996.0042. [DOI] [PubMed] [Google Scholar]
- Watson NV, Kimura D. Nontrivial sex differences in throwing and intercepting: Relation to psychometrically-defined spatial functions. Personality & Individual Differences. 1991;12:375–385. [Google Scholar]
- Williams JHG, Greenhalgh KD, Manning JT. Second to fourth finger ratio and the possible precursors of developmental psychopathology in preschool children. Early Human Development. 2003;72:57–65. doi: 10.1016/s0378-3782(03)00012-4. [DOI] [PubMed] [Google Scholar]
- Williams KE, Koran LM. Obsessive-compulsive disorder in pregnancy, the puerperium, and the premenstruum. Journal of Clinical Psychiatry. 1997;58:330–334. doi: 10.4088/jcp.v58n0709. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Pepitone ME, Christensen ME, Cooke BM, Huberman AD, Breedlove NJ, et al. Finger-lengths ratios and sexual orientation. Nature. 2000;404:455–456. doi: 10.1038/35006555. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Kidner CL. Sex differences in anxiety disorders. In: Lewis-Hall F, Williams TS, et al., editors. Psychiatric illness in women: Emerging treatments & research. Washington, DC: American Psychiatric Publishing; 2002. pp. 5–30. [Google Scholar]