Abstract
Carbon nanotubes, owing to their electrical, chemical, mechanical, and thermal properties, are one of the most promising nanomaterials for the electronics, computer, and aerospace industries. More recently, these unique materials are finding their niche in neuroscience. Here, we discuss the use of carbon nanotubes as scaffolds for neuronal growth. The chemical properties of carbon nanotubes can be systematically varied by attaching different functional groups. Such functionalized carbon nanotubes can be used to control the outgrowth and branching pattern of neuronal processes. We also discuss electrical interactions between neurons and carbon nanotubes. The electrical properties of nanotubes can provide a mechanism to monitor or stimulate neurons through the scaffold itself. The ease of which carbon nanotubes can be patterned makes them attractive for studying the organization of neural networks and has the potential to develop new devices for neural prosthesis. We note that additional toxicity studies of carbon nanotubes are necessary so that exposure guidelines and safety regulations can be set.
Keywords: Carbon Nanotubes, Modification, Substrates/Scaffolds for Neuronal Growth, Electrical Recordings, Interface
INTRODUCTION
Nanoneuroscience is a recently coined term that describes the convergence of the existing but separate worlds of nanotechnology, chemistry, engineering, and neurobiology. A large body of research is emerging that hints at the potential applications of nanotechnology in neuroscience, although basic scientific and clinical progress is limited by the intrinsic complexities of the mammalian central nervous system (CNS; Silva, 2006). Nevertheless, recent studies interfacing nanomaterials with neural systems have provided a foundation for generating a new class of multifunctional devices and hybrid systems that could help in the repair of damaged CNS tissue, and that have paved the road to nanoneuroscience as a new discipline that can aid in unveiling functional properties of the brain.
Carbon nanotubes (CNTs) have been at the forefront of nanotechnology due to their unique electrical, mechanical, and chemical characteristics, which allow the development of a variety of miniaturized devices with outstanding properties (Krishnan et al., 1998). CNTs are cylindrically shaped carbon nanostructures, discovered in the 1990s (Iijima, 1991; Iijima and Ichihashi, 1993; Bethune et al., 1993). The simplest geometry of CNT is that of a single-walled nanotube (SWNT). Here, a single graphene sheet is rolled up and closed at each end by a hemispherical fullerene cap; SWNT diameters typically range between 0.8 and 2 nm. Multi-walled nanotubes (MWNT), on the other hand, are composed of numerous concentric graphite cylinders and can reach diameters of up to 100 nm. Interestingly, depending on its hexagonal lattice structure, the resulting electronic conduction within an SWNT approaches the properties of an insulating, conducting, or semiconductive material (Krishnan et al., 1998). CNTs can be systematically manipulated by attaching different chemical groups and can be functionalized to display, for example, a variety of surface charges, such as positive, neutral, or negative. The ease of such chemical modifications, combined with their innate characteristics, make CNTs an excellent candidate for interfacing with neural systems. Indeed, there have been developments of biocompatible, durable, and robust substrates/scaffolds and devices that affect neuronal growth and potentially provide therapies for CNS disorders (reviewed in Malarkey and Parpura, 2007). Approaches toward the characterization of the interactions between CNTs and brain cells and their circuitry offer great opportunities for nanotechnological applications for the nervous system. This review article briefly summarizes recent advances in the study of the effects that CNTs exert on neural cells when used as substrates for cellular growth. We also discuss the inherent capacity of such substrates for interfacing with neuronal electrical activity.
CNTs as Substrates/Scaffolds for Neuronal Growth
Recently, CNTs have attracted tremendous attention due to their use as scaffolds for neuronal growth; the extension of this is their potential use for reestablishing intricate connections between neurons after injury. Possible future applications in tissue engineering require an understanding of mechanisms and chemical modifications that could alter the interactions of biological cells and tissues with the nanomaterials (Fortina et al., 2005). During the past decade, several research groups have assessed CNTs as substrates for neuronal growth, demonstrating the biocompatibility of these materials. The application of CNTs in neuroscience research has been oriented toward the use of both MWNTs and SWNTs.
Mattson et al. (2000) applied MWNTs dispersed in ethanol onto polyethyleneimine (PEI)-coated glass coverslips and then allowed evaporation of ethanol to generate an MWNT layer on top of the PEI. They demonstrated that rat hippocampal neurons plated on such a substrate survived and continued to grow for at least eight days in culture. These results indicated for the first time that CNTs support relatively long-term cell survival in culture (Mattson et al., 2000). Other researchers confirmed these findings by characterizing the presence of growth cones, neurite outgrowth, and branching in rat hippocampal neurons grown on MWNTs (Hu et al., 2004). Since then, MWNTs have been considered biocompatible substrates, although they have not supported as much neurite outgrowth and branching as other known permissive substrates for neuronal growth, such as PEI (Mattson et al., 2000; Hu et al., 2004). SWNTs also have been tested for their biocompatibility (Liopo et al., 2006). The material allowed long-term survival of NG108 neuroblastoma cells in culture. NG108 cells grown on SWNT films displayed attachment and growth, albeit at a reduced rate compared to their growth on more permissive substrates, such as tissue-treated polystyrene (Liopo et al., 2006).
When combined, these reports support the notion that CNTs, MWNTs, and SWNTs could be used as biocompatible materials that would permit cell viability. However, they also indicate some limits in respect to the ability of CNTs to support neurite elongation and branching. Such parameters were quantified in neurons and/or NG108 cells grown on CNTs and appeared reduced when compared to those gathered from neurons grown on widely accepted permissive substrates (Hu et al., 2004; Liopo et al., 2006). This issue was addressed by Galvan-Garcia and coworkers (Galvan-Garcia et al., 2007). They reported that directionally oriented CNTs in the form of sheets or yarns offer an alternative presentation of CNTs to cells. These CNT forms promoted cell attachment, differentiation, and cell growth. Additionally, when highly purified, these CNTs also allowed neurons to extend processes of which the number and length were comparable to those of neurons grown on a permissive substrate, represented by polyornithine pretreated glass (Galvan-Garcia et al., 2007). Thus, the interaction between neurons and CNTs might be affected by the purity of CNTs, as well as by the three-dimensional organization of the CNT substrate/scaffold.
Although highly informative, these studies, which assess the viability of neurons grown on CNTs and their morphology, have not investigated the presence of functional synaptic transmission. Indeed, the electrophysiological properties of neurons grown on CNTs and the functional status of their synaptic connections were addressed by Lovat et al. (2005). These authors reported for the first time the effects of CNT substrates on the electrical behavior of neurons and neuronal networks in culture. The CNTs used for neuronal growth were first functionalized, allowing uniform solvation, and then deposited onto a glass substrate. Following evaporation of the solvent, CNTs were defunctionalized by thermal treatment—generating glass coverslips covered by a film (i.e., a nanomeshwork) of native/nonfunctionalized CNTs. This strategy allowed a long-term and stable retention of CNT films on glass and, moreover, long-term neuronal cell survival in culture. When compared to control plain glass surfaces, MWNT substrates boosted neuronal network activity under culturing conditions (Lovat et al., 2005).
Similar effects were found when using SWNTs (Mazzatenta et al., 2007). CNTs increased the frequency of spontaneous postsynaptic currents in hippocampal neurons; the recording of these currents provide clear evidence of the existence of functional synapses. Additionally, neurons grown on CNTs displayed increased excitability as evidenced by the increased frequency of action potential discharges, or the so-called “firing rate” (Fig. 1). Thus, the growth of neurons on a CNT meshwork was accompanied by a significant increase in network activity. Because there is a lack of evidence for specific chemical interactions between neurons and nonfunctionalized CNT, it is likely that the electrical conductivity of the CNT substrate might be underlying this specific electrophysiological effect (Lovat et al., 2005; also, see below).
Fig. 1.

Carbon nanotube substrate affects synaptic activity and neuronal excitability. Neurons grown on MWNT-based substrate display increase in the frequency of (a) spontaneous post-synaptic currents and, (b) “firing rate” when compared to neurons grown on glass coverslips. Modified from Lovat et al., 2005.
Much attention has been directed toward generating CNT scaffolds that could guide nerve tissue regeneration after injury. Conceptually, this aim could be achieved by designing substrates able to promote controlled neurite outgrowth and branching. Toward that direction, several research groups modified CNT substrates/scaffolds by conjugating them with biologically active compounds or with molecules yielding various charges at the surface of modified CNTs. Mattson et al. (2000) reported that rat hippocampal neurons elaborated multiple neurites, with extensive branching, when they were grown on precoated MWNTs. MWNTs were precoated via physiosorption with the bioactive molecule 4-hydroxynonenal, a lipid peroxidation product that in a biological environment controls neurite outgrowth (Mattson et al., 2000). In addition, it has been observed that neuron-like differentiated PC12 cells display improved attachment to the substrate when grown on MWNTs precoated with a thin layer of type IV collagen, an extracellular matrix protein (Nguyen-Vu et al., 2006). These results indicate that modifications of CNTs by physiosorption of functional groups can be used to affect the interaction between neurons and nanomaterials. It should be noted, however, that molecules attached by physiosorption do not exhibit a stable and long-lasting retention to the CNTs.
The issue of retention can be resolved by chemical modification of CNTs using covalent attachments of functional groups to CNTs (Hu et al., 2004). A down side of covalent modification using biologically active compounds is the possibility of losing their specific activity. However, Matsumoto et al. (2007) demonstrate that neurotrophin, a key protein in differentiation, maintains its biological activity, promoting the neurite outgrowth of chick dorsal root ganglion neurons (Matsumoto et al., 2007), despite its covalent attachment to CNTs. Covalent modifications of CNTs have also been used to manipulate the charge carried by functionalized CNTs. Namely, it is accepted that neuronal growth and development on glass or plastic is enhanced by treating their surfaces with positively charged polymer molecules such as polylysine or polyornithine. Hence, Hu et al. (2004) chemically functionalized MWNT with carboxyl group, poly(m-aminobenzene sulfonic acid) (PABS), and ethylenediamine to obtain CNTs that exhibited negative, neutral, and positive surface charges, respectively, at the physiological pH of extracellular medium. They observed that rat hippocampal neurons grown on positively charged MWNTs showed longer neurites with more extensive branching and a larger number of growth cones when compared with neurons grown on neutral or negatively charged CNTs (Hu et al., 2004; Fig. 2). Similar results have been reported for neuronal growth on chemically modified SWNT, in which their functionalization with the positively charged PEI promoted neurite branching and outgrowth in comparison with that of neurites extending from neurons grown on native CNTs (Hu et al., 2005). On the contrary, the functionalization of SWNT substrates with 4-benzoic acid or 4-tert-butylphenyl to obtain negatively charged or neutral SWNTs, respectively, reduced both attachment and survival of cells grown on these modified substrates (Liopo et al., 2006). Chemical modification of CNTs is therefore emerging as a powerful tool to design substrates that can control the growth and morphology of neuronal cells.
Fig. 2.
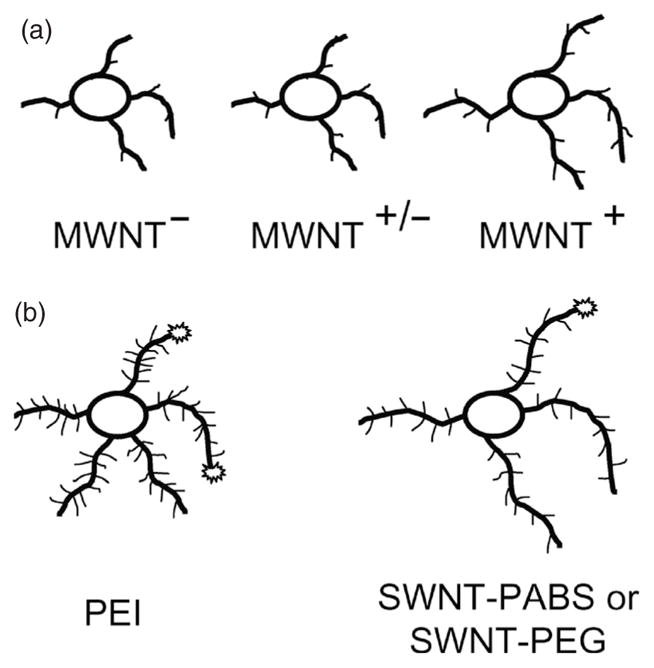
The effects of carbon nanotubes on growth cones, neurite outgrowth, and branching. (a) Manipulating the charge carried by functionalized MWNTs can be used to control the outgrowth and branching pattern of neuronal processes. (b) Water-soluble SWNT-PABS and SWNT-PEG copolymers when added to the culturing medium can increase the length of selected neuronal processes while reducing the number of growth cones. (a) Modified from Hu et al., 2004, and (b) modified from Ni et al., 2005.
One corollary of the ability to specifically affect neurite outgrowth and branching by modification of CNTs is to apply them in the future to neurite regeneration after injury. To achieve such an ambitious goal there are at least several additional requirements to be fulfilled:
the substrate would have to be a freestanding structure without support by glass or plastic,
CNTs would need to be patterned,
water-dispersible CNTs should be available, and
these materials should be tolerated by cells.
There have been advances in all of the above categories:
Freestanding SWNT composite cylinders formed by rolling up poly(N-cetyl-4-vinylpyridinium bromide-co-N-ethyl-4-vinylpyridinium bromide-co-4-vinylpyridine)-SWNT copolymers were made and used to support the growth of NG108 cells; neurite outgrowth was prominently displayed (Gheith et al., 2005).
Micropatterned arrays of CNT islands were generated and used to study self-organization of neural networks (Gabay et al., 2005; Sorkin et al., 2006). This advance makes it possible to grow cells in defined regions. More generally, they can be used for biocomputational purposes and/or for studying neural networks.
Chemically functionalized water-dispersible soluble SWNTs, SWNT-PABS, or SWNT-polyethylene glycol copolymers were designed and applied to neurons, which displayed enhanced outgrowth of selected neurites (Ni et al., 2005; Fig. 2). Such SWNTs could be injected at the local site of nerve injury to increase the chance of bridging the damaged site.
CNTs turned out to be substantially less toxic than carbon black when applied to cultured cells (Magrez et al., 2006).
Therefore, CNTs have the potential to be the next generation of materials for use in implantable neuroprosthetic devices and in injectable local treatment to promote nerve regeneration after injury.
Electrical Interactions Between Neurons and CNTs
An exciting development at the juncture between neuroscience and (nano)technology is that of designing neural interfaces. For example, devices for CNS implantation were developed to control motor disorders (Benabid et al., 2005) and drug-resistant psychiatric conditions (Nuttin et al., 2003; Mayberg et al., 2005), and to translate willful brain processes into specific actions through the control of external devices (Llinas et al., 2005; Mussa-Ivaldi and Miller, 2003; Lebedev et al., 2006). A prerequisite for exploiting CNTs in such biomedical devices is to understand their actions on neurons, especially in respect to neuronal excitability, changes in ionic conductances, and in intercellular signaling via synaptic transmission. Such knowledge would help in designing CNT-based neuronal interfaces, while minimizing unwanted interactions.
The first evidence that CNTs can affect ionic conductance came from the ability of SWNTs to block ion fluxes through potassium ion channels expressed in a cell line (Park et al., 2003). The authors speculated that the CNTs blocked the channel pore and interrupted ion permeability. Similarly, SWNTs caused a significant impairment in cytoplasmic Ca2+ elevation when neurons were depolarized; this might be due to CNTs interfering with the functioning of Ca2+ channels (Ni et al., 2005). Thus, these results caution that CNTs used as a structural component in biological applications could inadvertently affect the activity of cells with which they come into contact.
As mentioned earlier, Lovat et al. (2005) and Mazzatenta et al. (2007) studied the electrical properties of neurons grown on CNTs. These studies strongly suggest that growing neurons on conductive CNTs platforms promoted a significant increase in network operation. Such an effect was not related to an increased number of surviving neurons in the presence of CNTs. These authors determined that the cellular composition of eight-day-old hippocampal cultures, using immunocytochemical markers for astrocytes and neurons (Lovat et al., 2005; Mazzatenta et al., 2007), was similar to cells grown either on CNT-coated or plain glass coverslips. The authors speculated that CNTs might provide a bidirectional electrotonic current transfer, causing a redistribution of charge along the surface of the membrane, ultimately increasing neuronal excitability (Lovat et al., 2005). It is interesting to note that several electrophysiological membrane properties measured on the cultured hippocampal neurons did not indicate the occurrence in these cells of changes in ionic conductance brought about by the CNTs layers directly (Lovat et al., 2005; Mazzatenta et al., 2007).
Liopo et al. (2006) suggested that the conductive nature of CNTs could be used to stimulate neurons. They created a stimulation chamber containing dorsal root ganglion neurons grown on top of an SWNT film. Application of a current through the CNTs resulted in an inward transmembrane current, measured in neurons by a whole-cell patch clamp. Such current was indistinguishable from those induced by direct patch-clamp electrode-mediated depolarizing voltage steps (Liopo et al., 2006). Similarly, the electrical properties of SWNT films made by the layer-by-layer method have been used for external stimulation of NG108 cells in culture (Gheith et al., 2006). Again, such external stimulation via SWNTs evoked inward currents in NG108 cells that were indistinguishable from those evoked by intracellular stimulation using a whole-cell voltage clamp. These rapid inward currents were reminiscent of those usually associated with the ion flux through voltage-gated Na+ channels. Taken together, these reports indicate the possibility of stimulating neurons in culture using SWNTs.
Mazzatenta et al. (2007) employed scanning electron microscopy (SEM), electrophysiology, and computational modeling to understand the nature of electrical coupling between neurons and CNTs. SEM showed intimate contacts between hippocampal neurons and the substrate, composed of purified SWNTs, used to support cellular growth. Such contacts might represent a physical conduit for electrical coupling between SWNTs and neurons, because stimulation via SWNTs reliably evoked postsynaptic responses in neurons. The recordings suggested that coupling between neurons and SWNTs is in part resistive; further studying involved mathematical modeling. This combined approach implicated that any resistive coupling between biomembranes and SWNTs is qualitatively indistinguishable from a coupling between SWNT and the patch-pipette through the patch-seal path to the ground (Mazzatenta et al., 2007). Thus, whole-cell patch clamp recordings from neurons stimulated by SWNTs (see also Liopo et al., 2006; Gheith et al., 2006) could yield deceiving results. Hence, due to the non-idealities of the single-electrode voltage clamp, eliciting Na+ currents in neurons through SWNT stimulation does not conclusively prove a resistive coupling between SWNTs and neurons. Rather, this can be accomplished by detecting synaptic responses, evoked by action potentials elicited in not-clamped neurons using the electrical stimulus delivered via SWNTs (Mazzatenta et al., 2007).
The use of a combination of electrophysiology and computational/mathematical modeling perhaps represents an entry into the realm of computational (nano)neuroscience. Owing to the recent availability of powerful computer simulation software (Carnevale and Hines, 2006; Markram, 2006), this emerging field has a promising future.
The studies discussed here demonstrate the ability to stimulate neurons via CNTs. In many cases, electrical stimulation of neurons employed in neural prosthesis and neurological therapies requires microelectrode arrays. Indeed, CNT-based micro arrays have been constructed (Gabay et al., 2005; McKnight et al., 2006; Nguyen-Vu et al., 2006; Nguyen-Vu et al., 2007; Yu et al., 2007; Wang et al., 2006) by growing vertically aligned carbon nanofibers (VACNFs). Such electrodes offer higher limits for charge injection than presently used metal-based devices. Thus, not only can VACNF arrays be used for electrochemical applications (Wang et al., 2006; Gabay et al., 2005; McKnight et al., 2006), but they can also be used for repetitive stimulation of, for example, rat hippocampal neurons grown on them (Wang et al., 2006). Recently, these VACNF devices were also employed for extracellular recordings and stimulations in cultured hippocampal organotypic slices (Yu et al., 2007). Taken together, it appears that CNT-based arrays offer an improved platform for interfacing with neurons and neural tissue, and hold great promise for studying networks and medical applications.
CONCLUSION
The intent of this review is to put forward recent advances in the generation of CNT scaffolds for neuronal growth. We also discuss the effects that CNTs exert on neural cells when used as substrates for cellular growth, and their potential as an electrical interface with neural cells. It is apparent from the body of work presented in this review that CNTs have great potential in biotechnology and medicine. Some of the initial concerns regarding their toxicity (Service, 2003; Service, 2004) have been somewhat alleviated by the fact that they appear to be less hazardous than carbon black, a different form of carbon that has been widely used and has defined exposure guidelines. Because it is inevitable that widespread commercialization of CNTs will occur, we still need to stay alert to the potential harm that these new nanomaterials could exert on human health, and we must employ additional toxicity testing. For now, it appears, however, that the potential benefit warrants further investigation in research laboratories from a biological standpoint.
Acknowledgments
Financial support from NEURO-NANO-NMP4-CT-2006-031847 (to LB, MP, and MG) and from the National Institute for Mental Health R01 MH 069791 (to VP) is gratefully acknowledged. We thank Dr. Erik B. Malarkey for comments on earlier versions of this manuscript.
Biographies
 Antonietta Sucapane received a Ph.D. in Neurophysiology from University of Rome “La Sapienza” in 2006. She is currently Post-doc at the Department of Physiology and Pathology at the University of Trieste, Italy, where she employs electrophysiology to characterize the effects of CNT substrate on neuronal activity.
Antonietta Sucapane received a Ph.D. in Neurophysiology from University of Rome “La Sapienza” in 2006. She is currently Post-doc at the Department of Physiology and Pathology at the University of Trieste, Italy, where she employs electrophysiology to characterize the effects of CNT substrate on neuronal activity.
 Giada Cellot received a laurea-degree in Medical Biotechnology from the University of Trieste, Italy, in 2006. She’s currently a Ph.D. Student at the Department of Physiology and Pathology at the University of Trieste, where she employs electrophysiology to characterize neuronal responses in CNT—neuronal interfaces.
Giada Cellot received a laurea-degree in Medical Biotechnology from the University of Trieste, Italy, in 2006. She’s currently a Ph.D. Student at the Department of Physiology and Pathology at the University of Trieste, where she employs electrophysiology to characterize neuronal responses in CNT—neuronal interfaces.
 Maurizio Prato graduated in Chemistry at the University of Padova, Italy, where he was appointed Assistant Professor in 1983. He moved to Trieste, Italy, first as an Associate Professor in 1992 and then as Full Professor in 2000. He was a Post-doc at Yale University, Visiting Scientist at the University of California, Santa Barbara and Professeur Invité at the Ecole Normale Supérieure, Paris. His research focuses on the functionalization chemistry of fullerenes and carbon nanotubes for applications in materials science and medicinal chemistry. Professor Prato is in the International Advisory Board of Chemical Communications and the Journal of Materials Chemistry, both of the Royal Society of Chemistry since 1994. Since 2003, he is Chairman of the Editorial Board of the Journal of Materials Chemistry.
Maurizio Prato graduated in Chemistry at the University of Padova, Italy, where he was appointed Assistant Professor in 1983. He moved to Trieste, Italy, first as an Associate Professor in 1992 and then as Full Professor in 2000. He was a Post-doc at Yale University, Visiting Scientist at the University of California, Santa Barbara and Professeur Invité at the Ecole Normale Supérieure, Paris. His research focuses on the functionalization chemistry of fullerenes and carbon nanotubes for applications in materials science and medicinal chemistry. Professor Prato is in the International Advisory Board of Chemical Communications and the Journal of Materials Chemistry, both of the Royal Society of Chemistry since 1994. Since 2003, he is Chairman of the Editorial Board of the Journal of Materials Chemistry.
 Michele Giugliano graduated in Electronic Engineering at the University of Genova, Italy, in 1997, and obtained a Ph.D. in Bioengineering by the Polytechnic of Milan, Italy, in 2001. He was trained in electrophysiology as a HFSP Post-doc at the Department of Physiology of the University of Bern, and in 2005 became Group Leader at the Brain Mind Institute, EPFL, Lausanne, Switzerland. In 2008, he was appointed Assistant Professor at the University of Antwerp, Belgium. His research focuses on cortical cellular and network electrophysiology, computational neuroscience and neuroengineering. Since 2008, he is in the Editorial Board of Frontiers in Neuroengineering and he is Contributing Editor for the European Journal of Neuroscience.
Michele Giugliano graduated in Electronic Engineering at the University of Genova, Italy, in 1997, and obtained a Ph.D. in Bioengineering by the Polytechnic of Milan, Italy, in 2001. He was trained in electrophysiology as a HFSP Post-doc at the Department of Physiology of the University of Bern, and in 2005 became Group Leader at the Brain Mind Institute, EPFL, Lausanne, Switzerland. In 2008, he was appointed Assistant Professor at the University of Antwerp, Belgium. His research focuses on cortical cellular and network electrophysiology, computational neuroscience and neuroengineering. Since 2008, he is in the Editorial Board of Frontiers in Neuroengineering and he is Contributing Editor for the European Journal of Neuroscience.
 Vladimir Parpura holds both a medical degree, awarded from the University of Zagreb in Croatia in 1989, and a doctorate, received in Neuroscience and Zoology from Iowa State University in 1993. He has held faculty appointments at the Department of Zoology and Genetics, Iowa State University and the Department of Cell Biology and Neuroscience, University of California Riverside. He is presently an Associate Professor in the Department of Neurobiology, University of Alabama Birmingham.
Vladimir Parpura holds both a medical degree, awarded from the University of Zagreb in Croatia in 1989, and a doctorate, received in Neuroscience and Zoology from Iowa State University in 1993. He has held faculty appointments at the Department of Zoology and Genetics, Iowa State University and the Department of Cell Biology and Neuroscience, University of California Riverside. He is presently an Associate Professor in the Department of Neurobiology, University of Alabama Birmingham.
 Laura Ballerini graduated (M.D.) at the University of Florence, Italy, in 1988. She was a Post-doc at UCL, UK, and Assistant Professor in Physiology at the Biophysics Sector of the International School for Advanced Studies (SISSA-ISAS) of Trieste, Italy. In 2002, she became Associate Professor in Physiology at the Università di Trieste. Professor Ballerini has provided important contributions to the understanding of spinal network physiology, plasticity and development. Recently, she has been working on the interactions between living neurons and micro-nano fabricated substrates or bioactive-composite containing carbon nanotubes, demonstrating for the first time that carbon nanotubes substrates boost neuronal network activity. Professor Ballerini is coordinating an EU project (www.neuronano.net) designed to exploit the convergence of nanotechnology and neurobiology in view of the development of novel neuro-implantable devices to treat neurological traumatic and degenerative lesions. Since 2008, she is in the Editorial Board of Frontiers in Neuroscience and acts as Chief-Editor for Frontiers in Neuroengineering.
Laura Ballerini graduated (M.D.) at the University of Florence, Italy, in 1988. She was a Post-doc at UCL, UK, and Assistant Professor in Physiology at the Biophysics Sector of the International School for Advanced Studies (SISSA-ISAS) of Trieste, Italy. In 2002, she became Associate Professor in Physiology at the Università di Trieste. Professor Ballerini has provided important contributions to the understanding of spinal network physiology, plasticity and development. Recently, she has been working on the interactions between living neurons and micro-nano fabricated substrates or bioactive-composite containing carbon nanotubes, demonstrating for the first time that carbon nanotubes substrates boost neuronal network activity. Professor Ballerini is coordinating an EU project (www.neuronano.net) designed to exploit the convergence of nanotechnology and neurobiology in view of the development of novel neuro-implantable devices to treat neurological traumatic and degenerative lesions. Since 2008, she is in the Editorial Board of Frontiers in Neuroscience and acts as Chief-Editor for Frontiers in Neuroengineering.
References
- Benabid AL, Wallace B, Mitrofanis J, Xia C, Piallat B, Fraix V, Batir A, Krack P, Pollak P, Berger F. Therapeutic electrical stimulation of the central nervous system. Comptes Rendus Biologies. 2005;328:177–186. doi: 10.1016/j.crvi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Bethune DS, Kiang CHM, de Vries S, Gorman G, Savoy R, Vazquez J, Beyers R. Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls. Nature. 1993;363:605–607. [Google Scholar]
- Carnevale NT, Hines ML. The NEURON Book. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- Fortina P, Kricka LJ, Surrey S, Grodzinski P. Nanobiotechnology: the promise and reality of new approaches to molecular recognition. Trends in Biotechnology. 2005;23:168–173. doi: 10.1016/j.tibtech.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Gabay T, Jakobs E, Ben-Jacob E, Hanein Y. Engineered self-organization of neural networks using CNT clusters. Physica A. 2005;350:611–621. [Google Scholar]
- Galvan-Garcia P, Keefer EW, Yang F, Zhang M, Fang S, Zakhidov AA, Baughman RH, Romero MI. Robust cell migration and neuronal growth on pristine carbon nanotube sheets and yarns. J Biomaterials Science, Polymer Edition. 2007;18:1245–1261. doi: 10.1163/156856207782177891. [DOI] [PubMed] [Google Scholar]
- Gheith MK, Sinani VA, Wicksted JP, Matts RL, Kotov NA. Single-walled carbon nanotube polyelectrolyte multilayers and freestanding films as a biocompatible platform for neuroprosthetic implants. Adv Mater. 2005;17:2663–2667. [Google Scholar]
- Gheith MK, Pappas TC, Liopo AV, Sinani V, Shim BS, Motamedi M, Wicksted JP, Kotov NA. Stimulation of neural cells by lateral layer-by-layer films of single-walled currents in conductive carbon nanotubes. Adv Mater. 2006;18:2975–2978. [Google Scholar]
- Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4:507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J Phys Chem B. 2005;109:4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1 nm diameter. Nature. 1993;363:603–605. [Google Scholar]
- Krishnan A, Dujardin E, Ebbesen TW, Yianilos PN, Treacy MMJ. Young’s modulus of single-walled nanotubes. Phys Rev B. 1998;58:14013–14019. [Google Scholar]
- Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present, and future. Trends in Neurosciences. 2006;29:536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Liopo AV, Stewart MP, Hudson J, Tour JM, Pappas TC. Biocompatibility of native functionalized single-walled carbon nanotubes for neuronal interface. J Nanosci Nanotechnol. 2006;6:1365–1374. doi: 10.1166/jnn.2006.155. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Walton KD, Nakao M, Hunter I, Anquetil PA. Neurovascular central nervous recording/stimulating system: Using nanotechnology probes. J Nanopart Res. 2005;7:111–127. [Google Scholar]
- Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5:1107–1110. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Applications of carbon nanotubes in neurobiology. Neuro-Degenerative Diseases. 2007;4:292–299. doi: 10.1159/000101885. [DOI] [PubMed] [Google Scholar]
- Markram H. The blue brain project. Nat Rev Neurosci. 2006;7:153–160. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Sato C, Naka Y, Kitazawa A, Whitby RL, Shimizu N. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J Bioscience and Bioengineering. 2007;103:216–220. doi: 10.1263/jbb.103.216. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes use as substrates for neuronal growth. J Molecular Neuroscience. 2000;14:175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mazzatenta A, Giugliano M, Campidelli S, Gambazzi L, Businaro L, Markram H, Prato M, Ballerini L. Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. J Neurosci. 2007;27:6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight TE, Melechko AV, Fletcher BL, Jones SW, Hensley DK, Peckys DB, Griffin GD, Simpson ML, Ericson MN. Resident neuroelectrochemical interfacing using carbon nanofiber arrays. J Phys Chem B. 2006;110:15317–15327. doi: 10.1021/jp056467j. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Miller LE. Brain-machine interfaces: computational demands clinical needs meet basic neuroscience. Trends in Neurosciences. 2003;26:329–334. doi: 10.1016/S0166-2236(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Nguyen-Vu TD, Chen H, Cassell AM, Andrews R, Meyyappan M, Li J. Vertically aligned carbon nanofiber arrays: an advance toward electrical-neural interfaces. Small. 2006;2:89–94. doi: 10.1002/smll.200500175. [DOI] [PubMed] [Google Scholar]
- Nguyen-Vu TD, Chen H, Cassell AM, Andrews RJ, Meyyappan M, Li J. Vertically aligned carbon nanofiber architecture as a multi-functional 3-D neural electrical interface. IEEE Transactions on BioMedical Engineering. 2007;4:1121–1128. doi: 10.1109/TBME.2007.891169. [DOI] [PubMed] [Google Scholar]
- Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, Haddon RC, Parpura V. Chemically functionalized water-soluble single-walled carbon nanotubes modulate neurite outgrowth. J Nanosci Nanotechnol. 2005;10:707–712. doi: 10.1166/jnn.2005.189. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriels L, van Kuyck K, Cosyns P. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurgery Clinics of North America. 2003;14:267–274. doi: 10.1016/s1042-3680(02)00117-1. [DOI] [PubMed] [Google Scholar]
- Park KH, Chhowalla M, Iqbal Z, Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem. 2003;278:50212–50216. doi: 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- Service RF. Nanotechnology. Sorting technique may boost nanotube research. Science. 2003;300:2018. doi: 10.1126/science.300.5628.2018. [DOI] [PubMed] [Google Scholar]
- Service RF. Nanotoxicology. Nanotechnology grows up. Science. 2004;304:1732–1734. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- Silva GA. Neuroscience nanotechnology: progress, opportunities, challenges. Nat Neurosci Rev. 2006;7:65–74. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
- Sorkin R, Gabay T, Blinder P, Baranes D, Ben-Jacob E, Hanein Y. Compact self-wiring in cultured neural networks. J Neural Engineering. 2006;3:95–101. doi: 10.1088/1741-2560/3/2/003. [DOI] [PubMed] [Google Scholar]
- Yu Z, McKnight TE, Ericson MN, Melechko AV, Simpson ML, Ill BM. Vertically aligned carbon nanofiber arrays record electrophysiological signals from hippocampal slices. Nano Lett. 2007;7:2188–2195. doi: 10.1021/nl070291a. [DOI] [PubMed] [Google Scholar]
- Wang K, Fishman HA, Dai H, Harris JS. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 2006;6:2043–2048. doi: 10.1021/nl061241t. [DOI] [PubMed] [Google Scholar]


