Abstract
Calcium-independent phospholipase A2 (iPLA2) has been suggested to play an important role in the activation of caspase-1 induced by lipopolysaccharides (LPS). Here, we used pharmacological and genetic approaches to study the role of iPLA2 in the activation of caspase-1. Bromoenol lactone (BEL), an inhibitor that was originally used to support a role for iPLA2 in the secretion of IL-1β, prevented caspase-1 activation induced by LPS and ATP as described, and also activation triggered by Salmonella infection and cytosolic flagellin, which rely on the Nlrc4 inflammasome. Analysis of BEL enantiomers showed that the S-BEL form was more effective than R-BEL in inhibiting the inflammasome, suggesting a role for iPLA2β. However, caspase-1 activation and IL-1β secretion and their inhibition by BEL were unimpaired in macrophages deficient in iPLA2β. BEL was originally identified as an inhibitor of serine proteases. Consistent with the latter, the serine proteases inhibitors TPCK, TLCK and AAF-cmk prevented the activation of the Nlrc4 and Nlrp3 inflammasomes while pan-cathepsin inhibitors were ineffective. These results indicate that iPLA2β is not critical for caspase-1 activation as currently proposed. Instead, the results suggest that serine protease(s) targeted by BEL may play a critical role in the activation of the inflammasome triggered by microbial stimuli.
Key Words: Macrophage, Inflammation, Phospholipase A2
Introduction
IL-1β is a proinflammatory cytokine produced by macrophages in response to microbes or danger signals. IL-1β acts synergistically with other cytokines in the orchestration of the host response, and contributes to the development of inflammatory disease, fever and septic shock. The production of IL-1β is under tight and complex regulation. Unstimulated phagocytes express undetectable or low levels of the pro-IL-1β precursor. In response to diverse microbial stimuli, pro-IL-1β is induced in monocytes and macrophages via a transcriptional mechanism involving NF-κB activation [1]. Subsequently, pro-IL-1β is processed into the biologically active IL-1β molecule by caspase-1 [2]. The protease caspase-1 is also expressed in phagocytes as an inactive zymogen. Recent studies have identified several members of the NOD-like receptor (NLR) family of proteins as crucial mediators of caspase-1 activation by linking the recognition of microbial molecules to protease activation [3, 4, 5]. In response to microbial stimuli, NLR family members such as Nlrc4 and Nlrp3 assemble a large multi-protein complex, called the ‘inflammasome’, which induces the processing of pro-caspase-1 into the enzymatically active enzyme [2]. The NLR protein Nlrc4 (also called Ipaf) has been implicated in the activation of caspase-1 in response to several intracellular bacteria through the recognition of cytosolic flagellin [2, 6]. Similarly, Nlrp3 (also called cryopyrin/Nalp3) is critical for caspase-1 activation and secretion of IL-1β in macrophages stimulated with several microbial molecules and ATP, as well as with endogenous urate crystals, silica or asbestos particles [2, 6]. Although Nlrc4 and Nlrp3 can sense microbial stimuli, there is no evidence that they interact directly with their microbial activators. Furthermore, the regulatory events that link microbial stimuli to the inflammasome remain poorly understood.
The regulation of caspase-1 has been suggested to be influenced by other classes of host factors. Among these are phospholipases A2 (PLA2), a family of proteins that catalyzes the hydrolysis of the sn-2 fatty acid substituent of membrane phospholipids to generate free fatty acids (e.g. arachidonic acid) and lysophospholipids (e.g. lysophosphatidylcholine). There are several groups of PLA2 enzymes including the first recognized cytosolic PLA2 (cPLA2), which is induced to associate with its substrates in membranes by rises in cytosolic Ca2+, and PLA2 enzymes that do not require Ca2+ for catalytic activity and are therefore designated Ca2+-independent PLA2 (iPLA2). The first recognized mammalian members of each of these 2 families have been designated cPLA2α and iPLA2β, respectively. While cPLA2α and iPLA2β are cytosolic enzymes active inside the cell, members of the secretory PLA2 (sPLA2) family require millimolar concentrations of Ca2+ for catalytic activity, and are thought to function as extracellular secreted enzymes [7].
Studies involving the pharmacological inhibition of iPLA2 with the suicide substrate bromoenol lactone (BEL) have suggested that the enzyme participates in lipopolysaccharide (LPS)- and ATP-induced caspase-1 activation and maturation of pro-IL-1β [8, 9, 10, 11]. BEL inhibits iPLA2 at sub-micromolar to low micromolar concentrations, concentrations at which the activity of cPLA2 and sPLA2 enzymes are little affected [12]. Although BEL can distinguish among these PLA2 classes, it also inhibits other classes of hydrolases [13], and was first identified as a serine protease inhibitor [14]. Studies involving the pharmacologic inhibition of iPLA2 with BEL are thus ambiguous, and require confirmation by experiments involving genetic manipulation of iPLA2 expression where possible. Although BEL inhibits LPS- and ATP-induced caspase-1 activation mediated by the Nlrp3 inflammasome, its effects on events induced by microbial stimuli (such as infection with the intracellular bacteria Salmonella, which is mediated via the Nlrp4 inflammasome) have not yet been examined.
In the studies described here, we used both pharmacological and genetic approaches to assess the role of BEL and iPLA2 on caspase-1 activation induced by microbial stimuli that activate the Nlrc4 or Nlrp3 inflammasome. We provide conclusive evidence that both caspase-1 activation and IL-1β secretion are independent of iPLA2β activity. Thus, these results challenge the accepted notion that iPLA2β has a critical role in caspase-1 activation and suggest a role for serine protease(s) targeted by BEL in the regulation of the inflammasomes.
Materials and Methods
Mice and Cells
Mice deficient in iPLA2β have been previously described [15]. The animal studies were conducted under approved protocols by the University of Michigan Committee on the Use and Care of Animals. Mice were housed in a pathogen-free facility. Bone-marrow-derived macrophages were isolated as previously described. Briefly, femurs and tibias were removed from the euthanized mice and sterilized in 70% ethanol. Iscove's modified Dulbecco's medium was used to wash out the marrow cavity plugs, and bone marrow cells were resuspended in L-cell-conditioned medium containing mononuclear phagocyte colony-stimulating factor to stimulate proliferation and differentiation of the bone marrow progenitors into macrophages. After 5–6 days, the resulting bone-marrow-derived macrophages were replated. Macrophages were seeded at 1 × 106 cells per well in 12-well dishes the day before the experiment.
Reagents and Bacterial Infection
Racemic BEL, S-BEL and R-BEL, were from Cayman Chemicals. ATP and LPS from E. coli (O111B) were from Sigma. Nα-Tosyl-Lys-chloromethylketone·HCl (TLCK), Na-Tosyl-Phe-chloromethylketone (TPCK), benzyloxycarbonyl-Phe-Ala-fluoromethylketone (z-FA), and Ala-Ala-Phe-chloromethylketone (AAF-cmk) were from Biomol. Z-Phe-chloromethylketone was from Bachem. S. enterica serovar typhimurium strain SL1344 was a gift from A. Aderem, University of Washington. The bacteria were grown as previously described [13], and diluted to the desired concentration to infect macrophages at different bacterial/macrophage ratios. For the gentamicin protection assay and evaluation of IL-1β secretion, macrophages were infected for 30 min with bacteria, washed twice with PBS, and Iscove's modified Dulbecco's medium containing 100 μg/ml of gentamicin was added to limit the growth of extracellular bacteria. In the gentamicin protection assay, the number of live Salmonella was calculated at 1 or 4 h after infection by lysing the cells with 1 ml of 1% Triton X-100 for 10 min and counting the number of bacterial colonies after serial dilution on agar plates.
Immunoblotting
Cells were lysed together with the cell supernatant by the addition of 1% NP-40 complete protease inhibitor cocktail (Roche) and 2 mM dithiothreitol. Clarified lysates were resolved by SDS-PAGE and transferred to polyvinylidene fluoride membranes by electro-blotting. The rabbit anti-mouse caspase-1 was a kind gift from Dr. Vandanabeele (Ghent University, Belgium). Anti-IL-1β antibody was from R&D.
In vitro Caspase-1 Processing Assay
MonoMac6 cells (10 × 106 for each experimental condition) were primed with 100 ng/ml LPS overnight. Macrophage lysates for in vitro caspase-1 processing were isolated as described previously [9]. Briefly, cells were collected (2,000 rpm, 10 min), washed twice in PBS and resuspended in buffer W (20 mM, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1.0 mM EGTA and 1.0 mM EDTA) supplemented with protease inhibitor cocktail (Roche) and 20 μM BEL, 100 μM YVAD (Calbiochem) or 0.2 % (v/v) DMSO (Sigma). The final protein concentration was 22 mg/ml. The cells were allowed to swell for 15 min on ice, and were subsequently lysed by 15 passages through a 23-gauge needle. Lysates were spun at 14,000 rpm for 30 min, and the supernatant was transferred to a new tube and kept on ice. Lysates were incubated at 30°C to trigger caspase-1 processing, and the reactions were stopped by addition of 20 μl of 5× SDS-PAGE buffer. Immunoblotting was performed using 1 μg/ml of the caspase-1 p10 antibody (Santa Cruz Biotechnology).
Stimulation of Macrophages with Liposomes
DOTAP cationic lipid was purchased from Roche and used according to the manufacturer's instructions. Briefly, 50 μl DOTAP cationic lipid was incubated for 30 min in serum-free medium with 4 μg of purified flagellin (Invivogen) in a final volume of 500 μl. After the incubation, 1.5 ml of serum-free medium was added, and 1 ml was used to stimulate 2 × 106 macrophages seeded in 6-well plates for 3–5 h.
Macrophage Cytotoxicity Assay
The percentage of macrophage cell death was determined by measuring the release of macrophage LDH 16 h after infection. In the assay, the supernatants were collected, and the release of LDH was quantified using the CytoTox 96 non-radioactive cytotoxicity assay (Promega). The absorbance at 490 nm was measured, and the percentage of cell death was calculated as [(experimental release – spontaneous release)/(maximum release – spontaneous release)] × 100. The spontaneous release represents the level of LDH released from the cytoplasm of uninfected macrophages, whereas the maximum release is the value obtained by lysis of macrophages with a solution of 0.1% Triton X-100.
Results
BEL Inhibits Nlrc4-Dependent Activation of Caspase-1
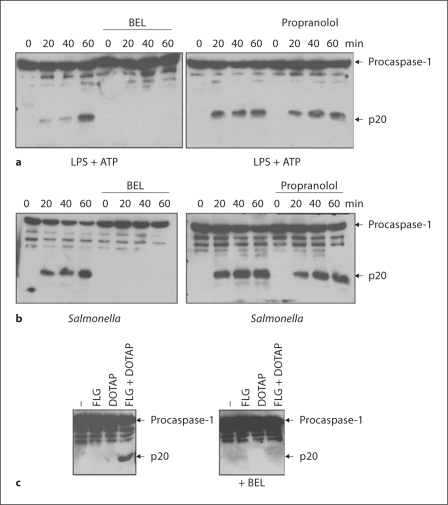
To gain insight into the mechanism of inflammasome activation, we initially tested the effect of the iPLA2 inhibitor BEL on LPS- and ATP-induced caspase-1 activation mediated by the Nlrp3 inflammasome [16, 17]. In LPS-primed macrophages, ATP induced the activation of caspase-1, and this was blocked by pretreatment with BEL (fig. 1a). BEL also inhibits the magnesium-dependent phosphatidate phosphohydrolase-1 (PAP-1), another enzyme that regulates the metabolism of phospholipids and is also inhibited by propranolol [18]. Therefore, we determined what effect propranolol exerted on Nlrp3-dependent caspase-1 activation. Propranolol treatment did not block caspase-1 activation induced by ATP in LPS-primed macrophages, suggesting that inhibition of PAP-1 does not account for the blocking effect of BEL upon Nlrp3-dependent caspase-1 activation (fig. 1a). These results are consistent with previous observations [8, 9, 10, 11], suggesting that BEL pretreatment inhibits Nlrp3-dependent activation of caspase-1 induced by LPS and ATP.
Fig. 1.
BEL inhibits Nlrc4-dependent activation of caspase-1. a Macrophages were primed with LPS for 3 h and then stimulated with ATP (5 mM) for 20, 40 or 60 min. In pretreated samples, BEL (10 μM) or propranolol (150 μM) were added 1 h before ATP stimulation. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. b Macrophages were pretreated with BEL (25 μM) or propranolol (150 μM) for 1 h or left untreated, and then infected with Salmonella for the indicated time. Extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. c Macrophages were left untreated or stimulated with flagellin (FLG), the cationic lipid DOTAP, or a combination of FLG + DOTAP for 5 h. Extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Results are representative of at least 3 separate experiments (a-c).
We then investigated the effect of BEL in the activation of caspase-1 by infection with Salmonella typhimurium, which triggers IL-1β secretion through Nlrc4 [19, 20]. We first assessed the number of viable intracellular bacteria over time, and found that pretreatment with BEL did not affect bacterial uptake or early macrophage bactericidal activity (data not shown). Next, we assessed the activation of caspase-1 in macrophages infected with Salmonella for 30, 60 or 90 min. As shown in figure. 1b, treatment with BEL completely prevented Salmonella-induced caspase-1 activation, although propranolol did not, and thus the effect of BEL cannot be attributed to PAP-1 inhibition. Salmonella-induced caspase-1 activation is dependent on the cytosolic detection of bacterial flagellin by Nlrc4 [19, 20]. To confirm the inhibitory effect of BEL on the Nlrc4 inflammasome, we stimulated macrophages with flagellin alone or with flagellin in combination with a cationic lipid (DOTAP) that allows delivery of flagellin into the host cytosol [19]. We found that cytosolic flagellin, but not extracellular flagellin, induced caspase-1 activation, and this was inhibited by pretreatment with BEL (fig. 1c). Therefore, BEL efficiently blocks both Nlrc4-dependent and Nlrp3-dependent activation of caspase-1, raising the possibility that iPLA2 could be a general regulator of the inflammasome.
Enantiomer of BEL Specific for iPLA2β Inhibits Nlrc4-Dependent Activation of Caspase-1
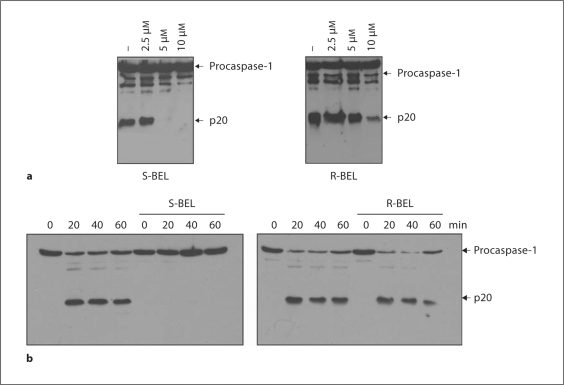
BEL, a member of a family of compounds known as haloenol lactones, is a suicide substrate for iPLA2 that targets critical cysteine residue(s) in iPLA2β [13]. BEL inhibits iPLA2 activity at sub-micromolar to low micromolar concentrations at which the activity of cPLA2 and sPLA2 enzymes are unaffected [12]. Racemic BEL inhibits both iPLA2β and iPLA2γ, but the S- and R-BEL enantiomers preferentially inhibit iPLA2β and iPLA2γ, respectively [21, 22]. We thus pretreated macrophages with either S-BEL or R-BEL before stimulation with LPS and ATP or infection with Salmonella. We found that S-BEL was more effective than R-BEL in preventing caspase-1 activation induced by stimulation with LPS and ATP, suggesting a role of iPLA2β in caspase-1 activation (fig. 2a). Similar concentration-responses with S-BEL versus R-BEL on caspase-1 activation were observed in BALB/c macrophages and the Bac1.2F5 macrophage line stimulated with LPS + ATP (not shown). In addition, activation of caspase-1 triggered by Salmonella infection was more effectively inhibited by S-BEL than it was by R-BEL (fig. 2b).
Fig. 2.
Enantiomer of BEL specific for iPLA 2β inhibits Nlrc4-dependent activation of caspase-1. a Macrophages were primed with LPS for 3 h, and then stimulated with ATP (5 mM) for 30 min. In pretreated samples, S-BEL or R-BEL at the indicated concentrations were added 1 h before ATP stimulation. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. b Macrophages were pretreated with S-BEL (25 μM) or R-BEL (25 μM) for 1 h, and then infected with Salmonella at a macrophage/bacterial ratio of 1/5 for 20, 40 or 60 min. Extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Results are representative of 3 separate experiments (a, b).
In Salmonella-infected macrophages, caspase-1 activation leads to the production of IL-1β and the induction of cell death. Therefore, we next investigated the effect S-BEL and R-BEL on those events. S-BEL inhibited both IL-1β production and cell death as effectively as racemic BEL, but R-BEL was ineffective (data not shown). Collectively, these results indicate that S-BEL, but not R-BEL, blocks the Nlrc4 and Nlrp3 inflammasomes, which would be consistent with participation of iPLA2β, rather than IPLA2γ, in caspase-1 activation.
iPLA2β Is Not Required for Caspase-1 Activation
Based on the relative specificity of S-BEL for iPLA2β among PLA2, the above experiments suggest that iPLA2β may be required for caspase-1 activation. To determine the role of iPLA2β more directly, we tested caspase-1 activation using macrophages from genetically modified mice that do not express iPLA2β because the gene encoding the enzyme has been disrupted by homologous recombination [15]. Wild-type mouse macrophages have been shown to express iPLA2β, while macrophages from iPLA2β-null mice fail to do so and exhibit several phenotypic abnormalities [23, 24].
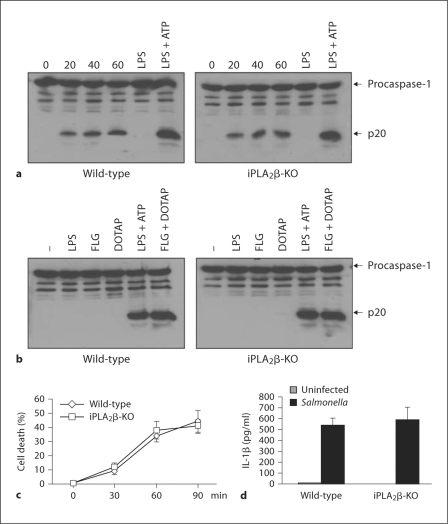
We first assessed the number of viable bacteria inside the cell in wild-type or iPLA2β-deficient macrophages after infection with Salmonella. At 1 and 4 h after infection, both wild-type and iPLA2β-null macrophages had comparable numbers of viable intracellular bacteria (data not shown), indicating that iPLA2β is not involved in either the initial bacterial uptake or early bactericidal activity. Next, we assessed caspase-1 activation in wild-type and iPLA2β-null macrophages induced by Salmonella infection or stimulation with LPS and ATP. Surprisingly, caspase-1 activation induced by these stimuli, which are mediated through Nlrc4 and Nlrp3, respectively, was comparable in wild-type and iPLA2β-deficient macrophages (fig. 3a). Consistent with this observation, the activation of caspase-1 induced by cytosolic flagellin (flagellin + DOTAP) was also unimpaired in iPLA2β-null macrophages (fig. 3b). To further verify these results, we determined whether the absence of iPLA2β affects the production of IL-1β or the induction of cell death. Consistently, both IL-1β secretion and cell death induced by Salmonella were comparable in wild-type and iPLα2β-deficient macrophages (fig. 3c, d). These results demonstrate that iPLA2β is not required for caspase-1 activation induced via the Nlrc4 and Nlrp3 inflammasomes.
Fig. 3.
Unimpaired caspase-1 activation in iPLA2 β-deficient macrophages. a Wildtype and iPLA2 β-null macrophages were infected with Salmonella at a macrophage/bacterial ratio of 1/5 for the indicated time point, or primed with LPS for 3 h and then stimulated with ATP (5 mM) for 30 min. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. b Macrophages were stimulated with LPS, flagellin (FLG), the cationic liposomal reagent DOTAP, or a combination of DOTAP with LPS or flagellin. Extracts were immunoblotted with caspase-1 antibody. c Wild-type and iPLA2 β-null macrophages were infected with Salmonella at a macrophage/bacterial ratio of 1/5 for the indicated time points. The induction of macrophage cell death was evaluated by the release of LDH of triplicate cultures. d Wild-type and iPLA2 β-null were primed with LPS and then infected with Salmonella at a macrophage/bacterial ratio of 1/5. Cell-free supernatants were analyzed for production of IL-1β 6 h after infection. Values represent means ± SD of triplicate cultures. Results are representative of at least 3 separate experiments (a-d).
BEL Inhibits Salmonella-Induced Caspase-1 Activation Independently of iPLA2β
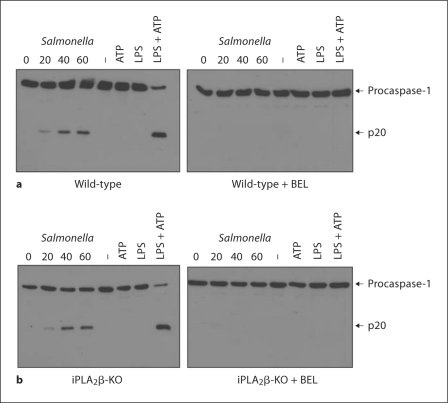
To demonstrate conclusively that inhibition of caspase-1 activation by BEL occurs through a mechanism independent of iPLA2β, wild-type or iPLA2β-null macrophages were pretreated with BEL and infected with Salmonella or stimulated with LPS and ATP. Activation of caspase-1 induced by LPS + ATP or Salmonella infection was effectively blocked by BEL in both wild-type and iPLA2β-null macrophages (fig. 4). Together, these results demonstrate that BEL mediates its inhibitory effect on the Nlrc4 and Nlrp3 inflammasomes by a mechanism other than inhibition of iPLA2β activity.
Fig. 4.
BEL inhibits Salmonella-induced caspase-1 activation independently of iPLA2β. Wild-type (a) and iPLA2β-null (b) macrophages were pretreated, or not, with BEL (25 μM) for 1 h and then infected with Salmonella at a macrophage/bacterial ratio of 1/5 for the indicated time point. Alternatively, macrophages were primed with LPS for 3 h or left untreated and then stimulated, or not, with ATP (5 mM) for 30 min, with or without BEL treatment. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Results are representative of at least 3 separate experiments.
BEL Acts Upstream of Caspase-1 to Inhibit the Inflammasome
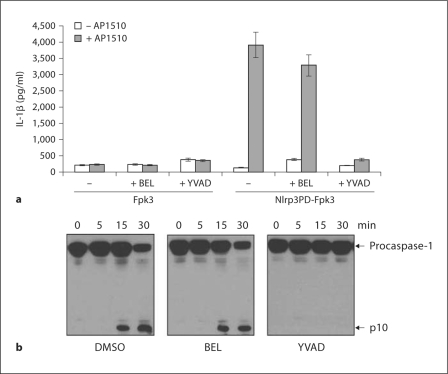
The activation of caspase-1 occurs induced via Nlrc4 and Nlrp3 in response to microbial stimuli is mediated by self-processing of the protease [25, 26]. To gain further insight into the mechanism whereby BEL inhibits caspase-1 activation, we determined whether the inhibitory effect of BEL occurs upstream or downstream of the inflammasome. To examine this issue, we used THP-1 macrophages that stably express a chimeric protein composed of the effector PD of Nlrp3 fused to 3 tandem Fkbp domains (Nlrp3PD-Fpk3) that can be oligomerized by the cell-permeable ligand AP1510 [27]. Figure 5a shows that forced oligomerization of the pyrin domain of Nlrp3-induced production of IL-1β, and this was prevented by the caspase-1 inhibitor YVAD-cmk. In contrast, incubation with BEL was ineffective (fig. 5a). Fpk3 alone did not induce the secretion of IL-1β, thus confirming the specificity of the assay (fig. 5a). These results indicate that BEL inhibits the inflammasome upstream of caspase-1. To further investigate the inhibitory effect of BEL, we used a previously described system in which cell rupture induces Nlrp3-dependent activation of caspase-1 [28]. LPS-stimulated MonoMAC6 macrophages were lysed, and the cell lysates were incubated in a hypotonic buffer containing low concentration of K+ for 5, 15 or 30 min at 37°C, a signal that induces Nlrp3-dependent caspase-1 activation [28]. After 15 min incubation, caspase-1 activation was detectable as measured by the appearance of the p10 subunit cleavage product (fig. 5b). As expected, induction of the p10 subunit was absent when cell lysates were treated with a specific caspase-1 inhibitor, YVAD-cmk (fig. 5b). In contrast, pretreatment of cell lysates with BEL was unable to block caspase-1 activation, excluding the possibility that BEL acts directly on caspase-1 (fig. 5c). These results are consistent with previous results obtained with THP-1 cells, and collectively suggest that BEL inhibits caspase-1 activation upstream of the inflammasome [9, 11].
Fig. 5.
BEL inhibits caspase-1 activity upstream of the inflammasome. a 1 × 106 THP-1 cells stably expressing Nlrp3PDFpk3 or control Fpk3 plasmid were stimulated with the Fpk3 ligand AP1510 for 6 h. Cell-free supernatants were analyzed for production of IL-1β. The results are given as the mean ± SD of triplicate cultures. b MonoMac6 cell lysates were incubated at 30°C in hypotonic buffer for the indicated times. Activation of caspase-1 was evaluated by immunoblotting with an antibody that recognizes the p10 subunit. Results are representative of 3 separate experiments (a, b).
Serine Protease Inhibitors Block Nlrc4-Dependent and the Nlrp3-Dependent Activation of Caspase-1
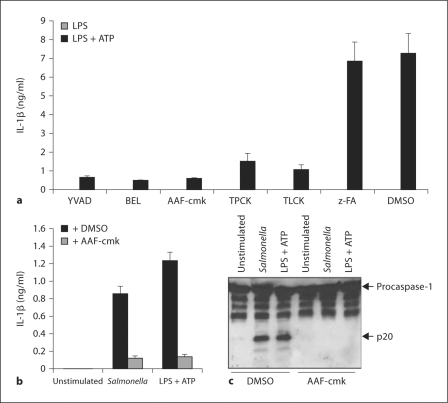
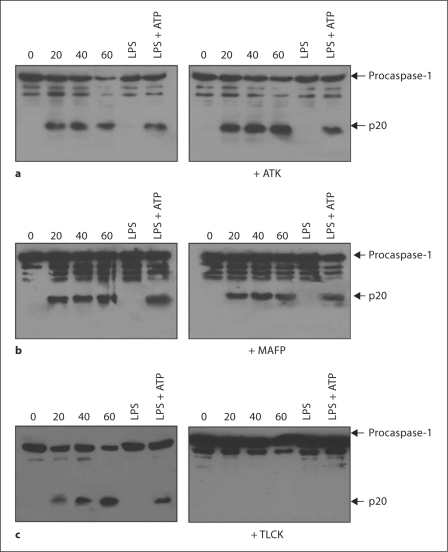
Our results demonstrate that BEL blocks inflammasome-mediated caspase-1 activation in a iPLA2β-independent manner. Because BEL was initially identified as a serine protease inhibitor [14], we reasoned that the activity of BEL might be explained by its ability to target serine proteases. We therefore tested 2 unrelated serine protease inhibitors, TPCK and TLCK, to block IL-1β production in macrophages stimulated with LPS and ATP. We found that BEL and the serine protease inhibitors efficiently reduced IL-1β secretion as effectively as the caspase-1 inhibitor YVAD-cmk (fig. 6a) In contrast, the pan-cathepsin inhibitor z-FA was unable to reduce IL-1β production in response to LPS and ATP (fig. 6a). CA-074-methyl ester, a chemically distinct membrane-permeable cathepsin B inhibitor, also had no inhibitory effect on LPS + ATP-induced caspase-1 maturation (data not shown). These results suggest that serine protease(s), rather than phospholipases, regulate the activation of the Nlrc4 and Nlpr3 inflammasomes. Next, we tested the serine protease inhibitor AAF-cmk, which was previously implicated in the activation of caspase-1 in response to Shigella flexneri infection in macrophages [29] and like Salmonella is mediated by the Nlc4 inflammasome [30]. The results showed that AAF-cmk inhibited both IL-1β secretion (fig. 6b) and caspase-1 activation (fig. 6c) induced by LPS + ATP or Salmonella. To confirm these results, we pretreated macrophages either with broad spectrum phospholipase inhibitors, ATK (arachidonyl trifluoromethyl ketone) or MAFP (methyl arachidonyl fluorophosphonate), or with the serine protease inhibitor TLCK and assessed the activation of caspase-1 in macrophages infected with Salmonella or stimulated with LPS + ATP. We found that treatment with TLCK efficiently inhibits caspase-1 activation. In contrast, neither phospholipase inhibitor blocked caspase-1 activation (fig. 7). These results suggest that serine protease(s), rather than phospholipases, regulate the activation of the Nlrc4 and Nlpr3 inflammasomes.
Fig. 6.
Serine protease(s) regulates IL-1β production and caspase-1 activation. a Macrophages were stimulated for 3 h with LPS and then treated for 1 h with YVAD-cmk (50 μM) or BEL (10 μM), TLCK (100 μM), TPCK (100 μM) and zFA (50 μM). Where indicated ATP (5 mM) was added for the last 30 min. Cell-free supernatants were analyzed for production of IL-1β. Values represent means ± SD of triplicate cultures. Results are representative of 3 separate experiments. b, c Macrophages were stimulated for 3 h with LPS and then treated, or not, with AAF-cmk (20 μM). The cells were then stimulated with ATP (5 mM) or infected with Salmonella for 30 min. b Cell-free supernatants were analyzed for production of IL-1β. Values represent means ± SD of triplicate cultures. c Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Results are representative of 3 separate experiments.
Fig. 7.
Serine protease(s), but not phospholipases, regulates caspase-1 activation. Macrophages were pretreated, or not, with ATK (a; 25 μM) or MAFP (b; 25 μM) or TLCK (c; 100 μM) and then infected with Salmonella at a macrophage/bacterial ratio of 1/5 for the indicated time point. Alternatively, macrophages were primed with LPS for 4 h and then stimulated, or not, with ATP (5 mM) for the last 30 min. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Results are representative of 2 separate experiments.
Discussion
Recent studies have revealed a crucial role for NLR proteins, such as Nlrc4 and Nlrp3, in caspase-1 activation and IL-1β production. While much progress has been made regarding the microbial stimuli that are sensed by Nlrc4 and Nlrp3 [2, 31, 32, 33, 34, 35], the regulatory events that link microbial molecules to inflammasome activation remain poorly understood. Earlier work using BEL suggested an important role for iPLA2 in the activation of caspase-1 and IL-1β secretion induced by LPS and ATP [8, 9, 10, 11]. In this report, we demonstrate that BEL inhibits not only caspase-1 activation induced by LPS and ATP, but also the Nlrc4 inflammasome that is triggered by Salmonella infection and cytosolic flagellin [19, 20]. More importantly, we have shown that the inhibition of both inflammasomes by BEL is not mediated by inhibition of iPLA2β as previously proposed [11]. Instead, our findings suggest that serine protease(s) targeted by BEL are important in inflammasome activation. Consistent with this notion, BEL was originally described as a serine protease inhibitor, although iPLA2β has previously been considered as the critical target underlying its suppression of caspase-1 activation and IL-1β release [14].
The regulation of the inflammasome is complex and still poorly characterized [2]. Previous studies suggested that intracellular K+ efflux is important or contributes to the activation of the Nlrp3 inflammasome [23]. Furthermore, BEL does not inhibit the ATP-induced release of intracellular K+ in macrophages [9]. Here, we found that BEL also inhibits Nlrc4-dependent activation of caspase-1 which is independent of K+ efflux [36, 37]. These new results indicate that BEL targets a common signal(s) that intersects both the K+-sensitive Nlrp3 inflammasome and the K+-insensitive Nlrc4 inflammasome. The finding that the 4 serine protease inhibitors, TPCK, TLCK, P-cmk and AAF-cmk, blocked inflammasome activity suggests that serine proteases are important for caspase-1 activation. Future studies will address the identity of the serine protease(s) targeted by BEL which are critical for the activation of the Nlrc4 and Nlrp3 inflammasomes in response to microbial stimuli. It is worth mentioning that cathepsin B, a lysosomal cysteine protease, has been previously implicated in the activation of the Nlrp3 inflammasome induced by silica, but not LPS + ATP [38]. In agreement with these observations, we found that a pan-cathepsin inhibitor, as well as specific cathepsin B inhibitors, did not impair caspase-1 activation induced by LPS + ATP or Salmonella (fig. 6 and data not shown). Collectively, these results suggest that the activation of the Nlrp3 or the Nlrc4 inflammasome induced by microbial stimuli depend on serine protease activity. One possibility is that serine protease(s) activated by microbial stimuli may degrade an inhibitor or alternatively induce proteolytic activation of a cellular factor that activates the inflammasome. This model is consistent with the indirect mechanism of activation of plant NLR homologs by pathogens [39, 40]. For instance, the bacterial effector AvrRpt2 is a protease that cleaves the plant RIN4, a step that leads to activation of the NLR homolog RPS2 [39, 40]. Similarly, the microbial cysteine protease AvrPphB cleaves the host protein PBS1, and this triggers activation of the plant NLR homolog RPS5 [39, 40]. Another possibility is that serine protease(s) form a complex with Nlrp3 and Nlrc4 or other components of the inflammasome and directly promote the activation of caspase-1. A better understanding of the role of serine proteases in inflammasome activation awaits the identification of the serine protease involved and of its substrates.
Acknowledgements
We would like to thank the all members of the Nuñez laboratory for their comments and suggestions. This research was supported by a fellowship from the Arthritis Foundation to L.F. and NIH grants AI063331 and AI064748 to G.N. and GM36387 to G.R.D.
References
- 1.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 4.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Warner N, Viani K, Nunez G. Function of NOD-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 9.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 10.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H, Weichel O, Bhakdi S. Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2. J Immunol. 2000;164:5120–5124. doi: 10.4049/jimmunol.164.10.5120. [DOI] [PubMed] [Google Scholar]
- 12.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Ramanadham S, Bao S, Hsu FF, Turk J. A bromoenol lactone suicide substrate inactivates group via phospholipase A2 by generating a diffusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry. 2006;45:1061–1073. doi: 10.1021/bi052065q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels SB, Cooney E, Sofia MJ, Chakravarty PK, Katzenellenbogen JA. Haloenol lactones: potent enzyme-activated irreversible inhibitors for alpha-chymotrypsin. J Biol Chem. 1983;258:15046–15053. [PubMed] [Google Scholar]
- 15.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group via phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 17.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Fuentes L, Perez R, Nieto ML, Balsinde J, Balboa MA. Bromoenol lactone promotes cell death by a mechanism involving phosphatidate phosphohydrolase-1 rather than calcium-independent phospholipase A2. J Biol Chem. 2003;278:44683–44690. doi: 10.1074/jbc.M307209200. [DOI] [PubMed] [Google Scholar]
- 19.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 20.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 21.Moran JM, Buller RM, McHowat J, Turk J, Wohltmann M, Gross RW, Corbett JA. Genetic and pharmacologic evidence that calcium-independent phospholipase A2beta regulates virus-induced inducible nitric-oxide synthase expression by macrophages. J Biol Chem. 2005;280:28162–28168. doi: 10.1074/jbc.M500013200. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2gamma, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells: enantioselective mechanism-based discrimination of mammalian iPLA2S. J Biol Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- 23.Bao S, Li Y, Lei X, Wohltmann M, Jin W, Bohrer A, Semenkovich CF, Ramanadham S, Tabas I, Turk J. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group via phospholipase A2. J Biol Chem. 2007;282:27100–27114. doi: 10.1074/jbc.M701316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Gong MC, Su W, Turk J, Guo Z. Group via phospholipase A2 (iPLA2beta) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J Biol Chem. 2007;282:25278–25289. doi: 10.1074/jbc.M611206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 27.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 28.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 29.Hilbi H, Puro RJ, Zychlinsky A. Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect Immun. 2000;68:5502–5508. doi: 10.1128/iai.68.10.5502-5508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 32.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 34.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 35.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 36.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 37.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 38.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 40.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]