Abstract
Keratinocytes undergo apoptosis in a variety of physiological and pathological conditions. Galectin-3 is a member of a family of β-galactoside-binding animal lectins expressed abundantly in keratinocytes and other epithelial cells. Here we have studied the regulatory role of galectin-3 in keratinocyte apoptosis by using cells from gene-targeted galectin-3 null (gal3−/−) mice. We showed that galectin-3 mRNA was transiently upregulated in ultraviolet-B (UVB)-irradiated wild-type keratinocytes. We found that gal3−/− keratinocytes were significantly more sensitive to apoptosis induced by UVB as well as various other stimuli, both in vitro and in vivo, than wild-type cells. Moreover, we demonstrated that increased apoptosis in gal3−/− keratinocytes was attributable to higher extracellular signal-regulated kinase (ERK) activation and lower AKT activation after UVB irradiation. We conclude that endogenous galectin-3 is an anti-apoptotic molecule in keratinocytes functioning by suppressing ERK activation and enhancing AKT activation and may play a role in the development of apoptosis-related skin diseases.
Introduction
Apoptosis is a tightly regulated process that enables removal of surplus, aged, or damaged cells. During apoptosis, a complex program becomes initiated that ultimately leads to the fragmentation of nuclear DNA. The epidermis forms the outermost component of the body. Epidermal keratinocytes are continuously exposed to many environmental stresses that are capable of inducing apoptosis, including a variety of physical (ultraviolet (UV) irradiation), biological (cytokines, hormones), and chemical (drugs, toxins) factors. Keratinocyte apoptosis has been recognized as an important control mechanism in the maintenance of epidermal homeostasis and the elimination of cells with damaged DNA. Accumulating evidence suggests that keratinocyte apoptosis is associated with various skin disorders, including skin cancers and eczematous dermatoses (Raj et al., 2006; Trautmann et al., 2000).
Galectins are a family of animal lectins defined by their affinity for β-galactosides and the presence of consensus amino-acid sequences in the carbohydrate-recognition domain (CRD). They exhibit a high degree of evolutionary conservation and family members are found in nematodes, insects, and mammals (Cooper, 2002; Leffler et al., 2004). Presently, fifteen members have been identified in mammals. Galectins exhibit extracellular functions as one might expect from the abundance of β-galactosides-containing glycoproteins oriented extracellularly, but they also function intracellularly in accordance with their localization within the cells. While these proteins participate in fundamental tasks associated with the lectin activity, they also function by interacting intracellularly with other proteins in a carbohydrate-independent manner (Liu et al., 2002).
Galectin-3 is the only chimeric galectin that consists of a flexible non-lectin domain made of tandem repeats linked to a C-terminal CRD. It is widely distributed in normal and disease tissues. It is highly expressed in a variety of inflammatory cells and epithelial cells including keratinocytes (Liu and Rabinovich, 2005). Galectin-3 is known to be involved in various biological events, including pre-mRNA splicing, cell adhesion, cell cycle regulation, and apoptosis (Liu et al., 2002; Yang and Liu, 2003).
Considerable evidence has accumulated showing that galectins are a family of apoptosis regulators. Galectin-3 has been shown to inhibit apoptosis induced by different stresses in a number of cell types through intracellular mechanisms (Liu et al., 2002; Nakahara et al., 2005). Other galectins, such as galectin-1, -7 and -9, on the other hand, possess proapoptotic activities (Hernandez and Baum, 2002; Liu and Rabinovich, 2005; Rabinovich, 1999). Recent reports showed that extracellular galectin-3 can also directly induce cell death in human thymocytes and T cells (Fukumori et al., 2003; Stillman et al., 2006). In addition, it is known that intracellular galectin-3 does not function as an anti-apoptotic protein in some cell types (Nakahara et al., 2005).
The mechanism underlying galectin-3’s anti-apoptotic activity is not fully understood, and the role of this protein in keratinocytes is unknown. Moreover, most of the studies were performed with transfectants overexpressing the protein. The availability of gene-targeted mice lacking galectin-3 (gal3−/− mice) has made possible the studies of the functions of endogenous galectin-3 (Colnot et al., 1998; Hsu et al., 2000). The aim of the present study is to gain insight into the function of galectin-3 in keratinocyte apoptosis, by using keratinocytes from gal3−/− mice. Our study shows that galectin-3 mRNA is transiently upregulated in UVB-irradiated keratinocytes. Importantly, gal3−/− keratinocytes is significantly more sensitive to apoptotic stimuli than wild-type (gal3+/+) cells both in vitro and in vivo. Moreover, we demonstrate that increased apoptosis in gal3−/− keratinocytes is attributable to increased extracellular signal-regulated kinase (ERK) activation and decreased AKT activation after UVB irradiation.
Results
Galectin-3 mRNA increases in keratinocytes exposed to UVB-irradiation
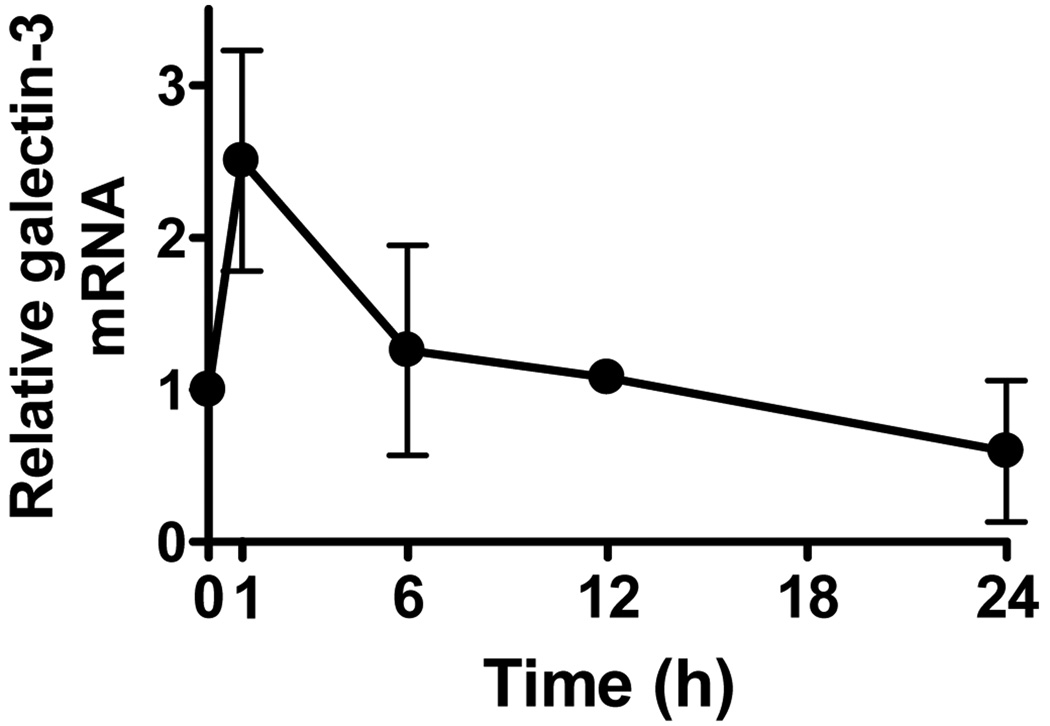
To study the role of galectin-3 in UVB-induced apoptosis, we first determined whether galectin-3 gene expression in keratinocytes was affected by UVB-irradiation. As shown in Fig. 1, real-time PCR analysis revealed that galectin-3 mRNA in cultured mouse keratinocytes increased significantly 1 h after UVB-irradiation (100 J/m2), but subsequently returned to the basal levels (Fig. 1). We measured the levels of galectin-3 protein in wild-type mouse keratinocytes after UVB irradiation, but did not notice a significant change at 0.5, 1, 2, 6 and 24 h compared to the baseline (data not shown).
Figure 1. Galectin-3 mRNA increases in normal mouse keratinocytes exposed to VB radiation.
Total RNA was extracted from cultured mouse keratinocytes exposed to UVB (100 J/m2) at the indicated time points. Real-time PCR was performed to quantify galectin mRNA. The results were normalized to HPRT. Bars represent mean ± SD. Similar results were obtained in two additional experiments.
Gal3−/− keratinocytes are more sensitive to UVB-induced apoptosis in vitro
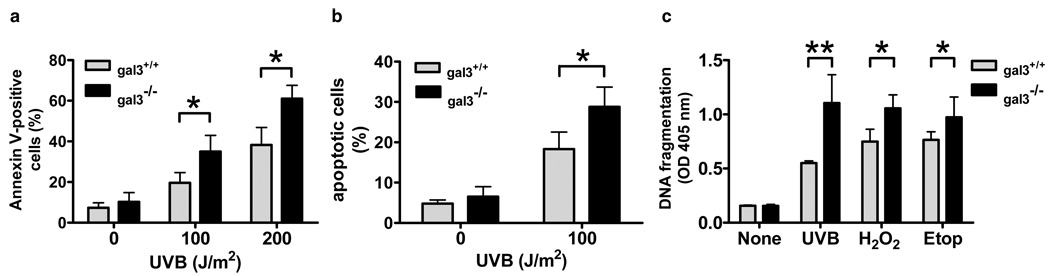
In order to determine whether galectin-3 plays a role in UVB-induced apoptosis in keratinocytes, we studied keratinocytes from gal3+/+ or gal3−/− mice treated with UVB in vitro and assessed the degree of apoptosis. We first quantitated by flow cytometry keratinocytes that were positively stained by FITC-labeled annexin V, at 24 h after UVB-irradiation, when the apoptotic response is known to peak. Both gal3+/+ and gal3−/− keratinocytes showed an increase in the number of positively stained cells as the dosage of UVB increased. There were significantly more positively stained cells in irradiated gal3−/− keratinocytes compared to irradiated gal3+/+ counterparts (Fig. 2a).
Figure 2. Gal3−/− keratinocytes are more sensitive to UVB-induced apoptosis in itro.
(a) Flow cytometric analysis of annexin-V stained keratinocytes. Keratinocytes from gal3+/+ and gal3−/− mice were untreated or irradiated with UVB at the indicated doses in vitro. Cells were harvested 24 h later and apoptosis was measured by flow cytometry with annexin-V-FITC and PI counterstaining. (b) Analysis of keratinocytes exposed to UVB for pycnotic nuclei. Keratinocytes were plated on coverslips and mock treated or irradiated with UVB at 100 J/m2, followed by incubation for 24 h. The cells were fixed, stained with Hoechst 33342, and analyzed for nuclear morphology of apoptosis by fluorescence microscopy. Cells with condensed or fragmented nuclei were determined as apoptotic cells. Data are expressed as percentage of apoptotic cells based on counting at least 150 cells. (c) Quantitative detection of histone-associated DNA fragments. Keratinocytes were irradiated with UVB (100 J/m2) or cultured with hydrogen peroxide (25 µM) or etoposide (25 µM). Twenty-four h later, histone-DNA complex was detected in an ELISA-based assay. Bars represent mean ± SD. *P<0.05. **P<0.001. Similar results were obtained in two additional experiments.
Then we analyzed the nuclear morphology of UVB-treated cells by Hoechst 33342 staining. Exposure of cells to UVB resulted in chromatin condensation and nuclear fragmentation, which are characteristic of apoptotic changes. Consistent with the previous report, about 5 % of unirradiated cells displayed apoptotic morphology (Grossman et al., 2001). As shown in Fig. 2b, gal3−/− keratinocytes contained more apoptotic cells compared to gal3+/+ keratinocytes after UVB-irradiation (Fig. 2b).
Another hallmark of apoptosis is the endonuclease-mediated cleavage of DNA into histone-associated fragments, which can be detected by specific antibodies. By an ELISA-based assay, we observed that UVB-induced DNA fragmentation was significantly enhanced by galectin-3 deficiency (Fig. 2c).
Gal3−/− keratinocytes are more susceptible to apoptosis induced by various agents
We also treated cells with hydrogen peroxide and etoposide to determine whether galectin-3 deficiency renders cells more susceptible to other apoptotic stimuli. The difference in apoptosis susceptibility between gal3+/+ and gal3−/− keratinocytes was also observed when cells were exposed to these agents (Fig. 2c). These results confirm that galectin-3 deficiency makes keratinocytes more sensitive to apoptosis in vitro.
A larger number of keratinocytes in the skin of gal3−/− mice undergo apoptosis when irradiated by UVB ex vivo
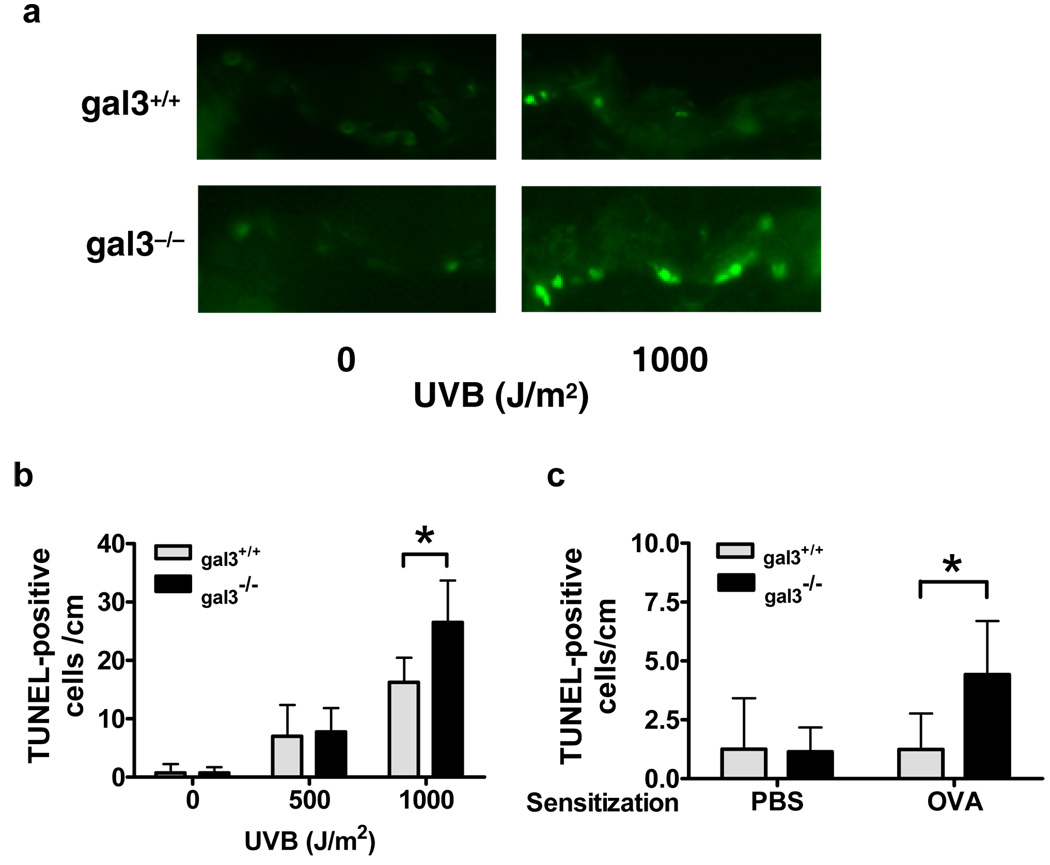
We also evaluated UVB-induced apoptosis in the skin ex vivo. Skin sections from gal3+/+ and gal3−/− mice were irradiated with UVB and, 24 h later, apoptotic keratinocytes were detected by TUNEL assay for internucleosomal DNA fragmentation. UVB-irradiation induced the appearance of TUNEL-labeled apoptotic keratinocytes in the epidermis in a dose-dependent manner (Fig. 3, a and b). The number of TUNEL-positive keratinocytes was significantly higher in the skin from gal3−/− mice compared to gal3+/+ mice at 24 h after exposure to 1000 J/m2 of UVB, although there was no significant difference between the two groups exposed to a lower dosage (500 J/m2) (Fig. 3b). TUNEL-positive cells were detected predominantly in the basal layer of epidermis in gal3−/− skin (Fig. 3a).
Figure 3. Higher numbers of apoptotic keratinocytes are induced by UVB rradiation and epicutaneous ovalbumin sensitization in gal3−/− mice.
(a and b) Skin sections from gal3+/+ and gal3−/− mice were placed on gauze soaked in RPMI medium and irradiated with UVB ex vivo at the indicated doses. Twenty-four h after irradiation, samples were processed for TUNEL reaction. (c) Gal3+/+ and gal3−/− mice were treated with three one-week periods of epicutaneous application of ovalbumin or PBS, each two weeks apart. Skin sections were obtained from the treated areas 24 h after the third sensitization period and processed for TUNEL reaction. Apoptotic cells were counted per cm in the skin sections. Bars represent mean ± SD. *P<0.05.
More apoptotic keratinocytes are found at allergic skin inflammation sites in gal3−/− mice
Trautmann et al. (Trautmann et al., 2000) demonstrated that keratinocyte apoptosis caused by skin-infiltrating T cells is a crucial event in the formation of eczematous lesions in both atopic dermatitis and allergic contact dermatitis. Thus, we evaluated keratinocyte apoptosis in a mouse model of atopic dermatitis that displays many of the features of human atopic dermatitis, including acanthosis of the epidermis and inflammatory cell infiltration in the dermis (Spergel et al., 1998; Wang et al., 1996). Significantly more apoptotic keratinocytes were found at the ovalbumin (OVA)-sensitized skin sites in gal3−/− mice (Fig. 3c), compared to gal3+/+ mice. TUNEL-stained keratinocytes were barely detected at the PBS-sensitized skin sites in gal3+/+ and gal3−/− mice.
Galectin-3 deficiency does not alter the expression of other galectins in keratinocytes
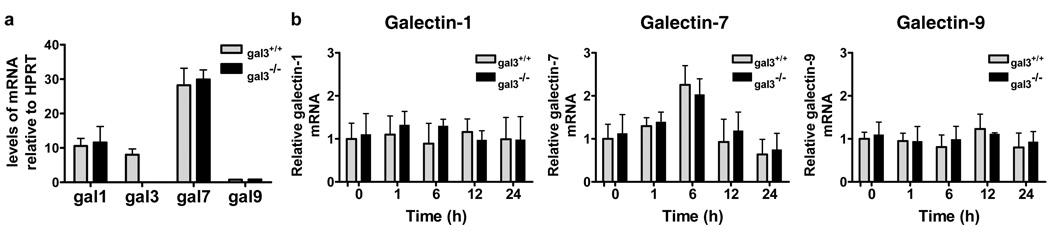
The outcome of cell survival/apoptosis may be dependent on the expression profile of different galectin members, some of which are pro-apoptotic and others are anti-apoptotic (Hsu et al., 2006). A recent report has shown that healing corneas of gal3−/− mice contained significantly reduced levels of galectin-7 compared to those of gal3+/+ mice (Cao et al., 2002). This suggests that altered expression of one galectin may affect the expression of others. We therefore studied gene expression patterns of galectin family members in keratinocytes from gal3+/+ and gal3−/− mice. cDNA microarray analysis showed that galectin−1, −3 and −7 are three major galectins in wild-type mouse keratinocytes, as well as normal human keratinocytes (data not shown). These results were further confirmed by real-time PCR analysis (Fig. 4a). There were no differences in the expression levels of galectin−1, −7, and −9 between gal3+/+ and gal3−/− keratinocytes (Fig. 4a). These results suggest that galectin-3-deficiency does not alter the expression of other galectins.
Figure 4. Galectin-3 deficiency does not alter the expression of other galectins.
(a) Total RNA was extracted from keratinocytes from gal3+/+ and gal3−/− mice and cDNA was synthesized. Real-time PCR was performed to quantify mRNA. The results were normalized to HPRT. (b) Total RNA was extracted from keratinocytes exposed to UVB at the indicated time points. Real-time PCR was performed to quantify galectin−1, −7 and −9 mRNA. The results were normalized to HPRT. Bars represent mean ± SD. Similar results were obtained in two additional experiments.
UVB-induced galectin-7 response is comparable in gal3+/+ and gal3−/− keratinocytes
Bernerd et al. (Bernerd et al., 1999) demonstrated that the amount of galectin-7 mRNA and protein increased in keratinocytes after ultraviolet-B (UVB) irradiation, paralleling p53 stabilization, and they provided evidence that galectin-7 contributes to UVB-induced apoptosis in keratinocytes. Thus, we next compared the induction of galectin-7 in UVB-treated gal3+/+ and gal3−/− keratinocytes, to determine whether the higher sensitivity of gal3−/− keratinocytes to UVB-irradiation could be due to higher levels of galectin-7. Consistent with the finding by Bernerd et al., the amount of galectin-7 mRNA peaked around 6 h after irradiation. However, there was no significant difference in the galectin-7 response to UVB between the two genotypes of keratinocytes (Fig. 4b). In addition, the levels of other galectins, galectin−1, and −9, did not change significantly after UVB-irradiation in both gal3+/+ and gal3−/− keratinocytes (Fig. 4b). Thus, galectin-3 deficiency does not alter the galectin−1, −7, and −9 responses to UVB in mouse keratinocytes.
UVB-induced JNK and ERK phosphorylation is higher in gal3−/− keratinocytes
To illustrate the mechanism of galectin-3’s anti-apoptotic activity in keratinocytes, we next focused on the signal transduction pathways in UVB-induced keratinocytes. Mitogen-activated protein kinases (MAPKs) have been shown to form the core signaling unit of UVB-induced stress responses in keratinocytes (Assefa et al., 1997). The three major families of the MAPKs are extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK.
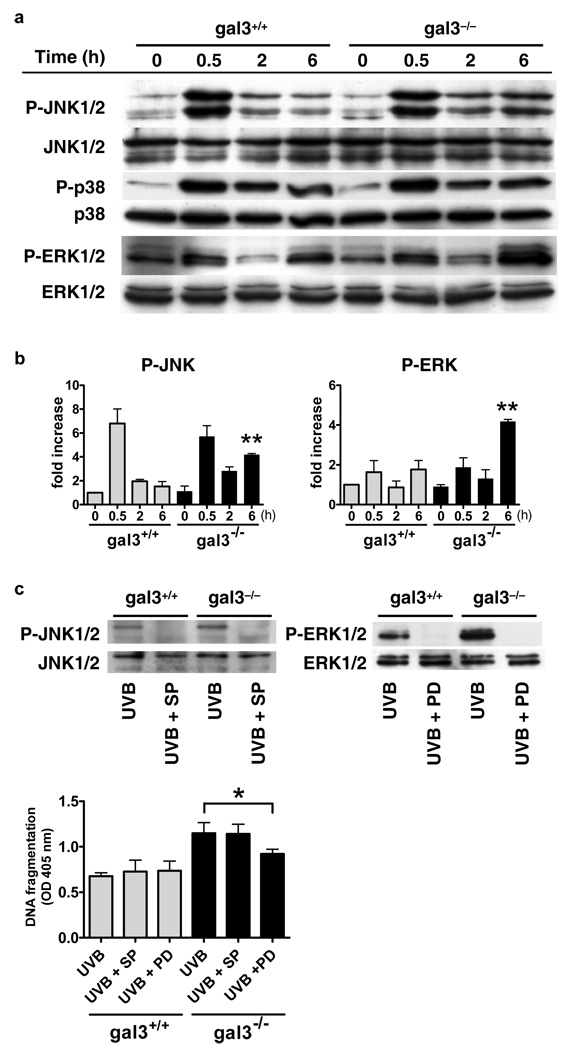
Comparable basal levels of JNK 1/2, p38 and ERK 1/2 activity were detected in unirradiated gal3+/+ and gal3−/− keratinocytes by immunoblot analysis (Fig. 5a and b). JNK 1/2 and p38 have been shown to be activated within 30 min after UVB, and then gradually return to the basal levels in normal human epidermal keratinocytes (Mantena and Katiyar, 2006). We noted that JNK 1/2 activity was significantly higher in gal3−/− keratinocytes compared to gal3+/+ cells at 6 h after irradiation (Fig. 5a and b).
Figure 5. Gal3−/− keratinocytes exhibit higher ERK phosphorylation upon VB-irradiation and ERK inhibitor decreases UVB-induced apoptosis in these ells.
(a) Keratinocytes from gal3+/+ and gal3−/− mice were irradiated with UVB (100 J/m2). Cells were collected at indicated times after irradiation, whole cell homogenates were prepared, and total and phosphorylated JNK 1/2, p38, and ERK 1/2 were determined by immunoblotting using specific antibodies. (b) Fold activation was calculated based on phosphorimager analysis. Bars represent mean ± SD from three independent experiments. **P<0.01, vs. wild-type at the same time point. (c) Cells were pre-incubated with 25 µM SP600125 (SP) or 50 µM PD98059 (PD) for 1 h before exposure to UVB (100 J/m2). Six h after irradiation, cell lysates were subjected to immunoblotting with each antibody. Twenty-four h later, histone-DNA complex was detected in an ELISA-based assay. Bars represent mean ± SD. *P<0.05.
Consistent with the previous reports (Nakamura et al., 2001; Zhang et al., 2004), there was a slight increase in phosphorylation of ERK1/2 occurring within 0.5 h after UVB irradiation. Phospho- ERK1/2 levels returned to the baseline, but were then strongly induced at 6 h after irradiation. While comparable levels of phosphorylated ERK1/2 were detected between the two genotypes of cells at 0.5 h after UVB irradiation, the level was significantly higher in gal3−/− keratinocytes compared to gall3+/+ cells at 6 h after irradiation (Fig. 5a and b).
ERK inhibitor decreases UVB-induced apoptosis in gal3−/− keratinocytes
The roles of JNK 1/2 and ERK 1/2 in UVB-induced apoptosis are still controversial. Previous studies have shown that JNK 1/2 and ERK 1/2 may be involved in both cell survival and cell apoptosis, depending on the cell type and/or the nature of the stimuli (Wada and Penninger, 2004). To assess the relationship between the increase in JNK 1/2 and ERK 1/2 phosphorylation to enhanced apoptosis in gal3−/− keratinocytes after UVB irradiation, we treated cells with kinase inhibitors before UVB irradiation. SP600125 (JNK inhibitor) and PD98059 (ERK inhibitor) inhibited the activation of JNK and ERK 1/2, respectively, after UVB irradiation, as expected (Fig. 5c). Importantly, while both did not affect the degree of UVB-induced apoptosis in gal3+/+ keratinocytes, PD98059, but not SP600125, significantly suppressed UVB-induced apoptosis in gal3−/− keratinocytes (Fig. 5c). Both inhibitors did not affect the viability of gal3+/+ and gal3−/− keratinocytes, when used alone in the absence of UVB irradiation (data not shown). The results suggest that enhanced ERK activation contributes to increased apoptosis in gal3−/− keratinocytes.
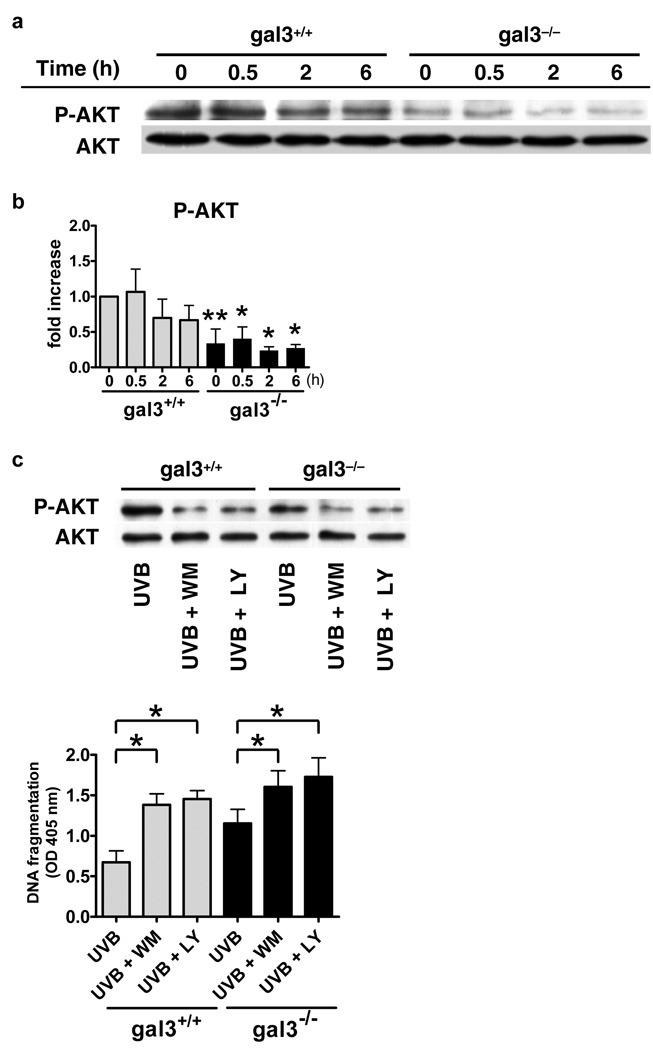
AKT phosphorylation is impaired in gal3−/− keratinocytes
AKT has been shown to promote cell survival in response to various stimuli (Datta et al., 1999). A recent report demonstrated that AKT activation is essential for protecting keratinocytes against UVB-induced apoptosis (Wang et al., 2003). We therefore studied the levels of AKT phosphorylation in gal3+/+ and gal3−/− keratinocytes, and found strikingly diminished levels in gal3−/− keratinocytes, both at the baseline and following UVB irradiation (Fig. 6a and b). Treatment of cells with phosphatidylinositol 3-kinase (PI3K)/AKT inhibitors, wortmannin and LY294002, significantly inhibited AKT activation, and resulted in increased apoptosis in both gal3+/+ and gal3−/− keratinocytes (Fig. 6c). Importantly, the difference in the degree of apoptosis between the two genotypes was diminished when the cells were treated with these inhibitors (Fig. 6c). Both inhibitors by themselves in the absence of UVB irradiation did not affect the viability of the cells (data not shown). The results suggest that reduced AKT activation contributes to increased apoptosis in gal3−/− keratinocytes.
Figure 6. AKT phosphorylation is markedly impaired in gal3−/− keratinocytes.
(a) Keratinocytes from gal3+/+ and gal3−/− mice were irradiated with UVB (100 J/m2). Cells were collected at indicated times after irradiation, whole cell homogenates were prepared, and total and phosphorylated AKT levels were determined by immunoblotting using specific antibodies. (b) Fold activation was calculated based on phosphorimager analysis. Bars represent mean ± SD from three independent experiments. *P<0.05, **P<0.01, vs. wild-type at the same time point. (c) Cells were pre-incubated with 500 nM Wortmannin or 20 µM LY294002 for 1 h before exposure to UVB (100 J/m2). Six h after irradiation, cell lysates were subjected to immunoblotting with each antibody. Twenty-four h later, histone-DNA complex was detected in an ELISA-based assay. Bars represent mean ± SD. *P<0.05.
Discussion
In this study, we have established a role for galectin-3 in the protection of keratinocytes from apoptosis. First, galectin-3 mRNA increases transiently in keratinocytes exposed to UVB-irradiation. Second, gal3−/− keratinocytes are more sensitive both in vitro and ex vivo to apoptosis induced by UVB, as well as various other stimuli. This was demonstrated by flow cytometry analysis of FITC-labeled annexin V assay, nuclear staining with Hoechst 33342, ELISA for DNA-fragmentation, and TUNEL assay. Third, galectin-3 deficiency is associated with alterations in the activation of signaling molecules known to be involved in apoptosis-regulating pathways. These include the increased activation of JNK and ERK and decreased activation of AKT.
Activation of MAPKs by UV irradiation represents one of the early cellular responses and displays a dependence on the dose and wavelength of UV light as well as the duration of the exposure (Assefa et al., 1997). We found that UVB-induced activation of JNK and ERK, but not p38 kinase, was enhanced in gal3−/− keratinocytes. There are a number of reports demonstrating the involvement of the JNK signaling pathway in UVB-induced apoptosis (Chen et al., 1999; Davis, 2000; Xia et al., 1995). However, we demonstrated that the JNK inhibitor SP600125 did not affect the degree of UVB-induced apoptosis in both gal3+/+ and gal3−/− keratinocytes. We thus conclude that the difference in the JNK activation between these two genotypes does not contribute significantly to the difference in the degree of apoptosis observed.
Substantial evidence supports that activation of the ERK signaling pathway contributes to cell survival. Peus et al. (Peus et al., 1999) have shown that inhibition of ERK resulted in enhanced cell death after UVB-irradiation. However, evidence also exists for a pro-apoptotic role of the ERK pathway. Another recent report has demonstrated that ERK activation facilitated UV-induced apoptosis in various cell types (Tang et al., 2002). The pro-apoptotic function for the ERK pathway has also been suggested in several other model systems (Wang et al., 2000) (Sutherland et al., 1996) (Alessandrini et al., 1999). With regard to galectin-3, both a positive correlation (in a galectin-3-overexpressing human breast carcinoma cell line (Takenaka et al., 2004)) and a negative correlation (in galectin-3 overexpressing human embryonic kidney cell line (Elad-Sfadia et al., 2004)) between the levels of galectin-3 and phosphorylated ERK exist. These varying results may be due to variation of galectin-3’s influence on the ERK pathway depending on the cell type and/or the nature of the stimuli, as galectin-3 is capable of interacting with different intermediates under different conditions. Here we show that treatment with the ERK inhibitor PD98059 significantly suppressed UVB-induced apoptosis in gal3−/− keratinocytes, but not gall3+/+ keratinocytes. Thus, in the presence of this inhibitor, the difference in the degree of apoptosis between the two genotypes was diminished. Our results suggest that galectin-3’s anti-apoptotic effect in keratinocytes can be explained by its ability to suppress the ERK signaling pathway.
AKT is a well-established anti-apoptotic protein. It is activated by phospholipid products of phosphatidylinositol 3-kinase (PI3K) and is a prominent downstream target of PI3K in cell survival signaling (Datta et al., 1999). It has been shown that AKT activation is essential for the protection of keratinocytes against UVB-induced apoptosis by abolishing cytochrome c release and activation of caspase-9, -8, and -3 (Wang et al., 2003). Furthermore, anti-apoptotic signals leading to the suppression of UVB-induced apoptosis by various factors have been shown to be mediated mainly through AKT activation (Decraene et al., 2002). In this study, we show that AKT activity substantially decreased in gal3−/− keratinocytes. Furthermore, the difference in the degree of apoptosis between the two genotypes was diminished when cells were treated with PI3K/AKT inhibitors. Our results strongly suggest that galectin-3’s anti-apoptotic effect in keratinocytes is associated with its ability to activate the AKT signaling pathway.
The significant albeit transient increase in the galectin-3 mRNA level at 1 h after UVB irradiation without a subsequent increase in the protein level requires an explanation. This could be due to the transient nature of the surge in the mRNA level, which either does not lead to a significant increase in the protein level, or makes the increase harder to detect. It is also possible that galectin-3 protein is degraded in cells undergoing apoptosis, thus offsetting the increase in protein translation. Another possibility is related to the regulation of galectin-3 by p53. p53 is induced by UVB irradiation and plays a critical role in UVB-induced keratinocyte apoptosis (Oren, 2003). We found p53 protein accumulation as early as 6 h after UVB irradiation in both gal3+/+ and gal3−/− keratinocytes (and there was no significant difference in its level between gal3+/+ and gal3−/− keratinocytes (data not shown)). It has been shown recently that p53 down regulates galectin-3 expression by transcriptional repression of the galectin-3 gene promoter activity (Cecchinelli et al., 2006). Thus, we propose that the early upregulation of galectin-3 mRNA at 1 h is not dependent on p53, but as the p53 protein is subsequently independently induced, it suppresses the expression galectin-3 mRNA and protein. This leads to the transient nature in the induction of the galectin-3 mRNA, as we noted, and the lack of a readily observable increase in the protein level.
There is evidence that galectin-3 exerts its anti-apoptotic activity by interacting with molecular components of apoptosis-regulating pathways (Hsu et al., 2006; Liu et al., 2002; Nakahara et al., 2005). These include the well-established anti-apoptotic protein Bcl-2 (Yang et al., 1996) and synexin, a Ca2+- and phospholipid-binding protein known to regulate intracellular vesicle fusion and membrane trafficking (Yu et al., 2002). Moreover, Fukumori et al. (Fukumori et al., 2004) demonstrated that galectin-3 is complexed with Fas-receptor (CD95), a receptor known to play an important role in the extrinsic pathway of apoptosis. In addition, galectin-3 becomes associated with membranous structures in apoptotic cells, especially mitochondria, which are known to be critically involved in the apoptotic process, and exhibit their anti-apoptotic activities therein (Matarrese et al., 2000; Yu et al., 2002). In keratinocytes undergoing apoptosis, whether galectin-3 binding to these molecules/structures contributes to regulation of ERK and AKT activation remains to be determined.
Galectin-3 has been shown to play an important role in tumor development and progression, including regulation of tumor cell growth as well as cell motility and invasion, in addition to inhibition of apoptosis (Liu and Rabinovich, 2005). AKT has also been demonstrated to play a critical role in tumorigenic processes. It is overexpressed in many cancer cells and has bee shown to contribute to enhanced cell proliferation and reduced apoptosis in tumor cells (Cheng et al., 1996). In addition, it has been shown to promote tumor cell motility and invasion (Toker and Yoeli-Lerner, 2006). In view of our demonstration that galectin-3 upregulates AKT expression in keratinocytes under conditions that can lead to development of skin cancers, galectin-3 may contribute to the development of skin cancers by regulating the AKT pathway.
Several reports illustrated the differences between cultured mouse and human keratinocytes. Mouse keratinocytes has been shown to be more readily immortalized than human keratinocytes (Greenberg et al., 1998). Chaturvedi et al. (Chaturvedi et al., 2004) reported that mouse keratinocytes exhibited increased expression of keratin-1 and reduced p53 levels compared with human keratinocytes. On the other hand, significant similarities in intracellular adhesion, structural integrity, and immune function-related molecules have been shown between mouse and human keratinocytes (Chan, 2004). Thus, although additional investigations using human keratinocytes are necessary, we believe that our results suggest that galectin-3 may play an important role in the response of keratinocytes to UVB-irradiation in humans.
In conclusion, endogenous galectin-3 is an anti-apoptotic molecule in keratinocytes. It likely exerts its anti-apoptotic activity by suppressing the activation ERK as well as promoting the activation of the PI3K/AKT pathway. Galectin-3 may play an important role in regulating keratinocyte apoptosis in diverse physiological and pathological processes occurring in the skin, including skin cancers and eczematous dermatoses.
Materials and Methods
Animals
Gal3−/− mice were developed as described (Hsu et al., 2000). These mice were backcrossed to C57BL/6 mice for nine generations and interbreeding of gal3+/− F9 resulted in gal3+/+ and gal3−/− mice in the C57BL6 background, which were used throughout this study. All experiments with mice were approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Sacramento, CA).
Primary cultures of mouse keratinocytes and mouse skin sections
Mouse epidermal cells were isolated from neonatal C57BL/6 mice (12 to 48 h after birth) and used to establish primary cultures of the keratinocytes according to the described procedures (Hager et al., 1999). Briefly, trunk skin was removed, flattened on 10 mg/ml of Dispase II (Roche Molecular Biochemicals, Indianapolis, IN) at 4°C overnight. Then epidermis was separated from the dermis, and minced with scissors. The cells were cultured in Epilife Medium (Cascade Biologics, Portland, OR) plus 0.06 mM CaCl2 supplemented with 10 ng/ml mouse epidermal growth factor (Sigma, St. Louis, MO), 10−10 M cholera toxin (Calbiochem, San Diego, CA), and Human Keratinocyte Growth Supplement-V2 (Cascade Biologics).
For ex vivo experiments, full-thickness skin of approximately 3 × 4 cm was dissected from the back of euthanized mice (7–9 weeks of age) after the hairs were first removed. The samples were cut into pieces of approximately 10 mm square each.
UV irradiation
Cultured keratinocytes and skin sections were exposed to UVB (312 nm), using BLE-8T312 UVB lamps (SPECTRONICS CORP., Westbury, NY). The intensity of the UV light was measured with a calibrated UVB-Meter model UVB-500C (National Biological Corporation, Twinsburg, OH). For the in vitro experiments, the dosage used was 100 – 200 J/m2 and for the ex vivo experiments, the dosage used was 500 – 1000 J/m2. For the in vitro experiments, equal numbers of gal3+/+ and gal3−/− keratinocytes were seeded, and the next day cells at 70–80% confluency were treated with UVB. Before UVB-irradiation, cells were washed and the media were replaced with PBS. After irradiation, the cells were immediately reconstituted with fresh medium as described above and incubated for indicated time periods. In some experiments, cells were treated with the ERK inhibitor PD98059 (50 µM; Calbiochem), JNK inhibitor SP600125 (25 µM; Calbiochem) or PI3K/AKT inhibitors, Wortmannin (500 nM; MP Biomedicals, Irvine, CA) and LY294002 (20 µM; Sigma), for 1 h prior to exposure to UVB. After UVB irradiation, the medium was also supplemented with the kinase inhibitors. Twenty-four h after irradiation, the cells were assayed for apoptosis.
Flow cytometry
After UVB-irradiation, both detached and attached cells were harvested and combined. Mild trypsinization was used to collect the attached cells. Cells were centrifuged at 200 × g for 5 min, washed once with PBS, stained with FITC-conjugated annexin V (BD Pharmingen) and propidium iodide (PI), and subjected to two-color analysis using FACS Calibur (Becton Dickinson, Mountain View, CA).
Nuclear staining with Hoechst 33342
Twenty-four h after UVB-irradiation, cells plated on coverslips were washed with PBS, fixed with 4% paraformaldehyde, and incubated for 10 min with 5 µg of Hoechst 33342 in the dark at room temperature. The morphological characteristics of apoptotic cells were identified with the aid of a fluorescence microscope using the excitation wavelength of 540 nm. Cells with fragmented and/or condensed nuclei were considered as apoptotic cells.
Quantitative estimation of DNA fragmentation using an enzyme-linked immunosorbent assay (ELISA)
Histone-associated DNA fragmentation was quantitatively estimated using an ELISA kit according to the manufacturer's protocol (Cell Detection ELISAPLUS, Roche Diagnostics Co., Indianapolis, IN). Briefly, keratinocytes were plated on flat-bottomed 6-well plates (Falcon, Becton Dickinson, Oxnard, CA), and irradiated with UVB (100 J/m2), or cultured with hydrogen peroxide (25 µM) or etoposide (25 µM). Twenty-four h later, the plates were centrifuged and the cell pellets were lysed in buffer provided with the kit. Soluble histone-DNA complex in the lysates as a result of DNA fragmentation was measured by ELISA. Absorbance at 405 nm (reference at 492 nm) was measured in each well.
In situ terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay
Skin sections were placed on a piece of sterile gauze soaked with RPMI-1640 supplemented with 10% fetal bovine serum (FBS), and then either not treated or irradiated with the indicated doses of UVB. Twenty-four h later, the samples were embedded in Tissue Tek oxacalcitriol (OCT) compound (Sakura Finetek, Torrance, CA) and quickly frozen in liquid nitrogen. TUNEL assay was performed on 6–8 µm-thick frozen sections using the In Situ Cell Death Detection Kit (Rohce Diagnostics Co.) according to the manufacture’s protocol. Apoptotic cells, which appear as fluorescent cells, were enumerated under a fluorescence microscope.
Mouse model of atopic dermatitis
Epicutaneous sensitization of 6- to 10-week-old female BALB/c mice was performed as described previously (Spergel et al., 1998). Briefly, an area on the trunk of anesthetized mice was shaved with an electric razor and tape-stripped six times. One hundred µg of OVA (Grade V; Sigma, St. Louis, MO) in 100 µl of PBS or 100 µl of PBS alone was placed on a patch of sterile gauze (1 × 1 cm). The gauze was secured to the back with Tegaderm (3M Health Care Ltd, St. Paul, MN), which was reinforced with BAND-AID (Johnson & Johnson Medical Inc, Arlington, TX). The patches were placed for a one-week period and then removed. Two weeks later, an identical patch was reapplied to the same skin sites. Each mouse had a total of three one-week exposures to the antigen separated from each other by two-week intervals. Skin samples were obtained 24 h after removal of the skin patch and frozen sections were prepared for TUNEL assay.
Western blot analysis
Cells were solubilized in lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) containing 1mM PMSF, 10 µg/ml aprotinin, and 10 µg/ml leupeptin at 4°C for 20 min. After centrifugation at 10,000 × g for 15 min, the supernatants were removed and the protein concentrations were determined by using the BCA Protein Assay Reagent (Pierce Chemical Company, Rockford, IL). Samples containing 10–30 µg of proteins were boiled for 5 min in SDS sample buffer. The activation of JNK1/2, p38, ERK1/2, and AKT was determined by immunoblot analysis using phosphorylation-specific antibodies (Cell Signaling Technology, Beverly, MA). Density of bands was quantified with Quantity One software (Bio Rad laboratories, Hercules, CA).
Real-time PCR analysis
Total RNA was extracted from cells by the RNeasy mini protocol (Qiagen, Valencia, California, USA). Total RNA (1 µg) was converted to cDNA by the reverse transcriptase enzyme reaction (Powerscript, BD Biosciences, San Jose, CA). Real-time quantitative PCR were carried out in an ABI Prism 7700 sequence detection system (Applied Biosystems) in a 25 µl volume. The reaction mixture consisted of 50 ng of cDNA template, 1x SYBR Green PCR master mix (TAKARA, Tokyo, Japan) and 200 nM primer mix (forward and reverse primers combined). Cycling parameters were 95°C for 10 min to activate DNA polymerase, then 40 cycles of 95°C for 20 sec and 60°C for 1 min. Primer sets used are as follows: Galectin-1, GAAAAGACAGCAACAACCTGTGCCTAC and GAGGGCTACAGGCTGGCTGGC; Galectin-3, GACAGTCAGCCTTCCCCTTTGAGAG and AGGCACACAGGGCCGGTTTCGG; Galectin-7, GGCACTGTCATGAGAATTCGAGGC and CGGTGGTGGAAGTGGAGATATTCGTC; Galectin-9, GTGATATCCAGCTGACCCACGTGC and ATGATATCAGGGTGGCTCTCCTG; hypoxanthine ribosyltransferase (HPRT), TGGATATGCCCTTGACTATAATGAGTACTTCAG and GTCTGGGGACGCAGCAACTGAC.
Statistical analysis
Statistical analysis was accomplished by Student’s t test using the software GraphPad Prism ver 4. Values of P<0.05 were considered significant.
Acknowledgements
The microarray analysis was conducted by the Gene Microarray (E) Core of The Consortium for Functional Glycomics funded by National Institute of General Medical Sciences Grant GM62116. The work was supported by NIH grants RO1 AI20958, RO1 AI39620, and R21 AR53116.
Abbreviations used in this paper
- CRD
carbohydrate-recognition domain
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- OVA
ovalbumin
- PI3K
phosphatidylinositol 3-kinase
- UVB
ultraviolet-B
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa Z, Garmyn M, Bouillon R, Merlevede W, Vandenheede JR, Agostinis P. Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes. J Invest Dermatol. 1997;108:886–891. doi: 10.1111/1523-1747.ep12292595. [DOI] [PubMed] [Google Scholar]
- Bernerd F, Sarasin A, Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc Natl Acad Sci U S A. 1999;96:11329–11334. doi: 10.1073/pnas.96.20.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Cecchinelli B, Lavra L, Rinaldo C, Iacovelli S, Gurtner A, Gasbarri A, et al. Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol Cell Biol. 2006;26:4746–4757. doi: 10.1128/MCB.00959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LS. Comparable Structure and Function of the Skin. In: Chan LS, editor. Animal Models of Human Inflammatory Skin Diseases. Florence: Informa Healthcare; 2004. pp. 3–18. [Google Scholar]
- Chaturvedi V, Bacon P, Bodner B, Nickoloff BJ. Proliferating cultured human keratinocytes are more susceptible to apoptosis compared with mouse keratinocytes. J Invest Dermatol. 2004;123:1200–1203. doi: 10.1111/j.0022-202X.2004.23514.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Ma W, Huang C, Dong Z. Translocation of protein kinase Cepsilon and protein kinase Cdelta to membrane is required for ultraviolet B-induced activation of mitogen-activated protein kinases and apoptosis. J Biol Chem. 1999;274:15389–15394. doi: 10.1074/jbc.274.22.15389. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Ripoche MA, Milon G, Montagutelli X, Crocker PR, Poirier F. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology. 1998;94:290–296. doi: 10.1046/j.1365-2567.1998.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Decraene D, Agostinis P, Bouillon R, Degreef H, Garmyn M. Insulin-like growth factor-1-mediated AKT activation postpones the onset of ultraviolet B-induced apoptosis, providing more time for cyclobutane thymine dimer removal in primary human keratinocytes. J Biol Chem. 2002;277:32587–32595. doi: 10.1074/jbc.M111106200. [DOI] [PubMed] [Google Scholar]
- Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–34930. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64:3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- Grossman D, Kim PJ, Blanc-Brude OP, Brash DE, Tognin S, Marchisio PC, et al. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J Clin Invest. 2001;108:991–999. doi: 10.1172/JCI13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager B, Bickenbach JR, Fleckman P. Long-term culture of murine epidermal keratinocytes. J Invest Dermatol. 1999;112:971–976. doi: 10.1046/j.1523-1747.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–273. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, et al. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic Biol Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Matarrese P, Tinari N, Semeraro ML, Natoli C, Iacobelli S, Malorni W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000;473:311–315. doi: 10.1016/s0014-5793(00)01547-7. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takahashi H, Kinouchi M, Manabe A, Ishida-Yamamoto A, Hashimoto Y, et al. Differential phosphorylation of mitogen-activated protein kinase families by epidermal growth factor and ultraviolet B irradiation in SV40-transformed human keratinocytes. J Dermatol Sci. 2001;25:139–149. doi: 10.1016/s0923-1811(00)00123-7. [DOI] [PubMed] [Google Scholar]
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- Peus D, Vasa RA, Beyerle A, Meves A, Krautmacher C, Pittelkow MR. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J Invest Dermatol. 1999;112:751–756. doi: 10.1046/j.1523-1747.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA. Galectins: an evolutionarily conserved family of animal lectins with multifunctional properties; a trip from the gene to clinical therapy. Cell Death Differ. 1999;6:711–721. doi: 10.1038/sj.cdd.4400535. [DOI] [PubMed] [Google Scholar]
- Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol. 2006;126:243–257. doi: 10.1038/sj.jid.5700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24:4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Quan T, He T, Franke TF, Voorhees JJ, Fisher GJ. Epidermal growth factor receptor-dependent, NF-kappaB-independent activation of the phosphatidylinositol 3-kinase/Akt pathway inhibits ultraviolet irradiation-induced caspases-3, -8, and -9 in human keratinocytes. J Biol Chem. 2003;278:45737–45745. doi: 10.1074/jbc.M300574200. [DOI] [PubMed] [Google Scholar]
- Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–4082. [PubMed] [Google Scholar]
- Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cell Mol Life Sci. 2003;60:267–276. doi: 10.1007/s000180300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pelech S, Uitto VJ. Bacterial GroEL-like heat shock protein 60 protects epithelial cells from stress-induced death through activation of ERK and inhibition of caspase 3. Exp Cell Res. 2004;292:231–240. doi: 10.1016/j.yexcr.2003.08.012. [DOI] [PubMed] [Google Scholar]