Abstract
Individuals with epilepsy are at increased risk of having psychotic symptoms that resemble those of schizophrenia. More controversial and less searched is if schizophrenia is a risk factor for epilepsy. Here we review overlapping epidemiological, clinical, neuropathological and neuroimaging features of these two diseases. We discuss the role of temporal and other brain areas in the development of schizophrenia-like psychosis of epilepsy. We underline the importance of ventricular enlargement in both conditions as a phenotypic manifestation of a shared biologic liability that might relate to abnormalities in neurodevelopment. We suggest that genes implicated in neurodevelopment may play a common role in both conditions and speculate that recently identified causative genes for partial complex seizures with auditory features might help explain the pathophysiology of schizophrenia. These particularly include the leucine-rich glioma inactivated (LGI) family gene loci overlap with genes of interest for psychiatric diseases like schizophrenia. Finally, we conclude that LGI genes associated with partial epilepsy with auditory features might also represent genes of interest for schizophrenia, especially among patients with prominent auditory hallucinations and formal thought disorder.
Keywords: schizophrenia, epilepsy, temporal lobe, susceptibility, neurodevelopment, LGI genes
INTRODUCTION
The relationship between schizophrenia and epilepsy has been of interest for many years. Kraepelin (1919) noted “As in dementia praecox epileptiform seizures occur, the malady may be taken for epilepsy…” (p. 274). Gibbs and Gibbs (Gibbs, Gibbs, 1952b) reported an increased frequency of interictal psychoses in patients with complex partial seizures. They suggested that schizophrenia and epilepsy might share some common pathology of the medial temporal lobe, where epileptiform potentials that underlie complex partial seizures most often originate. In this paper we review overlapping epidemiological, clinical, neuropathological and neuroimaging features of these conditions. We then discuss the recent identification of genes that are causally related to partial epilepsy with auditory features and their potential relevance to schizophrenia research.
1. Schizophrenia and Epilepsy
1.1 Epidemiology of the Association between Schizophrenia and Epilepsy
Prevalence rates of psychoses in epilepsy vary depending on how they are defined (Hyde, Weinberger, 1997). Imprecise terminology and lack of uniform psychiatric assessment appear to be a limitation in most of the studies conducted till the 70’s. Because epilepsy is defined by recurrent seizures, postictal confusional states and postictal paranoia must be differentiated from more enduring interictal psychoses (Flor-Henry, 1972). In the literature on the association of schizophrenia and epilepsy, the term “schizophrenia-like psychosis of the epilepsy” or SLPE is usually used to indicate signs and symptoms that resemble those experienced or displayed by patients with schizophrenia. Assessment of SLPE rates is done using diagnostic systems (i.e. DSM) that do not include a distinct diagnostic category of SLPE which would acknowledge the existence of a clinical condition similar to schizophrenia but not identical to it. In this review the term schizophrenia in patients with epilepsy will be used as synonym of SLPE to indicate the presence of a clinical picture characterized by symptoms that are present also in the “idiopathic” forms of schizophrenia.
Rates of “unclassified” psychoses range from 7% to 25% in patients with complex partial seizures (Gibbs, Gibbs, 1952a; Matsuura, et al., 2003) and from 2.4% to 9.4% when both complex partial and generalized seizures are considered suggesting that generalized seizures may weaken the frequency of the association (Bruens, 1971). Prevalence rates of schizophrenia in clinically mixed epilepsy population (i.e. partial and generalized forms) vary from 0.8% to 70% using unstructured clinical interview (Slater, et al., 1963), 9.25% using DSM-III criteria (Mendez, et al., 1993), and 50% in one study of patients with epilepsy of various types, including temporal lobe epilepsy, based on Present State Examination criteria (Perez, Trimble, 1980). More recently, the prevalence of schizophrenia was found to range from 9% to 52% when criteria from the Operational Criteria Checklist for Psychotic Illness were applied (Matsuura, et al., 2004). Finally, in a study of 87 children with temporal lobe seizures who were followed for 10 years, 10% developed a schizophreniform psychosis at some point (Lindsay, et al., 1979). Epidemiologic research based on clinical samples suggests that “psychoses” occur in patients with epilepsy more frequently than expected by chance. One limitation of studies based on clinically referred samples is that unrecognized ascertainment biases can affect the observed rates of co-morbidity. Another is that different methods used to define and assess psychosis might explain the wide variation in reported rates of “unclassified” psychoses vs. schizophrenia.
Case-control studies conducted on people with epilepsy receiving disability benefits compared to people with other somatic diseases showed an excess of men in the epilepsy group with psychoses, 6.2% of the patients in the index group compared with 2.3% in the control group had a diagnosis of psychotic illness, particularly schizophrenia and paranoid states diagnosed using the ICD system (Stefansson, et al., 1998). This suggests that when psychopathology is present, psychotic illness, particularly schizophrenia-like psychosis, is more likely to be associated with epilepsy than other chronic diseases. In an epidemiological survey of epilepsy in Iceland, carried out from 1960 to the end of 1964 (Gudmundsson, 1966), 7% of 987 prevalence cases had a history of psychotic illness.
Patients with schizophrenia might be more prone to head injuries or other events that are independently associated with the development of seizures. Population-based cohort studies that exclude subjects with a psychiatric history before the development of epilepsy minimize this potential confounder. A recent study, that confirmed but extended previous data (Bredkiaer et al. 1998), used a population-based cohort that excluded people with a psychiatric history prior to seizure onset (Qin, et al., 2005; Vestergaard, et al., 2005)In this study, epilepsy was associated with significantly increased risk of schizophrenia (RR = 2.48) and schizophrenia-like psychoses (RR = 2.93). Not surprisingly, family histories of psychosis and epilepsy were risk factors for both schizophrenia (RRs = 7.57 and 1.11, respectively) and schizophrenia-like psychosis (RRs = 6.24 and 1.20, respectively). The risk of schizophrenia also increased with the number of hospital admissions for epilepsy, and it was significantly greater among persons first admitted for epilepsy after age 25. Although the risk of schizophrenia was slightly higher in persons with complex partial than other seizure types, the difference was not statistically significant. In short, this population-based study demonstrates an association between seizures and schizophrenia or schizophrenia-like psychosis, suggesting that they could share common genetic or environmental causes.
If rates of psychoses, mostly schizophrenia-like, occur in patients with epilepsy more frequently than by chance alone, the opposite is less clear. Few studies have been dedicated to answer the question if schizophrenia is a risk factor for epilepsy. One of such studies showed that the prevalence of epilepsy and acute symptomatic seizure in treated patients with a DSMIII-R diagnosis of schizophrenia and paranoid disorders (n=460; mean age 41.9, range 17–79) was not particularly increased compared to the general population (Gelisse, et al., 1999). The opposite has been reported in a 28-year follow-up study of the 1966 northern Finland general population birth cohort (Makikyro, et al., 1998). In this study, epilepsy was strongly associated with schizophrenia (OR= 11.1, 95% CI= 4.0–31.6). No firm conclusion can be drawn regarding the issue if schizophrenia is a risk factor for epilepsy based only on few studies limited also by methodological problems of their own including criteria of inclusion/exclusion. The previously reported observation made by Kraepelin that “as in dementia praecox epileptiform seizures occur, the malady may be taken for epilepsy” still needs to be confirmed with more rigorous studies. Indeed, we think that an increased prevalence of epilepsy in schizophrenia would add strength to the hypothesis of their shared susceptibility.
1.2 Clinical Characteristics of Schizophrenia-Like Psychosis of Epilepsy (SLPE)
Forty years after the clinical description of SLPE made by Slater and coll. (Slater, et al., 1963) the issue of its phenomenology and the existence of a distinct nosologic entity is still a matter of debate. In Slater’s view, the symptoms of his psychotic epileptic patients would have to be diagnosed as “schizophrenia” although, he thought, the “combination of symptoms shown by individuals differs slightly from the most usual schizophrenic pattern” (Perez, Trimble, 1980; Slater, et al., 1963). Slater described a clinical picture with an average onset of 14 years from the beginning of epilepsy and with a prevalence of hallucinations and persecutory and mystical delusions with three-quarters of the patients having a temporal lobe focus. Furthermore, he reported that affective responsiveness and personality were preserved. He observed also a decreased prevalence of schizophrenia in the families of patients with epilepsy and psychosis that he interpreted as a “proof” that SLPE was a distinct diagnostic entity from schizophrenia. Indeed Slater observed some degree of heterogeneity in his epileptic patients with psychosis resembling in this the clinical heterogeneity of schizophrenia. A small number of them presented with a predominance of hebephrenic and catatonic symptoms and with deterioration more “typical” of schizophrenia. Slater thought that SLPE was schizophrenic in form but not in “etiology” and that it occurs in individuals lacking any special predisposition (i.e. no increased family history of schizophrenia and lack of pre-morbid schizoid traits). Slater’s seminal observations and his proposal of SLPE as a “mock up” for schizophrenia have informed most of the literature that has followed. Subsequent studies, using structured assessments show findings that makes difficult in our opinion to sustain the hypothesis of a distinct nosologic entity for the SLPE with specific and recognizable symptoms or risk predictors. Clear differences in terms of symptoms between patients with SLPE and patients with schizophrenia without epilepsy have not been confirmed using structured diagnostic instruments like PSE and CATEGO though it is difficult to agree with the conclusion of the study by Toone and coll. (1982) that found in their results a reason to assign a specific diagnostic category to SLPE ((Perez, Trimble, 1980); (Toone, et al., 1982). When a separation between post-ictal and interictal periods has been attempted (see Table 1.), patients with chronic interictal psychosis have showed to have more first-rank Schneiderian symptoms and auditory hallucinations of voices commenting on their behavior than patients with post-ictal psychosis, while the latter have more grandiose and religious delusions, often with elevated mood and feelings of mystic fusion with the universe (Kanemoto, et al., 1996). Although this study needs replication, it confirms a previous one (Oyebode, Davison, 1989) on the presence of first-rank symptoms in SLPE and both studies appear to speak against a separate diagnostic entity for SLPE. Most of the studies, with the recent exception of Matsuura et al. (2004), have also found no differences on the presence of a family history of schizophrenia between the two groups (Adachi, et al., 2000; Perez, Trimble, 1980; Toone, et al., 1982). Other investigators have found deterioration for the SLPE patients to be similar to that of schizophrenia patients (Oyebode, Davison, 1989) (Kanemoto, et al., 2001) and others have also described a course of the interictal psychosis similar to the heterogeneous course of schizophrenia without epilepsy with a group showing remissions and relapses while another being chronically ill (Onuma, et al., 1991). We find of great interest for the heterogeneity of the clinical presentation of temporal lobe epilepsy a recent study that has described negative symptoms to be prominent in TLE but also that negative symptoms to be independent of age at onset of recurrent seizures, duration of epilepsy, and presence of early initial precipitating injuries as such describing a distinct subset of TLE patients.(Getz, et al., 2002). Of importance was also the finding that negative symptoms were associated with neuropsychological deficits exceeding the general cognitive morbidity associated with TLE and with brain atrophy not limited to the temporal lobe. The finding is relevant for speaks against the “organic” deterioration purported by Slater to be characteristic of SLPE as different from that of “true” schizophrenia. Indeed, low intellectual functioning has also been found to be associated with chronic interictal psychosis (Kanemoto, et al., 2001).
Table 1.
Characteristics of Schizophrenia-Like Psychosis of Epilepsy (SLPE)
| Study type & population | Findings | Reference |
|---|---|---|
| CLINICAL | ||
| Acute post-ictal (n=30) vs. acute inter-ictal (n=33) vs. chronic inter-ictal (n=25) psychosis | Chronic iner-ictal psychosis had ↑ perceptual delusions & commenting voices | (Kanemoto, et al., 1996) |
| Interictal vs. postictal psychosis | Medial TLE associated with increased frequency of SLPE; early onset of epilepsy and prolonged febrile seizures associated with increased interictal psychosis | (Kanemoto, et al., 2001) |
| Mixed epilepsy | SLPE associated with ↓ family history of SZ, more acute onset of psychosis, less deterioration from premorbid functioning and greater unemployment | (Matsuura, et al., 2004) |
| NEUROPSYCHOLOGY | ||
| SLPE (n=45) vs. non-SLPE (n=34) | ↓ WAIS IQ; ↓ performance on Stroop Test | (Kristensen, Sindrup, 1979) |
| SLPE (n=25) vs. schizophrenia(n=22) | Both groups: ↓ verbal > visual memory Similar Attention impairment | (Mellers, et al., 2000) |
| TLE without (n=12) vs. with (n=12) psychosis or schizophrenia (n=26) | TLE with psychosis ↓ performance on verbal learning and executive functions > TLE without psychois and < Schizophrenia | (Nathaniel-James, et al., 2004) |
| TLE without (n=20) vs. with (n=20) psychosis | TLE with psychosis more impaired on semantic and memory tasks | (Flugel, et al., 2006c) |
| NEUROPATHOLOGY | ||
| Temporal lobectomy (n=255) | 23% of 47 patients with hamartomas and focal dysplasia vs 5% of 41 with medial temporal sclerosis had TLE with psychosis; ♀ more likely to have TLE with psychosis | (Taylor, 1975) |
| Temporal lobectomy (n=249) | Of 16 patients with SLPE “all” had medial temporal sclerosis and 10 had lesions of embryonic origin | (Roberts, et al., 1990) |
| SLPE (n=10) vs. epilepsy (n=36) | SLPE had: ventricular enlargements, periventricular gliosis and perivascular white matter softness | (Bruton, et al., 1994) |
| NEUROIMAGING | ||
| SLPE (n=9) vs. schizophrenia(n=46) vs. TLE (n=18) vs. normal controls (n=57) | SLPE ↑ ventricles and ↓ gray matter concentration in temporal, fronto-parietal areas and superior temporal gyrus | (Marsh, et al., 2001) |
| TLE without(n=24) vs. TLE with (n=26) psychosis vs. normal controls (n=20) | No cortical gray matter differences between the two groups using voxel-based morphometry analysis | (Rusch, et al., 2004) |
| TLE without (n=24) vs. TLE with (n=26) psychosis vs. normal controls (n=20) | TLE with psychosis ↑ amygdala volume but no difference in hippocampus volume | (Tebartz Van Elst, et al., 2002) |
| TLE without (n=12) vs. with (n=12) psychosis or schizophrenia (n=26) vs. normal controls (n=38) | TLE with psychosis ↓ volume bilaterally in amygdala and hippocampus | (Maier, et al., 2000) |
| TLE without (n=20) vs. with (n=20) psychosis | No volumetric differences between the two groups using voxel-based morphometry analysis but ↓ magnetic transference ratio in left superior and middle temporal gyri | (Flugel, et al., 2006a) |
Other elements appear important to underline in the context of the clinical characterization of SLPE. The first is the interval onset of seizures to onset of psychosis. There appears to be agreement in the literature that early onset of seizures and duration of epilepsy is positively related to onset of psychosis (Adachi, et al., 2002). Although a “kindling” hypothesis has been invoked to explain the positive correlation between duration of epilepsy and onset of psychosis, the mechanism remains to be determined..
“Bimodal” psychosis that characterizes those patients who initially manifest recurrent post-ictal psychosis and later on develop chronic interictal psychosis is a model worthwhile considering in view of the not yet settled relation between acute and chronic psychosis associated with epilepsy even though a relatively small number of subjects show this type of presentation (Adachi, et al., 2002). Indeed, chronologic subcategories of psychosis associated with epilepsy maybe indispensable to evaluate the effects of seizures on psychosis. The second is the type of epilepsy related to the presence of SLPE. The majority of the studies seem to agree also on a positive relation between TLE and SLPE. The majority of the patients in the studies had either complex partial seizures or temporal epileptiform activity on EEG.
Few investigations of psychopathology among patients with SLPE due to frontal lobe epilepsy (FLE) have been reported. One study compared two groups of patients with epilepsy and interictal psychosis (FLE vs. TLE) using the Brief Psychiatric Rating Scale (Adachi, et al., 2000). In this study, the groups showed equally severe psychomotor excitement, hostility, suspiciousness, and hallucinations, but patients with FLE showed more severe emotional withdrawal and affective blunting.
In summary, the clinical status of SLPE as it comes out of 40 plus years of clinical studies shows that SLPE patients are likely a heterogeneous group of subjects with a predominance of them showing positive symptoms not distinguishable from those of schizophrenia without epilepsy. A subset with predominant negative symptoms might also exist. SLPE patients seem to have a similar family history of schizophrenia and possibly similar deterioration of their personality in comparison to schizophrenia subjects without epilepsy. They seem to have predominance of temporal lobe involvement and importantly for the pathophysiology of the psychotic manifestations the beginning of symptoms follows the start of epilepsy by at least a decade. Is it important then to maintain a distinction between SLPE and schizophrenia? It seems difficult to do it on the ground of phenomenology alone or some of the risk factors related to psychosis like the presence of a family history. We shall see, in the rest of this review, if neuropsychology, neuroimaging and genetic studies offer support to the notion that SLPE is a “mock up” for schizophrenia (Roberts, et al., 1990; Slater, et al., 1963)
1.3 Neuropsychology of SLPE
Cognitive impairment is common in epilepsy, and studies have linked cognitive deficits to clinical aspects of the illness. As in schizophrenia, there is evidence that cognitive dysfunction precedes the onset of seizures in epilepsy (Austin, Dunn, 2002). In adults with epilepsy, cognitive decline progresses slowly over decades (Dodrill, 2002). While cross-sectional comparisons of chronic epileptic patients with normal controls have documented impaired memory, language, executive function and motor speed (Martin, et al., 2005; Oyegbile, et al., 2004), deficits in verbal and visual memory appear to be most conspicuous in longitudinal studies. Overall, cognitive profiles in epilepsy are as diverse as the epileptic syndromes. Patients with focal seizures generally have deficits in the cognitive functions subserved by the affected circuits, as exemplified by memory deficits in TLE.
Though absent from the diagnostic criteria for schizophrenia listed in standard diagnostic nomenclature, cognitive deficits precede, accompany, and outlast the positive symptoms (e.g., hallucinations and delusions) of schizophrenia and their treatment. Despite limitations inherent in different approaches to identifying cognitive domains that are most impaired in schizophrenia, there is converging evidence that impairments of verbal learning and memory, psychomotor speed, and sustained attention are prominent (Nuechterlein, et al., 2004; Schretlen, et al., 2007). Attention span, perceptual discrimination, and basic linguistic abilities, such as naming and receptive vocabulary, probably are least affected by schizophrenia.
TLE is thought to share some anatomical bases and clinical features with schizophrenia. Consequently, investigators have compared the two groups not only to describe commonalities and differences but also because the cognitive impairments seen in TLE might provide model for understanding impaired cognition in schizophrenia. One such comparison found that patients with schizophrenia showed greater impairment of attention than patients with TLE, whereas patients with TLE (regardless of hemisphere affected) showed better verbal, visual and delayed memory than patients with schizophrenia (Gold, et al., 1994; Gold, et al., 1995). However, patients with schizophrenia showed better delayed recall after controlling for attention, leading these investigators to infer that the memory impairment seen in schizophrenia might stem from extra-temporal brain dysfunction, and that TLE provides a poor model of neuropsychological impairment in schizophrenia. However, the Gold et al. study did not compare TLE patients with versus without psychosis. When the neuropsychological performance of SLPE patients was compared to that of patients with schizophrenia only, patients with epilepsy only, and healthy controls, no differences were found between the SLPE and schizophrenia groups (Mellers, et al., 2000). Both the SLPE and schizophrenia groups demonstrated memory deficits, especially for verbal material, compared to epilepsy patients without psychosis and healthy controls. This study pointed to dominant temporal lobe dysfunction in schizophrenia and SLPE, along with more general cerebral dysfunction in both. Similar results were obtained in another study wherein patients with SLPE demonstrated cognitive deficits that were intermediate in severity between those of patients with schizophrenia and those of patients with TLE only on tests of verbal learning and executive functioning (Nathaniel-James, et al., 2004). These investigators concluded that the findings did not support the existence of separate nosologic entities because the profiles of patients with SLPE and schizophrenia were so similar.
1.4 Neuropathology of SLPE
Despite the many controversies (Harrison, 1999), including lack of specificity and small sample size, there are now established facts about the neuropathology of schizophrenia. The disease is associated with a number of macroscopic and histologic abnormalities that include lateral and third ventricle enlargement, and small but significant reductions in brain volume, weight and length. There is disproportionate volume loss from temporal lobe structures including the hippocampus. Entorhinal dysplasia and “disarray” of hippocampal neurons have been also reported. The pathology is neither focal or uniform, being most convincingly demonstrated in the hippocampus, prefrontal cortex and dorsal thalamus (Harrison, 1999). Although there is considerable evidence for preferential involvement of the temporal lobe, there are also negative post-mortem reports for each of these parameters and some lack diagnostic specificity.
Turning to epilepsy, Bruton et al. (Bruton, et al., 1994) reported findings that in part overlap with those observed in schizophrenia. In this retrospective study, brains from patients with SLPE were compared to brains of patients with epilepsy who were confined to a hospital and to community-dwelling patients with epilepsy. These investigators found no association of temporal lobe pathology or medial temporal sclerosis with SLPE. Nor was a history of TLE more common in SLPE, all of whom had generalized seizures with or without other types of seizures. Four features distinguished the brains of patients with SLPE from those of patients with nonpsychotic epilepsy: ventricular enlargement, periventricular gliosis, greater focal brain damage, and minute perivascular white matter softening. These authors concluded that psychoses associated with epilepsy do not result from epileptic pathology of the temporal lobe but from other changes in the brain. The study’s finding of ventricular enlargement in SLPE indeed overlaps with that in schizophrenia and could be associated with the psychosis of the epilepsy. Periventricular gliosis is notably at odds with its absence in post-mortem brain of schizophrenia patients and finally the greater focal brain damage of the SLPE may represent a coincidental finding unlikely associated with the psychosis of epilepsy.
Two other studies have examined tissue resected from patients who underwent temporal lobectomies for treatment of intractable seizures. These studies found that “alien tissue” such as hamartomas and focal dysplasias (Taylor, 1975) or medial temporal sclerosis (Roberts, et al., 1990) was more prevalent among patients with SLPE than those with nonpsychotic epilepsy. These investigators also classified lesions according to the time of their development, and found that the majority of lesions in patients with SLPE were of embryonic or perinatal origin, findings that seem to support the role of neurodevelopmental abnormalities in schizophrenia. Furthermore, the findings about the presence of “dysplastic” tissue appear to partially overlap with the findings of entorhinal cortex dysplasia in schizophrenia (Falkai, et al., 2000). The two studies nonetheless are limited, in view of their surgical nature, by the analysis of only one anatomical region (i.e. temporal lobe). In conclusion, as shown in Table 1, the neuropathology of SLPE suffers from a limited number of studies with relatively small sample size. The results of Bruton et al. (Bruton, et al., 1994) about the lack of alien tissue or medial temporal sclerosis in SLPE post-mortem brains are not easily reconciled with those from Taylor (1975) and Roberts (1990) who found opposite results. The presence of enlarged ventricles in SLPE underlines once more the importance of this finding in its association with schizophrenia. With the limitations above described, the data of the SLPE literature indicate also that the temporal lobe plays a role in mediating the association with psychosis in SLPE.
1.5 Neuroimaging of SLPE
The last 30 years have seen an explosion of neuroimaging studies in schizophrenia research from the original observation of enlarged ventricles on computed tomography scan, as a consequence of the advent of magnetic resonance imaging (MRI) techniques. Imaging studies have helped to better define the neuroanatomy of the disease and by identifying brain areas involved in the pathophysiology of schizophrenia have complemented the neuropathology findings. Published reviews (Harrison, 1999; Pearlson, Marsh, 1999) and meta-analyses (Konick, Friedman, 2001; Nelson, et al., 1998; Wright, et al., 2000) agree that relative to healthy controls, patients with schizophrenia have whole-brain volume reduction of about 3% in gray matter and increased ventricular size. The volume reduction is most notable in fronto-temporal regions especially the prefrontal cortex and superior temporal gyrus. Medial temporal structures and particularly the hippocampus and amygdala are reduced by a greater amount than the whole-brain. There is evidence that the thalamus is also reduced in volume to a greater extent than the whole-brain. Specificity of the above reported findings especially in relation to mood disorders has been questioned. As the literature on depression and bipolar disorder accrues, it seems that the neuro-anatomy of affective disorder is qualitatively similar to that in schizophrenia though less marked in quantitative terms. A central issue for SLPE is, then, whether its pathogenesis is associated with localized temporal lobe abnormalities or more generalized disruptions of brain structure.
As in the neuropathology studies, most neuroimaging studies of SLPE have been driven by the hypothesis that the co-occurrence of epilepsy and psychosis implicate temporal lobe pathology. As shown in Table 1, one notable exception include a structural magnetic resonance imaging (sMRI) study (Marsh, et al., 2001). This sMRI study compared patients with SLPE to three control groups: TLE patients without psychosis, patients with schizophrenia, and healthy adults. Results showed that structural brain abnormalities in SLPE were not restricted to the left temporal lobe. All three patient groups showed ventricular enlargement and reduced gray matter volume in the temporal lobe, frontoparietal cortex, and superior temporal gyrus, but these abnormalities were most prominent in the SLPE group. Only TLE patients without psychosis had smaller hippocampi and temporal lobe white matter deficits. The changes in cortical gray matter, including those in the frontoparietal cortex, common to the SLPE and schizophrenia groups indicated the importance of those brain regions to the pathophysiology of psychosis. Their finding of enlarged ventricles in all 3 groups of patients studied (SLPE, simple TLE and schizophrenia) contrasts with the post-mortem study by Bruton et al. (Bruton, et al., 1994), who found enlarged ventricles only in the SLPE group. This is interesting because of the importance attributed to enlarged ventricles to explain the neurodevelopmental hypothesis of schizophrenia. Neuronal migration defects have been proposed as a mechanism related to enlarged ventricles, and this defect could be common to both schizophrenia and epilepsy, perhaps representing another shared susceptibility. Indeed, disorders of neuronal migration like lissencephaly, are associated with epileptic seizures (Dobyns, et al., 1985) and lissencephaly has been thought as a molecular model for schizophrenia (Ayala, et al., 2007). Also interesting in this regard is the finding that enlarged ventricles are common to first episode of schizophrenia and TLE subjects without psychosis (Barr, et al., 1997). Most other neuroimaging studies have focused on temporal lobe abnormalities in SLPE (Flugel, et al., 2006a; Flugel, et al., 2006b; Guarnieri, et al., 2005; Maier, et al., 2000; Rusch, et al., 2004; Tebartz Van Elst, et al., 2002), albeit with contrasting findings. Using automated voxel-based morphometric approaches, two studies found no cortical gray matter abnormalities in TLE patients with psychosis compared to TLE patients without psychosis and healthy controls (Flugel, et al., 2006a; Rusch, et al., 2004). The findings clearly contrast with those obtained in schizophrenia using the same technique and reported in a recent meta-analysis that shows the most consistent volume reduction in the left superior temporal gyrus and middle temporal lobe (Honea, et al., 2005). Conversely, TLE patients with psychosis showed decreased total brain volume together with enlarged amygdala bilaterally but not changes in the hippocampus compared to simple TLE patients and healthy controls using a region of interest analysis approach (Tebartz Van Elst, et al., 2002), prompting these investigators to conclude that the pathophysiology of SLPE in TLE is distinctly different from that of schizophrenia because the intact hippocampus volume clearly contrasted with established volume reduction found in schizophrenia. However, another study (Maier, et al., 2000) found bilaterally reduced hippocampus/amygdala volumes in TLE patients with psychosis compared to patients with simple TLE or chronic schizophrenia where the reduced volume was observed only on the left side.
In summary, these studies appear to be adequately powered to identify statistically significant differences between groups. Nonetheless, they are biased toward the hypothesis that SLPE is related to temporal lobe pathology, which limits the comparison with findings obtained in schizophrenia. The contradictory results about the hippocampus/amygdala size, important brain areas for the understanding of the pathophysiology of schizophrenia, in the studies by Tebartz Van Elst et al. (Tebartz Van Elst, et al., 2002) and Maier et al. (Maier, et al., 2000), are difficult to reconcile given similar characteristics of the SLPE subjects in both studies. The results of the imaging studies raise the possibility that SLPE stems from a combination of temporal lobe pathology and more extensive generalized brain abnormalities as the study by Marsh et al. (2001) seems to suggest. In this regard, brain volume abnormalities, found in SLPE and schizophrenia, could reflect alterations that predispose to epileptogenesis especially in medial temporal regions and to schizophrenia, with the added contribution of extra-temporal and subcortical regions. Alternatively, temporal lobe pathology, stressed in the majority of the imaging studies, could be viewed as important to the genesis of positive symptoms of schizophrenia. Analogously several studies of schizophrenia relate severity of positive symptoms to left superior temporal gyrus volume changes. Finally, the presence of ventricular enlargement in both conditions might indicate a common neurodevelopmental mechanism that predisposes to epileptogenesis and schizophrenia.
2. Do Schizophrenia and Epilepsy Share A Common Genetic Susceptibility?
Genetic aspects of SLPE have been generally ignored since Slater and coll. (1963) reported no correlation on the basis of a negative family history. As we have reported in previous sections of this review there is evidence in the epidemiologic and clinical literature to support a role for genetic factors in the development of SLPE.
Like cancer and diabetes, schizophrenia is a complex genetic disease not characterized by a single causative gene and not showing simple patterns of inheritance. The genetic architecture of schizophrenia susceptibility is almost certainly heterogeneous, meaning that no particular constellation of genes will characterize most ill individuals. Genetic linkage and association studies have recently revealed several candidate susceptibility genes for schizophrenia. These include regulator of G-protein signaling-4 (RGS4), CAPON, Disrupted-In-Schizophrenia-1 (DISC1), Dysbindin, Neuregulin-1, D-amino acid oxidase (DAAO), G72, and catechol-o-methyltransferase (COMT) (Harrison, Weinberger, 2005; Owen, et al., 2005). Very interestingly, most of the gene products have either functions associated with neurotransmission or those involved in neurodevelopment. Indeed, a neurodevelopmental hypothesis of schizophrenia is currently the most accepted model to explain pathogenesis and pathophysiology of the disease (Crow, et al., 1989).
Abnormalities in neurodevelopment including those that affect neuronal migration have also been related to epilepsy. Gross disorders of neuronal migration are invariably associated with the presence of early onset of seizure disorder like in lyssencephaly (Dobyns, et al., 1984). The clinical picture of lyssencephaly though lacks symptoms of schizophrenia and this critical aspect would make the case for considering abnormal neurodevelopment as a shared diathesis for the common occurrence of epilepsy and schizophrenia less strong. Nonetheless there is evidence that lissencephaly gene expression is abnormal in brains of patients with schizophrenia (Lipska, et al., 2006). Intriguingly, LIS1, the causal gene product for lissencephaly 1, has been found to interact with a number of proteins including DISC1 within the neuron and their interaction has been thought to regulate axonal elongation during central nervous system development (Taya, et al., 2007). On the other side, focal migration disorders like focal heterotopias have also been associated with epileptic seizures (Flint, Kriegstein, 1997). Indeed, we have previously reported in the Neuropathology section of this review that the presence of focal dysplasia was found to be more prevalent in patients with SLPE (Taylor, 1975) and that those findings seem to support the role of neurodevelopmental abnormalities in schizophrenia. Furthermore, the case of temporal lobe epilepsy with auditory hallucinations, whose genetic underpinning has been recently elucidated (Kalachikov, et al., 2002; Morante-Redolat, et al., 2002) and whose altered protein is likely to play a role in the pathogenesis of epilepsy by interfering with axon guidance and/or synaptogenesis (Scheel, et al., 2002) is particularly worthwhile considering in the context of an abnormal neurodevelopmental hypothesis for the common occurrence of epilepsy and schizophrenia. We shall discuss here this type of TLE with auditory hallucinations in more detail because, as we propose, it might help us better understand the pathophysiology of schizophrenia with prominent positive symptoms such as hallucinations and thought disorders.
Autosomal dominant partial epilepsy with auditory features (ADPEAF) is a benign and rare form of epilepsy characterized by auditory auras that in part resemble the auditory hallucinations experienced by patients with schizophrenia (Winawer, et al., 2000). The auditory hallucinations usually range from elementary sounds to fully formed voices talking to patients (Winawer, et al., 2000). Leucine-rich glioma inactivated 1 (LGI1), a member of the LGI/epitempin family that includes LGI2, 3 and 4 and a similarly structured protein thrombospondinepitempin1 (TNEP1), has been shown to be mutated in glioma and autosomal dominant lateral temporal epilepsy (Kalachikov, et al., 2002). Initially mapped to a 10 cM region on chromosome 10q24 in a single extended pedigree, linkage was subsequently reported narrowing the minimal genetic region to 3cM. Finally, presumptive mutations were identified in all tested affected individuals and the obligate carriers (Kalachikov, et al., 2002). The expression pattern of the LGI1 protein, in the mouse, is predominantly neuronal and is consistent with the anatomic regions involved in temporal lobe epilepsy (Kalachikov, et al., 2002). In humans, LGI1 is expressed primarily in brain (cerebellum, cortex, medulla, occipital pole, frontal lobe, temporal lobe and putamen) and at lower level in the spinal cord (Chernova, et al., 1998). Recent data show that LGI1 is secreted and its N-terminal leucine-rich repeat region is structurally similar to Slit proteins which are involved in axonal path finding (Senechal, et al., 2005). Thus, LGI1 might also function as a developmental morphogen or provide migration cues in the developing central nervous system (Senechal, et al., 2005). An involvement in neurodevelopment for LGI1 is also suggested by the role of its C-terminal EAR (epilepsy associated repeat) region (Scheel, et al., 2002). The known portion of the human genome encodes six EAR proteins, some of which map to chromosome regions associated with seizure disorders, including the very large G-protein coupled receptor or VLGR1 with its fragment monogenic audiogenic seizure-susceptible or MASS1 related to the febrile seizures in humans (Scheel, et al., 2002) and considered a risk factor in schizophrenia as reported in the Epidemiology section of this review. A number of observations provide circumstantial evidence for a role of EAR proteins in neural development. Firstly, the message of VLGR1 is most prominent in the developing nervous system, with very little expression found in adult tissues (McMillan, et al., 2002). Secondly, the overall VLGR1 architecture shows considerable resemblance to other very long G-protein-coupled receptors, including the flamingo family, the latrophilins and the brain angiogenesis inhibitors. A common feature of those proteins is a role in neural development. Finally, two proteins predicted from the Drosophila genome show a distant sequence relationship to singular EAR copies, which do not appear to cluster into groups of seven. While the functional significance of this similarity is not clear, one of those Drosophila proteins (CG11411) was recently found in a screen for axon guidance and synaptogenesis mutants (Kraut, et al., 2001) providing a further link between EAR proteins and developmental processes. It is conceivable that an increase in excitatory synapses, or a lack of functional inhibitory synapses, can also contribute to seizure disorders. To this regard a receptor for LGI1 has been very recently identified in ADAM22, a transmembrane protein that when mutated causes itself seizures (Fukata, et al., 2006). Furthermore, the LGI1/ADAM22 complex is scaffolded by post-synaptic domain 95 or PSD-95 that plays a critical role in synaptogenesis and synaptic plasticity. Many of the schizophrenia susceptibility factors interact with PSD-95 (Hashimoto, et al., 2007). LGI1 applied to hippocampal slices significantly increases the synaptic AMPA/NMDA ratio influencing glutamate transmission by increasing AMPA receptor surface expression (Fukata, et al., 2006).
Although mutations of the LGI2, 3 and 4 genes have not been described yet in relation to their neurological phenotypes, the distributions of the proteins in mammals and their potential function have been recently reported (Senechal, et al., 2005). Their distribution in mouse brain partially overlaps (Senechal, et al., 2005), though some authors (Lee, et al., 2006) consider LGI3 expression to occur mostly in the brain of the adult mouse with a distribution that include olfactory bulb, cerebellum and occipital cortex suggesting for LGI3 a distinct role in neural development, plasticity and synaptic regulation than LGI1.
In summary, genes (i.e. LGI1) not directly coding for ionic channels have been for the first time linked to a phenotype characterized by auditory hallucinations related to the epileptic discharge of neurons mostly from the left temporal lobe. Although the function of the LGI family of proteins remain to be fully understood, it appears that they may be involved in crucial aspects of CNS development raising the possibility that some forms of epilepsy might be neurodevelopmentally based. It is important to also note the role that LGI1 might play in regulating glutamatergic synaptic transmission (Fukata, et al., 2006), a process that is involved in the pathophysiology of schizophrenia.
2.1 Genetics
Human LGI genes have been mapped, as showed in Table 2, to chromosomal loci that have previously been identified to be linked or associated with schizophrenia (Blouin, et al., 1998; Christoforou, et al., 2007; Fallin, et al., 2003; Lerer, et al., 2003; Lewis, et al., 2003; Liu, et al., 2005; Macgregor, et al., 2004; Murtagh, et al., 2005). The overlap in loci raises the hypothesis that LGI genes might be new genes of interest for schizophrenia. It is conceivable that a gene like LGI1 located on 10q24 with its implied role in neurodevelopment and regulation of glutamate transmission might be implicated in the phenotypic manifestations of schizophrenia with predominance of auditory hallucinations given the relation between LGI1 mutations and partial temporal lobe epilepsy with auditory features. The role of temporal lobe structures in the pathophysiology of schizophrenia has been previously described either in relation to reversal of physiologic asymmetries as well as hallucinations, disorder of language, and thinking (Barta, et al., 1990). LGI1 mutation has also been associated with speech-induced aphasic seizures (Brodtkorb, et al., 2005), and although speculative at this stage, the finding may indicate that the LGI1 gene may have a physiologic function connected to the human capacity for speech and language. The search for mutations in the LGI family of genes in schizophrenia might call for a selection of patients based on the predominance of hallucinations and or thought disorder given that in the past replication of linkage or association studies in schizophrenia has been hampered by the heterogeneity of the illness.
Table 2.
Overlapping Loci of interest between LGI gene family and Schizophrenia
| Gene | Loci for LGI Genes | Schizophrenia Loci | References |
|---|---|---|---|
| LGI1 | 10q24 | 10q22-24, 10q21-24 | (Fallin, et al., 2003; Lerer, et al., 2003) |
| LGIL2 | 4p15.2 | 4p15-16 | (Christoforou, et al., 2007; Lerer, et al., 2003) |
| LGIL3 | 19q13.11(D19S220) | 19q (D19S224) | (Macgregor, et al., 2004) |
| LGIL4 | 8p21-22 | 8p21-22 | (Blouin, et al., 1998; Lewis, et al., 2003) |
| TNEP1 | 21q22.3 | 21q22.3 | (Liu, et al., 2005; Murtagh, et al., 2005) |
3. Concluding Remarks
The literature on the imaging and genetics of schizophrenia and epilepsy suggests that neurodevelopment and its abnormalities might represent an organizing framework to understand the co-occurrence of epilepsy and schizophrenia. Specific forms of epilepsy, whose genetic underpinning have been above described, and in particular ADPEAF, can lead the way to a novel understanding of the molecular pathology of positive symptoms of schizophrenia. The old hypothesis that epilepsy with chronic psychotic symptoms might represent a model for understanding the pathophysiology of schizophrenia still retains its validity today and can help shed light on some causes of a yet obscure disease like schizophrenia.
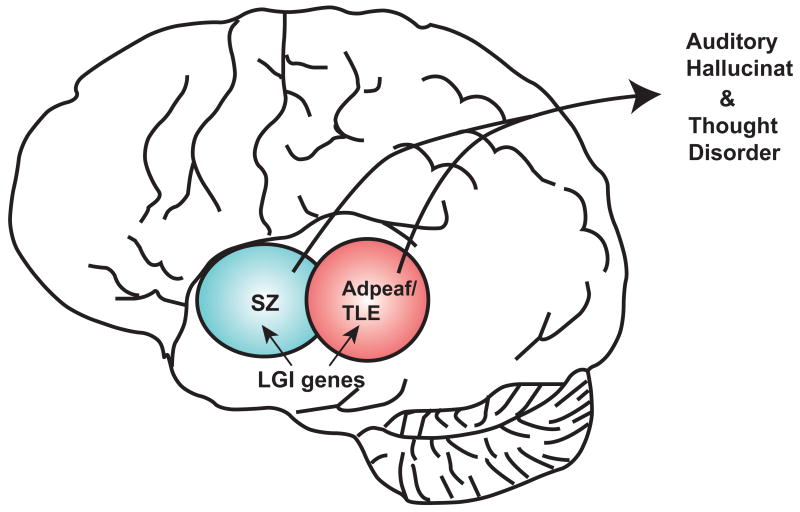
Figure 1.
Area of overlap between schizophrenia (SZ) and autosomal dominant epilepsy with auditory features (ADPEAF) for the LGI gene family.
Acknowledgments
We thank Yukiko Lema for preparation of the figures and manuscript. This work was supported by grants from U.S. Public Health Service Grant MH-69853 (AS), and MH-071473 (NC), as well as foundation grants from Stanley (AS), NARSAD (AS, DS), and S-R (AS).
FINANCIAL DISCLOSURES
The authors have no financial disclosure to make in relation to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi N, Onuma T, Nishiwaki S, Murauchi S, Akanuma N, Ishida S, Takei N. Inter-ictal and post-ictal psychoses in frontal lobe epilepsy: a retrospective comparison with psychoses in temporal lobe epilepsy. Seizure. 2000;9:328–335. doi: 10.1053/seiz.2000.0413. [DOI] [PubMed] [Google Scholar]
- Adachi N, Matsuura M, Hara T, Oana Y, Okubo Y, Kato M, Onuma T. Psychoses and epilepsy: are interictal and postictal psychoses distinct clinical entities? Epilepsia. 2002;43:1574–1582. doi: 10.1046/j.1528-1157.2002.22402.x. [DOI] [PubMed] [Google Scholar]
- Austin JK, Dunn DW. Progressive behavioral changes in children with epilepsy. Prog Brain Res. 2002;135:419–427. doi: 10.1016/S0079-6123(02)35039-8. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Barr WB, Ashtari M, Bilder RM, Degreef G, Lieberman JA. Brain morphometric comparison of first-episode schizophrenia and temporal lobe epilepsy. Br J Psychiatry. 1997;170:515–519. doi: 10.1192/bjp.170.6.515. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. The American journal of psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ullrich G, McGrath J, Kasch L, Lamacz M, Thomas MG, Gehrig C, Radhakrishna U, Snyder SE, Balk KG, Neufeld K, Swartz KL, DeMarchi N, Papadimitriou GN, Dikeos DG, Stefanis CN, Chakravarti A, Childs B, Housman DE, Kazazian HH, Antonarakis S, Pulver AE. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nature genetics. 1998;20:70–73. doi: 10.1038/1734. [DOI] [PubMed] [Google Scholar]
- Brodtkorb E, Michler RP, Gu W, Steinlein OK. Speech-induced aphasic seizures in epilepsy caused by LGI1 mutation. Epilepsia. 2005;46:963–966. doi: 10.1111/j.1528-1167.2005.47104.x. [DOI] [PubMed] [Google Scholar]
- Bruens JH. Psychoses in epilepsy. Psychiatria, neurologia, neurochirurgia. 1971;74:175–192. [PubMed] [Google Scholar]
- Bruton CJ, Stevens JR, Frith CD. Epilepsy, psychosis, and schizophrenia: clinical and neuropathologic correlations. Neurology. 1994;44:34–42. doi: 10.1212/wnl.44.1.34. [DOI] [PubMed] [Google Scholar]
- Chernova OB, Somerville RP, Cowell JK. A novel gene, LGI1, from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene. 1998;17:2873–2881. doi: 10.1038/sj.onc.1202481. [DOI] [PubMed] [Google Scholar]
- Christoforou A, Le Hellard S, Thomson PA, Morris SW, Tenesa A, Pickard BS, Wray NR, Muir WJ, Blackwood DH, Porteous DJ, Evans KL. Association analysis of the chromosome 4p15-p16 candidate region for bipolar disorder and schizophrenia. Molecular psychiatry. 2007;12:1011–1025. doi: 10.1038/sj.mp.4002003. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Archives of general psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Stratton RF, Greenberg F. Syndromes with lissencephaly. I: Miller-Dieker and Norman-Roberts syndromes and isolated lissencephaly. Am J Med Genet. 1984;18:509–526. doi: 10.1002/ajmg.1320180320. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Gilbert EF, Opitz JM. Further comments on the lissencephaly syndromes. Am J Med Genet. 1985;22:197–211. doi: 10.1002/ajmg.1320220119. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Progressive cognitive decline in adolescents and adults with epilepsy. Prog Brain Res. 2002;135:399–407. doi: 10.1016/S0079-6123(02)35037-4. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schneider-Axmann T, Honer WG. Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biological psychiatry. 2000;47:937–943. doi: 10.1016/s0006-3223(99)00250-4. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, Liang KY, Pulver AE. Genomewide linkage scan for schizophrenia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 10q22. American journal of human genetics. 2003;73:601–611. doi: 10.1086/378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Kriegstein AR. Mechanisms underlying neuronal migration disorders and epilepsy. Current opinion in neurology. 1997;10:92–97. doi: 10.1097/00019052-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Ictal and interictal psychiatric manifestations in epilepsy: specific or non-specific? A critical review of some of the evidence. Epilepsia. 1972;13:773–783. doi: 10.1111/j.1528-1157.1972.tb05162.x. [DOI] [PubMed] [Google Scholar]
- Flugel D, Cercignani M, Symms MR, Koepp MJ, Foong J. A magnetization transfer imaging study in patients with temporal lobe epilepsy and interictal psychosis. Biol Psychiatry. 2006a;59:560–567. doi: 10.1016/j.biopsych.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Flugel D, Cercignani M, Symms MR, O’Toole A, Thompson PJ, Koepp MJ, Foong J. Diffusion tensor imaging findings and their correlation with neuropsychological deficits in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia. 2006b;47:941–944. doi: 10.1111/j.1528-1167.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Flugel D, O’Toole A, Thompson PJ, Koepp MJ, Cercignani M, Symms MR, Foong J. A neuropsychological study of patients with temporal lobe epilepsy and chronic interictal psychosis. Epilepsy Res. 2006c;71:117–128. doi: 10.1016/j.eplepsyres.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313:1792–1795. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- Gelisse P, Samuelian JC, Genton P. Is schizophrenia a risk factor for epilepsy or acute symptomatic seizures? Epilepsia. 1999;40:1566–1571. doi: 10.1111/j.1528-1157.1999.tb02041.x. [DOI] [PubMed] [Google Scholar]
- Getz K, Hermann B, Seidenberg M, Bell B, Dow C, Jones J, Woodard A, Rutecki P, Sheth R, O’Leary D, Magnotta V. Negative symptoms in temporal lobe epilepsy. Am J Psychiatry. 2002;159:644–651. doi: 10.1176/appi.ajp.159.4.644. [DOI] [PubMed] [Google Scholar]
- Gibbs FA, Gibbs EL. Atlas of electroencephalography. Addison-Wesley Press; Cambridge, MA: 1952a. [Google Scholar]
- Gibbs FA, Gibbs EL. Atlas of electroencephalography. Addison-Wesley Press; Cambridge, Mass: 1952b. pp. 167–169. [Google Scholar]
- Gold JM, Hermann BP, Randolph C, Wyler AR, Goldberg TE, Weinberger DR. Schizophrenia and temporal lobe epilepsy. A neuropsychological analysis. Arch Gen Psychiatry. 1994;51:265–272. doi: 10.1001/archpsyc.1994.03950040009001. [DOI] [PubMed] [Google Scholar]
- Gold JM, Blaxton TA, Hermann BP, Randolph C, Fedio P, Goldberg TE, Theodore WH, Weinberger DR. Memory and intelligence in lateralized temporal lobe epilepsy and schizophrenia. Schizophr Res. 1995;17:59–65. doi: 10.1016/0920-9964(95)00030-p. [DOI] [PubMed] [Google Scholar]
- Guarnieri R, Wichert-Ana L, Hallak JE, Velasco TR, Walz R, Kato M, Alexandre V, Jr, Terra-Bustamante VC, Bianchin MM, Zuardi AW, Deakin JF, Sakamoto AC. Interictal SPECT in patients with mesial temporal lobe epilepsy and psychosis: a case-control study. Psychiatry Res. 2005;138:75–84. doi: 10.1016/j.pscychresns.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G. Epilepsy in Iceland. A clinical and epidemiological investigation. Acta Neurol Scand. 1966;43 Suppl 25:21–124. [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Tankou S, Takeda M, Sawa A. Postsynaptic density: a key convergent site for schizophrenia susceptibility factors and possible target for drug development. Drugs Today (Barc) 2007;43:645–654. doi: 10.1358/dot.2007.43.9.1088821. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23:611–622. doi: 10.1093/schbul/23.4.611. [DOI] [PubMed] [Google Scholar]
- Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, Choi C, Morozov P, Das K, Teplitskaya E, Yu A, Cayanis E, Penchaszadeh G, Kottmann AH, Pedley TA, Hauser WA, Ottman R, Gilliam TC. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto K, Kawasaki J, Kawai I. Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilepsia. 1996;37:551–556. doi: 10.1111/j.1528-1157.1996.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Kanemoto K, Tsuji T, Kawasaki J. Reexamination of interictal psychoses based on DSM IV psychosis classification and international epilepsy classification. Epilepsia. 2001;42:98–103. doi: 10.1046/j.1528-1157.2001.09000.x. [DOI] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox, Dementia praecox and paraphrenia. E. & S. Livingstone; Edinburgh: 1919. pp. 181–184. [Google Scholar]
- Kraut R, Menon K, Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol. 2001;11:417–430. doi: 10.1016/s0960-9822(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Kristensen O, Sindrup EH. Psychomotor epilepsy and psychosis. III Social and psychological correlates. Acta Neurol Scand. 1979;59:1–9. [PubMed] [Google Scholar]
- Lee SE, Lee AY, Park WJ, Jun DH, Kwon NS, Baek KJ, Kim YG, Yun HY. Mouse LGI3 gene: expression in brain and promoter analysis. Gene. 2006;372:8–17. doi: 10.1016/j.gene.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lerer B, Segman RH, Hamdan A, Kanyas K, Karni O, Kohn Y, Korner M, Lanktree M, Kaadan M, Turetsky N, Yakir A, Kerem B, Macciardi F. Genome scan of Arab Israeli families maps a schizophrenia susceptibility gene to chromosome 6q23 and supports a locus at chromosome 10q24. Molecular psychiatry. 2003;8:488–498. doi: 10.1038/sj.mp.4001322. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. American journal of human genetics. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Ounsted C, Richards P. Long-term outcome in children with temporal lobe seizures. III: Psychiatric aspects in childhood and adult life. Dev Med Child Neurol. 1979;21:630–636. doi: 10.1111/j.1469-8749.1979.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Mitkus SN, Mathew SV, Fatula R, Hyde TM, Weinberger DR, Kleinman JE. Functional genomics in postmortem human brain: abnormalities in a DISC1 molecular pathway in schizophrenia. Dialogues Clin Neurosci. 2006;8:353–357. doi: 10.31887/DCNS.2006.8.3/blipska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shi Y, Tang J, Guo T, Li X, Yang Y, Chen Q, Zhao X, He G, Feng G, Gu N, Zhu S, Liu H, He L. SNPs and haplotypes in the S100B gene reveal association with schizophrenia. Biochemical and biophysical research communications. 2005;328:335–341. doi: 10.1016/j.bbrc.2004.12.175. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Visscher PM, Knott SA, Thomson P, Porteous DJ, Millar JK, Devon RS, Blackwood D, Muir WJ. A genome scan and follow-up study identify a bipolar disorder susceptibility locus on chromosome 1q42. Molecular psychiatry. 2004;9:1083–1090. doi: 10.1038/sj.mp.4001544. [DOI] [PubMed] [Google Scholar]
- Maier M, Mellers J, Toone B, Trimble M, Ron MA. Schizophrenia, temporal lobe epilepsy and psychosis: an in vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol Med. 2000;30:571–581. doi: 10.1017/s0033291799001993. [DOI] [PubMed] [Google Scholar]
- Makikyro T, Karvonen JT, Hakko H, Nieminen P, Joukamaa M, Isohanni M, Jones P, Jarvelin MR. Comorbidity of hospital-treated psychiatric and physical disorders with special reference to schizophrenia: a 28 year follow-up of the 1966 northern Finland general population birth cohort. Public Health. 1998;112:221–228. doi: 10.1038/sj.ph.1900455. [DOI] [PubMed] [Google Scholar]
- Marsh L, Sullivan EV, Morrell M, Lim KO, Pfefferbaum A. Structural brain abnormalities in patients with schizophrenia, epilepsy, and epilepsy with chronic interictal psychosis. Psychiatry Res. 2001;108:1–15. doi: 10.1016/s0925-4927(01)00115-9. [DOI] [PubMed] [Google Scholar]
- Martin RC, Griffith HR, Faught E, Gilliam F, Mackey M, Vogtle L. Cognitive functioning in community dwelling older adults with chronic partial epilepsy. Epilepsia. 2005;46:298–303. doi: 10.1111/j.0013-9580.2005.02104.x. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Adachi N, Oana Y, Okubo Y, Kato M, Nakano T, Takei N. A polydiagnostic and dimensional comparison of epileptic psychoses and schizophrenia spectrum disorders. Schizophr Res. 2004;69:189–201. doi: 10.1016/s0920-9964(02)00492-9. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Oana Y, Kato M, Kawana A, Kan R, Kubota H, Nakano T, Hara T, Horikawa N. A multicenter study on the prevalence of psychiatric disorders among new referrals for epilepsy in Japan. Epilepsia. 2003;44:107–114. doi: 10.1046/j.1528-1157.2003.25202.x. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Kayes-Wandover KM, Richardson JA, White PC. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. The Journal of biological chemistry. 2002;277:785–792. doi: 10.1074/jbc.M108929200. [DOI] [PubMed] [Google Scholar]
- Mellers JD, Toone BK, Lishman WA. A neuropsychological comparison of schizophrenia and schizophrenia-like psychosis of epilepsy. Psychol Med. 2000;30:325–335. doi: 10.1017/s0033291799001786. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Grau R, Doss RC, Taylor JL. Schizophrenia in epilepsy: seizure and psychosis variables. Neurology. 1993;43:1073–1077. doi: 10.1212/wnl.43.6.1073. [DOI] [PubMed] [Google Scholar]
- Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, Saenz A, Poza JJ, Galan J, Gesk S, Sarafidou T, Mautner VF, Binelli S, Staub E, Hinzmann B, French L, Prud’homme JF, Passarelli D, Scannapieco P, Tassinari CA, Avanzini G, Marti-Masso JF, Kluwe L, Deloukas P, Moschonas NK, Michelucci R, Siebert R, Nobile C, Perez-Tur J, Lopez de Munain A. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–1128. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- Murtagh A, McTigue O, Ramsay L, Hegarty AM, Green AJ, Stallings RL, Corvin A. Interstitial deletion of chromosome 21q and schizophrenia susceptibility. Schizophrenia research. 2005;78:353–356. doi: 10.1016/j.schres.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James DA, Brown RG, Maier M, Mellers J, Toone B, Ron MA. Cognitive abnormalities in schizophrenia and schizophrenia-like psychosis of epilepsy. J Neuropsychiatry Clin Neurosci. 2004;16:472–479. doi: 10.1176/jnp.16.4.472. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Onuma T, Adachi N, Hisano T, Uesugi S. 10-year follow-up study of epilepsy with psychosis. Jpn J Psychiatry Neurol. 1991;45:360–361. doi: 10.1111/j.1440-1819.1991.tb02488.x. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O’Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Oyebode F, Davison K. Epileptic schizophrenia: clinical features and outcome. Acta Psychiatr Scand. 1989;79:327–331. doi: 10.1111/j.1600-0447.1989.tb10266.x. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Marsh L. Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry. 1999;46:627–649. doi: 10.1016/s0006-3223(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Perez MM, Trimble MR. Epileptic psychosis--diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245–249. doi: 10.1192/bjp.137.3.245. [DOI] [PubMed] [Google Scholar]
- Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Done DJ, Bruton C, Crow TJ. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biological psychiatry. 1990;28:127–143. doi: 10.1016/0006-3223(90)90630-k. [DOI] [PubMed] [Google Scholar]
- Rusch N, Tebartz van Elst L, Baeumer D, Ebert D, Trimble MR. Absence of cortical gray matter abnormalities in psychosis of epilepsy: a voxel-based MRI study in patients with temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci. 2004;16:148–155. doi: 10.1176/jnp.16.2.148. [DOI] [PubMed] [Google Scholar]
- Scheel H, Tomiuk S, Hofmann K. A common protein interaction domain links two recently identified epilepsy genes. Human molecular genetics. 2002;11:1757–1762. doi: 10.1093/hmg/11.15.1757. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, Dickerson FB, Yolken RH, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senechal KR, Thaller C, Noebels JL. ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum Mol Genet. 2005;14:1613–1620. doi: 10.1093/hmg/ddi169. [DOI] [PubMed] [Google Scholar]
- Slater E, Beard AW, Glithero E. The schizophrenialike psychoses of epilepsy. Br J Psychiatry. 1963;109:95–150. doi: 10.1192/bjp.109.458.95. [DOI] [PubMed] [Google Scholar]
- Stefansson SB, Olafsson E, Hauser WA. Psychiatric morbidity in epilepsy: a case controlled study of adults receiving disability benefits. J Neurol Neurosurg Psychiatry. 1998;64:238–241. doi: 10.1136/jnnp.64.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci. 2007;27:15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC. Factors influencing the occurrence of schizophrenia-like psychosis in patients with temporal lobe epilepsy. Psychol Med. 1975;5:249–254. doi: 10.1017/s0033291700056609. [DOI] [PubMed] [Google Scholar]
- Tebartz Van Elst L, Baeumer D, Lemieux L, Woermann FG, Koepp M, Krishnamoorthy S, Thompson PJ, Ebert D, Trimble MR. Amygdala pathology in psychosis of epilepsy: A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125:140–149. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- Toone BK, Garralda ME, Ron MA. The psychoses of epilepsy and the functional psychoses: a clinical and phenomenological comparison. Br J Psychiatry. 1982;141:256–261. doi: 10.1192/bjp.141.3.256. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Pedersen CB, Christensen J, Madsen KM, Olsen J, Mortensen PB. Febrile seizures and risk of schizophrenia. Schizophr Res. 2005;73:343–349. doi: 10.1016/j.schres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Winawer MR, Ottman R, Hauser WA, Pedley TA. Autosomal dominant partial epilepsy with auditory features: defining the phenotype. Neurology. 2000;54:2173–2176. doi: 10.1212/wnl.54.11.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]