Abstract
Background
Instability of breathing control due to heart failure (HF) manifests as exercise oscillatory ventilation (EOV). Prior descriptions of patients with EOV have not been controlled and have been limited to subjects with left ventricular ejection fraction (LVEF) of ≤ 0.40. The aim of this study was to compare clinical characteristics including ventilatory responses of subjects with EOV to those of control subjects with HF matched for LVEF.
Methods
Subjects (n = 47) were retrospectively identified from 1,340 consecutive patients referred for cardiopulmonary exercise testing. Study inclusion required EOV without consideration of LVEF while control subjects (n = 47) were composed of HF patients with no EOV matched for LVEF. Characteristics for each group were summarized and compared.
Results
For EOV subjects, the mean LVEF was 0.37 (range, 0.11 to 0.70), and 19 subjects (41%) had an LVEF of ≥ 0.40. Compared to control subjects, EOV subjects had increased left atrial dimension, mitral E-wave velocity, and right heart pressures as well as decreased exercise tidal volume response, functional capacity, rest and exercise end-tidal carbon dioxide, and increased ventilatory equivalent for carbon dioxide and dead space ventilation (all p < 0.05). Multivariate analysis demonstrated atrial fibrillation (odds ratio, 6.7; p = 0.006), digitalis therapy (odds ratio, 0.27; p = 0.02), New York Heart Association class (odds ratio, 3.5; p = 0.0006), rest end-tidal carbon dioxide (odds ratio, 0.87; p = 0.005), and peak heart rate (odds ratio, 0.98; p = 0.02) were independently associated with EOV.
Conclusions
Patients with EOV have clinical characteristics and exercise ventilatory responses consistent with more advanced HF than patients with comparable LV systolic function; EOV may occur in HF patients with an LVEF of ≥ 0.40.
Keywords: exercise, heart failure, ventilation
Instability of ventilatory control is frequent in patients with heart failure (HF) and may manifest as exercise oscillatory ventilation (EOV).1–3 EOV detected by cardiopulmonary exercise testing is characterized by the regular alteration of tidal volume (Vt) with a crescendo-decrescendo pattern without interposed apnea, which distinguishes it from other forms of periodic breathing observed in HF patients including Cheyne-Stokes respiration4–6 and central sleep apnea.1,7–10 The frequency of EOV has been reported to range from 12 to 30% of ambulatory HF patients managed at tertiary centers.2,3 While EOV may be common in patients with advanced HF, it is seldom recognized or reported. Moreover, routine detection may be a worthwhile goal as EOV is associated with advanced HF and may resolve with therapeutic intervention.11–15
Prior reports1–3,6,16,17 of EOV have been limited to descriptions of patients with left ventricular ejection fraction (LVEF) of ≤ 0.40 with associated findings that included severely decreased LVEF, advanced New York Heart Association (NYHA) class, decreased functional capacity, and adverse prognosis. However, these prior studies did not utilize control subjects matched for LVEF in order to facilitate the identification of other clinical characteristics that might discriminate individuals with EOV from HF patients with stable ventilatory control. Accordingly, the aim of this study was to evaluate clinical characteristics, including ventilatory responses of consecutive, unselected ambulatory patients with EOV for comparison with control subjects with HF and no EOV matched for LVEF.
Materials and Methods
The study was approved by the Mayo Clinic College of Medicine Institutional Review Board. All subjects provided informed consent.
Subject Selection
Subjects were retrospectively identified from 1,340 consecutive ambulatory patients without inducible ischemia who had been referred for the evaluation of dyspnea, exertional fatigue, or functional capacity by cardiopulmonary exercise testing and included 626 patients with a diagnosis of HF. Study inclusion required EOV detected by cardiopulmonary exercise testing, defined as a ≥ 25% variation of the amplitude of minute ventilation (V̇e)18 persisting for ≥ 60% of exercise duration (Fig 1).3 The amplitude of oscillatory ventilation was defined as (peak V̇e – nadir V̇e/mean V̇e) × 100 and evaluated within single oscillatory cycles at 50% peak and peak exercise.18 Control subjects included HF patients from the same referral cohort with no EOV matched for LVEF.
Figure 1.

Oscillation of V̇e during exercise in a subject with chronic, stable NYHA class III HF. Ventilatory oscillation resolved during the final phase of exercise. Oscillation of Vt is also shown; in this individual the magnitude of breath-to-breath Vt oscillation varied by > 250% during a single oscillatory cycle.
Exercise Testing
Subjects underwent treadmill exercise testing to volitional fatigue following instrumentation for the measurement of heart rate, metabolic gas exchange, and oxygen saturation. The protocol used an initial treadmill speed and grade of 2.0 miles per hour and 0%, respectively, with speed and grade increased every 2 min to yield an approximate 2 metabolic equivalent increase per work level to a rating of perceived exertion of 18 to 20 on the Borg scale.19
Gas Exchange Measures and Physiologic Monitoring
Breath-by-breath measurements were obtained by a metabolic cart (Medical Graphics; St. Paul, MN). Measures included peak oxygen consumption (V̇o2), carbon dioxide output (V̇co2), partial pressure of end-tidal carbon dioxide (Petco2), Vt, V̇e, and breathing frequency. The data were collected continuously and reported as averages obtained over the final 30 s of each workload. Derived measures included the respiratory exchange ratio, defined as the ratio of V̇co2/V̇o2 and the ventilatory equivalent for CO2 (V̇e/V̇co2).20
Echocardiography
Chamber dimensions, right heart pressures, stroke volume, cardiac output, diastolic hemodynamics, and LVEF were assessed by echocardiography.21–24
Statistical Analysis
Characteristics, including ventilatory responses, were summarized with continuous data expressed as the mean ± SD or frequency expressed as a percentage. Characteristics of the study groups were compared by an unpaired two-tailed Student t test or Wilcoxon rank-sum test for continuous variables or χ2 test for categoric variables. A Student paired t test was used for the comparison of ventilatory responses within groups.
Linear regression was performed to evaluate the relationship between Petco2 and the V̇e/V̇co2. Multivariate regression using a backward selection procedure was used to identify characteristics independently associated with EOV; candidate variables for the multivariate model included those with p ≤ 0.2 by univariate analysis. For all analyses, p values ≤ 0.05 were considered to be significant.
Results
Subject Characteristics
The frequency of EOV for all ambulatory patients without ischemia who had been referred for cardiopulmonary exercise testing was 47 of 1,340 (3.5%) compared to 45 of 646 (7.0%) for those with a referral diagnosis of HF (p < 0.001). The study group (n = 47) and control group (n = 47) were not different with regard to LVEF, body mass index, gender, or therapy with β-blockers, digitalis, and diuretics. On univariate analysis, characteristics that discriminated EOV subjects from control subjects included older age, more advanced NYHA class, and increased frequency of atrial fibrillation. Subjects with EOV were also less likely to be receiving treatment with an angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker and were more likely to have had a history of hypertension (Table 1).
Table 1. Characteristics of Patients and Control Subjects*.
| Characteristics | Control Subjects (n = 47 |
EOV Patients (n = 47) |
p Value |
|---|---|---|---|
| Age, yr | 55 ± 13 | 61 ± 14 | 0.03† |
| NYHA class | 2.1 ± 0.9 | 2.6 ± 0.8 | 0.006† |
| BMI, kg/m2 | 29 ± 5.0 | 29 ± 6.8 | 0.49† |
| LVEF | 0.35 ± 0.14 | 0.37 ± 0.17 | 0.77† |
| Male gender | 33 (70) | 34 (72) | 0.82‡ |
| HF diagnosis | 47 (100) | 45 (96) | 0.49‡ |
| History of hypertension | 11 (23) | 21 (42) | 0.03‡ |
| Ischemic etiology | 14 (30) | 20 (49) | 0.07‡ |
| Atrial fibrillation | 6 (13) | 16 (35) | 0.01‡ |
| Pacemaker | 13 (28) | 11 (23) | 0.64‡ |
| Medications | |||
| ACE-I or ARB | 36 (78) | 28 (60) | 0.05‡ |
| Digoxin | 28 (60) | 19 (42) | 0.10‡ |
| β-blockers | 33 (70) | 33 (70) | 1.00‡ |
| Diuretics | 34 (72) | 37 (79) | 0.47‡ |
| Calcium blockers | 3 (6) | 8 (16) | 0.51‡ |
| Nitrates | 11 (22) | 8 (16) | 0.44‡ |
Values are given as the mean ± SD or No. (%), unless otherwise indicated. ACE-I = ACE-inhibitor; ARB = angiotensin-II receptor blocker; BMI = body mass index.
Determined by t test.
Determined by χ2 test.
Echocardiography
Cardiac output, index, and stroke volume were not different for subjects and control subjects, and values were within normal limits. For EOV subjects, the mean LVEF was 0.37 (range, 0.11 to 0.70), and 19 subjects (41%) had an LVEF of ≥ 0.40. Left atrial volume was significantly greater in subjects compared to control subjects as were estimates of right atrial and peak right ventricular systolic pressure. Mitral inflow peak E-wave velocity and peak velocity of pulmonary vein diastolic flow were also higher in subjects consistent with higher left atrial and pulmonary venous diastolic pressures (Table 2).22
Table 2. Echocardiographic Findings*.
| Variables | Control Subjects | No. | EOV Patients | No. | p Value |
|---|---|---|---|---|---|
| LVEF ≥ 0.40 | 19 (40) | 47 | 19 (41) | 46 | 0.93† |
| Stroke volume, mL/beat | 80 ± 22 | 46 | 77 ± 29 | 45 | 0.36‡ |
| Cardiac output, L/min | 5.5 ± 1.5 | 46 | 5.5 ± 1.8 | 45 | 0.62‡ |
| Cardiac index, L/min/m2 | 2.7 ± 0.17 | 45 | 2.7 ± 0.8 | 44 | 0.41‡ |
| LVEDd, mm | 62 ± 11 | 47 | 60 ± 12 | 45 | 0.38‡ |
| Left atrial volume, mL | 71 ± 27 | 42 | 90 ± 38 | 43 | 0.01‡ |
| Mitral E-wave velocity, m/s | 0.8 ± 0.3 | 44 | 1.0 ± 0.4 | 44 | 0.02‡ |
| Pulmonary vein diastolic velocity, m/s | 0.5 ± 0.2 | 39 | 0.6 ± 0.2 | 34 | 0.009‡ |
| Right atrial pressure, mm Hg | 7.6 ± 3.7 | 36 | 9.9 ± 5.2 | 30 | 0.04‡ |
| RV systolic pressure, mm Hg | 38 ± 15 | 36 | 46 ± 17 | 40 | 0.02‡ |
Values are given as No. (%) or the mean ± SD, unless otherwise indicated. LVEDd = left ventricular end-diastolic diameter; RV = right ventricular.
Determined by χ2 test.
Determined by t test.
Exercise and Ventilatory Responses
Subjects with EOV had significantly shorter exercise duration and lower peak heart rate compared to control subjects (Table 3). The breathing pattern of EOV subjects was also more rapid and shallower with significantly attenuated Vt response (Fig 2, top, A) and more limited functional capacity (Table 4). For EOV subjects, Petco2 was significantly lower compared to control subjects both at rest and during exercise, while breathing frequency and V̇e/V̇co2 were significantly higher at rest and at 50% peak exercise, which is consistent with greater hyperventilation due to increased ventilatory drive. In addition, for EOV subjects there was a significant further decrease of Petco2 during exercise consistent with increasing hyperventilation, which was not observed for control subjects (Table 4).
Table 3. Rest and Exercise Heart Rate, BP, and Symptoms*.
| Variables | Control Subjects (n = 47) |
EOV Patients (n = 47) |
p Value† |
|---|---|---|---|
| Rest | |||
| HR, beats/min | 74 ± 13 | 73 ± 14 | 0.58 |
| Systolic BP, mm Hg | 105 ± 16 | 110 ± 21 | 0.32 |
| Diastolic BP, mm Hg | 67 ± 11 | 69 ± 11 | 0.22 |
| Peak exercise | |||
| HR, beats/min | 131 ± 29 | 112 ± 25 | 0.002 |
| Systolic BP, mm Hg | 133 ± 34 | 130 ± 34 | 0.75 |
| Diastolic BP, mm Hg | 69 ± 15 | 64 ± 12 | 0.07 |
| RPE, Borg scale | 17 ± 2 | 18 ± 2 | 0.38 |
| Duration, min | 7.5 ± 2.9 | 5.9 ± 2.1 | 0.006 |
Values are given as the mean ± SD, unless otherwise indicated. HR = heart rate; RPE = rating of perceived exertion.
Determined by t test.
Figure 2.

Top, A: Vt from rest through peak exercise in control subjects and subjects with EOV; bars represent SEs at each stage. The difference of Vt between groups was significant at 50% peak (p = 0.03); however, at peak exercise the difference was not significant (p = 0.06). Bottom, B: subjects with EOV are portrayed in the following two subgroups: subjects in whom EOV resolved; and subjects in whom EOV persisted and compared to control subjects; bars represent SEs at each stage. In subjects in whom EOV persisted, Vt at 50% peak (p = 0.06) and peak exercise (p = 0.001) was lower than in subjects in whom EOV resolved. The mean increase in Vt from 50% to peak exercise in subjects for whom EOV resolved was also significantly greater (0.9 ± 0.4 vs 0.5 ± 0.2, respectively; p < 0.001) than that observed for the subgroup in whom EOV persisted.
Table 4. Measured Parameters of Ventilation and Gas Exchange at Rest and Exercise*.
| Variables | Control Subjects (n = 47) |
EOV Patients (n = 47) |
p Value† |
|---|---|---|---|
| Rest | |||
| V̇o2, mL/kg/min | 3.9 ± 1.2 | 4.1 ± 1.1 | 0.34 |
| V̇co2, L/min | 0.31 ± 0.11 | 0.31 ± 0.11 | 0.99 |
| V̇e, L/min | 12 ± 4.2 | 14 ± 5.1 | 0.23 |
| Vt, L | 0.79 ± 0.51 | 0.72 ± 0.29 | 0.84 |
| fb, breaths/min | 17 ± 4.7 | 20 ± 4.4 | < 0.001 |
| Petco2, mm Hg | 33 ± 3.3 | 31 ± 4.3‡ | 0.02 |
| V̇e/V̇co2 | 40 ± 4.7 | 45 ± 7.5 | 0.002 |
| 50% peak exercise | |||
| V̇o2, mL/kg/min | 10 ± 2.9 | 8.6 ± 3.0 | 0.005 |
| V̇co2, L/min | 0.71 ± 0.25 | 0.61 ± 0.22 | 0.04 |
| V̇e, L/min | 24 ± 7.5 | 25 ± 8.7 | 0.84 |
| Vt, L | 1.1 ± 0.4 | 1.0 ± 0.3 | 0.03 |
| fb, breaths/min | 21 ± 4.7 | 26 ± 5.8 | < 0.001 |
| Petco2, mm Hg | 35 ± 4.0 | 31 ± 4.5 | < 0.001 |
| V̇e/V̇co2 | 34 ± 5.6 | 41 ± 6.8 | < 0.001 |
| Peak exercise | |||
| V̇o2, mL/kg/min | 20 ± 7.5 | 16 ± 5.2 | 0.01 |
| V̇co2, L/min | 1.9 ± 0.87 | 1.4 ± 0.56 | 0.01 |
| V˙e, L/min | 61 ± 25 | 57 ± 20 | 0.66 |
| Vt, L | 1.9 ± 0.7 | 1.7 ± 0.6 | 0.06 |
| fb, breaths/min | 32 ± 5.5 | 35 ± 7.1 | 0.08 |
| Petco2, mm Hg | 34 ± 5.3 | 30 ± 5.0‡ | < 0.001 |
| V̇e/V̇co2 | 34 ± 6.6 | 40.2 ± 7.8 | < 0.001 |
| RER | 1.1 ± 0.12 | 1.1 ± 0.13 | 0.08 |
Values are given as the mean ± SD, unless otherwise indicated. fb = breathing frequency; RER = respiratory exchange ratio
Determined by t test.
p = 0.03 for comparison of Petco2 (within group) from rest to peak exercise.
Resolution of Oscillatory Ventilation
In 18 subjects (38%), oscillatory ventilation resolved during the final stage of exercise (Fig 1). For this subgroup, exercise duration and peak V̇o2 were not different from those values in control subjects; however, their exercise duration was significantly greater compared to the subgroup of subjects for whom EOV persisted (p = 0.01).
Subjects in whom EOV resolved were also compared to subjects in whom EOV persisted and control subjects with respect to changes in Vt and heart rate. For subjects in whom EOV resolved, Vt at peak exercise was similar to that in control subjects and was significantly higher than that for subjects in whom EOV persisted (Fig 2, bottom, B). The increase in Vt from rest to peak exercise and from 50% peak to peak exercise were also comparable to that in control subjects and was significantly higher than that for subjects in whom EOV persisted (Fig 2, bottom, B). For the subgroup in whom EOV resolved, there was also significantly greater increase of heart rate from 50% peak to peak exercise compared to that in the subgroup in whom EOV persisted (Δ heart rate, 29 vs 19 beats/min, respectively; p = 0.04).
V̇e/ V̇co2 and End-Tidal CO2
The relationship of V̇e/V̇co2 to Petco2 may be summarized by the following modified alveolar gas equation25 in which Petco2 measured by cardiopulmonary exercise testing has been substituted for Paco2:
V̇e/V̇co2 = 863/Petco2(1 − Vd/Vt)25
A strong inverse correlation between V̇e/V̇co2 and Petco2 for subjects and control subjects was observed, consistent with tight regulatory control of the Paco2 by ventilation (Fig 3).25 Though the slopes of the regression lines for the two study groups were not statistically different, the intercept for EOV subjects was shifted significantly upward, indicating that, at comparable Petco2 values, EOV subjects had higher V̇e/V̇co2, which is consistent with greater physiologic dead space ventilation (Vd/Vt) [p = 0.002].
Figure 3.
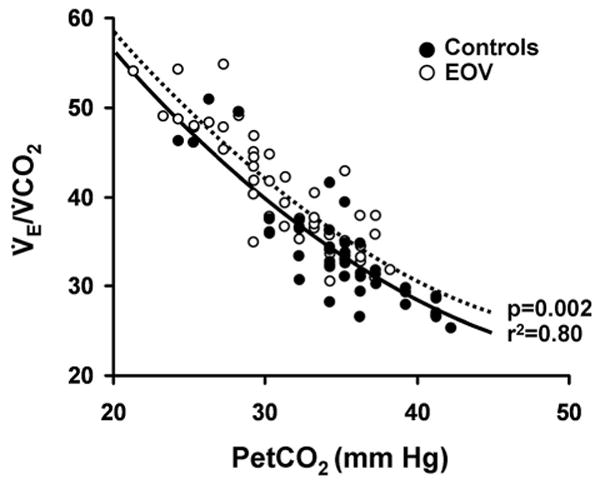
Relationship of Petco2 to V̇e/V̇co2 at 50% peak exercise in control subjects and subjects with EOV. The slopes of the regression lines for each group are similar; however, the significant upward shift of the intercept for EOV subjects is consistent with significantly increased Vd/Vt per the modified alveolar gas equation at comparable V̇e/V̇co2.
Multivariate Analysis
Subject characteristics independently associated with EOV included atrial fibrillation (odds ratio, 6.7; p = 0.006), digitalis therapy (odds ratio, 0.27; p = 0.02), NYHA class (odds ratio, 3.5 per one class change; p = 0.0006), resting Petco2 (odds ratio, 0.87 per one unit; p = 0.05), and peak exercise heart rate (odds ratio, 0.98 per one unit; p = 0.02).
Discussion
A unique and noteworthy finding of this study was that a substantial proportion of EOV subjects (41%) had an LVEF of ≥ 0.40. Indeed, on average, EOV subjects had normal stroke volume and cardiac index that were not different from control subjects, indicating that severe LV systolic dysfunction is not a prerequisite for EOV. Additional novel observations were that EOV subjects had larger left atrial volume, findings that are consistent with higher estimated left atrial, pulmonary venous and right heart pressures, attenuated Vt response to exercise, and increased Vd/Vt compared to control subjects. This study also demonstrated EOV subjects had greater hyperventilation as well as increased V̇e/V̇co2. The tachypneic and shallow breathing pattern, increased Vd/Vt, lower Petco2, more abnormal hemodynamics, increased V̇e/V̇co2, more limited exercise capacity, and higher NYHA class are each consistent with more advanced HF in subjects with EOV despite LVEF matched to control subjects.
For this study, oscillatory ventilation was defined as a ≥ 25% variation of V̇e amplitude18 persisting for ≥ 60% of exercise duration,3 whereas previous studies used less restrictive criteria, including a V̇e amplitude threshold of ≥ 15%1 or oscillations present for two or more consecutive cycles.18 Furthermore, subject selection required only EOV without consideration of LVEF or prior diagnosis of HF, whereas prior studies1–3,6,16–18 limited study enrollment to subjects with known HF and LVEF of ≤ 0.40. In addition, 70% of our subjects were treated with β-blockers compared to previous reports2,3 in which such therapy was less frequently used, which may be relevant as β-blocker therapy may reduce hyperventilation and thereby decrease the predisposition to EOV.26 Hence, the lower frequency of EOV observed in our study compared to that in prior reports1–3 may be related to differences in referral population, selection criteria, or therapy.
Other relevant methodological considerations were the use of control subjects matched for LVEF and multivariate analysis. Controlling for LVEF enabled the identification of characteristics other than LV systolic dysfunction, which distinguished EOV subjects from control subjects. One prior study1 of EOV used multivariate analysis and demonstrated that only an apnea-hypopnea index of > 30 events per hour, as determined by polysomnography, was independently associated with EOV. In contrast, we report the novel observations that factors independently associated with EOV also include the presence of atrial fibrillation, lack of digitalis therapy, lower resting Petco2, and lower peak exercise heart rate.
The mechanisms that account for EOV have not been fully elucidated.27–29 However, the breathing pattern of EOV is reminiscent of central sleep apnea, suggesting similar pathophysiology.9,30 Indeed, EOV and central sleep apnea frequently coexist.1 Increased cardiac-filling pressures with pulmonary congestion have been implicated as a primary factor in the genesis of periodic breathing due to central sleep apnea.9,30 Our findings of increased left atrial volume, mitral peak E flow velocity, pulmonary venous diastolic flow velocity, and right heart pressures by echocardiography are consistent with higher left atrial, pulmonary venous, and pulmonary arterial pressure in EOV subjects compared to those in control subjects. Hence, the observations reported herein also support a possible role for increased cardiac filling pressures with pulmonary congestion in the genesis of EOV.
Pulmonary congestion imposes reduced lung compliance, which may limit the increase of Vt with exercise, making increased breathing frequency necessary to maintain V̇e.31 The stretching of lung J-receptors by vascular congestion and interstitial edema also stimulates medullary respiratory centers via vagal afferents, which promotes rapid, shallow breathing32–35 and heightens chemosensitivity with consequent hyperventilation with hypocapnia.32,33 Oscillatory ventilation may then ensue as Paco2 is driven to values near or below the apnea threshold9,30,36,37; as Paco2 falls, hypoventilation occurs; as Paco2 subsequently rises, hyperventilation resumes, thereby completing an oscillatory cycle.
In contrast, a mechanism for the resolution of oscillatory ventilation during exercise has not been previously proposed.1–3 It is perhaps noteworthy that interventions that increase the difference between the ambient Paco2 and the apnea threshold may resolve the periodic breathing of central sleep apnea, and are achieved by administration of CO238 or acetazolamide, which increase the ambient Paco2.13 Alternatively, this same difference may be made greater by lowering of the Paco2 required to produce apnea (ie, the apnea threshold) by an increase of chemosensitivity such as achieved by treatment with phosphodiesterase inhibitors.39,40 In this regard, β-adrenergic agonists increase V̇e41 and may also lower the apnea threshold in patients with HF.42 In our study, EOV subjects had significantly lower Petco2 than control subjects at rest and with exercise. Furthermore, with exercise Petco2 decreased further in EOV subjects, while remaining stable in control subjects. As ventilatory responses may be enhanced by increased β-adrenergic stimulation, acting either centrally43 or via the influence of venous norepinephrine on peripheral chemoreceptors,41 our observations suggest further that the enhancement of chemosensitivity during exercise may have decreased the ambient Paco2 while also sufficiently lowering the apnea threshold such that EOV resolved (ie, because the apnea threshold was not approached, thereby preventing the initiation or maintenance of periodic breathing). In addition, the observation of a significantly greater increase in exercise heart rate for subjects in whom EOV resolved compared to that in subjects in whom it persisted is consistent with greater preservation of postsynaptic β-adrenergic function44 in the former subgroup, suggesting that superior exercise capacity may have been mediated by β-adrenergic effects on both heart rate and chemosensitivity.
The resolution of EOV with the stabilization of ventilatory control was associated with a nearly twofold increase of Vt from 50% peak to peak exercise compared to subjects in whom EOV persisted, which likely contributed to the greater functional capacity in the former subgroup. The magnitude of this difference seems unlikely to have been due to bronchodilation, though some role for this mechanism cannot be excluded. It seems more likely that the restoration of stable ventilatory control led to increased Vt by the resolution of episodic hypoventilation.
Clinical Implications
As diuretics,12,13 inotropes,11 cardiac resynchronization,14 and cardiac surgery11,15 may reduce or abolish periodic breathing, the presence of EOV suggests that an intensification of therapy be considered to optimize cardiac hemodynamics, improve symptoms, and increase functional capacity. The detection of EOV may also be useful for the assessment of patients with well-preserved LVEF in whom the diagnosis of HF is not certain. Hence, the recognition of EOV by cardiopulmonary exercise testing may be useful for the diagnosis and surveillance of HF while also identifying a high-risk patient subgroup and therapeutic target.
Study Limitations
The main limitation of this study was its retrospective design. Nevertheless, the validity of the observations is strengthened by the large study group size, the use of control subjects matched for LVEF, and the magnitude of differences between subjects and control subjects. There was a statistically significant difference in age between the subject and control groups, but it seems unlikely that this difference influenced ventilatory responses, as might be expected from the higher Vd/Vt associated with more advanced age, as age-associated increases have been estimated at < 0.1%/year.45
Conclusions
Patients with EOV have clinical characteristics and exercise ventilatory responses that are consistent with more advanced HF than control subjects with comparable left ventricular systolic function; EOV may occur in HF patients with an LVEF of ≥ 0.40.
Acknowledgments
We thank Kathy O'Malley and Angela Heydmann for assistance in data management, and Michelle Small for manuscript preparation.
This study was supported by the Mayo Foundation, and National Heart Lung and Blood Institute grants HL71478, HL71478S1, HL-65176, HL-70302, and HL-73211.
Abbreviations
- EOV
exercise oscillatory ventilation
- HF
heart failure
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- Petco2
end-tidal carbon dioxide
- V̇co2
carbon dioxide output
- Vd/Vt
physiologic dead space ventilation
- V̇e
minute ventilation
- V̇e/V̇co2
ventilatory equivalent for CO2
- V̇o2
oxygen consumption
- Vt
tidal volume
Footnotes
The authors have reported to the ACCP that no significant conflicts of intere conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Corra U, Pistono M, Mezzani A, et al. Sleep and exertional periodic breathing in chronic heart failure. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- 2.Leite JJ, Mansur AJ, de Freitas HFG, et al. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J Am Coll Cardiol. 2003;41:2175–2181. doi: 10.1016/s0735-1097(03)00460-1. [DOI] [PubMed] [Google Scholar]
- 3.Corra U, Giordano A, Bosimini E, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121:1572–1580. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 4.Hanley PJ, Zuberi-Khohhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 5.Andreas S, Hagenah G, Moller C, et al. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78:1260–1264. doi: 10.1016/s0002-9149(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 6.Mortara A, Sleight P, Pinna GD, et al. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation. 1997;96:246–252. doi: 10.1161/01.cir.96.1.246. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 9.Bradley TD, Floras JS. Sleep apnea and heart failure: part II. Central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 10.Molhotra A, Berry RB, White DP. Central sleep apnea: clinical sleep disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2005. pp. 331–346. [Google Scholar]
- 11.Ribeiro JP, Knutzen A, Rocco MB, et al. Periodic breathing during exercise in severe heart failure: reversal with milrinone or cardiac transplantation. Chest. 1987;92:555–556. doi: 10.1378/chest.92.3.555. [DOI] [PubMed] [Google Scholar]
- 12.Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 13.White DP, Zwillich CW, Pickett CK, et al. Central sleep apnea: improvement with acetazolamide therapy. Arch Intern Med. 1982;142:1816–1819. [PubMed] [Google Scholar]
- 14.Sinha AM, Skobel EC, Breithardt OA, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Tomcsany J, Karlocai K, Papp L. Disappearance of periodic breathing after heart operations. J Thorac Cardiovasc Surg. 1994;107:317–318. [PubMed] [Google Scholar]
- 16.Feld H, Priest S. A cyclic breathing pattern in patients with poor left ventricular function and compensated heart failure: a mild form of Cheyne-Stokes respiration. J Am Coll Cardiol. 1993;21:971–974. doi: 10.1016/0735-1097(93)90355-5. [DOI] [PubMed] [Google Scholar]
- 17.Kremser CB, O'Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:900–905. doi: 10.1016/0002-9149(87)91116-7. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Dov I, Sietsema KE, Casaburi R, et al. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145:776–781. doi: 10.1164/ajrccm/145.4_Pt_1.776. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Subjective effort and physical abilities. Scand J Rehabil Med. 1978;6:105–113. [PubMed] [Google Scholar]
- 20.Sun XG, Hansen JE, Garatachea N, et al. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Hurrell DG, et al. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–29. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Loutfi H, Nishimura RA. Quantitative evaluation of left ventricular systolic function by Doppler echocardiographic techniques. Echocardiography. 1994;11:305–314. doi: 10.1111/j.1540-8175.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RL., Jr Gas exchange efficiency in congestive heart failure. Circulation. 2000;101:2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 26.Wolk R, Johnson BD, Somers VK, et al. Effects of β-blocker therapy on ventilatory responses to exercise in patients with heart failure. J Card Fail. 2005;11:333–339. doi: 10.1016/j.cardfail.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro JP. Periodic breathing in heart failure. Circulation. 2006;113:9–10. doi: 10.1161/CIRCULATIONAHA.105.590265. [DOI] [PubMed] [Google Scholar]
- 28.Bradley TD. The ups and downs of periodic breathing. J Am Coll Cardiol. 2003;41:2182–2184. doi: 10.1016/s0735-1097(03)00470-4. [DOI] [PubMed] [Google Scholar]
- 29.Francis DP, Willson K, Davies C, et al. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 30.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 31.Agostoni P, Pellegrino R, Conca C, et al. Exercise hyperpnea in chronic heart failure: relationships to lung stiffness and expiratory flow limitation. J Appl Physiol. 2002;92:1409–1416. doi: 10.1152/japplphysiol.00724.2001. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AM, Bhattacharya J, Schultz HD, et al. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58:512–522. doi: 10.1161/01.res.58.4.512. [DOI] [PubMed] [Google Scholar]
- 33.Hatridge J, Haji A, Perez-Padilla JR, et al. Rapid shallow breathing caused by pulmonary vascular congestion in cats. J Appl Physiol. 1989;67:2257–2264. doi: 10.1152/jappl.1989.67.6.2257. [DOI] [PubMed] [Google Scholar]
- 34.Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol. 1969;203:511–532. doi: 10.1113/jphysiol.1969.sp008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd TC., Jr Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol. 1990;69:1973–1980. doi: 10.1152/jappl.1990.69.6.1973. [DOI] [PubMed] [Google Scholar]
- 36.Ponikowski P, Anker SD, Chua TP, et al. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 37.Solin P, Roebuck T, Johns DP, et al. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzi-Filho G, Rankin F, Bies I, et al. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159:1490–1498. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- 39.Javaheri S, Parker TJ, Wexler L, et al. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 40.Andreas S, Reiter H, Luthje L, et al. Differential effects of theophylline on sympathetic excitation: hemodynamics and breathing in heart failure. Circulation. 2004;110:2157–2162. doi: 10.1161/01.CIR.0000144356.39262.16. [DOI] [PubMed] [Google Scholar]
- 41.Heistad DD, Wheeler RC, Mark AL, et al. Effects of adrenergic stimulation on ventilation in man. J Clin Invest. 1972;51:1469–1475. doi: 10.1172/JCI106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson RL., Jr Gas exchange efficiency in congestive heart failure II. Circulation. 2001;103:916–918. doi: 10.1161/01.cir.103.7.916. [DOI] [PubMed] [Google Scholar]
- 43.Kaye DM, Lambert GW, Lefkovits J, et al. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- 44.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure: role of postsynaptic β-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 45.Raine JM, Bishop JM. A-a difference in O2 tension and physiological dead space in normal man. J Appl Physiol. 1963;18:284–288. doi: 10.1152/jappl.1963.18.2.284. [DOI] [PubMed] [Google Scholar]


