Abstract
Recruitment of PMNs into the lungs in response to inhaled pathogens is initiated by epithelial signaling, the activation of toll-like receptors (TLR) and IL-8 production. As PMNs must be mobilized through epithelial junctions to reach the site of infection, we postulated that TLR signaling includes a mechanism to modulate the epithelial barrier to accommodate PMN migration. We demonstrate that Ca2+ fluxes generated by TLR2 signals activate calpains which cleave the transmembrane proteins occludin and E-cadherin. Calpain inhibitors decrease PMN transmigration in response to TLR2 agonists both in vitro and in a mouse model of P. aeruginosa infection. TLR2 signaling in the airway not only induces chemokine expression, but also initiates cleavage of junctional proteins to accommodate transmigration of recruited PMNs.
Airway epithelial cells are an important component of the mucosal immune system providing both signaling and barrier function to protect the lungs from inhaled pathogens. All of the toll-like receptors (TLR) are expressed by airway cells (Muir et al., 2004) and can participate in pathogen recognition. TLR2 is displayed on the exposed, apical surface of airway epithelial cells and is broadly responsive to PAMPS (pathogen associated molecular patterns) from clinically important respiratory pathogens including the Gram negative opportunist Pseudomonas aeruginosa. TLR2 signaling results in epithelial production of GM-CSF and IL-8 that are important in recruiting and activating polymorphonuclear leukocytes (PMNs). For these phagocytes in the bloodstream to reach bacteria in the airway lumen, they must migrate across both the endothelial barrier and the epithelial barrier which are normally tightly apposed via adherens and tight junction proteins (Wagner and Roth, 2000).
The cascade of adhesive, stimulatory and guidance factors involved in PMN extravasation across endothelial cells and subsequent migration within the interstitial space has been studied in some detail (Burns et al., 2003). In comparison less is known about the transepithelial migration of PMNs. Since the major function of the epithelial tight junctions is to create a barrier to maintain sterility in the lung, they are more substantial and are 10 times less leaky than endothelial junctions (Burns et al., 2003). Disruption of the tight junction in the presence of infection in the airway lumen could facilitate bacterial invasion and disseminated infection. Exactly how PMNs are able to squeeze between respiratory epithelial cells without breaching the barrier provided by tight junctions is not well understood, yet is critically important in controlling inflammation in the airway which if excessive, blocks air exchange and causes respiratory failure.
Tight junctions (TJ) are maintained through a complex network of interacting proteins of several different types (Harhaj and Antonetti, 2004). Some of the junctional complex proteins such as occludin and E-cadherin span the plasma membrane and associate through homotypic interactions with corresponding domains on adjacent cells (Feldman et al., 2005; Mege et al., 2006). Occludin, one of the first TJ proteins to be identified has two extracellular loops linking adjacent cells. These extracellular domains are proposed to affect PMN migration between endothelial cells (Feldman et al., 2005). In response to various signals such as MAPKs and oxidative stress, occludin undergoes endocytosis through a caveolin-1 mediated process (Shen and Turner, 2005). Although not essential to maintain the tight junction (Saitou et al., 2000; Schulzke et al., 2005), occludin is thought to have a critical regulatory function in that it interacts with several other junctional proteins including ZO-1 and, indirectly, with actin and the cytoskeleton (Muller et al., 2005).
E-cadherin is also critical for maintenance of the epithelial barrier and spans the paracellular space through five extracellular domains (Bryant and Stow, 2004). In response to various stimuli, E-cadherin undergoes endocytosis as part of the dynamic process of membrane homeostasis (Bryant and Stow, 2004; Mege et al., 2006). The distribution of these junctional proteins could be altered to facilitate phagocyte recruitment across the epithelium as part of the epithelial response to bacterial stimuli. Accumulation of PMNs into the airway lumen occurs very rapidly following bacterial exposure (Reutershan et al., 2005; Wagner and Roth, 2000) suggesting that changes in the permeability characteristics of the paracellular junctions are an immediate consequence of the epithelial proinflammatory signaling cascade.
TLR2 functions as an epithelial receptor responding to components of bacterial pathogens in the airway lumen (Constantin et al., 2004; Fournier and Philpott, 2005; Soong et al., 2004). TLR2 is actively mobilized to the apical surface of the airway cell in response to ligands where it is phosphorylated by c-Src, recruits PI3K and PLCγ thereby releasing Ca2+ from intracellular stores and stimulates MAPK activity and NF-κB translocation to initiate chemokine and cytokine expression (Chun and Prince, 2006). We reasoned that the signaling cascade that initiates chemokine expression must also include a mechanism to facilitate PMN mobilization across the epithelial barrier. In endothelial cells, Ca2+ signaling was shown to promote transendothelial migration of PMNs by opening their intercellular junctions (Huang et al., 1993). The Ca2+ flux was not required for PMN adhesion but was required for PMN migration across HUVEC monolayers (Huang et al., 1993). The exact mechanism of Ca2+ dependent PMN migration, however, remains to be examined. As Ca2+ functions as a second messenger, we postulated that Ca2+ dependent proteases are involved in modulating junctional proteins to accommodate PMN transmigration to the airway lumen. Calpain 1 (mu-calpain) and calpain 2 (m-calpain) are Ca2+ dependent cysteine proteases that target cytoskeletal proteins in a number of cell types including the lung. They are known to be involved in cellular motility, apoptosis and inflammation (Fettucciari et al., 2006; Goll et al., 2003). In the experiments described in this report, we demonstrate that Ca2+ fluxes initiated by TLR2 signaling activate calpains, which cleave both occludin and E-cadherin to promote recruitment of PMNs into the airway.
Results
Bacterial stimulation alters epithelial cell junctions
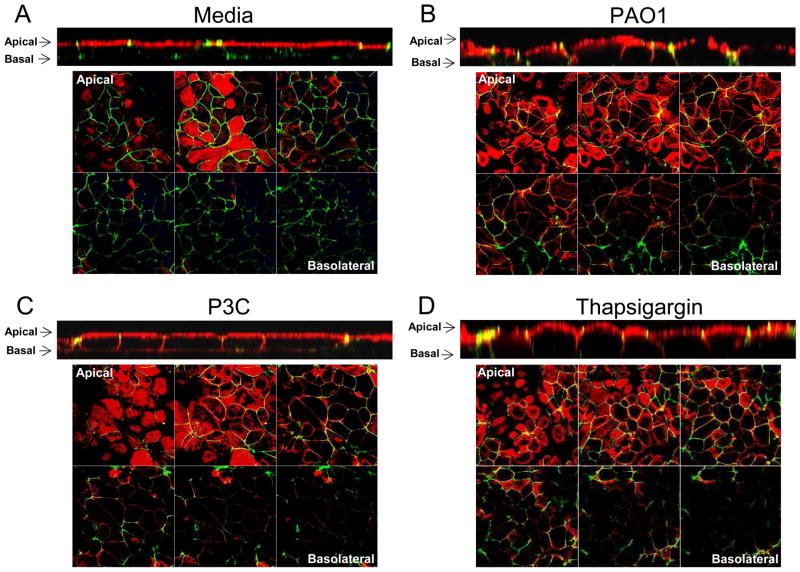
Most of the organisms that cause pneumonia, especially opportunists such as P. aeruginosa, gain access to the lung by inhalation and are first encountered by the mucosal epithelium. To determine how the initial exposure to bacteria affects the “tightness” of the epithelial barrier, we used biotin detected with Alexa Fluor 555 conjugated streptavidin to outline the exposed surfaces of polarized airway epithelial monolayers and followed its ability to intercalate into the paracellular spaces following bacterial challenge or exposure to a TLR2 agonist (Guttman et al., 2006). In control monolayers, biotin (red) was limited to the most apical surfaces of the monolayer and failed to intercalate between cells (Figure 1). In contrast, exposure to heat killed P. aeruginosa PAO1, to Pam3Cys-Ser-Lys4 (P3C), a TLR2 agonist, or to thapsigargin, a sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) pump inhibitor that stimulates release of Ca2+ from intracellular stores, resulted in the penetration of biotin (red) between the cells in a polarized monolayer both in z-sections and images obtained from planes below the top of the monolayers (Figure 1).
Figure 1.
Epithelial cell junctions are affected by bacterial stimulation. Polarized 16HBE cells were incubated for 4 h with (A) media, (B) heat killed PAO1, (C) P3C or (D) thapsigargin. Cells were incubated with biotin detected with Alexa Fluor 555 conjugated streptavidin (red) and stained for ZO-1 (green). Apical to basolateral x-y scans are shown with corresponding z-sections below. Data is representative of at least three separate experiments.
P. aeruginosa stimulates the redistribution of occludin and E-cadherin without loss of barrier function
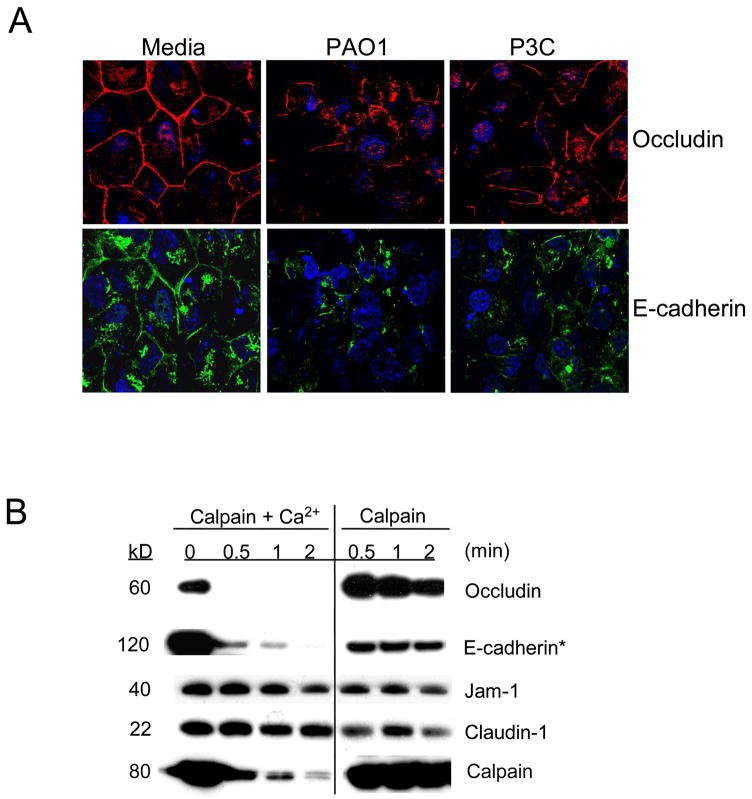
To demonstrate that TLR2 or Ca2+ signals induce changes in epithelial junctions, the distribution of the membrane spanning junctional proteins occludin and E-cadherin were imaged. The expected “chicken wire” distribution of both occludin and E-cadherin (Figure 2A) was substantially altered following 6 h exposure of airway cells to P. aeruginosa PAO1 or P3C. Yet, despite loss of occludin and E-cadherin at the cell borders, there was no concomitant decrease in the transepithelial resistance measured across the monolayers over this time period; nor was there an increase in permeability to fluorescent dextran, or to bacteria across the paracellular space indicating that the barrier function of the monolayer remained intact (Figure S1).
Figure 2.
Occludin and E-cadherin are targets for calpain cleavage. (A) Distribution of occludin (red) and E-cadherin (green) is altered following 6 h incubation with media, heat killed PAO1 or P3C. Cells were stained with DAPI (blue). Data is one representative of at least three separate experiments. (B) In the presence of 20 mM CaCl2, 1 μg of exogenous human calpain 1 cleaved occludin and E-cadherin but not JAM-1 or claudin-1 immunoprecipitated from 1HAEo- cell lysates. The exogenous calpain 1 in these reactions is autolysed upon activation in the presence of Ca2+. * Shorter exposure time on the three right bands compared to the three left bands. Data is one representative of at least three separate experiments.
Occludin and E-cadherin are targets for calpain proteolysis
Occludin and E-cadherin are both substrates for proteases known to be responsible for their dissociation from the cell junctions (Bojarski et al., 2004; Rios-Doria et al., 2003; Zhu et al., 2006). Among the many cellular proteases that could target these proteins, we focused on the Ca2+-dependent calpains, postulating that the TLR2 induced Ca2+-flux would initiate activity. In an in vitro experiment, exogenous calpain activated by the addition of Ca2+, degraded occludin and E-cadherin but not claudin-1 or JAM-1 (Figure 2B), indicating that calpains target specific junctional proteins. Autolysis of calpain is readily detectable in the presence of Ca2+ and confirms calpain activation in vitro (Figure 2B).
Calpain is activated by TLR2 signaling
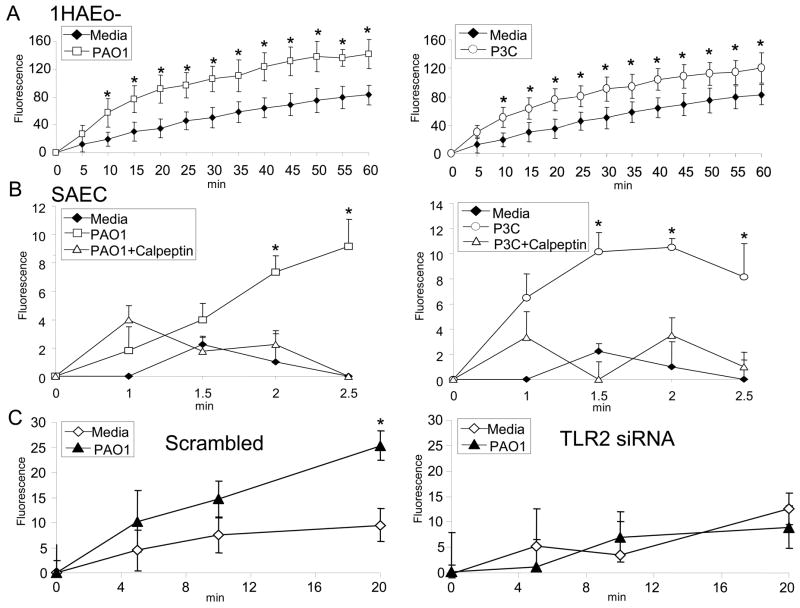
Calpains 1 and 2 as well as the endogenous inhibitor calpastatin are present in human airway cells and transcription of calpain 2 is increased following epithelial stimulation by either bacteria or P3C (Figure S2). To test whether calpain is activated in response to TLR2 signaling, 1HAEo- cells and human small airway cells in primary culture (SAEC) were loaded with a cell permeable fluorogenic calpain substrate. 1HAEo- and SAEC were selected because they have lower background fluorescence compared to 16HBE- cells. Calpain activity in 1HAEo- and SAEC was significantly increased following bacterial or P3C exposure (Figure 3A) and was inhibited in the presence of calpeptin, a selective calpain inhibitor (Figure 3B). Calpain activity was dependent upon TLR2 signaling and was not increased by bacterial stimulation of cells expressing TLR2 siRNA, as compared with a scrambled siRNA control (Figure 3C). The TLR2 ligand, P3C by itself was sufficient to activate calpain and the presence of its receptor was necessary for bacterial activation of the protease.
Figure 3.
TLR2 mediated activation of calpain. (A)1HAEo- cells or (B) Human small airway epithelial cells in primary culture (SAEC) were loaded with a fluorogenic calpain substrate, t-BOC L-leucine L-methionine, and incubated with heat killed PAO1 or P3C in the presence of 20 μM calpeptin or DMSO vehicle control at the indicated times. (C) 1HAEo- cells expressing scrambled or TLR2 siRNA were stimulated with heat killed PAO1 at the indicated times. The fluorescence was quantified with ex 360 nm, em 465 nm and baseline fluorescence was subtracted. Data represents the mean ± s.d. of quadruplicate samples and is one representative of three independent experiments. (* P<0.05 compared with media alone controls; Student’s t-test).
Calpain mediated cleavage of occludin is activated by bacterial ligands
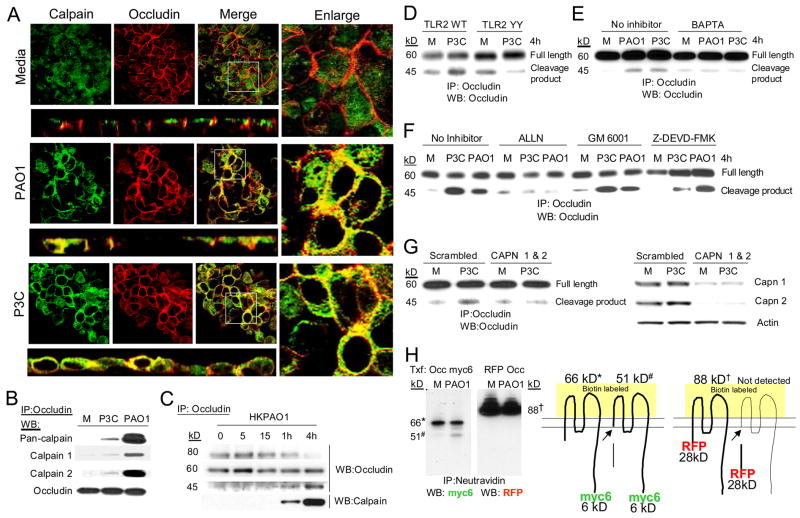
Having observed that calpain targets occludin in vitro and that bacterial stimulation induces the redistribution of occludin and the activation of endogenous calpain, it seemed likely that occludin is a calpain substrate in airway cells. Calpain was found throughout the cytoplasm in confocal images of unstimulated airway cells, whereas occludin was concentrated at the plasma membrane (Figure 4A). By one hour after bacterial or P3C exposure, calpain assumed a peripheral distribution co-localizing with membrane-associated occludin, visualized in confocal images (Figure 4A). Immunoprecipitation experiments further confirmed this interaction and also demonstrated that both calpain 1 and calpain 2 associate with occludin (Figure 4B). The association of occludin and calpain after 1 and 4 h stimulation with P. aeruginosa PAO1 corresponded to a loss of the 80 kD hyperphosphorylated form of occludin and subsequent appearance of a 45 kD occludin cleavage fragment predicted from studies of occludin processing in other model systems (Wan et al., 2000; Wu et al., 2000) (Figure 4C). This result was also confirmed in human small airway epithelial cells in primary culture (SAEC) (data not shown). Generation of the occludin cleavage product was substantially decreased in cells unable to activate TLR2 signaling, expressing a dominant negative TLR2 mutation lacking the tyrosine residues necessary for TLR2 phosphorylation (Figure 4D) (Chun and Prince, 2006); or in cells treated with an intracellular Ca2+ chelator BAPTA/AM (Figure 4E). Neither the general matrix metalloproteinase inhibitor GM6001 nor the caspase inhibitor Z-DEVD-FMK blocked generation of the occludin cleavage product, in contrast to the calpain inhibitor, ALLN (Figure 4F). To further confirm the role of calpain in occludin cleavage, calpain 1 and 2 expressions were silenced by siRNA. The occludin cleavage product was detected in cells expressing scrambled oligos in response to P3C stimulation but not in cell expressing both calpain 1 and 2 siRNA (Figure 4G). Knockdown of calpain 1 or calpain 2 individually was not sufficient to block occludin cleavage in response to P3C stimulation (data not shown).
Figure 4.
Occludin is a calpain substrate. (A) Co-localization of calpain (green) and occludin (red) in polarized 16HBE cells following 1 h stimulation with heat killed PAO1 or P3C is shown in xy and xz sections. The adjacent panel shows an enlargement of white boxed area of the xy image. (B) Immunoprecipitation of occludin from 1HAEo- cell following stimulation with heat killed PAO1 or P3C and detection of occludin, pan-calpain, calpain 1 and calpain 2 by immunoblot. Data are representative of at least three separate experiments. (C) Immunoprecipitation of occludin from 1HAEo- cells following heat killed PAO1 stimulation and detection of the 80 kD hyperphosphorylated form of occludin, 60 kD full length form of occludin and 45kD cleavage fragment of occludin. (D) Detection of occludin cleavage in 1HAEo- cells overexpressing TLR2 WT or TLR2 Y616A/Y761A (TLR2YY). (E, F) Detection of occludin cleavage in cells treated with 6 μM BAPTA/AM, 20 μM ALLN, 20 μM GM6001, or 25 μM Z-DEVD-FMK. (G) Detection of occludin cleavage in P3C stimulated scrambled control or calpain 1 and 2 siRNA (CAPN 1 & 2) expressing cells. Silencing of calpain 1 and 2 expressions in scrambled control and siRNA expressing cells is shown in the adjacent panel. (H) Neutravidin immunoprecipitation (IP) from biotinylated 1HAEo- cells that were transfected (Txf) with C-terminal myc6 tagged occludin (Occ myc6) or N-terminal RFP tagged occludin (RFP Occ) and incubated with media alone (M) or heat killed PAO1 (PAO1) were immunoblotted (WB) with anti-myc or anti-RFP antibodies. Cartoon illustrates the forms of occludin that correspond with the bands on the immunoblot. (Data are representative of at least three separate experiments).
To locate where calpain cleaves occludin, a series of mapping experiments were done. Since the 45 kD cleavage fragment of occludin was immunoprecipitated and immunoblotted using an antibody that recognizes the intracellular C-terminal tail of occludin, we speculated that the calpain cleaves at the intracellular N-terminal tail of occludin. To further map exactly where calpain cleaves occludin, we took advantage of occludin contructs with N-terminal RFP (RFP Occ) (Shen and Turner, 2005) and C-terminal myc6 labels (Occ myc6) (Bojarski et al., 2004). By biotinylating 1HAEo- cells expressing these labeled occludins and performing immunoblots on neutravidin precipitates with anti-RFP and anti-myc antibodies, we were able to identify the region of occludin targeted by calpain at the cell surface. Occ myc6 is a 66 kD protein which generates a 51 kD myc-tagged fragment in heat-killed PAO1 treated cells (Figure 4H). The generation of this tagged fragment suggests that the cleavage site is in the region of the intracellular N-terminal domain which is illustrated in Figure 4H. Expression of the N-terminal RFP tagged occludin in airway cells results in a 88 kD product that does not generate an RFP tagged fragment, indicating that the RFP label has been cleaved and is not detectable at the cell surface (Figure 4H). These studies are consistent with calpain targeting the N-terminal portion of occludin.
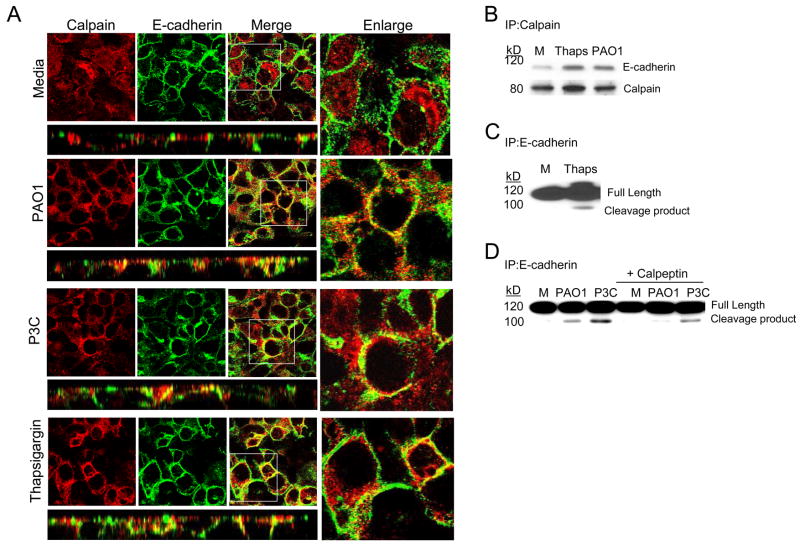
Calpain mediated cleavage of E-cadherin
To determine if calpain similarly targets E-cadherin, confocal imaging and co-immunoprecipitation experiments were performed. E-cadherin was predominantly membrane –associated in unstimulated airway cells (Figure 5A). Calpain was mobilized to the membrane, colocalizing with E-cadherin following bacterial or P3C stimulation (Figure 5A). Treatment of the cells with thapsigargin to generate the release of Ca2+ from intracellular stores also induced mobilization and co-localization of calpain with E-cadherin (Figure 5A). The association of calpain and E-cadherin was further confirmed by immunoprecipitation (Figure 5B) and calpain activity demonstrated by the generation of a 100 kD E-cadherin cleavage product in thapsigargin, bacterial or P3C treated airway cells, which was substantially decreased in cells treated with the calpain inhibitor calpeptin (Figures 5C and D). Thus, calpain activity induced by bacteria, or by intracellular Ca2+ fluxes results in E-cadherin cleavage.
Figure 5.
E-cadherin is a calpain substrate. (A) Co-localization of calpain (red) and E-cadherin (green) in polarized 16HBE cells following 1 h stimulation with heat killed PAO1, P3C amd Thaps is shown in xy and xz sections. The adjacent panel shows an enlarged version of the xy merged image. (B) Immunoprecipitation (IP) of E-cadherin from 1HAEo-cells incubated with media alone (M) thapsigargin (Thaps) or heat killed PAO1 and detection of E-cadherin and calpain by immunoblot. (C) Identification of the E-cadherin cleavage product in 1HAEo- cells following 4 h stimulation with thapsigargin as compared with media (M) control. (D) Detection of E-cadherin cleavage products in 1HAEo- cells following 4 h stimulation with heat killed PAO1 or P3C in the presence or absence of 20 μM calpeptin. Data is representative of at least three separate experiments.
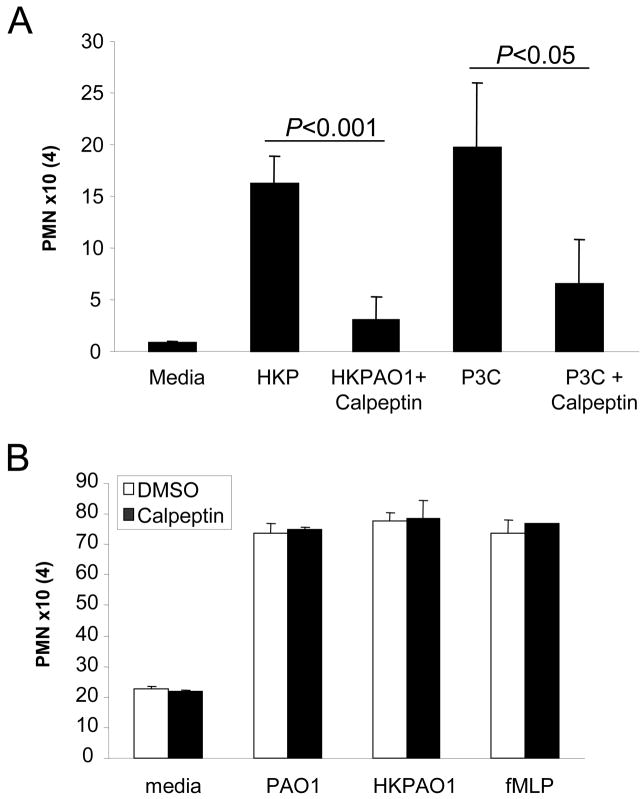
Calpain activation facilitates PMN transmigration and pulmonary inflammation
As a major function of TLR2 signaling is stimulating chemokine expression and PMN recruitment, we postulated that calpain modified junctional proteins must facilitate migration of PMNs across the epithelial barrier. To demonstrate the role of calpain in PMN transmigration from the basal to the apical surface of polarized human airway epithelial cells, monolayers were stimulated with heat killed P. aeruginosa PAO1 or P3C in the presence of the calpain inhibitor calpeptin and PMN migration monitored by measuring myeloperoxidase activity in the apical compartment. Both heat killed P. aeruginosa PAO1 (P<0.001) and P3C (P<0.05) induced PMN transmigration were significantly inhibited in airway cells treated with calpeptin (Figure 6A). Calpeptin did not directly inhibit migration of PMNs across a porous Transwell (without epithelial cells) in response to live P. aeruginosa PAO1, heat-killed PAO1 or the chemoattractant fMLP (Figure 6B), nor did calpeptin inhibit the activity of myeloperoxidase or the production of myeloperoxidase by PMNs (Figure S3A, B).
Figure 6.
PMN transmigration across airway epithelial monolayer is facilitated by calpain. (A) The number of PMNs that have migrated into the apical compartment of 16HBE monolayers stimulated with heat killed PAO1 or P3C in the presence or absence of calpeptin. (B) The migration of 106 calcein-AM labeled PMNs across a Transwell in response to 108 CFU/ml live PAO1, 108 CFU/ml HKPAO1 or 10 nM fMLP was not affected by the presence of 20 μM calpeptin. Data is presented as number of PMNs multiplied by 104 and represents mean ± s.d. of sextuplicate samples of one representative from three independent experiments. (P< 0.05, P<0.001; Student’s t-test).
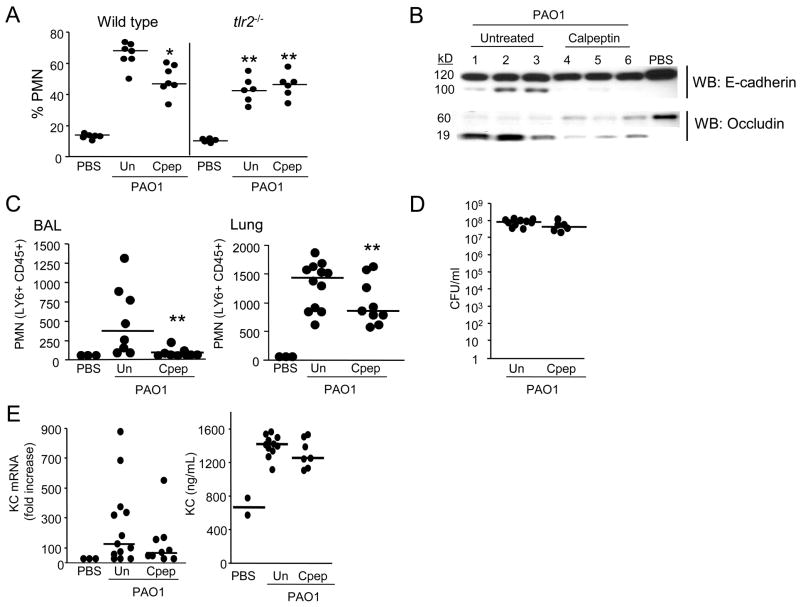
The biological importance of TLR2 induced calpain activity was tested in both neonatal and adult murine models of infection. In 7 day old C57BL/6 pups intraperitoneal (i.p.) calpeptin treatment resulted in 31% fewer PMNs recruited into the lung (P<0.05; Figure 7A) 4 h following intranasal P. aeruginosa inoculation as compared to vehicle treated mice (Figure 7A). Significantly fewer PMNs (P<0.01; Figure 7A) were mobilized into the lungs in Tlr2 −/− mice in response to infection as compared with wild type infected mice; a response equivalent to that of the calpeptin treated mice. PMN recruitment in the tlr2 −/− animals was not further diminished by calpain inhibition, consistent with a requirement for TLR2 signaling to activate calpain. The involvement of calpain-dependent cleavage of junctional proteins in vivo was verified by identifying occludin and E-cadherin cleavage products in whole lung lysates of the infected wild type mice, but not in uninfected controls and substantially decreased cleavage products in the calpeptin treated animals (Figure 7B).
Figure 7.
Calpain activity contributes to PMN recruitment in response to P. aeruginosa infection. (A) The % of PMNs in a single cell suspension of lung was quantified in wild type or tlr2−/− pups treated with i.p. calpeptin or vehicle (Un) and intranasally inoculated with 108 CFU PAO1 or PBS. (B) Lung suspensions from representative mice in (A) were immunoblotted for occludin and E-cadherin cleavage products. (C) Adult wild type mice treated with i.p calpeptin or vehicle (Un) were intranasally inoculated with 109 CFU PAO1 or PBS for 2 h. BAL and lung cell suspensions were obtained and absolute numbers of PMNs enumerated by flow cytometry. (D) Bacterial counts in CFU/ml were determined from whole lung suspension. (E) KC mRNA expression was quantified from lung suspensions and KC protein levels determined from BAL. (A,C) Individual mouse values are shown, and the short horizontal lines indicate the median values of each group. (*P <0.05, ** P <0.01 compared with wild type PAO1 infected mice; non-parametric Mann-Whitney test).
To test the effects of calpain inhibition on the transmigration of PMNs into P. aeruginosa infected airways, bronchoalveolar lavage (BAL) experiments were performed on adult C57BL/6 mice. Mice were treated with i.p. calpeptin or vehicle and intranasally infected with P. aeruginosa for 2 h. PMNs in the airway lumen (BAL) or whole lung suspension was quantified by flow cytometry. Compared to vehicle treated controls, calpeptin treated mice had 37% fewer PMNs recruited in the whole lung and 90% fewer into the airway lumen suggesting a more pronounced effect on the epithelial junctions (Figure 7C). Calpeptin treatment did not affect the bacterial load or KC levels in the lung at this time point (Figure 7D,E). Thus, recruitment of PMNs into the murine lung and airway lumen in response to P. aeruginosa is facilitated by calpain activity and especially its affects on junctional proteins at the mucosal surface.
Discussion
Mucosal epithelial cells initiate the host response to inhaled pathogens through chemokine and cytokine production that recruit PMNs into the airway lumen to eradicate the infecting bacteria. The toll-like receptors are critical in the host defenses against pulmonary infection and P .aeruginosa infection specifically (Skerrett et al., 2004). The data presented herein indicate that TLR2 signaling also initiates the efferent limb of the inflammatory pathway by effecting changes in epithelial junctions to accommodate PMN egress into the airways. TLR2 is especially important in the airway; it is apically displayed on airway cells and broadly responsive to diverse bacterial ligands including lipoproteins, cell wall components and pili, through its association with lipid co-receptors (Soong et al., 2004). While TLR2 is not the only TLR involved in sensing luminal pathogens, it appears to be especially important in the initial stages of the innate immune response. An immediate consequence of TLR2 activation is a rapid local generation of Ca2+ fluxes which are both sufficient and necessary to evoke the distal proinflammatory signaling cascade, NF-κB activation, and IL-8 production (Chun and Prince, 2006). We propose that this TLR2 mediated Ca2+ release also promotes PMN transepithelial migration into the airway lumen.
Although extensive work has focused on understanding the migration of PMNs across endothelial cells, the mechanisms involved in PMN migration across epithelial cells remains unclear. The major PMN chemokine, IL-8, was shown to recruit PMNs from the bloodstream to the basolateral surface of airway epithelial cells, but was not involved in further mediating PMN transmigration into the airway lumen (Hurley et al., 2004). In this regard, the secretion of an arachidonic acid metabolite, hepoxilin A3, by airway epithelial cells has been proposed to direct PMN migration across airway epithelial cells (Hurley et al., 2004). Although guided by chemokines and chemoattractants, PMNs still need to migrate across a complex network of tight and adherens junction proteins. We demonstrate a dramatic loss in occludin and E-cadherin localization at the membrane in response to P3C and PAO1. Interestingly, there are no changes in transepithelial resistance, dextran permeability or bacterial invasion suggesting that these modifications to the junctions are subtle but sufficient to facilitate PMN transmigration. A qualitative change in the junction in response to P3C, PAO1 and thapsigargin is demonstrated by the increase in accessibility of the junctions to biotin, a 557 Da molecule. Much of what is known about PMN transepithelial migration comes from studies, using intestinal epithelial cells, which suggest that focal disruption of epithelial tight junctions facilitates PMN egress (Burns et al., 2003). Consistent with this model, we propose that TLR2 dependent Ca2+ fluxes signal the activation of calpains which subsequently target junction proteins to promote PMN transepithelial migration.
Calpains are ubiquitously expressed Ca2+-dependent proteases, usually activated by ATP or PKC induced Ca2+ fluxes in the micromolar (mu-calpain or calpain 1) or millimolar (m-calpain or calpain 2) range (Goll et al., 2003). When membrane associated this Ca2+ requirement is diminished to a more physiological range (Shao et al., 2006) as occurs when calpains are mobilized to the epithelial junctions. Calpains have been implicated in a number of physiological processes through their effects on integrins, platelet activity and especially cell adhesion and migration (Cuvelier et al., 2005; Franco and Huttenlocher, 2005; Kuchay and Chishti, 2007; Nuzzi et al., 2007). Calpains target cytoskeletal proteins in migrating cells such as talin, ezrin and focal adhesion kinase (Franco and Huttenlocher, 2005) and participate in cellular detachment and polarity during migration (Franco and Huttenlocher, 2005; Nuzzi et al., 2007). EGFR and ERK signaling also activate calpains in specific settings (Shao et al., 2006). The involvement of calpain 2 in regulating the cytoskeletal architecture of the lung was reported over a decade ago in cells exposed to phorbol esters (Dwyer-Nield et al., 1996). We find a similar role for calpains in response to more physiological stimuli, the generation of Ca2+ fluxes associated with TLR2 activation. Other known cytoskeletal targets of calpain, such as ezrin or talin, may also be targeted in airway epithelial cells as a consequence of TLR2 activity. The importance of proteases, especially the matrix metalloproteinases, in inflammatory processes is well established (Parks et al., 2004). MMP7 targets several epithelial components (Li et al., 2002) including E-cadherin and contributes to the shedding of its ectodomain and endocytosis (McGuire et al., 2003). MMP9 facilitates translocation of PMNs from endovascular spaces causing MMP9−/− mice to have defective PMN trafficking in response to infection (Ichiyasu et al., 2004). Occludin proteolysis is also reported to be a component of PMN-dependent inflammation (Wachtel et al., 1999). However, the observation that occludin proteolysis remains unaltered in the MMP9−/− mouse (Ichiyasu et al., 2004) indicated that additional protease(s) must target this tight junction protein. The failure of a general MMP inhibitors to block either occludin or E-cadherin cleavage in airway cells, as we demonstrate, also suggests the involvement of a different protease. While bacterial proteases or toxins could affect epithelial junctions, the ability of heat killed organisms or the synthetic ligand P3C to induce modifications of the junctions indicates that epithelial not bacterial proteases are sufficient.
Our data indicate that epithelial calpain has a major role in targeting junctional proteins and facilitating PMN transmigration. In both our in vitro and in vivo models of infection, inhibition of epithelial calpain activity blocked the ability of PMNs to traverse the epithelium. To document that this result was not due to inhibition of PMN movement, we demonstrate no effect of calpeptin on PMN chemotaxis across porous Transwells. It has been shown that constitutive calpain activity in resting PMNs blocks their ability to migrate; and calpain inhibition was shown to promote PMN movement by initiating MAPK and Rac GTPase activation (Katsube et al., 2008; Lokuta et al., 2003). Thus, epithelial calpain activity is independent from that of the PMN itself
The contribution of calpain activity in the epithelium seems especially important in the egress of PMNs from the lung into the airway lumen. While TLR2 mediated calpain activation appears to account for only 30% to 40% of PMN recruitment into the lung, calpain inhibition blocked the majority (90%) of PMN mobilization into the airway lumen demonstrating the importance of calpain activity in PMN egress across the airway epithelium. The remaining TLR2 independent signaling may be due to the TLR5 ligand, flagella, which also activate Ca2+ fluxes in airway cells (Ratner et al., 2001).
Occludin was one of the first components of tight junctions to be identified but its precise role in maintaining or regulating the epithelial barrier remains incompletely defined (Feldman et al., 2005). Occludin interacts with many components of the junctional complex (Feldman et al., 2005), binds directly to the ZO-1 scaffold (Muller et al., 2005), diffuses rapidly within the tight junctions at steady state (Shen et al., 2008), and internalizes in response to various stimuli (Yu and Turner, 2008). We observed a decrease in the phosphorylated form of occludin which is the predominate form of occludin expressed at the tight junctions (Feldman et al., 2005). The kinetics of this decrease coincided with the association of occludin with calpain and the appearance of a 45 kD fragment of occludin. We speculate that the phosphoryated 80kD form of occludin is the main target for calpain as the levels of the non-phosphorylated 60kD form of occludin are unchanged in response to stimulation. The occludin null mouse has pleotropic alterations in inflammatory responses (Saitou et al., 2000); paracellular permeability to specific compounds is affected but TER is maintained and there is no real defect in the tight junction barrier (Yu et al., 2005). Loss of occludin affects Rho GTPase activity and actin polymerization (Hopkins et al., 2003; Shen and Turner, 2005). Our studies are consistent with these observations and demonstrate displacement of occludin from junctions in response to TLR2 signals without defects in TER or permeability to dextrans. These observations suggest that calpain mediated processing of occludin may interrupt interactions with cytoskeletal binding partners and influence the ability of the cytoskeleton to accommodate PMN transmigration without breaching the integrity of the epithelial barrier. Our data also suggests that calpain targets the N-terminal intracellular domain of occludin. This is consistent with previous studies which demonstrate that occludin mutations in the N-terminal cytoplasmic domains increase PMN migration across MDCK cells, whereas deletion of the C-terminal cytoplasmic domain did not have an effect (Huber et al., 2000). Furthermore, expression of an occludin N-terminal truncation mutant was shown to decrease barrier function in murine epithelial cells (Bamforth et al., 1999), consistent with our mapping data.
E-cadherin is a known calpain substrate with calpain cleavage sites on its cytoplasmic tail between residues 782 and 787 (Rios-Doria et al., 2003). The 100 kD truncated forms of E-cadherin generated by epithelial calpain in airway cells have been reported previously in other model systems (Rios-Doria and Day, 2005). E-cadherin processing can be accomplished by a number of proteases (D’Souza-Schorey, 2005) and is an important component of constitutive E-cadherin endocytosis and recycling (Bryant et al., 2007; Bryant and Stow, 2004). There are numerous cadherin binding partners in the junctional complex and an extensive literature details these interactions and effects on endothelial permeability (Mehta and Malik, 2006). E-cadherin associates directly with β-catenins and hence to actin and the cytoskeleton (Mehta and Malik, 2006). Mutant VE-cadherin lacking the extracellular domain results in impaired PMN migration in response to chemotactic stimuli (Orrington-Myers et al., 2006). Interactions of cadherins and the small GTPases that regulate actin polymerization affect paracellular permeability in endothelial cells (Hordijk, 2003). Calpain mediated cleavage of epithelial E-cadherin, along with occludin, may ultimately affect the deformability of the cytoskeleton to enable the paracellular junction to accommodate migrating PMNs.
While TLR2 is not usually considered the major innate immune effector for Gram negative organisms, at least for P. aeruginosa, TLR2 activation in the airway epithelium coordinates both the afferent and efferent limbs of the initial inflammatory response. Not only does the airway epithelial cell produce IL-8 to direct PMN recruitment, the same signaling cascade modulates the tight junction to accommodate PMN egress without breaching the epithelial barrier. This pathway may provide a useful pharmacologic target in pulmonary infection to selectively limit PMN recruitment into the lung, without entirely compromising host defenses to bacterial infection.
Methods
Cell lines and bacteria
1HAEo- and 16HBE cells (D. Gruenert, California Pacific Medical Center Research Institute, San Francisco, CA), were grown as previously detailed (Ratner et al., 2001). Human small airway epithelial cells in primary culture (SAEC) were obtained from (Lonza/Clonetics) and grown as directed. The TLR2 WT, TLR2 Y616A/Y761A (TLR2 YY) mutant expressing cells and TLR2 siRNA cell lines were generated as previously described (Chun and Prince, 2006). pCS2+MT h Occludin (C-terminal myc6 tagged occludin) was provided by Dr. Otmar Huber (Charite Universita, Berlin) and pmRFP1-h Occludin (N-terminal tagged RFP occludin) was provided by Dr. Jerrold Turner (University of Chicago, IL). 1HAEo- cells were transiently transfected with pCS2+MT h Occludin or pmRFP1-h Occludin using an Amaxa Nucleofector following manufacturer’s instructions. P. aeruginosa PAO1 resuspended in minimum essential media (MEM) at a density of 108 CFU/ml was heat killed by incubating at 60 °C for 1 h.
Confocal microscopy
16HBE cells were grown on 3 μm pore size Transwell-Clear filters (Corning-Costar) with an air–liquid interface to form polarized monolayers. For confocal imaging cells were fixed with 4% paraformaldehyde, blocked with 5% normal donkey serum and incubated at 4º C overnight with the polyclonal pan-calpain (Santa Cruz Biotechnology), monoclonal occludin (Invitrogen/Zymed) and/or monoclonal E-cadherin (BD Pharmingen) antibodies. Alexa Fluor 594 conjugated and Alexa Fluor 488 conjugated secondary antibodies (Invitrogen/Molecular Probes) were added at room temperature for 1 h. After washing, filters were removed from Transwells using a scalpel, mounted with Vectashield with DAPI (Vector Laboratories) onto glass slides and imaged using a Zeiss LSM 510 Meta scanning confocal microscope. For biotin labeling experiments 1 mg/ml EZ-Link Sulfo-NHS-LC-Biotin was added apically for 30 min at 4°C before fixing the cells. Alexa Fluor 555 conjugated streptavidin (Molecular Probes) was added for detection of biotin (Guttman et al., 2006).
In vitro calpain assay
Occludin, E-cadherin, JAM-1 and claudin-1 were immunoprecipitated (IP) as described below. IP were washed using a high salt wash buffer (500 mM NaCl, 50 mM Tris and 1%NP40) and resuspended in 100 μl of calpain assay buffer (100 mM Hepes pH 8.0, 50 mM NaCl, 10 mM EGTA, and 0.1% Triton X100). 1 μg of exogenouse calpain 1 (Calbiochem) was added to 7 μl of the IP reaction and incubated with 20 mM CaCl2 for 0, 0.5, 1, or 2 min at room temperature. An equivalent volume of calpain assay buffer was added to reactions that did not receive CaCl2. The reaction was terminated by adding 2X Laemmli buffer containing 10 mM ALLN, resolved on a gradient protein gel and analyzed by immunoblot using anti-occludin, anti-JAM-1 and anti-claudin-1 antibodies from Zymed, anti-calpain antibody from Santa Cruz Biotechnologies or a monoclonal anti- E-cadherin antibody from BD Pharmingen.
Calpain activity assay
Confluent monolayers of 1HAEo- cells or SAECs were loaded with 20 μM t-BOC-L-leucine-L-methionine amide (Boc-LM-CMAC), a calpain specific membrane permeable fluorogenic substrate. Cells were stimulated with heat killed PAO1 (108 CFU) or P3C (15 μg/ml) and fluorescence was quantified at ex 360 nm and em 465 nm with a Spectrafluor Plus fluorimeter (Tecan). For the indicated experiments, cells were preincubated and stimulated in the presence of 20 μM calpeptin and compared with DMSO vehicle control.
Immunoprecipitation and Immunoblotting
1HAEo- cells or SAECs were grown to confluence on 10 cm plates, stimulated with heat killed PAO1 (108 CFU) or P3C (15 μg/ml), and whole cell lysates made using 60 mM n-octyl-β-D-glucopyranoside (OGP) in TBS (0.1 M Tris-HCl and 0.15 M NaCl, pH 7.8) containing Complete Mini protease inhibitor tablets (Roche), 100 μM ALLN, 1 mM sodium orthovanadate and 100 μM sodium fluoride. To detect phosphorylated occludin, higher concentrations of phosphatase inhibitors, 2 mM sodium orthovanadate and 200 mM sodium fluoride were added to the lysis buffer. Monoclonal anti-E-cadherin (BD Pharmingen), polyclonal anti-pan calpain (Santa Cruz Biotechnologies) or monoclonal (mouse) anti-occludin (Invitrogen/Zymed) antibodies were used for immunoprecipitations followed by the addition of Protein G Agarose beads (Invitrogen)(Chun and Prince, 2006). For biotin labeling experiments 1 mg/ml EZ-Link Sulfo-NHS-LC-Biotin was added for 30 min at 4°C before OGP cell lysis. NeutrAvidin Agarose Resin (Thermo Scientific) was used for biotin immunoprecipitations according to manufacturer’s instructions. Immunodetection was performed using a monoclonal anti-E-cadherin antibody from BD Pharmingen, polyclonal (rabbit) anti-occludin antibody from Invitrogen, anti-RFP conjugated to HRP and anti-myc conjugated to HRP antibodies from Abcam, and anti-pan calpain, anti-calpain 1 and anti-calpain 2 antibodies from Santa Cruz Biotechnology.
Calpain siRNA
1HAEo- cells were transiently transfected with scrambled (control) or calpain 1 and 2 siRNA Smart pool oligos (Dharmacon). Transfection was carried out using an Amaxa Nucleofector following manufacturer’s instructions. Briefly, 106 1HAEo- cells were electroporated in 100 μl Buffer L with 1.5 μg of scrambled or siRNA oligo and transferred into pre-warmed 6 well tissue culture dishes. Cells were incubated at 37 °C, 5% CO2 for 48 h before performing experiments.
Neutrophil isolation and Migration Assays
Human PMNs were isolated according to standard techniques from venous blood from healthy consenting adults in accordance with a protocol approved by the Institutional Review Board of Human Subjects at Columbia University (IRB-AAAC5450). PMNs were isolated using dextran sedimentation and Hypaque-Ficoll (Sigma) density-gradient separation following hypotonic lysis of erythrocytes as previously described(Boyum, 1968). Purified PMNs were counted on a hemocytometer and incubated in DMEM to a concentration of 107 cells/ml. Migration assays were performed across 16HBE monolayers plated on the underside of 3 μm transwells which were coated with minimal essential media containing 0.01 mg/ml fibronectin, 0.03 mg/ml bovine collagen type I, 0.1 mg/ml BSA. After allowing the cells to adhere overnight, transwells were flipped over and inserted into 24 well plates. 500 μl of media was added to the lower chamber (apical surface of the cell) and 100 μl of media added to the transwell (basolateral surface of the cell). When the cells became confluent, they were polarized with an air liquid interface for 5 days by removing the 500 μl of media in the lower chamber. Selected wells were preincubated for 30 min with 20 μM calpeptin in the apical and basolateral chambers. Untreated controls received equal volume of DMSO. Monolayers were stimulated with 250 μl heat killed PAO1 (108 CFU) or P3C (15 μg/ml) at the apical surface (lower chamber) for 4 h at 37° C. Media in the basolateral chamber (in the transwell) containing calpeptin was removed and 106 neutrophils (in 100 μl) were then added to the basolateral surface for 2 h. Simultaneously, calpeptin was added to the apical chamber to a final concentration of 20 μM to maintain epithelial exposure to inhibitor. Neutrophils from the apical chamber were collected and centrifuged for 6 min at 1500 rpm at 4 °C. Supernatant was discarded and the cell pellet resuspended in 50 mM sodium phosphate buffer pH 7.0. The cell suspension was freeze thawed three times and stored at −80 °C until used for myeloperoxidase assay.
For migration assays in the absence of an epithelial monolayer, 106 calcein loaded PMNs were added in fresh DMEM to the top chamber of a 3 μm pore Transwell coated with minimal essential media containing 0.01 mg/ml fibronectin, 0.03 mg/ml bovine collagen type I, 0.1 mg/ml BSA. PMNs were induced to migrate for 1 h toward live PAO1 (108 CFU), heat-killed PAO1 (108 CFU) or the chemoattractant fMLP (10 nM) which were added to the lower chamber of the tissue culture well in the presence or absence of 20 μM calpeptin. Fluorescence of the migrated PMNs was quantified on a fluorescent plate reader (Tecan Infinite M200) at Ex 490, Em 515. A standard curve was generated by counting labeled PMNs on a hemacytometer, serially diluting the cells and measuring fluorescence.
Myeloperoxidase Assay
Myeloperoxidase (MPO) activity was measured using the human MPO activity assay as described by manufacturer (R&D Systems). Briefly, 20 μl of the cell suspension was added to 30 μl of 0.00667% H2O2 and 50 μl of 100 mM guaiacol in a 96 well plate and the oxidized guaiacol was read on a microplate reader at 470 nm. PMNs were counted on a hemacytometer, serially diluted, and analyzed for MPO activity. A standard curve was generated to determine the number of neutrophils from MPO activity.
Mouse Models of Infection
Seven day old C57BL/6 wild type or tlr2−/− pups (Jackson Laboratories) were intranasally inoculated with 10 μl PAO1 (108 CFU) (Tang et al., 1996). Control mice received 10 μl of PBS. Mice were pretreated with 20 mg/kg calpeptin or DMSO in PBS by intraperitoneal route 16 h and 2 h before intranasal inoculation and a third dose was administered by i.p. route 30 min post infection. Lungs were harvested 4 h after infection and cell suspensions used for PMN detection and immunoblotting. Immunoblots were performed on 10 μg of protein obtained by lysing lung cells in OGP buffer as described above for 1HAEo- cells. Adult C57BL/6 wild type mice (Charles River Laboratories) were anesthetized with ketamine/xylazine and intranasally inoculated with 25 μl PAO1 (109 CFU) or PBS. Mice were pretreated with 20 mg/kg calpeptin or DMSO in PBS by intraperitoneal route 2 h and immediately before intranasal inoculation. BAL was obtained and lungs harvested 2 h after infection and used for PMN detection, determination of bacterial CFU/ml, and KC protein levels. Animal experiments were performed in accordance with the guidelines of the IACUC at Columbia University (protocol number AAAA5999).
Neutrophil Detection
BAL or lung cell suspensions were double stained with phycoerythrin (PE)-labeled anti-CD45 (to detect leukocytes) and fluorescein isothiocyanate (FITC)-labeled anti-Ly6C/Ly6G antibodies (to detect neutrophils) (BD Pharmingen) and analyzed by flow cytometry with a FACSCalibur using Cell Quest software (Becton Dickinson)(Gomez et al., 2004). Irrelevant, isotype-matched antibodies were used as a control. Cells were gated on the basis of their forward scatter and side scatter profile and analyzed for double expression of CD45 and Ly6C/Ly6G.
KC expression
For KC mRNA quantification, the upper right quadrant of the mouse lungs were obtained and stored in RNAlater (Qiagen). RNA was isolated using the Qiagen RNeasy mini kit. cDNA was made from 1 μg RNA using the iScript synthesis kit (Bio-Rad). For quantitative real-time PCR, amplification was done in an Applied Biosystems Step One Plus using the Power SYBR Green master mix (Applied Biosystems). Primers used for KC amplification were 5’-CCGCGCCTATCGCCAATGAGCTGCGC-3’ and 5’-CTTGGGGACACCTTTTAGCATCTTTTGG-3’, and 35 cycles were run with denaturing at 95 ºC for 8 s, amplification at 56 ºC for 10 s, and extension at 72 ºC for 12 s. Actin was amplified on each individual sample and used as control for standardization. Primers used for actin amplification were 5’-GTGGGGCGCCCCAGGCACCA-3’ and 5’-CGGTTGGCCTTGGGGTTCAGGGGGG-3’, and 35 cycles were run with denaturing at 95 ºC for 8 s, amplification at 63 ºC for 10 s, and extension at 72 ºC for 12 s. KC protein levels in BAL were determined by ELISA according to the manufacturer’s instructions (R&D Systems).
Statistical analysis
Samples with normal distribution were analyzed by Student’s t test. Mouse samples that did not follow normal distribution were compared using the non-parametric Mann-Whitney test. Differences between groups were considered significant at P < 0.05. Statistical analysis was determined using GraphPad Instat version 3.0 (GraphPad).
Supplementary Material
Acknowledgments
We thank G. Soong (Columbia University) for excellent technical assistance. Confocal microscopy was performed at the Herbert Irving Optical Microscopy facility at Columbia University. This study was supported by NIH grant HL73989 to AP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112(Pt 12):1879–1888. doi: 10.1242/jcs.112.12.1879. [DOI] [PubMed] [Google Scholar]
- Bojarski C, Weiske J, Schoneberg T, Schroder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- Chun J, Prince A. Activation of Ca2+-dependent signaling by TLR2. J Immunol. 2006;177:1330–1337. doi: 10.4049/jimmunol.177.2.1330. [DOI] [PubMed] [Google Scholar]
- Constantin D, Cordenier A, Robinson K, Ala’Aldeen DA, Murphy S. Neisseria meningitidis-induced death of cerebrovascular endothelium: mechanisms triggering transcriptional activation of inducible nitric oxide synthase. J Neurochem. 2004;89:1166–1174. doi: 10.1111/j.1471-4159.2004.02393.x. [DOI] [PubMed] [Google Scholar]
- Cuvelier SL, Paul S, Shariat N, Colarusso P, Patel KD. Eosinophil adhesion under flow conditions activates mechanosensitive signaling pathways in human endothelial cells. J Exp Med. 2005;202:865–876. doi: 10.1084/jem.20041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trend Cell Biol. 2005;15:19–26. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Dwyer-Nield LD, Miller AC, Neighbors BW, Dinsdale D, Malkinson AM. Cytoskeletal architecture in mouse lung epithelial cells is regulated by protein-kinase C-alpha and calpain II. Am J Physiol. 1996;270:L526–534. doi: 10.1152/ajplung.1996.270.4.L526. [DOI] [PubMed] [Google Scholar]
- Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Fettucciari K, Fetriconi I, Mannucci R, Nicoletti I, Bartoli A, Coaccioli S, Marconi P. Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18:521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- Guttman JA, Samji FN, Li Y, Vogl AW, Finlay BB. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect Immun. 2006;74:6075–6084. doi: 10.1128/IAI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- Hordijk P. Endothelial signaling in leukocyte transmigration. Cell Biochem Biophys. 2003;38:305–322. doi: 10.1385/cbb:38:3:305. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Balda MS, Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem. 2000;275:5773–5778. doi: 10.1074/jbc.275.8.5773. [DOI] [PubMed] [Google Scholar]
- Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- Ichiyasu H, McCormack JM, McCarthy KM, Dombkowski D, Preffer FI, Schneeberger EE. Matrix metalloproteinase-9-deficient dendritic cells have impaired migration through tracheal epithelial tight junctions. Am J Respir Cell Mol Biol. 2004;30:761–770. doi: 10.1165/rcmb.2003-0370OC. [DOI] [PubMed] [Google Scholar]
- Katsube M, Kato T, Kitagawa M, Noma H, Fujita H, Kitagawa S. Calpain-mediated regulation of the distinct signaling pathways and cell migration in human neutrophils. J Leuk Biol. 2008 doi: 10.1189/jlb.0907664. [DOI] [PubMed] [Google Scholar]
- Kuchay SM, Chishti AH. Calpain-mediated regulation of platelet signaling pathways. Curr Opin Hematol. 2007;14:249–254. doi: 10.1097/MOH.0b013e3280ef68f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Lokuta MA, Nuzzi PA, Huttenlocher A. Calpain regulates neutrophil chemotaxis. Proc Nat Acad Sci USA. 2003;100:4006–4011. doi: 10.1073/pnas.0636533100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- Nuzzi PA, Senetar MA, Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell. 2007;18:795–805. doi: 10.1091/mbc.E06-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrington-Myers J, Gao X, Kouklis P, Broman M, Rahman A, Vogel SM, Malik AB. Regulation of lung neutrophil recruitment by VE-cadherin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L764–771. doi: 10.1152/ajplung.00502.2005. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA, Day ML. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- Rios-Doria J, Day ML. Truncated E-cadherin potentiates cell death in prostate epithelial cells. Prostate. 2005;63:259–268. doi: 10.1002/pros.20179. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Shao H, Chou J, Baty CJ, Burke NA, Watkins SC, Stolz DB, Wells A. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008 doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against P. aeruginosa but not S. aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Contribution of specific P. aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112(Pt 23):4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Wan H, Winton HL, Soeller C, Stewart GA, Thompson PJ, Gruenert DC, Cannell MB, Garrod DR, Robinson C. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur Respir J. 2000;15:1058–1068. doi: 10.1034/j.1399-3003.2000.01514.x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta. 2008;1778:709–716. doi: 10.1016/j.bbamem.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Li X, Zeng R, Gorodeski GI. Changes in tight junctional resistance of the cervical epithelium are associated with modulation of content and phosphorylation of occludin 65-kD and 50-kD forms. Endocrinol. 2006;147:977–989. doi: 10.1210/en.2005-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.