Abstract
Despite intensive efforts for its eradication, addiction to both legal and illicit drugs continues to be a major worldwide medical and social problem. Drug addiction is defined as a disease state in which the body relies on a substance for normal functioning and develops physical dependence leading to compulsive and repetitive use despite negative consequences to the user’s health, mental state or social life. Psychoactive substances such as cocaine, nicotine, alcohol, and amphetamines are able to cross the blood-brain barrier once ingested and temporarily alter the chemical balance of the brain. Current medications used for the treatment of dependence are typically agonists or antagonists of the drugs of abuse. The complex interrelations of the neuronal circuits have made it difficult to accurately predict the actions of potential agonist/antagonist drugs and have led to undesirable side effects within the central nervous system. Nearly forty years ago, a handful of groups began to explore the possibility of utilizing an individual’s own immune machinery to counteract the effects of drug exposure in an approach later termed by our laboratory, immunopharmacotherapy. Immunopharmacotherapy aims to use highly specific antibodies to sequester the drug of interest while the latter is still in the bloodstream. Thus, creation of the antibody-drug complex will blunt crossing of the blood brain barrier (BBB) not only counteracting the positive reinforcing effects of the drug but also preventing any detrimental side effects on the CNS. In the present mini-review we aim to present a focused summary, including relevant challenges and future directions, of the current state of cocaine and nicotine vaccines as these two programs have been the most successful to date.
Keywords: nicotine, cocaine, vaccination, passive immunization, catalytic antibodies, monoclonal antibodies
Introduction
Despite intensive efforts for its eradication, addiction to both legal and illicit drugs continues to be a major worldwide medical and social problem. Drug addiction is defined as a disease state in which the body develops physical dependence leading to compulsive and repetitive use despite negative consequences to the user’s health, mental state or social life. Psychoactive substances such as cocaine, nicotine, alcohol, and amphetamines are able to cross the blood-brain barrier when ingested and once there hijack common reward-dependent learning circuitry that originally evolved to respond to basic, rudimentary and fundamental stimuli such as food, water and sex. Addiction involves multiple, complex neural adaptations that develop with different time courses depending on the substance, frequency of use, means of ingestion, intensity of pleasure and the individual’s genetic and psychological susceptibility (Kauer & Malenka, 2007).
Addictions often have both physical and psychological components and are typically handled as part of a psychosocial and rehabilitation program. Yet, the high relapse rates amongst drug abusers seeking treatment has made it imperative to develop new treatment options for this disease. Current medications used for the treatment of dependence are typically direct and indirect agonists or antagonists of the drugs of abuse. Despite a deeper understanding of the molecular basis of addiction; the generation of targeted “designer drugs” for the treatment of drug dependency has remained elusive. The complex interrelations of the neuronal circuits have made it difficult to accurately predict the actions of agonist/antagonist drugs and have led to undesirable side effects within the central nervous system (CNS). As early as the 1970s, several groups had been exploring the possibility of utilizing the individual’s own immune machinery to counteract the effects of drug exposure (Spector, 1971; Berkowitz & Spector, 1972; Bonese et al, 1974) in an approach later termed by our laboratory, immunopharmacotherapy.
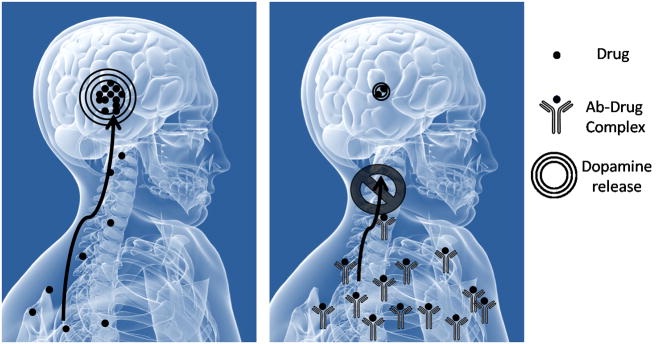
Immunopharmacotherapy aims to use highly specific antibodies to sequester the drug of interest while the latter is still in the bloodstream. Thus, creation of the antibody-drug complex will blunt crossing of the blood brain barrier (BBB) not only counteracting the reinforcing effects of the drug but also preventing any detrimental side effects on the CNS (see Fig. 1). In addition, presence of circulating antibodies could generate a gradient towards removal of the drug from the brain, as drug can freely cross the BBB. One of the first indications that this technology could be successfully applied came in 1974, when rhesus monkeys in a heroin self-administration model showed how treatment with a morphine-bovine serum albumin (BSA) conjugate could significantly reduce their self-administration patterns (Bonese et al, 1974). Additionally, it was noted that higher heroin dosages would restitute the behavioral patterns seen before immunization and thus were able to overcome the protective effects of the anti-heroin antibodies. At the time of the Bonese study, the scientific community, unfortunately, did not embrace this seminal study and it has only been in the last twenty years that the field of immunopharmacotherapy has flourished.
Figure 1.
The principle of Immunopharmacotherapy. Neutralizing antibodies bind the drug in the periphery and prevent crossing of the blood-brain-barrier thus minimizing reinforcing effects.
It has quickly become apparent that success of an immunological strategy greatly relies on a few parameters; the magnitude of the antibody concentration, termed titer, elicited as well as the affinity and specificity of the antibodies towards a certain molecular structure. The immune system has not evolved to generate a response for small molecules less than about 10 kDa, a limit that is well beyond the molecular weight of any drug of abuse. Thus, small molecule drugs must be covalently linked to a carrier protein in order to trigger an immune response. This is typically achieved by the synthesis of a chemical derivative of the drug, hereby termed hapten, which incorporates a linker arm with a terminal reactive moiety used for protein appendage. The intensity of response, measured as antibody concentration, is susceptible to variations in carrier protein identity, as well as the type of adjuvant used in the antigenic formulation. Additionally, antibody affinity and specificity are directly related to successful hapten presentation which is impacted by hapten design, linker length and attachment site. Once the identity of hapten design, carrier protein and adjuvant has been ascertained protection may be conferred either via an active or passive immunization strategy.
In an active immunization schedule, the appropriate antigenic drug-protein conjugate is directly administered, which causes T and B cell activation, leading to generation of specific antibodies. Traditionally, this approach is able to confer longer lasting protection through immunological memory with minimal treatment compliance and thus is very cost effective. Nonetheless, limitations of active immunity include first that some exposure time, ranging from weeks to months, is required for interaction of the antigen and immune system before protection is conferred and second that a significant amount of individual variability will be present in the type of mounted response.
In contrast, passive immunization offers immediate protection via injection of pre-formed high-affinity antibodies, typically of monoclonal identity (mAbs), making this approach particularly relevant in a drug overdose scenario as well as during the critical time points in the addiction-relapse cycle. Nonetheless, passive immunity is an expensive technique and its effects are shorter lasting depending on the antibody half-life and are limited to the amount of antibodies supplied. Multiple reviews have been published on the use of immune therapy against different drugs of abuse such as cocaine, nicotine, phencyclidine, methamphetamine, and heroin (Bunce et al, 2003; Kantak, 2003; Meijler et al, 2004; Kosten & Owens, 2005). In the present mini-review we aim to present a focused summary, including relevant challenges and future directions, of the current state of cocaine and nicotine vaccines as these two programs have been the most successful to date.
Cocaine
(−)-Cocaine is a powerful psycho-stimulating substance listed as a Schedule II agent by the Drug Enforcement Agency, and may well be the most reinforcing of all addictive drugs (Withers et al, 1995; Meijler et al, 2004). It is a tropane alkaloid extracted from the leaves of the South American Erythroxylon coca plant and commonly abused either as the hydrochloride salt or free base “crack” form. According to the 2007 National Survey on Drug Use and Health, an estimated 2.1 million Americans (0.8% of population) are current abusers of cocaine, of which 0.9 million are recent initiates, numbers that have remained stable if unacceptably high since 2002 (US Substance Abuse and Mental Health Services Administration, 2008; National Institute on Drug Abuse, 2004). While the cocaine addicted population is much smaller than that of other drugs, the social impact is disproportionately large. Chronic consumption of cocaine leads to social incapacitation and is accompanied by serious physical and psychological consequences such as cardiac arrhythmia, stroke, seizures, heart attack, respiratory failure, hallucinations, mood disturbances and paranoia (National Institute on Drug Abuse, 2004). In rare instances, deaths have been reported after the first use. In addition, crack-cocaine is one of the most abused substances among pregnant women, which puts users at an increased risk for contracting new HIV and hepatitis infections via needle-sharing and heightened risk behavior. Cocaine is able to cross the placenta barrier and interfere with fetal development. The full extent of the effects of prenatal drug exposure is not completely known, yet there is a consensus that “crack-babies” are prone to be premature, present smaller body weight/length at birth as well as deficits in cognitive performance, information processing and attention to tasks later in life (National Institute on Drug Abuse, 2004; Chasnoff et al, 1985; Singer et al, 2002).
Cocaine is the most common drug problem cited by patients entering treatment for illicit drug use and thus has stimulated extensive efforts to develop successful therapies. Yet, to date no medication has been approved for specific treatment of cocaine addiction. A number of serotonin and dopaminergic agonists/antagonists have been used with limited success and adverse side effects (European Monitoring Centre for Drugs and Drug Addiction, 2007). Additionally, due to the hedonic dysregulation (Koob & Le Moal, 1997) experienced, general antidepressants have shown some benefit. Retention rates in behavioral programs are low, and relapse rates among primary cocaine users are generally high (Preti, 2006). Since the mid 1990s, several research groups have actively pursued an immunopharmacotherapeutical approach to address the cocaine epidemic with significant promise leading to human phase I and II clinical trials. Both active and passive immunization strategies against cocaine have been extensively investigated. Active immunization strategies have been solely tailored towards prevention of relapse for individuals in the process of quitting, whereas passive immunization offers the additional possibility of rescuing survival rates of individuals after overdose and acute intoxication.
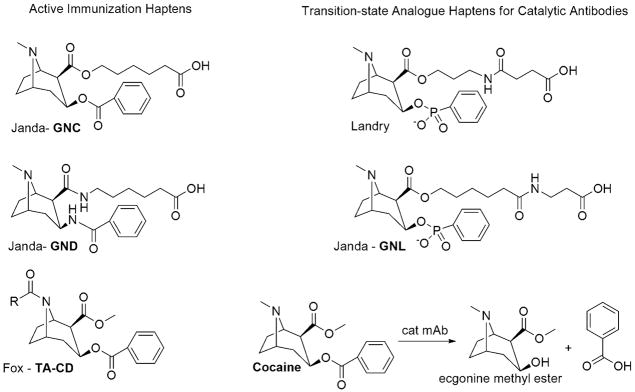
The first report of a stable cocaine immunoconjugate, termed GNC, with the specific intent to treat cocaine addiction was published by our group in 1995. In this study, active immunization with GNC, (see Fig. 2), coupled to the carrier protein Keyhole Limpet Hemocyanin (KLH) elicited elevated antibody titers with high affinity for cocaine (Kd ~1μM), which were able to significantly reduce cocaine levels in the striatum and cerebellum while also suppressing locomotor activity and stereotyped behaviors in rats (Carrera et al, 1995). Later studies indicated GNC-KLH antibody titers were sufficient to block reinstatement induced by a single dose of drug, yet were overcome by increasing the dose or frequency of cocaine intake (Carrera et al, 2000). A second generation design strategy, termed GND, (Fig. 2), aimed to increase hapten stability via use of amide bonds (Carrera et al, 2001). Excitingly, GND-KLH conjugate provided greater and longer-lasting protection against cocaine, yet antibody titers remained in the 25 000 range suggesting that high and repeated doses of administered drug would also overcome their protective effects. Further studies aiming to improve hapten design via variation of the linker attachment site were not fruitful; eliciting antibody titers in the same region as GNC, yet, showing significantly lower affinity for cocaine (Ino et al, 2007). An elegant variation by Schabacker et al. attempted to overcome hapten instability by exploring the use of an antibody molecule, whose internal configuration mimics that of the cocaine molecule, as the antigen to elicit anti-idiotypic antibodies (Ab2). In their study anti-cocaine monoclonal antibodies (Ab1) were produced by active immunization of a cocaine-protein conjugate. These Ab1 monoclonal antibodies were subsequently linked to KLH and vaccination of this conjugate induced formation of Ab2β which presented anti-cocaine effects upon drug challenge in mice (Schabacker et al, 2000). Yet, behavioral and pharmacokinetic tests have not been conducted to date.
Fig. 2.
Cocaine like haptens used for active vaccines/monoclonal antibody production and transition-state analogue haptens for the generation of catalytic antibodies for the degradation of cocaine.
A later effort complementing our own studies was carried out by Fox et al. who conjugated norcocaine, (Fig. 2), to BSA; a strategy that eventually led to human clinical trials. In mice, antibody titers over 100 000 were observed and held for up to 4 months (Fox et al, 1996). Competition ELISA showed that cocaine, norcocaine, and cocaethylene (a toxic derivative produced with combined use of ethanol and cocaine) were exclusively bound. In further studies norcocaine-recombinant cholera toxin B conjugates were used to examine the cocaine self-administration behavior of rats (Kantak et al, 2000). Drug seeking behavior was significantly affected by immunization, but only for animals with total serum antibody amounts greater than 0.05 mg/mL. In these animals, even drug infusions typically high enough to produce convulsions and death produced only mild stereotypic locomotor activity and low drug-seeking behavior. Furthermore, there was no evidence of surmountability of the conferred protective effects even at high doses.
On the basis of these findings, TA-CD was carried into human clinical testing by Xenova (now Celtic Pharma). Results from phase I clinical trial showed that TA-CD was well tolerated both locally and systemically after three injections over a 2-month period (Kosten et al, 2002). Detectable amounts of anti-cocaine antibodies appeared after one month, reached maximum levels after the third vaccination and remained high for approximately 4 months before completely disappearing after one year. As expected, substantial amounts of subjective variability in the magnitude of immune response was observed, yet higher doses of TA-CD correlated with higher antibody levels. For the lowest vaccine dose the amount of specific anti-cocaine antibodies was only 0.003 mg/mL, well below the target level predicted to be minimal to antagonize typical cocaine doses. Further clinical trials included an improved dose administration schedule. As of 2008, four phase II trials including 161 patients have been completed (Celtic Pharma TA CD). Trial IIb was the largest study to date, consisting of a double-blinded placebo controlled study in 114 patients, and assessed the efficacy of vaccination against cocaine users undergoing methadone therapy for treatment of heroin addiction. While some indications of efficacy were present, the primary end-point defined as abstinence from cocaine use for three consecutive weeks was not met. It was hypothesized that a higher than expected placebo effect might be partly to blame for this shortcoming (Celtic Pharma, 2006). TA-CD was expected to enter a larger phase II study in 2007 and Celtic Pharma anticipates the submission of the vaccine dossier to the FDA and EMEA by 2010.
Passive immunization with high-affinity anti-cocaine monoclonal antibodies (mAbs) has the distinct benefit of using a well-defined, homogeneous antibody population to probe the advantages of vaccination. Murine mAbs have been used to demonstrate the correlation between Abs dosage and self-administration (Fox et al, 1996; Kantak et al, 2000) as well as to examine the reduced entry of cocaine into the brain and corresponding behavioral and pharmacokinetic effects (Carrera et al, 2000, 2001). Furthermore, studies with mAb GNC92H2 have shown positive results in blocking cocaine toxicity in an overdose model (Carrera et al, 2005). Humanized versions of mAb with modest cocaine affinity have also been reported (Redwan et al, 2003). Moreover, a powerful variation of passive immunization invokes the use of catalytic monoclonal antibodies which not only sequester the cocaine molecule in the bloodstream but also metabolize the drug into inactive products thereby diminishing the drug’s potential effect on the CNS. Due to their enzymatic nature, after cocaine is hydrolyzed to ecgonine methyl ester, the antibody becomes free for degradation of additional molecules. Landry et al were the first to generate a potent catalytic monoclonal antibody, 15A10, against cocaine using a phosphonate monoester hapten that mirrors the transition state of the hydrolysis reaction, (Fig. 2), (Landry et al, 1993; Yang et al, 1996). After passive immunization of 15A10, rats showed increased survival rates after lethal infusions of drug, as well as reduced self-administration behavior but only for a 48–72 hour window post-injection (Mets et al, 1998; Baird et al, 2000). Shortly after, our group published the discovery of several catalytic antibodies arising from several transition-state analogs, (Fig. 2), with mAb GNL3A6 in particular being the most efficient (Matsushita et al, 2001). As with 15A10, the kinetic parameters of GNL3A6 fell short from what would be required for therapeutic efficacy.
Cocaine is naturally hydrolyzed by butyrylcholinesterase (BChE) and liver carboxyesterase h-CE2 and has an in vivo elimination half-life of 30–60 min in humans. Therefore, for enzymatic therapy to be viable the catalytic efficiency, kcat/Km constant, must be ~104 M−1s−1 so that extensive clearance of cocaine is accomplished within minutes to blunt its pharmacological effects. Recently, new modeling efforts have been successfully directed towards the creation of high-activity mutants of BChE, naturally more effective against the biologically inactive (+)-cocaine, via a computational design strategy based on the modeling of rate determining steps for cocaine hydrolysis (Zheng et al, 2008). Additionally, we have spent significant efforts towards the optimization of the catalytic constants for GNL3A6 (McKenzie et al, 2007). However, an inability to over-express sufficient quantities of protein required for the monitoring of cocaine hydrolysis proved to be a significant hurdle. To circumvent these problems and, thus, identify novel anti-cocaine catalytic antibodies with inherent bacterial expression, a biased scFv phage library was panned against the transition state analog GNL, (Fig. 2). The key for this experiment relied in use of a pre-enriched antibody library generated from mice immunized with GNL. Six new single chain antibody fragments were identified, four of which exhibited catalytic activity. scFv 3F3 showed sequence and catalytic efficiency homology with GNL3A6, and its catalytic activity was improved via directed evolution techniques. A 3F3 double mutant specifically showed marked rate enhancement over wild-type. Most likely a combination of novel hapten design coupled with directed evolution will be needed to uncover catalytic antibodies with proficiencies needed to pharmacokinetically impact cocaine’s psychoactive properties.
Finally, our group has provided a new bioengineering way forward for immunopharmacotherapy wherein filamentous bacteriophage are administered to deliver cocaine-sequestering antibodies (Carrera et al, 2004b). Filamentous bacteriophage are viruses with enhanced genetic flexibility, which allow for foreign cDNA to be fused to the genes encoding surface proteins pIII and/or pVIII and thus be displayed. High titers of bacteriophage can be produced in bacterial culture. The filamentous phage is approximately 895nm long and 9nm in diameter and as such is able to penetrate the blood brain barrier without detrimentally affecting the host or eliciting phage specific immune responses.
In our pilot study cocaine-specific mAb GNC92H2 (Carrera et al, 2000, 2001) was displayed on the pVIII major coat protein of bacteriophage in hopes to generate a “sponge” able to absorb cocaine centrally, within the CNS. Affinity of GNC92H2-pVIII for cocaine was dependant on the number of copies displayed and was comparable to that of the mAb in solution. Rats administered twice daily intranasal doses of the phage-vaccine showed significant psychomotor differences especially for low and medium drug dose groups. Further studies assessing dosage as well as the effects of chronic phage administration are necessary. Nonetheless, simultaneous peripheral and central immunization could lead to potential synergistic and additive effects and makes this an exciting technology not only for cocaine but also for other drugs of abuse.
Nicotine
(S)-Nicotine is a legal drug, possessing an alkaloid structure, mainly present in the leaves of Solanaceae plants such as tobacco. It is the main agent responsible for causing addiction to cigarette smoking. Upon consumption, nicotine reaches the brain where it binds nicotinic acetylcholine receptors leading to a surge of adrenaline and dopamine providing feelings of enjoyment and reinforcing the behavior. Chronic use of tobacco has been linked to serious diseases such as coronary heart disease, chronic obstructive pulmonary disease (COPD), stroke, vascular disease, chronic lung diseases, and cancer. Cigarette smoking is considered to be the leading preventable cause of death in the United States (Mokdad et al, 2004). Despite knowledge of these adverse effects nicotine addiction has historically been one of the hardest to break. In 2007, 60.1 million Americans were current cigarettes smokers of which 70% expressed a desire to quit; in 2006 40% attempted to quit and only less than 5% were successful (U.S. Substance Abuse and Mental Health Services Administration, 2008; U.S. Department of Health and Human Services Center for Disease Control and Prevention).
Current pharmacological therapies available to curb nicotine addiction offer only limited success. Nicotine replacement products (NRP), such as gum, inhaler or dermal patches, seek to aid addiction by delivering nicotine over extended periods of time to minimize withdrawal and craving. Over the counter NRPs are efficacious; yet show only a modest success rate estimated at 8–11% past at least 6 months (Hughes et al, 2003). Other common smoking cessation pharmacotherapies include general antidepressants such as ZYBAN (bupropion hydrochloride; GlaxoSmithKline, USA) as well as nicotinic-receptor-specific drugs such as the recently launched CHANTIX (Varenicline; Pfizer, USA). Varenicline is nicotinic receptor partial agonist and acts by relieving craving while at the same time blocking the reinforcing effects of continued nicotine use. Results from the varenicline phase III clinical trials found a 23% success rate for smoking cessation for at least 13 months compared to 14.6% for bupropion and 10.5% for placebo groups (Jorenby et al, 2006). As exciting as these results appear to be, they must also be viewed with caution as varenicline was recently suspected to exacerbate depressed-mood, as well as erratic and possible suicidal behavior. In 2008, the FDA issued a warning linking CHANTIX to serious neuropsychiatric symptoms emphasizing the need for alternate therapy, such as immunopharmacotherapy, to aid smoking cessation.
Formation of nicotine-protein conjugates was first reported over 30 years ago; yet these studies were aimed at the creation of ELISA detection methods for nicotine in various media. Success with cocaine vaccines sparked interest to develop a nicotine counterpart and various research groups have produced a wealth of information over the past decades. Furthermore, it has been suggested that nicotine addiction could be a better candidate for immunotherapy as the maximum daily dose of nicotine consumed is significantly less than that of a serious cocaine addict and as such it would take considerably more effort to surmount the protective effects of immunization. A significant number of nicotine haptens, with five possible linker attachment sites, have been reported. Additionally, various types of delivery vehicles have been utilized ranging from traditional carrier proteins such as KLH, recombinant cholera toxin B subunit (Cerny et al, 2002) and pseudomonas exoprotein A (Pentel et al, 2000) to a 19-residue conformationally biased peptide that eliminates the need for external adjuvant (Sanderson et al, 2003). Finally, virus-like particles formed from Qb bacteriophage (Maurer et al, 2005) have been used.
A total of 9 different nicotine vaccine formulations have been tested in rodents. All vaccinations tested have produced anti-nicotine specific antibodies, albeit with low to moderate titers. Both active and passive immunization strategies have been explored. Rat immunization experiments demonstrated a significant increase in the concentration of nicotine in serum (Hieda et al, 1997; Pentel et al, 2000), thus reducing the amount of unbound drug able to enter the brain and stimulate locomotor activity (Carrera et al, 2004a; Cerny et al, 2002; Pentel et al, 2000). Experiments with high single doses or continuous administration of nicotine have shown little effect (~26% reduction) on chronic accumulation of nicotine in the brain. Nonetheless, single doses 28-fold higher than the estimated antibody binding capacity have been shown to reduce the number of nicotine induced seizures as well as nicotine distribution to brain (Hieda et al, 2000; Tuncok et al, 2001). Furthermore, nicotine challenge after passive immunization fails to alleviate nicotine withdrawal symptoms corroborating peripheral sequestration of the drug (Malin et al, 2001). Finally, nicotine-specific antibodies have been shown to reduce fetal brain exposure to the drug by 40–60% during pregnancy (Keyler et al, 2003; Nekhayeva et al, 2005). These studies are the first to demonstrate protection of both mother and fetus and could have important implications not only for nicotine but also for other drugs of abuse. Passive immunization experiments (Carrera et al, 2004a; Keyler et al, 2005) have established a direct link between amount of drug bound and antibody dose and affinity; thus protective effects are greatest in subjects with the highest antibody concentrations.
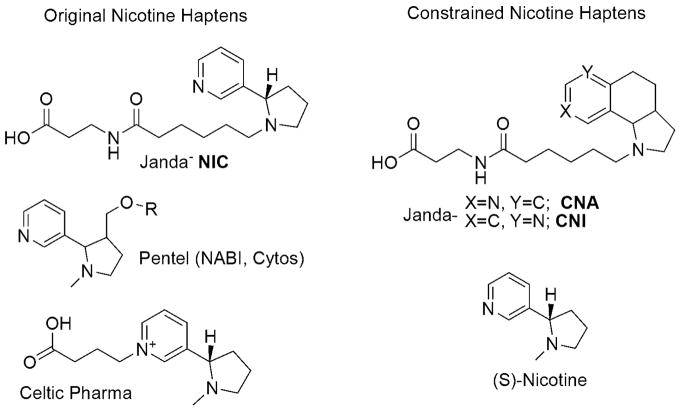
Currently, there are three nicotine vaccines in human phase I and II clinical trials; NicVAX (Hatsukami et al, 2005) by Nabi Pharmaceuticals, NicQb (Maurer et al, 2005) by Cytos Pharmaceutical and TA-NIC (St Clair Roberts et al, 2003) by Celtic Pharma, (for hapten design see Fig. 3). Active immunization was done using 2 to 6 doses over 2 to 4 weeks plus a later boost for NicVAX and TA-NIC. As expected, serum antibody levels increased after each subsequent dose and were maintained over a couple of months, however, only via subsequent booster dosage. Phase I results for all three vaccines deemed the formulations safe and well tolerated with only mild local and systemic reactions that subsided without medical intervention. Results from large scale phase II trials have been released for NicVAX and NicQb showing limited efficacy obtained to date. Thus, the NicVAX trial included 201 subjects and had a total combined success rate of 11% over 6% placebo after 12-months post-treatment. Most of the successful quitters (5%) were observed in the subjects that fell onto the high-titer category (Nabi Biopharmaceutical Clinical Trial Information, 2007). NicQb’s trial showed similar results. The trial included 159 subjects; and total combined success rate was 29.5% versus 21% of the placebo group. Again, the highest abstinence (up to 42% within their group) was observed in high-titer participants (Maurer et al, 2005; Cytos Biotechnology Clinical Development CYT002-NicQb, 2006). In both studies, maximum success was observed in the high-titer respondents further emphasizing that protective effects are directly related to amount of circulating antibodies. This data indicates that an active immunization strategy can give clinical effectiveness in treating nicotine addiction; yet, the efficacy of this approach could be greatly improved if reproducibly high antibody titers with potent and selective affinity to nicotine were obtained.
Fig. 3.
Relevant Nicotine Haptens
Several approaches aiming to improve the immunogenicity of nicotine vaccines in rodent models have been developed. Two separate strategies by Pentel et al. have recently been published. One effort included the use of a bivalent formulation where the antibody response of two nicotine immunogens with different linker attachment sites, and thus hapten presentation, was tested alone as well as combined (Keyler et al, 2008). The effects on serum antibody concentration proved to be additive in the bivalent group showing an enhanced antibody response over either vaccine alone. Additionally, a separate study tested the hypothesis of supplementing active inoculation with administration of mAbs to produce greater effects than vaccination alone (Roiko et al, 2008). Combined use of active and passive immunization produced higher total serum antibody levels, greater changes in nicotine pharmacokinetics, and a greater attenuation of locomotor sensitization than either treatment alone.
Our laboratory has an active immunopharmacotherapy program which has been focused on ways to maximize the immunogenicity of the nicotine vaccine via optimization of hapten design. In our initial foray into this area, we designed haptens so as to maximize both structural and stereochemical features as found within the nicotine nucleus (Isomura et al, 2001). However, titers and antibody affinity was modest in comparison with our cocaine immunopharmacotherapeutic effort. Upon further contemplation we realized that cocaine and nicotine, while chemically different, presented similar immune epitopes thus it was hypothesized that cocaine’s enhanced antigencity was due to its constrained tropane core. In nicotine, free-rotation along the single bond joining the two rings would allow the immune system to sample all possible conformers and thus generate a heterogeneous population of antibodies. However, if the molecule were to be frozen in space, the response would be focused and limited to one conformation leading to increased antibody affinity and specificity. Constraining a ligand is a well documented technique for improving receptor binding in medicinal chemistry efforts (Cowell et al, 2004), however, applying this to small molecule vaccines was unprecedented before our initial report. Initial haptens, CNA and CNI, (Fig. 3), were developed to mimic the energetically most stable conformations of nicotine at physiological pH (Meijler et al, 2003). Rodent immunization saw an increase in titer from 3 200 to 25 000 plus a two to three fold increase in binding affinity versus their unconstrained counterparts. Studies with second generation constrained haptens are ongoing.
Conclusions
Treatment retention and compliance are two critical factors in the recovery from substance abuse. Vaccination approaches have four key advantages; first they are safe and offer low cross-reactivity, second they only require a brief series of monthly injections which improves patient compliance, additionally their effects are relatively long-lived and lastly their unique mechanism of action makes them well suited to combination with other pharmacotherapies, such as anti-craving medication, which could be used to maximize efficacy (Le Sage et al, 2006). Immunopharmacotherapy exerts its effects via peripheral sequestration of drug which in turn limits concentrations able to reach the CNS. Both animal and human data to date has shown the efficacy of vaccination to be limited and dependant on the magnitude, affinity and specificity of the immune response elicited. The main current challenge for the field relies on improvement of the intrinsic immunogenicity of vaccine formulations. Current advances in adjuvant research will benefit the field as it will allow more focused and tailor responses (Guy, 2007). To date, the only adjuvant approved for human use in the US is alum salt which may not be optimal. Inclusion of new adjuvant technology in humans may experience delays as safety concerns are addressed, yet, offers a new possible direction. Nonetheless, an intrinsic limitation of the technology remains in the significant amount of subject-to-subject variability; though the current numbers remain to be optimized. In this respect, co-administration of passive and active immunization would provide greater benefits, however such an approach would be expensive, perhaps prohibitively so. Furthermore, concern for overcoming the protective effects of vaccination using higher doses of the drug has emerged. Animal behavioral work suggests that once a minimal threshold value for circulating antibody concentration is achieved even drug doses significantly higher than the anticipated binding capacity will not overcome antibody shielding effects. This threshold value, however, seems to vary greatly from drug to drug and the factors governing surmountability seem to be complex. Encouragingly, data from nicotine clinical trials suggest subjects did not attempt to override vaccination by increasing nicotine consumption (Nabi Biopharmaceutical Clinical Trial Information, 2007).
Acknowledgments
We thank the support provided by NIDA (DA008590), the State of California Tobacco-Related Disease Research Program (16RT-0115), the Skaggs Institute for Chemical Biology and Novartis Pharmaceuticals for a Graduate Fellowship in Organic Chemistry for Women and Minorities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Baird TJ, Deng SX, Landry DW, et al. Natural and artificial enzymes against cocaine. I: Monoclonal antibody 15A10 and the reinforcing effect of cocaine in rats. J Pharmacol Exp Ther. 2000;295:1127–1134. [PubMed] [Google Scholar]
- Berkowitz B, Spector S. Evidence for Active Immunity to Morphine in Mice. Science. 1972;178:1290–1292. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- Bonese K, Wainer B, Fitch F, Rothberg R, Schuster C. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252:708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- Bunce CJ, Loudon P, Akers C, Dobson J, Wood D. Development of vaccines to help treat drug dependence. Current Opinion in Molecular Therapeutics. 2003:58–63. [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection againste relapse in a rat model. Proc Natl Acad Sci. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Hoffman TZ, Isomura S, Wirsching P, Koob GF, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004a;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Kaufmann GF, Mee JM, Meijler MM, Koob GF, Janda KD. Treating cocaine addiction with viruses. Proc Natl Acad Sci USA. 2004b;101:10416–10421. doi: 10.1073/pnas.0403795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Trigo JM, Wirsching P, Roberts A, Janda KD. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol Biochem Behav. 2005;81:709–714. doi: 10.1016/j.pbb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Celtic Pharma. Celtic Pharma reports preliminary results of phase 2 clinical trials with TA-CD, cocaine addiction vaccine. 2006 Retrieved September 23, 2008, from http://www.celticpharma.com/news/pr/release_062106.pdf.
- Celtic Pharma TA CD. Retrieved September 23, 2008, from http://www.celticpharma.com/theportfolio/ta-cd.html.
- Cerny EH, Levy R, Mauel J, et al. Preclinical development of a vaccine "against smoking". Onkologie. 2002;25:406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Bruns WJ, Schnoll WJ, Burns KA. Cocaine use in pregnancy. New England Journal of Medicine. 1985;313:666–669. doi: 10.1056/NEJM198509123131105. [DOI] [PubMed] [Google Scholar]
- Cowell S, Lee Y, et al. Exploring Ramachandran and Chi Space: Confomrationally Constrained Amino Acids and Peptides in the Design of Bioactive Polypeptide Ligands. Current Med Chem. 2004;11:2785–2798. doi: 10.2174/0929867043364270. [DOI] [PubMed] [Google Scholar]
- Cytos Biotechnology Clinical Development CYT002-NicQb. 2006 Retrieved June 30, 2008, from http://www.cytos.com/doc/NicQb_June06_E_fv.pdf.
- Estroff TW. Manual of Adolescent Substance Abuse Treatment. American Psychiatric Pub; 2001. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Literature reviews-Treatment of problem cocaine use: a review of the literature. 2007 Lisbon: Section 3.1. Available online http://www.emcdda.europa.eu/?nnodeid=18945.
- Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- Guy B. The perfect mix: recent progress in adjuvant research. Nature Rev Microbio. 207(5):505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Rennard S, Jorenby DE, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Vandevoort JT, et al. Active immunization alters the plasma nicotine concntration in rats. J Pharmacol Exp Ther. 1997;283:1076–1081. [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22:809–819. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tobacco Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino A, Dickerson TJ, Janda KD. Positional linker effects in haptens for cocaine immunopharmacotherapy. Bioorg Med Chem Lett. 2007;17:4280–4283. doi: 10.1016/j.bmcl.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Isomura S, Wirsching P, Janda KD. An Immunotherapeutic Program for the Treatment of Nicotine Addiction: Hapten Design and Synthesis. J Org Chem. 2001;66:4115–4121. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of Varenicline, an a4b2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation: A Randomized Controlled Trial. J Amer Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, et al. Evalutaion of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacol. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kantak KM. Vaccines against drugs of abuse: A viable treatment option? Drugs. 2003:341–352. doi: 10.2165/00003495-200363040-00001. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic Plasticity and Addiciton. Nature Review Neuroscience. 2007:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Soeman D, LeSage MG, Calvin AD, Pentel PR. Maternal vaccination against nicotine reduces nicotine distribution to fetal brain in rats. J Pharmacol Exp Ther. 2003;305:587–592. doi: 10.1124/jpet.102.046805. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, et al. Monoclonoal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose and affinity response relationship. Drug Metab Dispos. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earlye CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008;8:1589–1594. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug Abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rosen M, Bond J, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- Kosten T, Owens M. Immunotherapy for the treatment of drug abuse. Pharmacology and Therapeutics. 2005:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Landry DW, Zhao K, Yang GX, et al. Antibody-catalyzed degradation of cocaine. Science. 1993;259:1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- Le Sage MG, Keyler DE, Pentel PR. Current Status of Immunologic Approaches to Treating Tobacco Dependence: Vaccines and Nicotine-specific Antibodies. The AAPS J. 2006;8(1):E65–E75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Lin A, et al. Passive immunization against nicotine prevents nicotine alleviation of nicotine abstinence syndrome. Pharmacol Biochem Behav. 2001;68:87–92. doi: 10.1016/s0091-3057(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hoffman TZ, Ashley JA, Zhou B, Wirsching P, Janda KD. Cocaine catalytic antibodies: the primary importance of linker effects. Bioorg Med Chem Lett. 2001;11:87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- Maurer P, Jennings GT, Willers J, et al. A therapeutic vaccine for nicotine dependece: preclinical efficacy and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- McKenzie KM, Mee JM, Rogers CJ, Hixon MS, Kaufmann G, Janda KD. Identification and Characterization of Single Chain Anti-cocaine Catalytic Antibodies. J Mol Bio. 2007;365:722–731. doi: 10.1016/j.jmb.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijler MM, Matsushita M, Altobell L, Wirsching P, Janda KD. A new strategy for improved nicotine vaccines using conformationally constrained haptens. J Am Chem Soc. 2003;125:7164–7165. doi: 10.1021/ja034805t. [DOI] [PubMed] [Google Scholar]
- Meijler MM, Matsushita M, Wirsching P, Janda KD. Development of Immunopharmacotherapy Against Drugs of Abuse. Current Drug Discovery Technologies. 2004;1:77–89. doi: 10.2174/1570163043484851. [DOI] [PubMed] [Google Scholar]
- Mets B, Winger G, Cabrera C, et al. A catalytic antibody against cocaine prevents cocaine’s reinforcing and toxic effects in rats. Proc Natl Acad Sci USA. 1998;95:10176–10181. doi: 10.1073/pnas.95.17.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States. J Amer Med Assoc. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nabi Biopharmaceutical Clinical Trial Information. 2007 Retrieved June 30, 2008, from http://www.nabi.com/pipeline/clinicaltrials.php#3.
- National Institute on Drug Abuse. NIDA research report-cocaine abuse and addiction. 2004. NIH Publication No. 99–4342. [Google Scholar]
- Nekhayeva IA, Nanovskaya TV, Pentel PR, Keyler D, et al. Effects of nicotine-specfiic antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta. Biochem Pharmacol. 2005;70:1664–1672. doi: 10.1016/j.bcp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Malin DH, Ennifar S, et al. A nicotine-conjugate vaccine reduces nicotine distribution to brian and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Preti A. The Treatment of Cocaine Abuse: Problems and Perspectives. Cocaine and Heroin Abuse Research. 2006:1–35. [Google Scholar]
- Redwan ER, Larsen NA, Zhou B, Wirsching P, Janda KD, Wilson IA. Expression and characterization of a humanized cocaine-binding antibody. Biotechnol Bioeng. 2003;82:612–618. doi: 10.1002/bit.10598. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, LeSage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325:985–993. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- Sanderson SD, Cheruku SR, Padmanilayam MP, et al. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137–146. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- Schabacker DS, Kirschbaum KS, Segre M. Exploring the feasibility of anti-idiotypic cocaine vaccine: analysis of the specificity of anticocaine antibodies (Ab1) capable of inducing Ab2beta anti-idiotypic antibodies. Immunology. 2000;100:48–56. doi: 10.1046/j.1365-2567.2000.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner L, et al. Cognititve and Motor Outcomes of Cocaine-Exposed Infants. J Am Med Assoc. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. Quantitative determination of morphine in serum by radioimmunoassay. J Pharm Exp Ther. 1971;178:253–258. [PubMed] [Google Scholar]
- St Clair Roberts J, Akers CV, Vanhinsbergh L, et al. Longitudinal safety and immunogenicity data of TA-NIC, a novel nicotine vaccine. Proceedings of the 9th Annual Meeting of the Society for Research on Nicotine and Tobacco; Feb 19–22, 2003; New Orleans, LA: Society for Reserach on Nicotine and Tobacco; 2003. [Google Scholar]
- Tuncok Y, Hieda Y, Keyler DE, et al. Inhibition of nicotine-induced seizures in rats by combining vaccination against nicotine with chronic nicotine infusion. Exp Clin Psychopharmacol. 2001;9:228–234. doi: 10.1037//1064-1297.9.2.228. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Center for Disease Control and Prevention. Retrieved June 30, 2008, from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/cessation2.htm.
- US Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2008. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- Withers NW, Pulvirenti L, Koob GF, Gillin JC. Cocaine Abuse and Dependence. J Clinical Psychopharmacol. 1995;15:63–78. doi: 10.1097/00004714-199502000-00010. [DOI] [PubMed] [Google Scholar]
- Yang G, Chun J, Arakawa-Uramoto H, et al. Anti-cocaine catalytic antibodies: a synthetic approach to improve antibody diversity. J Am Chem Soc. 1996;118:5881–5890. [Google Scholar]
- Zheng F, Yang W, Ko M-C, Liu J, Cho H, Gao D, et al. Most Efficient Cocaine Hydrolase Designed by Virtual Screening of Transistion States. J Am Chem Soc. 2008;130:12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]