Abstract
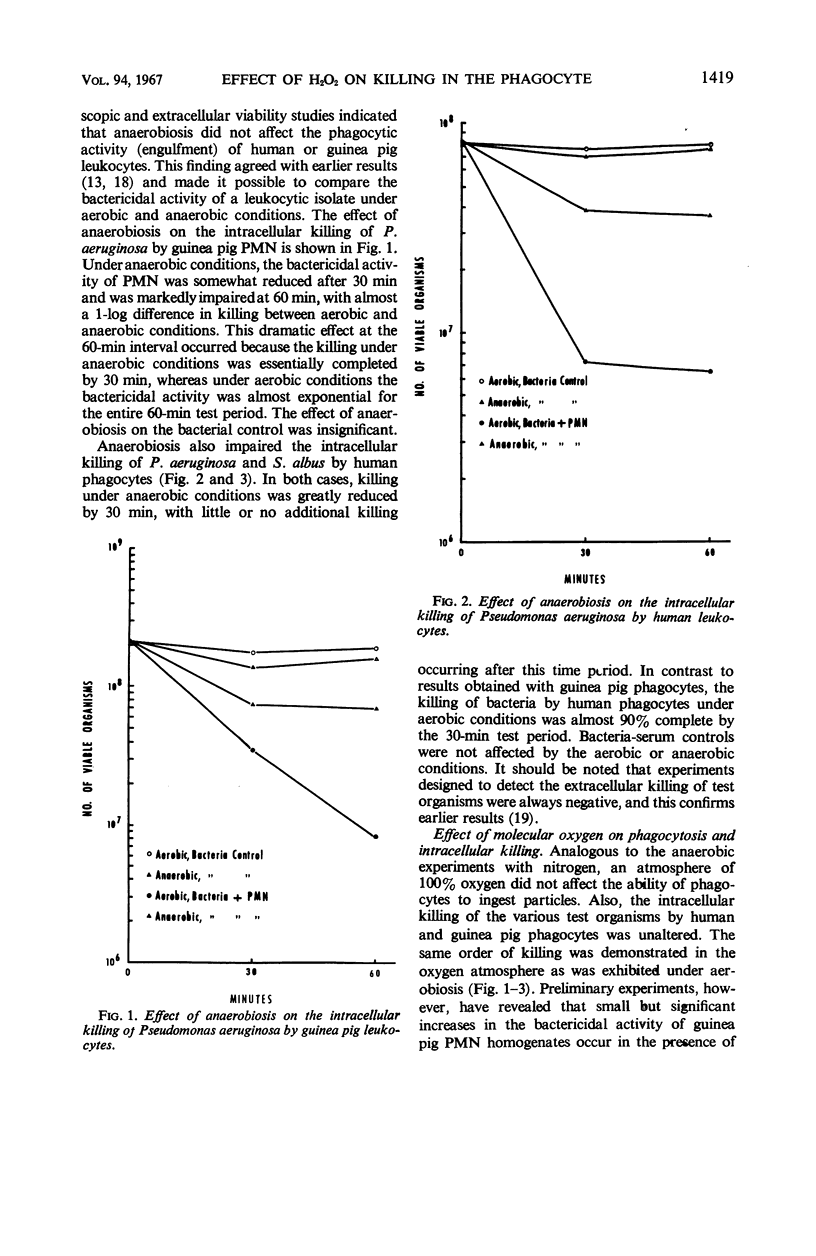
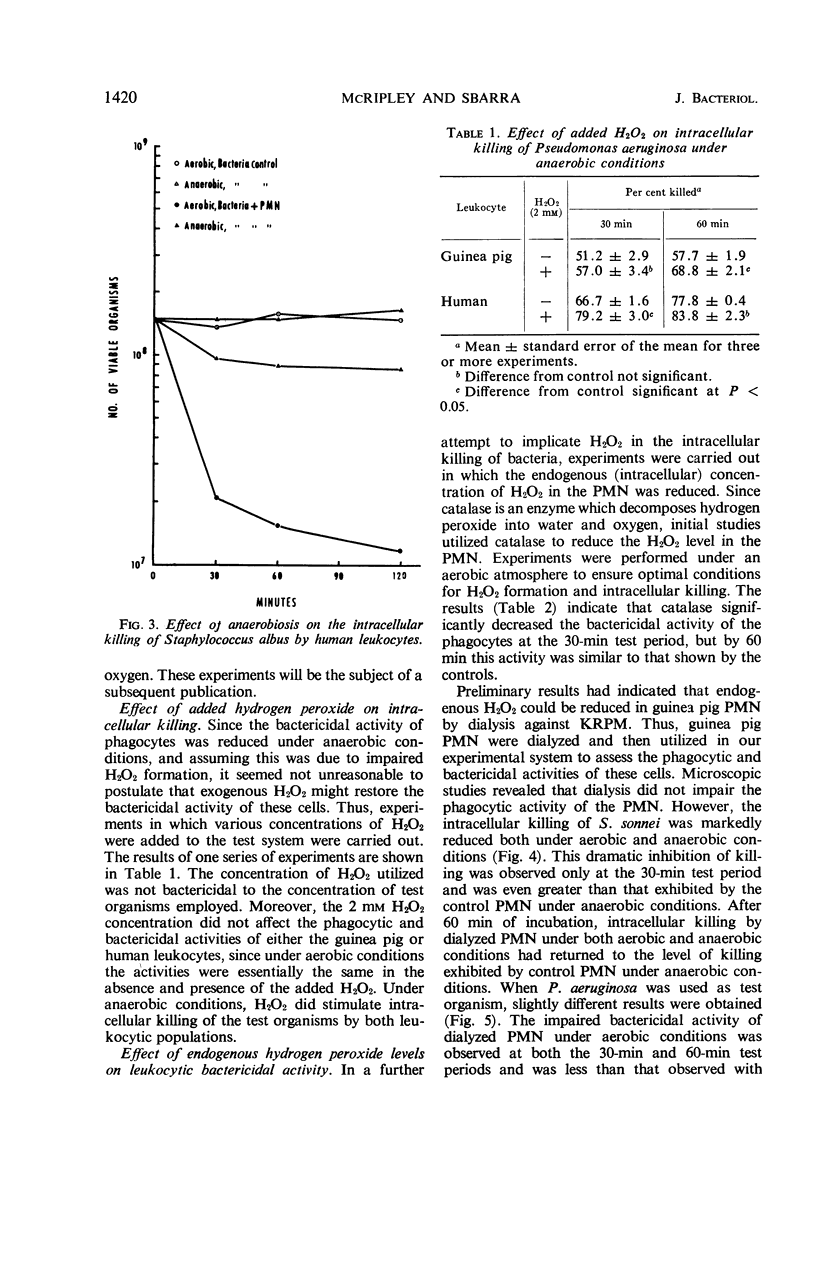
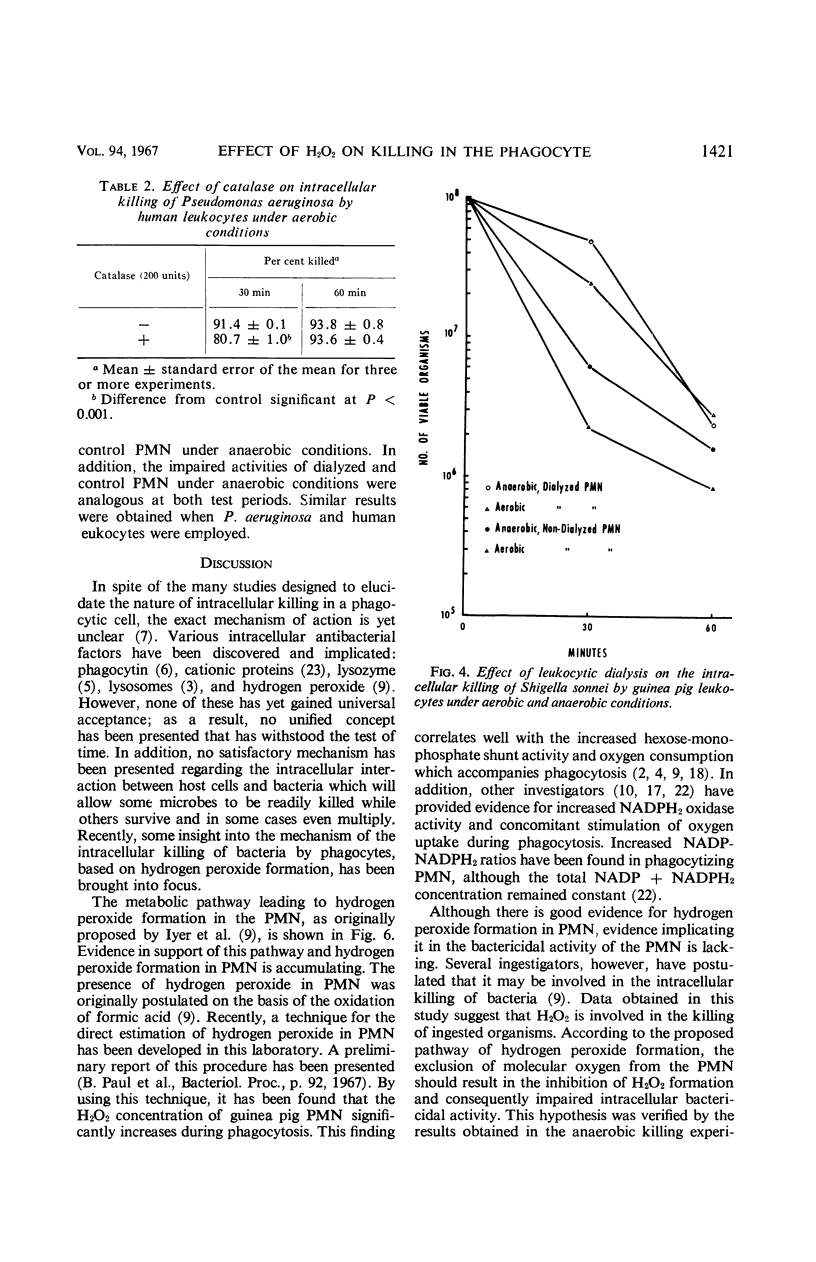
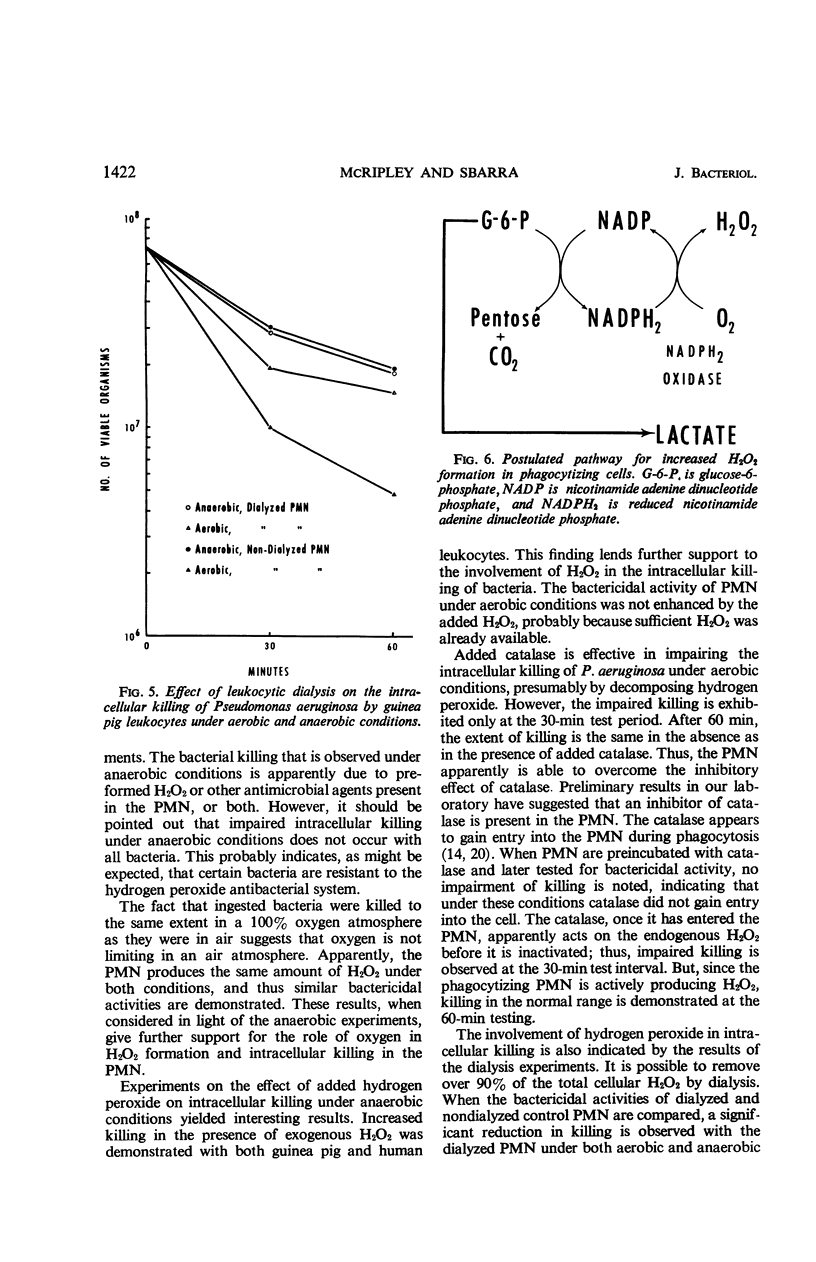
The increased respiratory and hexose monophosphate activities noted in phagocytizing cells results in the formation of hydrogen peroxide. This is brought about by the oxidation of reduced nicotinamide adenine dinucleotide phosphate by its oxidase. Evidence is presented which indicates that this H2O2 is involved in the intracellular killing of bacteria. When molecular oxygen was excluded from phagocytizing leukocytes by anaerobiosis, thus inhibiting H2O2 formation, reduced intracellular killing was observed. In some cases the impairment of leukocytic bactericidal activity by anaerobiosis could be partially reversed by the addition of H2O2. Exogenous catalase also could reduce intracellular killing. In addition, when leukocytic isolates were dialyzed so as to reduce endogenous H2O2, the bactericidal activity of the leukocytes was significantly decreased under both aerobic and anaerobic conditions. These results occurred with both guinea pig and human leukocytes and with several test microorganisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Nathan D. G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967 Feb 17;155(3764):835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Metabolism of the circulating leukocyte. Physiol Rev. 1965 Oct;45(4):674–720. doi: 10.1152/physrev.1965.45.4.674. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G. Phagocytosis. Annu Rev Microbiol. 1965;19:339–350. doi: 10.1146/annurev.mi.19.100165.002011. [DOI] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Good R. A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966 Jun 4;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- NUNGESTER W. J. Mechanisms of man's resistance to infectious diseases. Bacteriol Rev. 1951 Sep;15(3):105–129. doi: 10.1128/br.15.3.105-129.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman E., Bonventre P. F. Facilitated uptake of streptomycin by Kupffer cells during phagocytosis. Nature. 1967 Jan 21;213(5073):294–295. doi: 10.1038/213294a0. [DOI] [PubMed] [Google Scholar]
- RECHCIGL M., Jr, EVANS W. H. ROLE OF CATALASE AND PEROXIDASE IN THE METABOLISM OF LEUCOCYTES. Nature. 1963 Sep 7;199:1001–1002. doi: 10.1038/1991001b0. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BARDAWIL W. A. 'Piggy-back' phagocytosis. Nature. 1962 Apr 21;194:255–256. doi: 10.1038/194255a0. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., SELVARAJ R. J., IOUCHI E., ROSENBAUM E. THE ROLE OF THE PHAGOCYTE IN HOST-PARASITE INTERACTIONS. I. THE PHAGOCYTIC CAPABILITIES OF LEUKOCYTES FROM LYMPHOPROLIFERATIVE DISORDERS. Cancer Res. 1964 Dec;24:1958–1968. [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. The role of the phagocyte in host-parasite interactions. VII. Di- and triphosphopyridine nucleotide kinetics during phagocytosis. Biochim Biophys Acta. 1967 Jul 25;141(2):243–249. doi: 10.1016/0304-4165(67)90097-9. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



