Abstract
Overexpression of the adenine nucleotide translocase (ANT) has been shown to be cytotoxic in several cell types. Although ANT was originally proposed to be a critical component of the mitochondrial permeability transition (MPT) pore, recent data have suggested that this may not be the case. We therefore hypothesized that the cytotoxic actions of ANT are through an alternative mechanism, independent of the MPT pore. Infection of cultured neonatal cardiomyocytes with an ANT1-encoding adenovirus induced a gene dosage-dependent increase in cell death. However, ANT1 overexpression failed to induce MPT, and neither pharmacological nor genetic inhibition of the MPT pore was able to prevent ANT1-induced cell death. These data suggested that ANT1-induced death progressed through an MPT pore-independent pathway. Somewhat surprisingly, we observed that protein levels of Bax, a pro-apoptotic Bcl protein, were consistently elevated in ANT1-infected cardiomyocytes. Membranes isolated from ANT1-infected myocytes exhibited significantly increased amounts of membrane-inserted Bax, and immunocytochemistry revealed increased Bax activation in ANT1-infected myocytes. Co-expression with the Bax antagonist Bcl2 was able to greatly reduce the degree of ANT1-induced cell death. Furthermore, Bax/Bak-deficient fibroblasts were resistant to the cytotoxic effects of ANT1 overexpression. Interestingly, ANT1 overexpression was also associated with enhanced production of reactive oxygen species (ROS), and the antioxidant MnTBAP was able to significantly attenuate both the ANT1-induced upregulation of Bax and cell death. Taken together, these data indicate that ANT mediates cell death, not through the MPT pore, but rather via a ROS-dependent upregulation and activation of Bax.
Keywords: cardiomyocytes, mitochondrial permeability transition, cell death, Bax, reactive oxygen species
INTRODUCTION
The mitochondrion is critical for normal cardiac myocyte function. Mitochondria supply the energy required for contraction and are essential regulators of ion homeostasis. However, it is now well established that mitochondrial dysfunction and injury can contribute to the pathogenesis of several cardiac diseases [1–4]. Cardiac stresses, such as ischemia/reperfusion, oxidative stress, and cytotoxic drugs induce rapid increases in mitochondrial permeability and volume [3–7], a phenomenon known as the mitochondrial permeability transition (MPT). These increases in permeability cause dissipation of the mitochondrial membrane potential (ΔΨm), inhibition of ATP synthesis, swelling, and rupture of the outer membrane [5,8]. This in turn releases pro-apoptotic inter-membrane proteins, most notably cytochrome c and Smac, which in turn activate the caspase system [1,2,8]. Therefore, activation of the mitochondrial pathway initially induces apoptosis. However, if the stress is severe and/or prolonged, ATP will be depleted and the cell will instead undergo necrosis. The Bcl2 family members are critical regulators of this mitochondrial death machinery, and are divided into two subgroups: pro-survival, e.g., Bcl2, BclXL, and pro-death, e.g., Bax, Bak, [1,2,9]. In the case of the pro-death proteins, cardiac stimuli cause their translocation and integration into the outer mitochondrial membrane, causing the release of apoptogenic proteins and activation of the mitochondrial death pathway [1,2].
One mitochondrial protein that has received a large amount of attention as a potential mediator of mitochondrial-induced death, both in cardiac and non-cardiac cells, is the adenine nucleotide translocase (ANT). The ANT family mediates the exchange of ATP and ADP across the inner mitochondrial membrane. Two homologous isoforms, ANT1 and ANT2 are present in the mouse, with a third isoform, ANT3, found in humans [10]. The ANT1 isoform is found primarily in striated muscle, with ANT2 being more ubiquitously expressed. A third mouse isoform (ANT4) has been recently reported but appears to be restricted to testicular germ cells [11]. Regarding a specific role in cardiac cell death, biochemical and pharmacological data have implicated ANT, alongside the voltage-dependent anion channel (VDAC) and cyclophilin-D (CypD), as a component of the non-specific pore that mediates MPT [5,12,13]. For example, pharmacological manipulation of ANT with either atractyloside or bongkrekic acid can induce or inhibit, respectively, MPT and cell death in cardiac myocytes [14–18]. ANT1, but not ANT2, interacts with CypD at contact sites between the inner and outer mitochondrial membranes, where the pore is believed to localize [19], and reconstitution of ANT plus CypD can yield an MPT-like pore in proteoliposomes [20]. Moreover, overexpression of ANT1, but not ANT2, can induce mitochondrial-dependent death in non-cardiac cells [21–24]. However, genetic deletion of both mouse ANT isoforms did not appreciably alter MPT and cell death [25], and some ANT ligands induce mitochondrial dysfunction and cell death independent of MPT [26].
Consequently, despite a large body of research, the mechanisms by which ANT is capable of inducing cell death remain unclear. Given that shifts towards the potentially more lethal ANT1 isoform are observed in cardiomyopathic patients [27,28], delineation of the cellular pathways by which ANT1 invokes cardiomyocyte death could reveal novel molecular targets for the treatment of cardiac disease. The purpose of the present study, therefore, was to examine the specific mechanisms that mediate cardiomyocyte death induced by increased ANT1 expression. Surprisingly, we found that ANT1 initiates cell killing, not through induction of the MPT response, but rather through a mechanism involving a reactive oxygen species (ROS)-dependent upregulation and activation of the pro-death Bcl2 protein Bax.
EXPERIMENTAL PROCEDURES
Reagents
Tetramethylrhodamine ethyl ester (TMRE), calcein, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA), Mitotracker-CMXRos, propidium iodide, M199 medium, DMEM medium, penicillin/streptomycin, and fetal bovine serum were from Invitrogen; the In Situ Cell Death Detection (TUNEL) kit was from Roche; the PCR-based DNA laddering kit was from Maxim Biotech; Cyclosporine-A was from Calbiochem; Manganese (III) tetrakis (4-benzoic acid)porphyrin chloride (MnTBAP) and bongkrekic acid were from Alexis Biochemicals. All other chemicals/reagents were from Sigma-Aldrich.
Cell culture
Neonatal ventricular myocytes were isolated from 1–2 day-old Sprague-Dawley rats as previously described [29]. The cells were then cultured in serum-free M199 medium supplemented with penicillin (100 U/mL) and streptomycin (0.1 mg/mL). Primary cultured wildtype, Ppif, and Bax/Bak-null mouse embryonic fibroblasts (MEFs) were obtained from E13.5–15.5 embryos by trypsin digestion as previously described [30,31]. The MEFs were then maintained in DMEM medium supplemented with 10%v/v fetal bovine serum, penicillin (100 U/mL), and streptomycin (0.1 mg/mL).
Adenovirus construction and infection
Replication-deficient adenoviruses for β-galactosidase, mouse ANT1, mouse CypD or a R96G isomerase-deficient mutant of CypD, mouse mitofilin, and mouse citrin (with a c-terminal FLAG tag) were generated using the AdEasy adenoviral system (Stratagene). The Bcl2 adenovirus was a generous gift from Dr. Lorrie Kirshenbaum, University of Manitoba, Canada. Cardiomyocytes and MEFs were typically infected with adenovirus at a multiplicity of infection (MOI) of 30–120 plaque-forming units for 2 hrs at 37°C [29–31]. Cells were then cultured for another 48 hrs in virus-free media before analysis.
Membrane fractionation
To examine insertion of Bax into mitochondrial membranes, infected cardiomyocytes were incubated with 100 mM Na2CO3 (pH 11.5) for 30 min on ice [32]. The precipitated membranes (and integral proteins) were then pelleted by airfuge (Beckman) operating for 10 min at 30 psi. Both the membrane pellet and supernatant (soluble proteins) were then subjected to Western blotting. As a positive control for Bax activation uninfected myocytes were treated with 1 mM staurosporine for 4 h.
Western blotting
Cells were lysed in buffer containing 150 mM NaCl, 10 mM Tris (pH 7.4), 1mM EDTA, and 1% Triton-X100. Proteins were resolved by SDS-PAGE using 10–15% acrylamide, transferred onto PVDF membranes, and immunoblotted using the following commercially available antibodies: anti-ANT (1:100), anti-Bax (1:1000), anti-Bak (1:500), anti-Bcl2 (1:1000), anti-BclXL (1:1000) from Santa Cruz Biotechnology; anti-CypD (1:3000), anti-mitofilin (1:1000), and anti-VDAC (1:1000) from Mitosciences; anti-actin (1:4000) and anti-FLAG (1:1000) from Sigma-Aldrich; anti-cytochrome c (1:1000) from BD Biosciences; and anti-cytochrome oxidase IV (COX-IV, 1:4000) from Invitrogen. Membranes were then incubated with the appropriate alkaline phosphatase-linked secondary antibody (Santa Cruz) and visualized by enhanced chemifluorescence (Amersham).
Cell death assays
Apoptosis was assessed in cardiomyocytes and MEFs using commercially available TUNEL staining and PCR-based DNA laddering kits according to the manufacturers instructions. Necrosis was determined by propidium iodide (PI) exclusion as previously described [30,31].
Fluorescence microscopy
ΔΨm, MPT pore opening, and reactive oxygen species (ROS) were assessed by TMRE, calcein/CoCl2, and CM-H2DCFDA staining, respectively [30,31,33]. Briefly, infected cardiomyocytes were incubated with either 100 nM TMRE, 1 mM calcein-AM plus 1 mM CoCl2, or 100 nM CM-H2DCFDA in Hanks buffered saline solution (HBSS) for 30 min at 37°C. Dye-loaded cells were then washed twice with HBSS and fluorescence images collected using an inverted fluorescent microscope (Olympus IX70) connected to a digital SPOT camera (Diagnostic Instruments). For immunohistochemistry, infected myocytes were fixed in 4% paraformaldehyde, permeabilized, and then incubated overnight with either anti-ANT1 (1:100, Santa Cruz) or an antibody that recognizes the active form of Bax (clone 6A7, 1:100, BD Biosciences). The cells were then incubated with the appropriate fluorophore-conjugated secondary antibody (Alexa, Invitrogen) and visualized. For the co-localization experiments the myocytes were incubated with 100 nM Mitotracker for 30 min at 37°C prior to fixation.
Statistical analyses
Statistical significance was calculated by the Student’s t test. A P value <0.05 was considered statistically significant.
RESULTS
ANT1 induces cell death in cardiomyocytes
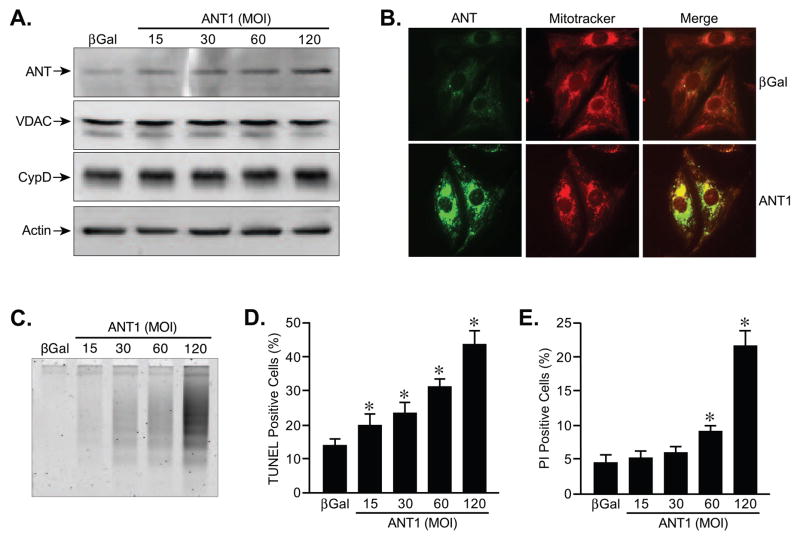
The purpose of this study was to investigate the mechanisms by which ANT induces cell death. Myocytes were selected as a primary model system given their known mechanisms of cell death and prominence of mitochondria. We generated a recombinant adenovirus-encoding mouse ANT1 to achieve uniform infectivity of all primary neonatal cardiomyocytes in culture, and a virus encoding β-galactosidase (βGal) was used as a control. Infection with increasing titres of the ANT1 virus elicited the expected gene dose-dependent increase in ANT1 protein levels (Fig. 1A). Importantly, protein levels of VDAC and CypD were unaffected by the changes in ANT1. We also confirmed that the exogenous ANT1 protein was correctly localized to the mitochondria of the myocytes (Fig. 1B). Concomitant with enhanced ANT1 levels, the cardiomyocytes exhibited a dose-dependent increase in apoptotic cell death, as determined by both DNA laddering (Fig. 1C) and TUNEL staining (Fig. 1D) analyses. Moreover, at the higher levels of ANT1 expression we also began to observe significant increases in cell membrane failure (Fig. 1E), as determined by propidium iodide exclusion. Translocase activity appeared to be required, at least in part, for cytotoxicity as co-incubation with bongkrekic acid (25 μM), an inhibitor of ANT, significantly attenuated ANT1-induced cell death (Supplementary Fig. 1A,B). For the remainder of the experiments we used the highest titre of the adenovirus (MOI of 120).
FIGURE 1. ANT1 overexpression induces cell death in cardiomyocytes.
A, Western blotting for ANT, VDAC1, and CypD in neonatal cardiomyocytes infected with βGal (control)- or increasing amounts of ANT1-expressing adenoviruses. Actin was used to demonstrate equivalent loading. B, Immunocytochemistry in AdβGal and AdANT1-infected cardiomyocytes. Mitochondria were stained using Mitotracker CMXRos (Red), whereas ANT1 was visualized using an anti-ANT polyclonal antibody (Green). The images were then overlapped to show co-localization (orange/yellow). C, DNA laddering in AdβGal and AdANT1-infected cardiomyocytes. D, TUNEL staining in AdβGal and AdANT1-infected cardiomyocytes. E, Propidium iodide (PI) staining in AdβGal and AdANT1-infected cardiomyocytes. All results represent the average values of at least 4 independent experiments performed in duplicate. Error bars indicate s.e.m, and * denotes P<0.05 versus βGal.
One concern with this approach is that we are simply increasing protein content in an already protein-saturated inner membrane, and that this will lead to a non-specific perturbation of inner membrane structure/function. To determine whether this was the case, we overexpressed two other inner mitochondrial membrane proteins, mitofilin and citrin, and examined their effect on cardiomyocyte death. Mitofilin is a structural protein that is believed to regulate the architecture of the cristae [34], whereas citrin is an aspartate-glutamate transporter, which, like ANT1, is a member of the SLC25 carrier family [35]. Infection of cardiomyocytes with adenoviruses encoding either mitofilin or citrin lead to a robust expression of the respective protein (Supplementary Fig. 2A,B). However, in contrast to ANT1, overexpression of either mitofilin or citrin was unable to induce myocyte death (Supplementary Fig. 2C). These data indicate that the pro-death actions of ANT1 are specific to that protein, and are not due to a generalized disruption of the inner membrane.
ANT1-induced cell death is not dependent on the MPT pore
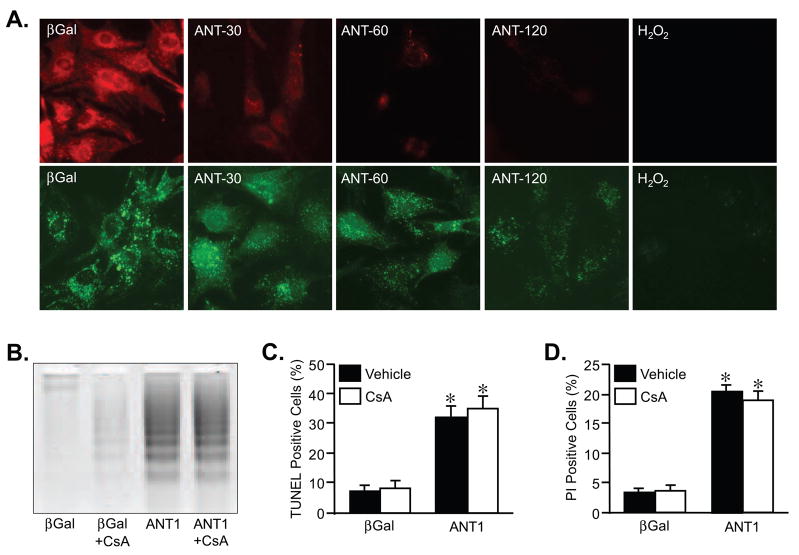
To test whether ANT1 overexpression induced cell killing through induction of MPT we stained the infected myocytes with TMRE or calcein plus CoCl2, which measure ΔΨm and MPT, respectively. Overexpression of ANT1 induced a gene dose-dependent loss of mitochondrial ΔΨ suggestive of mitochondrial dysfunction (Fig. 2A). However, changes in ΔΨm are not necessarily indicative of MPT. Therefore, we used the calcein/CoCl2 method to more directly assess MPT in cardiomyocytes. The combination of these two reagents results in selective staining of mitochondria (Fig. 2A). MPT allows efflux of calcein out into the cytosol, where the CoCl2 quenches it. Thus a loss of mitochondrial fluorescence is indicative of MPT. Increasing levels of ANT1 caused a small loss of calcein fluorescence when compared to control βGal-infected cells (Fig. 2A). However, the magnitude of the fluorescence decrease was substantially less than that observed with 200 μM H2O2, an excellent inducer of MPT [30], possibly suggesting that ANT1 mediated killing maybe distinct from MPT.
FIGURE 2. Pharmacological inhibition of MPT does not prevent ANT1-induced myocyte death.
A, Mitochondrial ΔΨM and MPT determined by TMRE (red) and calcein/CoCl2 (green) fluorescence, respectively, in AdβGal and AdANT1-infected cardiomyocytes. Exposure to H2O2 (200 μM for 1hr) was used as a positive control for MPT induction. B, DNA laddering in AdβGal and AdANT1-infected cardiomyocytes co-incubated with either vehicle or 1 μM cyclosporine-A (CsA). C, TUNEL staining in AdβGal and AdANT1-infected cardiomyocytes treated with either vehicle or CsA. D, Propidium iodide (PI) staining in AdβGal and AdANT1-infected cardiomyocytes treated with either vehicle or CsA. The results shown are representative of at least 3 independent experiments performed in duplicate. Error bars indicate s.e.m, and * denotes P<0.05 versus βGal.
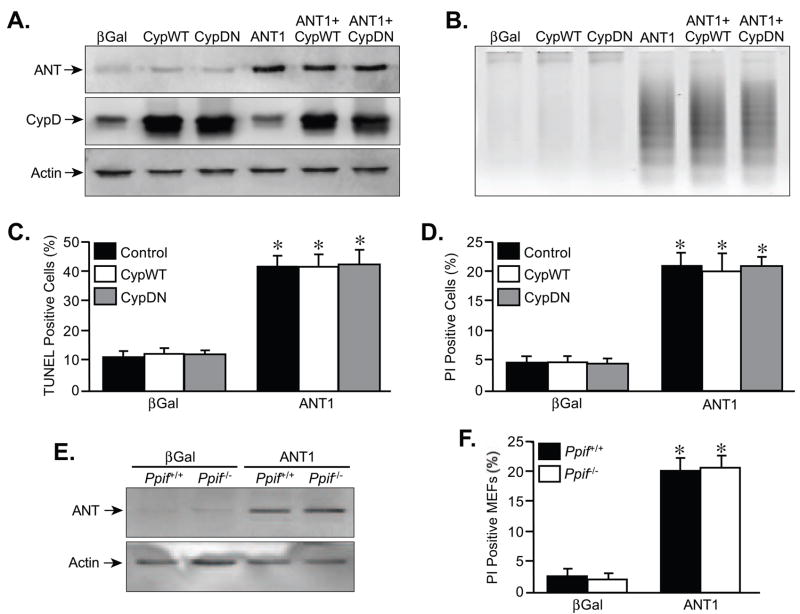
To more definitively examine if ANT1 mediates cell death independent of the MPT, we co-incubated cardiomyocytes with 1 μM cyclosporine-A for 48 hrs, an MPT pore inhibitor [5,14,30]. Cyclosporine-A failed to abrogate the induction of DNA laddering (Fig. 2B), TUNEL staining (Fig. 2C), and PI positivity (Fig. 2D) caused by ANT1 overexpression. We further tested the effects of ANT1 on the MPT pore by co-infecting cardiomyocytes with either wildtype CypD or a catalytically inactive mutant of CypD (Fig. 3A). If ANT was indeed acting through the pore then overexpression of CypD should sensitize the cells to ANTs effects. However, neither the wildtype nor the inactive CypD proteins had any effect on ANT1-induced myocyte cell death (Fig. 3B–D). Finally, overexpression of ANT1 in CypD-deficient fibroblasts, which are resistant to MPT (30,36,37), induced the same degree of cell killing as in wildtype fibroblasts (Fig. 3E,F).
FIGURE 3. Genetic manipulation of CypD does not affect ANT1-induced myocyte death.
A, Western blotting for ANT and CypD in neonatal cardiomyocytes infected with AdβGal or AdANT1 adenoviruses in conjunction with viruses expressing either wildtype (CypWT) or dominant-negative (CypDN) CypD. Actin was used as a loading control. B, DNA laddering in AdβGal and AdANT1-infected cardiomyocytes co-incubated with the AdCypWT or AdCypDN viruses. C, TUNEL staining in AdβGal and AdANT1-infected cardiomyocytes co-incubated with the AdCypWT or AdCypDN viruses. D, Propidium iodide (PI) staining in AdβGal and AdANT1-infected cardiomyocytes co-incubated with AdCypWT or AdCypDN viruses. E, Western blotting for ANT and actin in wildtype and CypD-deficient MEFs infected with AdβGal or AdANT1. F, PI staining in wildtype and CypD-deficient MEFs infected with AdβGal or AdANT1. The results shown are representative of at least 3 independent experiments performed in duplicate. Error bars indicate s.e.m, and * denotes P<0.05 versus βGal.
ANT1 causes the upregulation and activation of Bax
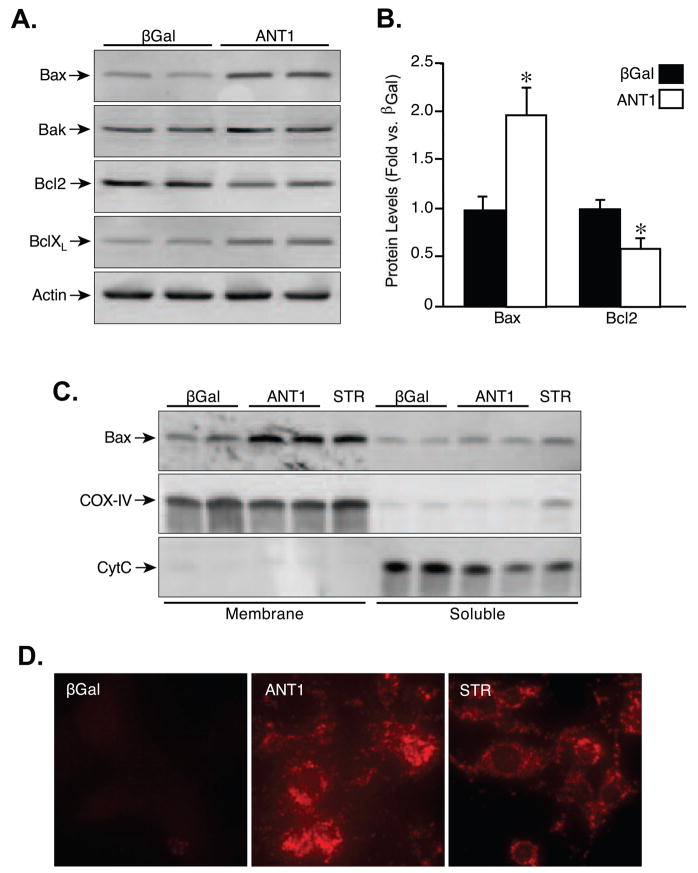
Given that the cell death induced by ANT1 was primarily apoptotic in nature, we investigated whether ANT overexpression lead to alterations in the levels of various pro- and anti-apoptotic Bcl2 proteins. Somewhat to our surprise we found that levels of Bax were elevated 2-fold in ANT1 compared to βGal-infected cardiomyocytes (Fig. 4A,B). In contrast, Bcl2 levels were reduced by nearly half. Neither Bak nor BclXL were significantly affected (Fig. 4A). We next tested whether Bax was also activated in the ANT1-infected cells. One of the hallmarks of Bax activation is its physical insertion into the outer mitochondrial membrane [32], which we subsequently examined by alkali precipitation of membranes and their integral proteins from cardiomyocytes. The proper separation of membrane and soluble mitochondrial proteins was confirmed by blotting for COX-IV and cytochrome c, respectively (Fig. 4C). Infection of myocytes with AdANT1 caused a robust increase in membrane-inserted Bax, similar to the Bax activator staurosporine (STR), compared to control cells (Fig. 4C). Similarly, immunocytochemistry of myocytes using an antibody that recognizes the open, active conformation of Bax [38] further demonstrated a significant increase in the activated form of this pro-apoptotic protein in ANT1-infected cells (Fig. 4D).
FIGURE 4. ANT1 increases Bax expression and activation.
A, Western blotting for Bax, Bak, Bcl2, and BclXL in neonatal cardiomyocytes infected with AdβGal or AdANT1. Actin was used to demonstrate equivalent loading between samples. B, Quantification of the changes in Bax and Bcl2 expression following ANT1 adenoviral infection. C, Western blotting for Bax, COX-IV, and cytochrome c (CytC) in alkali-extracted membrane and soluble fractions from AdβGal or AdANT1-infected cardiomyocytes. D, Immunocytochemistry for activated Bax in AdβGal and AdANT1-infected cardiomyocytes. Treatment with 1 mM staurosporine (STR) for 4 h was used as a positive control for Bax activation. The results shown are representative of at least 3 independent experiments performed in duplicate. Error bars indicate s.e.m, and * denotes P<0.05 versus βGal.
ANT1 cytotoxicity is Bax-dependent
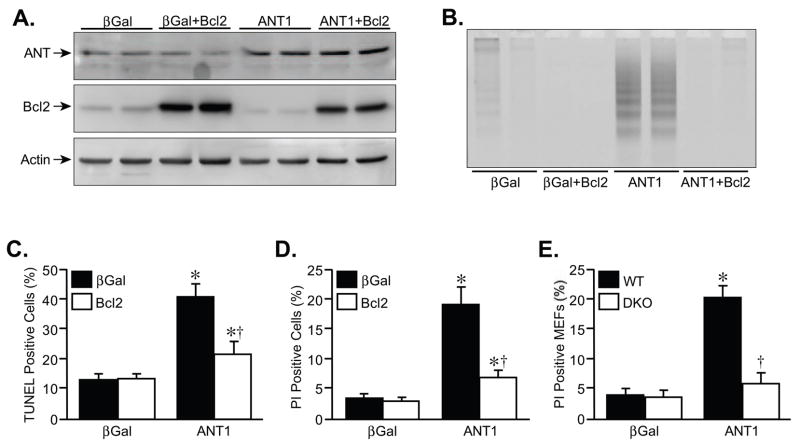
To test whether the upregulation and activation of Bax was necessary for ANT1-induced death, we co-infected cardiomyocytes with the Bax antagonist Bcl2. Overexpression of Bcl2 itself did not affect ANT1 expression (Fig. 5A). However, Bcl2 was extremely effective at attenuating the increases in DNA laddering, TUNEL, and PI staining induced by the ANT1 adenovirus (Fig. 5B–D, respectively). Perhaps more convincingly, overexpression of ANT1 was unable to induce cell killing in bax/bak-null MEFs as opposed to wildtype cells (Fig. 5E), further confirming a causative role for Bax in ANT1-induced cytotoxicity.
FIGURE 5. ANT1-induced myocyte death is inhibited by co-expression of Bcl2.
A, Western blotting for ANT and Bcl2 in neonatal cardiomyocytes infected with AdβGal or AdANT1 in conjunction with an adenovirus expressing Bcl2. Actin was used as a loading control. B, DNA laddering in AdβGal and AdANT1-infected cardiomyocytes co-incubated with the AdBcl2. C, TUNEL staining in AdβGal and AdANT1-infected cardiomyocytes co-incubated with AdBcl2. D, Propidium iodide (PI) staining in AdβGal and AdANT1-infected cardiomyocytes co-incubated with AdBcl2. E, PI staining in wildtype (WT) and Bax/Bak-deficient (DKO) MEFs infected with AdβGal or AdANT1. The results shown are representative of at least 3 independent experiments performed in duplicate. Error bars indicate s.e.m. * denotes P<0.05 versus βGal and † denotes P<0.05 versus ANT1 alone.
Bax upregulation is mediated by increased ROS production
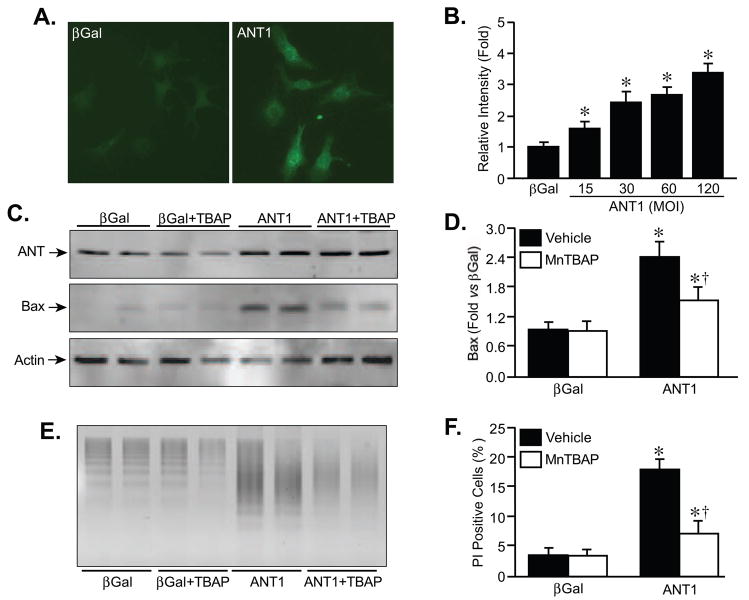
Previous studies have indicated that increases in mitochondrial ROS production can affect the transcription of several genes [39–41], including Bax [41,42]. We therefore examined whether ANT1 overexpression elicited an elevation in ROS production, and whether such increases in ROS output were responsible for the alterations in Bax expression and subsequent cell death. Staining of infected cardiomyocytes with the ROS-sensitive dye DCF (Fig. 6A), did indeed reveal an increase in ROS production in ANT1 versus βGal-infected cells that was gene dose-dependent (Fig. 6B). Moreover, the ANT1-induced increase in ROS was at least partially sensitive to 25 μM bongkrekic acid (Supplementary Fig. 1C). We then incubated cardiomyocytes with the manganese superoxide dismutase mimetic MnTBAP (100 μM) for the duration of the 48 hr infection period. MnTBAP did not interfere with the increase in ANT1 expression produced by the adenovirus (Fig. 6C). However, the antioxidant significantly reduced the upregulation of Bax in response to ANT1 overexpression (Fig. 6C,D). Concomitantly, MnTBAP was able to greatly attenuate cell death induced by ANT1 (Fig. 6E,F). Together, these data suggest that enhanced ROS production is a mechanism by which ANT1 induces Bax expression and cell death. Indeed, the ANT1-induced ROS production was still observed in bax/bak-null MEFs (data not shown), further indicating that the ROS are acting upstream of Bax upregulation and cell death in this mechanism.
FIGURE 6. ANT1-induced Bax upregulation and cell death are blunted by ROS scavenging.
A, ROS production determined by DCF fluorescence in AdβGal or AdANT1-infected neonatal cardiomyocytes. B, Quantification of changes in DCF fluorescence in myocytes following infection with increasing MOI of AdANT1. C, Western blotting for ANT, Bax, and actin in AdβGal or AdANT1-infected neonatal cardiomyocytes treated with either vehicle or the ROS scavenger MnTBAP (100 μM). D, Quantification of the changes in Bax expression following AdANT1 infection with or without MnTBAP. E, DNA laddering in AdβGal and AdANT1-infected cardiomyocytes co-incubated with MnTBAP. F, Propidium iodide (PI) staining in AdβGal and AdANT1-infected cardiomyocytes co-incubated with MnTBAP. The results shown are representative of at least 3 independent experiments performed in duplicate. Error bars indicate s.e.m. * denotes P<0.05 versus βGal alone and † denotes P<0.05 versus ANT1 alone.
DISCUSSION
The role of ANT in cell death has been the subject of considerable controversy. As the concept of the MPT pore was emerging several years ago, a large number of biochemical and pharmacological studies implicated ANT as a structural component of the pore [5,12,13]. Originally thought to be consistent with such a hypothesis, direct overexpression of the ANT1 (and human ANT3) isoform dominantly induced mitochondrial dysfunction and apoptosis in a variety of cell types [21–24]. However, simultaneous ablation of the ant1 and ant2 genes in mice yielded mitochondria and cells that remained sensitive to MPT and cell death-inducing stimuli [25]. Moreover, some ANT ligands have been shown to induce cytochrome c release and apoptosis independently of MPT [26]. Therefore the role of ANT in MPT has been seriously questioned, raising the possibility that ANT mediates cell death through an alternative mechanism.
In the present study we attempted to address the question of whether ANT induced-cell death depends on the MPT pore. Moreover, unlike previous studies we utilized a more clinically relevant cell type, namely primary cardiomyocytes. Mitochondrial dysfunction is a key mediator of numerous cardiac diseases and elevations in ANT1 are observed in human cardiomyopathies [27,28]. Consistent with previous studies, we observed a gene dose-dependent increase in cell death when ANT1 was ectopically expressed in neonatal cardiomyocytes. Moreover, this effect was specific to ANT1, as overexpression of other inner membrane proteins, such as mitofilin and citrin, failed to increase cell mortality. Interestingly, apoptosis was the primary form of cell death, with necrosis only being observed at the highest levels of ANT1 overexpression. Given that MPT appears to be primarily involved in necrosis rather than apoptosis [30,36,37], this initial observation suggested that the ability of ANT1 to elicit myocyte death might well be independent of MPT.
Using fluorescence-based changes in ΔΨm as their index of MPT, prior studies have concluded that ANT-induced cell death was MPT-dependent [21–23], consistent with the idea that ANT was an essential component of the MPT pore complex. Indeed, we too observed a gene dose-dependent reduction in ΔΨm in cardiomyocytes with ANT1 overexpression. However, alterations in mitochondrial potential can occur independently of MPT pore opening, and therefore are not the most reliable way of observing MPT in intact cells. In addition to the ΔΨm measurements, we also utilized the calcein/CoCl2 fluorescence technique developed by Paolo Bernardi’s group, which is a more dependable index of MPT [44]. Unexpectedly, we found that ANT1 overexpression in cardiomyocytes only elicited a small reduction in mitochondrial calcein fluorescence when compared to H2O2, a well-established inducer of MPT. Although this suggested that MPT was not required for ANT1-induced cytotoxicity, it was still conceivable that even the small degree of MPT observed was sufficient to elicit cell death. However, pharmacological blockade of the MPT pore with cyclosporine-A did not protect the myocytes. Similarly, genetic inhibition of CypD, by the use of CypD-deficient fibroblasts, which are resistant to MPT [30,36,37], did nothing to alter the death response to ANT1. Therefore, contrary to the previously published conclusions, the marked mitochondrial dysfunction and cell death associated with ANT1 overexpression is mostly independent of the MPT response, a conclusion consistent with the phenotype of ant1/ant2-null mice [25].
Ruling out MPT as a potential mediator of cell death due to ANT1 left us with the problem of ascribing an alternative mechanism of this protein’s cytotoxic actions. Subsequent analysis of known apoptotic regulators revealed a surprising increased in Bax protein levels and activity, together with a reduction in the pro-survival Bcl2 protein. Thus the Bax/Bcl2 ratio, often used as a measure of a cell’s apoptotic “potential”, was increased ~4-fold. In fact while this manuscript was in preparation, another study also reported that ANT overexpression induced a marked increase in Bax expression in cancer cells [24]. As such changes could easily account for our results observed with ANT1 overexpression, we resolved to further test the premise that Bax upregulation (and presumably activation) was a necessary event in ANT1-induced myocyte death. Upon activation, Bax undergoes a conformational change that enables it to physically integrate into the outer mitochondrial membrane [32,38]. Indeed, we were able demonstrate enhanced levels of the active Bax conformer and concomitant increases in membrane-embedded Bax in myocyte membranes following ANT1 infection. Therefore, in addition to simply increasing Bax protein, ANT1 expression also causes the activation and mitochondrial integration of this pro-apoptotic molecule. However, while these data were very suggestive, they still did not ascribe a causative role for Bax upregulation/activation in ANT1-induced cell death. The simplest approach to address this question was to replenish the lost Bcl2 pool with exogenously expressed protein, thereby antagonizing Bax. Co-expression of Bcl2 almost completely abrogated the cytotoxic effects of ANT1 expression in the myocytes, indicating that Bax does indeed play a role in this process. Although we cannot rule out that Bcl2 exerts its protective effects via a direct interaction with ANT1 [45,46] rather than neutralization of Bax, the fact that ANT1 was ineffective in Bax/Bak-null cells only helps to confirm a role for Bax as a critical mediator of ANT1-induced cell death.
The fact that overexpression of ANT1 specifically in the mitochondria induces the upregulation of the nuclear encoded Bax suggests that a mitochondrial-nuclear signal is being generated. One prominent way mitochondria communicate with other subcellular compartments is through the generation of ROS, and the release of sub-lethal amounts of oxygen radicals has been shown to influence the transcription of a variety of nuclear genes [39–43]. In the present study, we found that ANT1 overexpression in cardiomyocytes elicited a small but significant rise in ROS production. Moreover, the degree of oxidant generation correlated with the amount of ANT1 protein, suggesting that this phenomenon is specific to ANT1 and not just a generalized perturbation of the mitochondrion. One might expect that increased ROS could induce a robust MPT response, but since this was not observed it suggests that the level or micro-domain of the ROS generated by ANT1 expression served a signaling function instead. Importantly, scavenging this ROS significantly reversed the ability of ANT1 to upregulate Bax levels, and consequently reduced the degree of cardiomyocyte mortality. Therefore, ROS appear to be a relevant signaling mechanism by which ANT1 crosstalks with Bax. How exactly this ANT1-induced ROS production specifically alters Bax expression is still unclear, although it has been reported that ANT1 expression can inhibit NFκB signaling [24,47], which would relieve suppression of the Bax gene [48,49]. Alternatively, the possibility that ANT1 increases Bax levels through reduced protein degradation (as opposed to increased production) cannot be ruled out at this juncture.
Although the present study provides new molecular insight into the role ANT1 may be playing in the regulation of cardiac myocyte mortality, it is limited by the fact the experiments were conducted in cultured cells. Therefore, caution should be exercised when extrapolating these findings to the in vivo situation. Schultheiss’ group has reported that there is an increase in the ANT1 to ANT2 ratio in cardiomyopathic patients [27,28]. Ours, and others, data would suggest that this is deleterious because ANT1 is cytotoxic whereas ANT2 appears to be innocuous [21,23]. However, this is complicated by the fact that an ANT2 to ANT1 shift also decreases net ANT activity, which in of itself would be detrimental and potentially precipitate heart failure. Indeed, at least in a model of renin-induced cardiomyopathy, transgenic overexpression of ANT1 in mice was cardioprotective rather than cardiotoxic [50]. The discrepancy between these and our findings is not entirely clear. The different metabolic demands of the myocyte in vitro versus in vivo could certainly play a role. It is also conceivable that the chronic expression of ANT1 during development of the transgenics leads to compensatory changes that suppress ANT1’s detrimental facets. It may simply be that the role of ANT in cardiomyocyte death in vivo is context-specific. Further study is clearly warranted.
Supplementary Material
Acknowledgments
We are very grateful to Lorrie Kirshenbaum (University of Manitoba) for the generous gift of the Bcl2 adenovirus, and the late Stanley Korsmeyer (Dana-Farber Cancer Institute) for the Bax/Bak gene-targeted mice. The plasmids containing the mitofilin and citrin cDNAs were generous gifts from Jiping Zha (University of Texas Southwestern), and Jorgina Satrústegui (Universidad Autonoma de Madrid), respectively. This work was supported by grants from the National Institutes of Health (J.D.M), an American Heart Association Scientist Development Grant (C.P.B.), and a Children’s Hospital Research Foundation Trustee Grant (C.P.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 2.Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol. 2005;38:3–13. doi: 10.1016/j.yjmcc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–58. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 5.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 6.Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–15. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Fiore C, Trézéguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80:137–50. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 11.Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem. 2007;282:29658–66. doi: 10.1074/jbc.M704386200. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10:1507–25. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 13.Belzacq AS, Vieira HL, Kroemer G, Brenner C. The adenine nucleotide translocator in apoptosis. Biochimie. 2002;84:167–76. doi: 10.1016/s0300-9084(02)01366-4. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Wang Y, Hirai K, Ayub A, Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H899–908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 15.Rajesh KG, Sasaguri S, Suzuki R, Maeda H. Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am J Physiol Heart Circ Physiol. 2003;285:H2171–8. doi: 10.1152/ajpheart.00143.2003. [DOI] [PubMed] [Google Scholar]
- 16.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Gαq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ Res. 2000;87:1180–7. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 18.Akao M, O’Rourke B, Kusuoka H, Teshima Y, Jones SP, Marbán E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- 19.Vyssokikh MY, Katz A, Rueck A, Wuensch C, Dörner A, Zorov DB, Brdiczka D. Adenine nucleotide translocator isoforms 1 and 2 are differently distributed in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem J. 2001;358:349–58. doi: 10.1042/0264-6021:3580349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodfield K, Rück A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336:287–90. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer MK, Schubert A, Rocks O, Grimm S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J Cell Biol. 1999;147:1493–502. doi: 10.1083/jcb.147.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert A, Grimm S. Cyclophilin D, a component of the permeability transition-pore, is an apoptosis repressor. Cancer Res. 2004;64:85–93. doi: 10.1158/0008-5472.can-03-0476. [DOI] [PubMed] [Google Scholar]
- 23.Zamora M, Granell M, Mampel T, Viñas O. Adenine nucleotide translocase 3 (ANT3) overexpression induces apoptosis in cultured cells. FEBS Lett. 2004;563:155–60. doi: 10.1016/S0014-5793(04)00293-5. [DOI] [PubMed] [Google Scholar]
- 24.Jang JY, Choi Y, Jeon YK, Aung KC, Kim CW. Over-expression of adenine nucleotide translocase 1 (ANT1) induces apoptosis and tumor regression in vivo. BMC Cancer. 2008;8:160. doi: 10.1186/1471-2407-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–5. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machida K, Hayashi Y, Osada H. A novel adenine nucleotide translocase inhibitor, MT-21, induces cytochrome c release by a mitochondrial permeability transition-independent mechanism. J Biol Chem. 2002;277:31243–8. doi: 10.1074/jbc.M204564200. [DOI] [PubMed] [Google Scholar]
- 27.Dörner A, Schultheiss HP. The myocardial expression of the adenine nucleotide translocator isoforms is specifically altered in dilated cardiomyopathy. Herz. 2000;25:176–80. doi: 10.1007/s000590050004. [DOI] [PubMed] [Google Scholar]
- 28.Schultheiss HP, Schulze K, Dörner A. Significance of the adenine nucleotide translocator in the pathogenesis of viral heart disease. Mol Cell Biochem. 1996;163–164:319–27. doi: 10.1007/BF00408672. [DOI] [PubMed] [Google Scholar]
- 29.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–9. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 30.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 31.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–5. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–15. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell. 2005;16:1543–54. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Arco A, Agudo M, Satrústegui J. Characterization of a second member of the subfamily of calcium-binding mitochondrial carriers expressed in human non-excitable tissues. Biochem J. 2000;345:725–32. doi: 10.1042/0264-6021:3450725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 37.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–41. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 40.Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal. 2007;9:1317–28. doi: 10.1089/ars.2007.1708. [DOI] [PubMed] [Google Scholar]
- 41.Chandel NS, Vander Heiden MG, Thompson CB, Schumacker PT. Redox regulation of p53 during hypoxia. Oncogene. 2000;19:3840–8. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- 42.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–60. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 43.Park WH, Han YW, Kim SH, Kim SZ. An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J Cell Biochem. 2007;102:98–109. doi: 10.1002/jcb.21280. [DOI] [PubMed] [Google Scholar]
- 44.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–34. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC, Kroemer G. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–36. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- 46.Belzacq AS, Vieira HL, Verrier F, Vandecasteele G, Cohen I, Prévost MC, Larquet E, Pariselli F, Petit PX, Kahn A, Rizzuto R, Brenner C, Kroemer G. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 2003;63:541–6. [PubMed] [Google Scholar]
- 47.Zamora M, Meroño C, Viñas O, Mampel T. Recruitment of NF-kappaB into mitochondria is involved in adenine nucleotide translocase 1 (ANT1)-induced apoptosis. J Biol Chem. 2004;279:38415–23. doi: 10.1074/jbc.M404928200. [DOI] [PubMed] [Google Scholar]
- 48.Cianfrocca R, Muscolini M, Marzano V, Annibaldi A, Marinari B, Levrero M, Costanzo A, Tuosto L. RelA/NF-kappaB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008;15:354–63. doi: 10.1038/sj.cdd.4402264. [DOI] [PubMed] [Google Scholar]
- 49.Bentires-Alj M, Dejardin E, Viatour P, Van Lint C, Froesch B, Reed JC, Merville MP, Bours V. Inhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell lines. Oncogene. 2001;20:2805–13. doi: 10.1038/sj.onc.1204343. [DOI] [PubMed] [Google Scholar]
- 50.Walther T, Tschöpe C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE, Bader M, Shakibaei M, Schultheiss HP, Dörner A. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–44. doi: 10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.