Abstract
Background:
Metastatic melanoma is the most deadly form of skin cancer and with an overall 5-year survival rate of <11%, there is an acute need for novel therapeutic strategies. Activating mutations in the BRAF oncogene are present in 50–70% of cases and contribute to tumourigenesis, thus, defining downstream targets of oncogenic BRAF may help define novel targets for therapeutic intervention. The Ca2+/calcineurin-regulated transcription factor, Nuclear factor of activated T-cells (NFAT), is important in the pathogenesis of several human cancers, target genes of which are also known to contribute to melanoma progression. One such NFAT target gene is COX-2, increased expression of which correlates with poor prognosis; however, upstream regulators of COX-2 in melanoma remain undefined. Therefore, the aim of this study was to evaluate NFAT expression and activity in metastatic melanoma and establish whether or not oncogenic BRAF signalling modulates NFAT activity and determine if NFAT is a key upstream regulator of COX-2 in melanoma.
Methods:
Nuclear factor of activated T-cells transcriptional activity and protein expression were determined in three human metastatic melanoma cell lines with differing B-RAF mutational status. NFAT activation by oncogenic BRAFV600E was explored by BRAFV600E overexpression and application of the specific MEK inhibitor PD98059. Regulation of COX-2 expression by NFAT was investigated using NFAT-targeted siRNA, calcineurin inhibitors cyclosporin A and FK506, in addition to COX-2 luciferase reporter vectors that selectively lacked NFAT binding sites.
Results:
NFAT transcriptional activity was increased in BRAF-mutated melanoma cells compared with wild-type cells. Furthermore, in wild-type cells, overexpression of BRAFV600E increased NFAT activity, which was blocked by the MEK inhibitor PD98059. Using calcineurin inhibitors and siRNA-mediated knockdown of NFAT2 and 4, we show NFAT is required for COX-2 promoter activation and protein induction in metastatic melanoma cells.
Conclusion:
NFAT2 and 4 are expressed in human metastatic melanoma cell lines and are activated by oncogenic BRAFV600E via MEK/ERK signalling. NFAT is an important upstream regulator of COX-2 in metastatic melanoma. Furthermore, as the BRAF/MEK/ERK pathway is hyperactive in other malignancies and MEK/ERK are also activated by oncogenic RAS in 30% of all human cancers, the potential to exploit NFAT signalling for therapeutic benefit warrants further investigation.
Keywords: B-RAF, NFAT, melanoma, COX-2
Malignant melanoma (MM) is the most lethal form of skin cancer and represents an increasing world health problem currently responsible for around 48 000 deaths worldwide each year (www.WHO.int). MM is notoriously unresponsive to current chemotherapy and with an overall 5-year survival rate of <11%, there is an acute need for novel therapeutic strategies (Dhomen and Marais, 2007).
Perhaps the greatest recent advance in understanding risk factors predisposing to melanoma has been the discovery of activating mutations in the BRAF gene, occurring in 50–70% of all melanomas (Davies et al, 2002; Dhomen and Marais, 2007). NRAS mutations are also common in melanoma occurring in up to 30% of cases, and as the occurrence of NRAS or BRAF mutation in melanoma is mutually exclusive, up to 90% of melanomas harbour a mutated, hyperactive Ras–RAF signalling pathway (Davies et al, 2002). Understanding downstream effectors of oncogenic BRAF signalling may, therefore, facilitate the identification of novel therapeutic targets. B-RAF is a serine–threonine kinase belonging to the RAF-extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK pathway, which regulates diverse cellular processes including proliferation, differentiation and apoptosis (Dhomen and Marais, 2007). The BRAFV600E activating mutation accounts for approximately 90% of BRAF mutations in melanoma and BRAFV600E drives melanomagenesis in mice (Dhomen and Marais, 2007; Dankort et al, 2009). Inhibition of mutant BRAF signalling in melanoma cell lines reduces proliferation, migration, increases susceptibility to apoptosis induction and ablates tumour formation in mice (Karasarides et al, 2004; Sharma et al, 2005, 2006). Although BRAF is also mutated in up to 80% of benign melanocytic naevi (Pollock et al, 2003), the proportion of naevi harbouring a BRAF mutation that progress to melanoma is small, underscoring that further genetic events are required for the development of melanoma. Furthermore, it has now been established that after initial oncogenic activation and proliferation, naevi become senescent and events that overcome or evade senescence are crucial for melanoma development in mice (Michaloglou et al, 2005). Nevertheless, the mechanisms by which BRAF signalling promotes melanoma development and/or progression to metastatic disease are not well defined and effective melanoma therapy remains elusive.
Although pre-clinical studies indicate that blocking oncogenic BRAF signalling provides a promising strategy for MM treatment, current clinical trials are yet to demonstrate whether pharmacological targeting of the BRAF/MEK/ERK pathway represents an effective treatment for melanoma as a monotherapy. Although the efficacy of such agents may be improved by combination with conventional chemotherapeutics (Haass et al, 2008), the functional redundancy between pathways driving tumour progression and acquired chemoresistance, nevertheless, leaves a demand for alternative drug targets. For example, prolonged treatment of BRAFV600E-expressing melanoma cells with the BRAF inhibitor AZ628, leads to the development of clones that maintain high levels of phosphorylated ERK, which continue to proliferate in the presence of drug (Montagut et al, 2008). The identification of novel targets for use in combination chemotherapy may therefore help to combat acquired chemoresistance and oncogenic-signalling redundancy.
Nuclear factor of activated T cells (NFAT) proteins were originally defined as a family of Ca2+/calcineurin-dependent transcription factors required for T-cell activation (Hogan et al, 2003). Outwith the immune system, NFAT is functionally important in the muscle, the bone, the skin (Flockhart et al, 2008) and the neural tissue, involved in diverse functions including proliferation, angiogenesis and apoptosis (Hogan et al, 2003; Zaichuk et al, 2004). Four Ca2+/calcineurin-regulated NFAT proteins (NFAT1–4) have been identified that reside hyper-phosphorylated in the cytosol. Dephosphorylation by the Ca2+-dependent phosphatase calcineurin evokes NFAT nuclear translocation and transcriptional activation where NFAT drives gene transcription (Hogan et al, 2003).
Pertinent to tumour biology, deregulated or abnormal expression of NFAT has been reported in a number of haematological malignancies and solid tumours (Medyouf and Ghysdael, 2008), and we have recently shown that NFAT is preferentially activated by carcinogenic UVB radiation in keratinocytes (Flockhart et al, 2008). Furthermore, accumulating evidence suggests that increased NFAT transcriptional activity contributes to both the development and progression of cancer (Buchholz and Ellenrieder, 2007), and identifies NFAT signalling as a potential target for cancer therapy. In the context of melanoma, increased expression of the NFAT target gene cyclooxygenase-2 (COX-2), an inducible enzyme involved in the conversion of arachidonic acid to prostaglandins, correlates with poor prognosis (Kuzbicki et al, 2006; Chwirot and Kuzbicki, 2007), suggesting that NFAT signalling may be important in melanoma. However, upstream activators of COX-2 in melanoma and a role for oncogene-driven NFAT activation remain undefined.
Outwith the cancer context, an important role for the Ras/RAF/MEK/ERK signalling axis in regulating NFAT transcriptional activity has been established in immune cells and in myocytes. An effective adaptive immune response relies partly on the production of IL-2 by T cells after T-cell receptor (TCR) activation, which proceeds via MAPK/NFAT signalling. In T cells, dominant negative BRAF or MEK inhibition completely abolished activated TCR-driven ERK activation, IL-2 promoter activation and activation of NFAT1 and 2 indicating the importance of MAPK/NFAT signalling in immune cell biology (Tsukamoto et al, 2004). Similarly, stimulating cardiac myocytes with phenylephrine, a ligand that contributes to cardiac hypertrophy, induced NFAT activation that was blocked by MEK/ERK inhibition (Ichida and Finkel, 2001). NFAT therefore serves as a putative downstream target of activated MAPK signalling in non-cancerous cells, however, a role for oncogene-driven MAPK/NFAT signalling has not been described.
The aim of the present study was to test the hypothesis that NFAT may be an important downstream target of oncogenic BRAF in melanoma and that NFAT is involved in regulating COX-2 expression. We show that NFAT is activated by oncogenic BRAFV600E in human metastatic melanoma cell lines via canonical MEK/ERK signalling. In addition, we identify that COX-2 expression is regulated by NFAT in BRAF-mutated melanoma cell lines, therefore highlighting that NFAT can be activated by oncogene mutation and that NFAT regulates downstream factors, which are important in melanoma biology.
Materials and methods
Cell culture and chemicals
The human metastatic melanoma cell lines A375, CHL-1 and WM266-4 (American Type Culture Collection, Teddington, UK) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Basel, Switzerland) supplemented with 10% foetal bovine serum, penicillin and streptomycin in a humidified incubator at 37°C/5% CO2. Primary human melanocytes were identified in cultures of primary keratinocytes obtained from human foreskin samples and isolated by selective trypsinisation, as previously described (Lovat et al, 2008). Primary melanocytes were cultured in medium-254 supplemented with melanocyte growth supplements (Invitrogen, Paisley, UK) plus antibiotics. Chemicals purchased were as follows; 12-O-tetradecanoylphorbol-13-acetate (TPA), ionomycin, FK506 (Sigma, Poole, UK), Cyclosporin A (Merk Chemicals Ltd, Nottingham, UK) and PD98059 (Promega, Southampton, UK).
Luciferase assays
Nuclear factor of activated T-cells transcriptional activity was measured using an NFAT-dependent luciferase reporter (p(NFAT)9-luc) (Wilkins et al, 2004) (a gift from JD Molkentin, Children's Hospital Medical Center, Cincinnati, OH, USA). In all luciferase experiments cells were co-transfected with a renilla luciferase control vector (pRLTK, Promega) and reporter firefly values were normalised to renilla values. Assays were carried out using the dual luciferase assay system (Promega). Cells were seeded in 24-well culture plates and transfected with 0.5 μg of firefly reporter plus 0.0125 μg of renilla control using Lipofectamine 2000 reagent and Opti-MEM medium (Invitrogen) according to manufacturer's instructions. For BRAF overexpression experiments, cells were transfected with 0.5 μg firefly reporter, 0.25 μg BRAF expression vector (pEFmBRAFWT or pEFmBRAFV600E (Davies et al, 2002), kind gifts from R. Marais, Institute of Cancer Research, London, UK) and 0.0125 μg renilla. COX-2 luciferase reporters were transfected, as described above, and were gifts from M.A. Iniguez, Centro de Biologia Molecular, Madrid, Spain (Iñiguez et al, 2000).
siRNA-mediated gene silencing
Small interfering RNA (siRNA) targeting NFAT2 (cat. no. SI03057684) and NFAT4 (cat. no. SI03114118) (Qiagen, Crawley, UK) was transfected at a final concentration of 40 nM using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were incubated for 48 h after transfection then treated with TPA (50 nM) and ionomycin (1 μM) for 4 h and cell lysates were prepared for western blotting.
mRNA extraction and real-time qPCR
Total mRNA was extracted using an RNeasy kit (Qiagen) and converted to cDNA using a high capacity cDNA reverse transcriptase kit (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. Expression of NFAT mRNA was determined using a customised gene expression card (Applied Biosystems) and real-time qPCR was carried out using the ABI Prism 7900HT sequence detection system.
Western blotting
Total protein was extracted from cell pellets, resolved by SDS–PAGE (Web Scientific, Crewe, Cheshire, UK), and transferred to PVDF membranes, blocked and probed as previously described (Flockhart et al, 2008). In all, 30 μg of protein was loaded per lane. Antibodies used were as follows: COX-2 (1 : 100), BRAF (1 : 1000), NFAT3 (1 : 1000) and NFAT4 (1 : 500) (Santa Cruz Biotechnology, Autogen Bioclear, Calne, UK), ERK and phospho-ERK (1 : 1000, Cell Signalling Technology, New England Biolabs, Hitchin, UK), and NFAT2 (1 : 1000, BD Biosciences, Oxford, UK). Equal protein loading was confirmed by blotting for β-actin (1 : 40 000, Sigma) or GAPDH (1 : 2000, Cell Signalling Technology). Antibody signal was detected using HRP-conjugated secondary antibodies (1 : 2000, Millipore Ltd, Watford, UK) and membranes were visualised using ECL Plus reagent (Amersham Biosciences, Buckinghamshire, UK) and a Fujifilm FLA-3000 fluorescent image analyzer.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5. 00, GraphPad Software (San Diego, CA, USA).
Results
NFAT activity and expression in metastatic melanoma cell lines
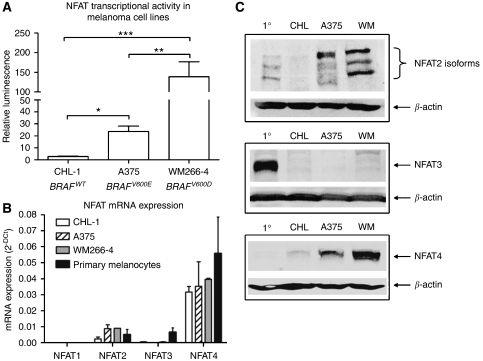
Basal NFAT transcriptional activity was measured in three human metastatic melanoma cell lines with different BRAF mutational status. CHL-1 cells are BRAF wild-type, whereas A375 and WM266-4 cells harbour BRAFV600E and BRAFV600D mutations respectively (Dhomen and Marais, 2007). NFAT transcriptional activity was approximately 10-fold higher in A375 cells and approximately 70-fold higher in WM266-4 cells compared with BRAF wild-type CHL-1 cells (Figure 1A), consistent with increased B-RAF activity of BRAFV600D compared with BRAFV600E (Wan et al, 2004). At the mRNA level, NFAT2 and to a greater extent NFAT4 were expressed in all the three cell lines and in primary melanocytes, whereas NFAT3 mRNA was expressed exclusively in primary melanocytes, and NFAT1 expression was not detected in any cell type (Figure 1B). We therefore examined the expression of NFAT2, 3 and 4 proteins in primary melanocytes and in the metastatic cell lines. Three previously described NFAT2 isoforms (Hogan et al, 2003) were expressed in primary melanocytes but were absent in CHL-1 cells, whereas their expression was higher in A375 cells and had further increased in WM266-4 cells (Figure 1C). NFAT3 protein was exclusively expressed in primary melanocytes (Figure 1C). NFAT4 protein was not detected in primary melanocytes and was expressed at low levels in CHL-1 cells, whereas expression had increased in A375 cells and elevated further in WM266-4 cells (Figure 1C). These data suggest that NFAT2 and NFAT4 are the active NFAT proteins that contribute to the NFAT activity shown in Figure 1A.
Figure 1.
Nuclear factor of activated T-cells (NFAT) transcriptional activity, mRNA expression and protein expression in melanoma cell lines. (A) NFAT transcriptional activity measured in three human metastatic melanoma cell lines. BRAF mutational status is indicated for reference. Values are means from four independent experiments carried out in triplicate (± s.e.m.). (B) NFAT mRNA expression in primary melanocytes and in three metastatic melanoma cell lines as determined by real-time qPCR. Values are mean 2−ΔCt from RNA derived from two independent cultures (± s.d.). (C) Western blots indicate the expression of three previously described NFAT2 isoforms, NFAT3 protein and NFAT4 protein. 1°=primary melanocytes, CHL=CHL-1 cells, WM=WM266-4 cells. Equal protein loading was confirmed by blotting for β-actin. Statistical analysis in (A) performed by one-way ANOVA, * P⩽0.05, ** P⩽0.01, *** P⩽0.001.
NFAT is activated by oncogenic BRAFV600E
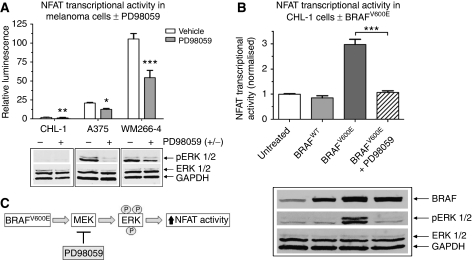
As data in Figure 1 indicated NFAT transcriptional activity was higher in BRAF-mutated melanoma cells; activation of NFAT by mutant BRAF was further investigated. Inhibition of BRAF/MEK/ERK signalling using the highly specific MEK inhibitor PD98059 resulted in a reduction in NFAT transcriptional activity among all three melanoma cell lines (Figure 2A). PD98059 also reduced the amount of phosphorylated-ERK1/2, thereby confirming inhibition of MEK/ERK signalling and that MEK/ERK signals regulate NFAT activity (Figure 2A). We next assessed the effect of overexpression of BRAFWT or the most common BRAF mutation in melanoma, BRAFV600E, on NFAT transcriptional activity in CHL-1 cells. Overexpression of BRAFWT had no effect on NFAT activity, whereas BRAFV600E induced a significant three-fold increase that was blocked by PD98059 (Figure 2B). Western blotting confirmed overexpression of BRAFWT and BRAFV600E, BRAFV600E-induced ERK phosphorylation and inhibition of BRAFV600E-induced ERK phosphorylation by PD98059 (Figure 2B). These data indicate that BRAFV600E increases NFAT transcriptional activity via MEK/ERK-dependent signalling (Figure 2C).
Figure 2.
BRAFV600E activates nuclear factor of activated T-cells (NFAT) through a extracellular signal-regulated kinase kinase (MEK)-dependent pathway. (A) NFAT transcriptional activity was measured 16 h after treatment with the MEK inhibitor PD98059 (10 μM). Values are means from three independent experiments carried out in triplicate (±s.e.m.). Identically treated samples were prepared for western blotting and membranes were probed for phosphorylated extracellular signal-regulated kinase (ERK) (pERK 1/2) and total ERK (ERK 1/2). (B) The effect of BRAFWT or BRAFV600E overexpression on NFAT transcriptional activity in CHL-1 cells was measured by co-transfecting cells with an NFAT luciferase reporter±vectors expressing BRAFWT or BRAFV600E. BRAFV600E-expressing cells were also treated with PD98059 where indicated. Untreated cells were transfected with NFAT reporter plus transfection reagent only and NFAT luciferase activity was measured 48 h later. Values are normalised to the untreated control and are means from four independent experiments carried out in triplicate (±s.e.m.). Identically treated samples were prepared for western blotting to confirm BRAFWT and BRAFV600E overexpression and downstream ERK phosphorylation. Equal protein loading was confirmed by blotting for GAPDH. Statistical analysis performed by t-test (A) or one-way ANOVA (B), ** P⩽0.01, *** P⩽0.001 vs vehicle control. (C) Schematic of BRAFV600E-induced NFAT activation.
NFAT regulates COX-2 protein induction in metastatic melanoma cell lines
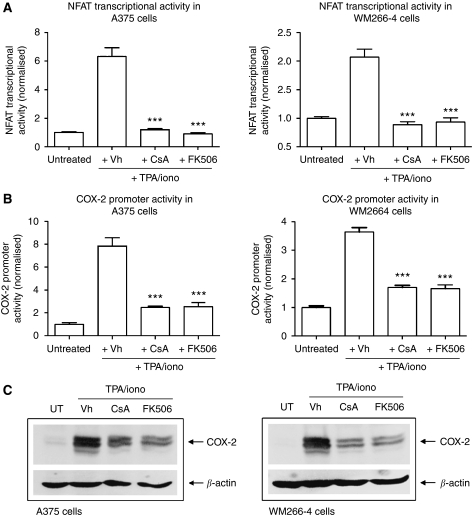
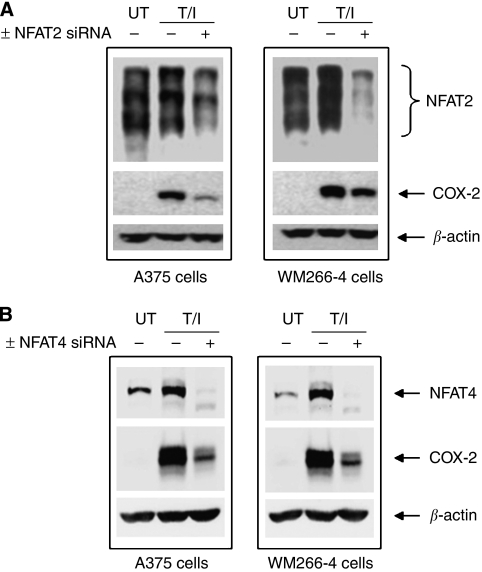
The importance of NFAT signalling in regulating COX-2 promoter activation and protein production was evaluated in BRAF-mutated melanoma cells using COX-2 luciferase reporter vectors that selectively lacked functional NFAT binding sites, calcineurin inhibitors and siRNA-mediated knockdown of NFAT2 and 4. Treating A375 and WM266-4 cells with the classical NFAT activators TPA and ionomycin (TPA/iono) (Hogan et al, 2003) increased both NFAT transcriptional activity (Figure 3A) and COX-2 promoter activity (Figure 3B) that was blocked by the calcineurin inhibitors CsA and FK506. This induction/inhibition approach was then employed to establish effects of NFAT inhibition on COX-2 protein induction. Treating A375 and WM266-4 cells with TPA/iono induced a profound increase in COX-2 protein expression that was markedly reduced by CsA and FK506 (Figure 3C). In addition, as NFAT2 and 4 were shown to be expressed in A375 and WM266-4 cells (Figure 1), we decided to ablate NFAT2 and 4 protein using siRNA to show that specific NFAT proteins regulate COX-2 protein induction. Indeed, knockdown of NFAT2 or NFAT4 reduced COX-2 protein induction in these cells (Figure 4).
Figure 3.
Nuclear factor of activated T-cells (NFAT) inhibition blocks cyclooxygenase-2 (COX-2) protein induction. Cells were transfected with either an NFAT luciferase reporter (A) or COX-2 luciferase reporter (B) and pretreated for 2 h with vehicle (DMSO, Vh), CsA (1 μM) or FK506 (1 μM) before TPA (50 nM)/ionomycin (iono) (1 μM) treatment. Luciferase activity was measured 4 h after TPA/iono treatment and results are shown for A375 and WM266-4 cells. (C) Western blot samples were treated exactly as in luciferase experiments and induction of COX-2 protein is shown for A375 and WM266-4 cells. The three immunoreactive bands present are previously described, glycosylated variants of COX-2 protein. Equal protein loading was confirmed by blotting for β-actin. In (A) and (B) values are means from three independent experiments carried out in triplicate (± s.e.m.). Statistical analysis performed by one-way ANOVA, *** P⩽0.001 vs cells treated with TPA/iono+Vh.
Figure 4.
Small interfering RNA (siRNA)-mediated knockdown of nuclear factor of activated T-cells (NFAT) 2 or NFAT4 reduces cyclooxygenase-2 (COX-2) protein induction. WM266-4 and A375 cells were transfected with siRNA targeting either NFAT2 (A) or NFAT4 (B). 48 h post-transfection, cells were treated with TPA (50 nM) and ionomycin (1 μM) (T/I). UT=untreated control sample. At 4 h after treatment, cell lysates were prepared for western blotting. Membranes were probed with antibodies to detect NFAT2 (A), NFAT4 (B) and COX-2. Equal protein loading was confirmed by blotting for β-actin.
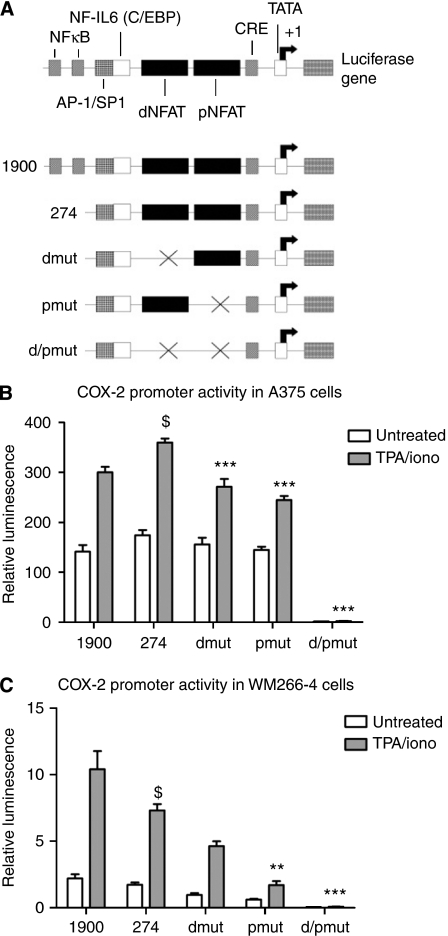
Additional COX-2 luciferase reporter experiments indicated functional NFAT binding sites in the COX-2 promoter were required for maximal COX-2 induction (Figure 5). The human COX-2 promoter contains two NFAT binding sites and COX-2 luciferase reporter vectors that selectively lacked either one or both of these sites were used to further assess the importance of NFAT as a regulator of the COX-2 promoter (schematic in Figure 5A). Basal COX-2 promoter activity and activity induced by TPA/iono was assessed. Treating A375 and WM266-4 cells with TPA/iono increased the COX-2 promoter activity and this response was reduced in vectors that contained mutated distal (dmut) or proximal (pmut) sites compared with the vector containing both functional NFAT binding sites (274) (Figure 5B, C). Basal promoter activity was slightly reduced in vectors with either dmut or pmut sites, whereas the vector with both NFAT sites mutated reported minimal activity. The reporter vector with both NFAT sites mutated (d/pmut) was not induced at all by TPA/iono in either cell type (Figure 5B, C). Collectively, these data provide evidence that NFAT regulates COX-2 promoter activity and induction of COX-2 protein in metastatic melanoma cells.
Figure 5.
Nuclear factor of activated T-cells (NFAT) regulates cyclooxygenase-2 (COX-2) promoter activity in melanoma cell lines. (A) Schematic of the human COX-2 promoter and the COX-2 luciferase reporter vectors used. Cells were transfected with the panel of reporter vectors and treated 24 h later with TPA (50 nM)/ionomycin (iono) (1 μM) for 4 h. Reporter activity is shown for A375 cells (B) and WM266-4 cells (C). Values are means from three independent experiments performed in triplicate (± s.e.m.). Statistical analysis performed by one-way ANOVA, **P⩽0.01, *** P⩽0.001 vs control vector containing all functional NFAT binding sites ($).
Discussion
Previous studies have shown that NFAT signalling is important in haematological malignancies and solid tumours (Buchholz and Ellenrieder, 2007; Medyouf and Ghysdael, 2008) and that NFAT is activated by environmental carcinogens such as ultraviolet radiation (Flockhart et al, 2008), but a role for oncogene mutation-driven NFAT signalling has not been previously described. Although α6β4 integrin signals stimulate NFAT activity in breast cancer (Jauliac et al, 2002), we now identify that NFAT is activated by oncogenic BRAF signalling, which is the most frequently mutated gene in melanoma and a gene frequently mutated in many other cancers. Activating BRAF mutations are present in 29–69% of papillary thyroid carcinomas (Wojciechowska and Lewinski, 2006), are also common in colorectal cancers (Ogino et al, 2009) and present in 4% of small cell lung cancers (Halilovic and Solit, 2008). Identifying that NFAT functions downstream of mutant BRAF in melanoma, raises the possibility that NFAT may be similarly activated in other BRAF-mutated cancers.
Mechanistically, our data indicate that activation of NFAT by oncogenic BRAFV600E involves canonical BRAF/MEK/ERK signalling. Studies in myocytes and T and B lymphocytes indicate that non-oncogenic Ras/RAF signalling increases NFAT activity via ERK activation concurrent with results from this study (Ichida and Finkel, 2001; Tsukamoto et al, 2004). Although MAPK signalling in a non-oncogenic context has been shown to be an important regulator of NFAT activity, the mechanism responsible for this is not completely understood. It is interesting to note that elevated MEK/ERK signalling stimulates AP-1 complex formation which is a well established binding-partner of NFAT that supports NFAT-mediated gene transcription (Macian et al, 2000, 2001). In addition, MEK, ERK, calcineurin and NFAT4 are able to form a large molecular weight protein complex in cardiac myocytes, and although active MEK/ERK signalling did not affect NFAT at the nuclear localisation level, MEK/ERK signalling did increase DNA binding of NFAT4 (Sanna et al, 2005). In this context, increased NFAT transcriptional activity required AP-1 complex formation (Sanna et al, 2005). It is therefore possible that oncogenic Ras/RAF signalling may increase DNA binding and transcriptional activity of NFAT proteins via a MEK/ERK-dependent mechanism that may also require AP-1 complex formation. Further studies to elucidate the mechanism by which MEK/ERK signals increase NFAT transcriptional activity are required particularly, since MEK/ERK/NFAT signalling may be important in other cancers.
Our observation that NFAT activity was higher in V600D-mutated WM266-4 cells compared with V600E-mutated A375 cells, which correlated with the amount of BRAF kinase activity evoked by each mutant is particularly noteworthy. The V600E mutation confers an increase in BRAF kinase activity of approximately 480 times compared with wild-type BRAF, whereas the V600D mutation increases this by approximately 700 times (Wan et al, 2004). The level of inhibition of NFAT activity by PD98059 also correlated with the ability of PD98059 to reduce levels of phospho-ERK consistent with the notion that NFAT activity is regulated in-part by MEK/ERK-dependent signalling in melanoma cells. Increased amounts of PD98059 were not used in an effort to further reduce phospho-ERK levels in WM266-4 cells, as increased concentrations of PD98059 can exert non-specific effects. Although MEK inhibition resulted in the downregulation of NFAT activity in BRAF mutant melanoma cells, this effect was also evident in BRAF wild-type CHL-1 cells, although the absolute levels of NFAT activation were substantially lower. This is not surprising as MEK/ERK signalling is still functional, albeit at lower levels in CHL-1 cells. Indeed, the low threshold level of pERK in these cells may promote their sensitisation to MEK inhibition.
The involvement of MEK/ERK activation raises the possibility that other upstream signals activating MEK/ERK may increase NFAT activity. This is highly significant as oncogenic RAS (active in approximately 30% of all human cancers) signals partly via MEK/ERK (Schubbert et al, 2007), thus further investigation of NFAT signalling in the context of oncogenic Ras/RAF is merited.
The identification of COX-2 as a direct transcriptional target of NFAT in melanoma suggests targeting NFAT/COX-2 signalling may offer a novel therapeutic strategy. COX-2 contributes to tumourigenesis through stimulation of cell proliferation, angiogenesis and tumour metastasis (Buchholz and Ellenrieder, 2007). Recent clinical trials of the specific COX-2 inhibitor celecoxib suggest that COX-2 inhibition may increase the clinical efficacy of temozolomide for melanoma treatment (Gogas et al, 2006). Furthermore, as COX-2 is more highly expressed in cutaneous melanoma and metastatic melanoma compared with benign naevi (Chwirot and Kuzbicki, 2007), defining upstream regulators could be valuable for drug development. Pertinent to cancer pathogenesis and metastasis, c-myc and VEGF are additional transcriptional targets of NFAT (Buchholz and Ellenrieder, 2007), further emphasising the relevance of NFAT signalling for melanoma therapy.
Although the classical calcineurin/NFAT inhibitors CsA and FK506 revolutionised transplant biology by inducing immunosuppression via NFAT inactivation in T cells, their potential application in cancer therapy remains untested. The value of CsA and FK506 per se may be limited as long term, systemic application in transplant patients causes renal toxicity and increases cancer risk because of reduced immunosurveillance (Botti et al, 1998; Sheil, 1998). Novel analogues of CsA and FK506 named ISATX247 and L732531 respectively, exhibit significantly less toxicity while retaining efficacy, and may have a place in future cancer therapy directed at NFAT inhibition (Dumont, 2000; Aspeslet et al, 2001; Stalder et al, 2003). Alternatively, novel small peptides that block calcineurin/NFAT interaction without inhibiting calcineurin phosphatase activity have exhibited efficacy for inhibiting NFAT in vivo in the absence of significant toxicity (Yu et al, 2007) and may prove to be valuable as therapeutic NFAT antagonists.
In summary, NFAT is expressed and is transcriptionally active in human metastatic melanoma cell lines and is activated by oncogenic BRAFV600E via canonical MEK/ERK signalling. Although NFAT is overexpressed in other cancers, activation has never previously been linked to the mutation of a specific oncogene. Data indicating that COX-2 expression in melanoma is regulated by NFAT further suggest that NFAT merits additional investigation as a transcription factor important in melanoma biology. Furthermore, given that Ras/RAF signalling is highly activated in many other human cancers, the potential to exploit NFAT signalling for therapeutic benefit clearly warrants further investigation.
Acknowledgments
We thank JD Molkentin (Howard Hughes Medical Institute, Cincinnati, OH, USA) for the NFAT luciferase reporter vector, M. A. Iniguez (Centro de Biologia Molecular, Madrid, Spain) for COX-2 luciferase reporter vectors and R. Marais (ICR, London, UK) for BRAF expression vectors. Financial support: The Newcastle Healthcare Charity and The British Skin Foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Aspeslet L, Freitag D, Trepanier D, Abel M, Naicker S, Kneteman N, Foster R, Yatscoff R (2001) ISA(TX)247: a novel calcineurin inhibitor. Transplant Proc 33(1–2): 1048–1051 [DOI] [PubMed] [Google Scholar]
- Botti C, Seregni E, Ferrari L, Martinetti A, Bombardieri E (1998) Immunosuppressive factors: role in cancer development and progression. Int J Biol Markers 13(2): 51–69 [DOI] [PubMed] [Google Scholar]
- Buchholz M, Ellenrieder V (2007) An emerging role for Ca2+/calcineurin/NFAT signaling in cancerogenesis. Cell Cycle 6(1): 16–19 [DOI] [PubMed] [Google Scholar]
- Chwirot BW, Kuzbicki L (2007) Cyclooxygenase-2 (COX-2): first immunohistochemical marker distinguishing early cutaneous melanomas from benign melanocytic skin tumours. Melanoma Res 17(3): 139–145 [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky Jr WE, You MJ, DePinho RA, McMahon M, Bosenberg M (2009) Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41(5): 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892): 949–954 [DOI] [PubMed] [Google Scholar]
- Dhomen N, Marais R (2007) New insight into BRAF mutations in cancer. Curr Opin Genet Dev 17(1): 31–39 [DOI] [PubMed] [Google Scholar]
- Dumont FJ (2000) FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem 7(7): 731–748 [DOI] [PubMed] [Google Scholar]
- Flockhart RJ, Diffey BL, Farr PM, Lloyd J, Reynolds NJ (2008) NFAT regulates induction of COX-2 and apoptosis of keratinocytes in response to ultraviolet radiation exposure. FASEB J 22(12): 4218–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogas H, Polyzos A, Stavrinidis I, Frangia K, Tsoutsos D, Panagiotou P, Markopoulos C, Papadopoulos O, Pectasides D, Mantzourani M, Middleton M, Vaiopoulos G, Fountzilas G (2006) Temozolomide in combination with celecoxib in patients with advanced melanoma. A phase II study of the Hellenic Cooperative Oncology Group. Ann Oncol 17(12): 1835–1841 [DOI] [PubMed] [Google Scholar]
- Haass NK, Sproesser K, Nguyen TK, Contractor R, Medina CA, Nathanson KL, Herlyn M, Smalley KS (2008) The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res 14(1): 230–239 [DOI] [PubMed] [Google Scholar]
- Halilovic E, Solit DB (2008) Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol 8(4): 419–426 [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17(18): 2205–2232 [DOI] [PubMed] [Google Scholar]
- Ichida M, Finkel T (2001) Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem 276(5): 3524–3530 [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Martinez-Martinez S, Punzón C, Redondo JM, Fresno M (2000) An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem 275(31): 23627–23635 [DOI] [PubMed] [Google Scholar]
- Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A (2002) The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 4(7): 540–544 [DOI] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R (2004) B-RAF is a therapeutic target in melanoma. Oncogene 23(37): 6292–6298 [DOI] [PubMed] [Google Scholar]
- Kuzbicki L, Sarnecka A, Chwirot BW (2006) Expression of cyclooxygenase-2 in benign naevi and during human cutaneous melanoma progression. Melanoma Res 16(1): 29–36 [DOI] [PubMed] [Google Scholar]
- Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, Brown AM, Piacentini M, Birch-Machin MA, Redfern CP (2008) Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res 68(13): 5363–5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F, Garcia-Rodriguez C, Rao A (2000) Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J 19(17): 4783–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F, Lopez-Rodriguez C, Rao A (2001) Partners in transcription: NFAT and AP-1. Oncogene 20(19): 2476–2489 [DOI] [PubMed] [Google Scholar]
- Medyouf H, Ghysdael J (2008) The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors. Cell Cycle 7(3): 297–303 [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS (2005) BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436(7051): 720–724 [DOI] [PubMed] [Google Scholar]
- Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A, Drew L, Haber DA, Settleman J (2008) Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 68(12): 4853–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS (2009) CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 58(1): 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS (2003) High frequency of BRAF mutations in nevi. Nat Genet 33(1): 19–20 [DOI] [PubMed] [Google Scholar]
- Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD (2005) Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 25(3): 865–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G (2007) Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 7(4): 295–308 [DOI] [PubMed] [Google Scholar]
- Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, Dong C, Robertson GP (2006) Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res 66(16): 8200–8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP (2005) Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res 65(6): 2412–2421 [DOI] [PubMed] [Google Scholar]
- Sheil AG (1998) Cancer in immune-suppressed organ transplant recipients: aetiology and evolution. Transplant Proc 30(5): 2055–2057 [DOI] [PubMed] [Google Scholar]
- Stalder M, Birsan T, Hubble RW, Paniagua RT, Morris RE (2003) In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant 22(12): 1343–1352 [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Irie A, Nishimura Y (2004) B-Raf contributes to sustained extracellular signal-regulated kinase activation associated with interleukin-2 production stimulated through the T cell receptor. J Biol Chem 279(46): 48457–48465 [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116(6): 855–867 [DOI] [PubMed] [Google Scholar]
- Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD (2004) Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94(1): 110–118 [DOI] [PubMed] [Google Scholar]
- Wojciechowska K, Lewinski A (2006) BRAF mutations in papillary thyroid carcinoma. Endocr Regul 40(4): 129–138 [PubMed] [Google Scholar]
- Yu H, van Berkel TJ, Biessen EA (2007) Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activated T cells, in cardiovascular disorders. Cardiovasc Drug Rev 25(2): 175–187 [DOI] [PubMed] [Google Scholar]
- Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV (2004) Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med 199(11): 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]