Abstract
Background:
Acquired resistance to endocrine therapy in breast cancer is poorly understood. Characterisation of the molecular response to aromatase inhibitors in breast cancer tissue may provide important information regarding development of oestrogen hypersensitivity.
Methods:
We examined the expression levels of nuclear receptor co-regulators, the orphan nuclear receptor liver receptor homologue-1 and HER-2/neu growth factor receptor using real-time RT-PCR before and after 13–16 weeks of primary medical treatment with the aromatase inhibitors anastrozole or letrozole.
Results:
mRNA expression of the steroid receptor co-activator 1 (SRC-1) and peroxisome-proliferator-activated receptor γ co-activator-1α (PGC-1α) was correlated (P=0.002), and both co-activators increased during treatment in the patient group as a whole (P=0.008 and P=0.032, respectively), as well as in the subgroup of patients achieving an objective treatment response (P=0.002 and P=0.006). Although we recorded no significant change in SRC-3/amplified in breast cancer 1 level, the expression correlated positively to the change of SRC-1 (P=0.002). Notably, we recorded an increase in HER-2/neu levels during therapy in the total patient group (18 out of 26; P=0.016), but in particular among responders (15 out of 21; P=0.008).
Conclusion:
Our results show an upregulation of co-activator mRNA and HER-2/neu during treatment with aromatase inhibitors. These mechanisms may represent an early adaption of the breast cancer cells to oestrogen deprivation in vivo.
Keywords: steroid receptor co-activator, AIB1, PGC-1α, HER-2, breast cancer, aromatase inhibitors
Hormonal manipulations through oestrogen deprivation or administration of anti-oestrogens have a key function in breast cancer therapy. The third-generation aromatase inhibitors (AIs) have shown improved outcome compared to tamoxifen when used as adjuvant therapy and in treatment of metastatic disease in postmenopausal women (Bonneterre et al, 2001; Mouridsen et al, 2001; Howell et al, 2005; Jakesz et al, 2005; Thurlimann et al, 2005; Coombes et al, 2007). However, although first-line hormonal therapy may cause an objective response among 20–50% of patients with metastatic breast cancer and stabilise disease in many patients (Bonneterre et al, 2001; Mouridsen et al, 2001), resistance and disease progression inevitably occur.
Patients receiving neo-adjuvant treatment with the AIs experience a profound suppression of oestrogen levels, and the suppression of oestrogen in tumour is comparable with the fall in plasma oestrogen levels and in vivo total body aromatase inhibition (Geisler et al, 2001, 2002, 2008). In vitro models of de novo resistance to endocrine therapy have indicated that breast cancer cells have the ability to adapt to low oestrogen levels by developing oestrogen hypersensitivity (Lippman et al, 1976; Masamura et al, 1995; Chan et al, 2002) through changes in gene expression and activation of growth factor pathways (Kuang et al, 2005; Santen et al, 2005). Thus, characterisation of the molecular response to AIs in breast cancer tissue may provide important information regarding development of oestrogen hypersensitivity.
The transcriptional activity of the oestrogen receptor (ER) is regulated not only by its ligands, but also by the levels of ER co-regulators. High levels of co-activators may force ER into an active conformation that stimulates oestrogen-induced gene expression (Jordan and O’Malley, 2007). Thus, changes in the levels of ER co-activators may be of importance for the response to endocrine therapy. The expression of the steroid receptor co-activator-1 (SRC-1) has been associated with nodal positivity and endocrine resistance (Fleming et al, 2004; Myers et al, 2004). Furthermore, SRC-3/AIB1 is over-expressed in more than 30% of breast cancers with gene amplification in 5–10% of the tumours (Anzick et al, 1997; Murphy et al, 2000; List et al, 2001). Growth factor pathways may activate the co-activators at the posttranscriptional level (Rowan et al, 2000; Fleming et al, 2004; Wu et al, 2007; O’Malley et al, 2008), and over-expression of these co-activators, similar to over-expression of HER-2/neu, has been associated with inferior response to endocrine therapy (Osborne et al, 2003; Shin et al, 2006). It has been reported that status of some patients converts from HER-2/neu negative at the initiation of endocrine therapy to positive serum HER-2/neu at the time of progression (Lipton et al, 2005). However, the knowledge of HER-2/neu expression and the levels of ER co-regulators in tumour tissue during treatment with AIs is limited.
The orphan nuclear receptor liver receptor homologue-1 (LRH-1) is a specific activator of aromatase gene expression in human breast pre-adipocytes and a regulator of oestrogen biosynthesis (Clyne et al, 2002; Zhou et al, 2005). Although LRH-1 is transcriptionally regulated by ER (Annicotte et al, 2005) and is stimulated by co-activators, such as the peroxisome-proliferator-activated receptor γ co-activator-1α (PGC-1α), to execute its function (Safi et al, 2005), little is known about the regulation of LRH-1 and PGC-1α in breast tumour tissue during aromatase inhibition.
Although several studies have reported the different NR co-regulators to be over-expressed in breast cancer (Anzick et al, 1997; Bautista et al, 1998; Berns et al, 1998; Bouras et al, 2001; List et al, 2001; Hudelist et al, 2003; Iwase et al, 2003; Osborne et al, 2003; Fleming et al, 2004; Myers et al, 2004, 2005), little is known about the levels of co-regulators during oestrogen deprivation through treatment with AIs. Of interest, the level of the ER co-repressor NCoR has been shown to be downregulated in breast cancer cells resistant to anti-oestrogens (Wang et al, 2006). In this study, we examined the mRNA levels of the ER co-regulators SRC-1, SRC-3/AIB1, PGC-1α, NCoR, the nuclear receptor LRH-1 as well as the HER-2/neu growth factor receptor, together with other potential markers of endocrine response (pS2 and Ki67) and tissue oestrogen levels from the same tumours before and during treatment with the third-generation AIs anastrozole and letrozole.
Materials and methods
Study population
This material was collected in three similar protocols performed in Bergen, Norway (B) and Edinburgh, UK (E) as previously reported (Geisler et al, 2001, 2008; Miller et al, 2006). In summary, a total of 31 breast cancer patients treated with a non-steroidal AI (anastrozole or letrozole) as primary medical treatment (previously termed neo-adjuvant therapy) were enrolled. All patients provided written informed consent, and each protocol was approved by the local regulatory authorities.
Treatment and tissue collection
Of 31 patients, 12 received anastrozole as an oral dose of 1 mg daily for 15 weeks (B), whereas letrozole was given as an oral dose of 2.5 mg daily with a treatment period of 16 weeks for 12 patients (B) and 13 weeks for 7 patients (E). Treatment was administered up to the day of surgery.
Tumour size was estimated by calculating the product of the largest diameter and its perpendicular. The patients were classified as responders or non-responders depending on more or less than 50% reduction in tumour size, respectively. In two of the studies (Geisler et al, 2001, 2008) clinical response was assessed by clinical tumour measurement using a calliper. In the third study (Miller et al, 2006) tumour assessment was carried out by ultrasound. Breast tumour tissue available for gene expression analysis was collected by core-cut (E) or incisional biopsy (B) before treatment and during final surgery.
Tissue oestrogen measurements and histochemical methods
Intra-tumoural levels of E1, E2 and E1S were measured in the Norwegian materials using a highly sensitive HPLC-RIA method (Geisler et al, 2000), and are previously reported (Geisler et al, 2001, 2008).
ER, progesterone receptor (PR), HER-2/neu, Ki67 and the oestrogen-regulated gene pS2 were analysed using standard immunohistochemical methods as already published (Geisler et al, 2001; Miller et al, 2006). ER and PR were reported as percentage of positively stained cells and the tumours were considered positive if ⩾10% of the cells stained for ER/PR (B), or as Allred score (E) where the first number represents an estimation of ER- or PR-positive tumour cells (0, none; 1, <1%; 2, 1–10%; 3, 10–33%; 4, 33–66% and 5, >66%) and the second number represents the average intensity of ER- or PR-positive tumours cells (0, none; 1, weak; 2, intermediate and 3, strong) (Harvey et al, 1999). Apoptosis was estimated using the TUNEL method (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling).
Quantitative real-time RT-PCR
Tumour tissue was homogenised using a MagNA Lyser (50–100 mg; Roche, Basel, Switzerland) or manually (∼25 mg) and total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. Total RNA was resuspended in PCR-grade water, and RNA quality and concentration were estimated by optical density measurement using the Nanodrop (Saveen Werner, Copenhagen, Denmark) and a Bioanalyzer (Applied Biosystems, Lincoln, CA, USA). Each sample of 1 μg total RNA was reverse transcribed using the First Strand cDNA Synthesis Kit (Roche). The cDNA was stored at −20°C until use.
Real-time PCR reactions were carried out on a LightCycler 3 (Roche) using the SYBR Green detection format. Because of a marked variation in expression levels of our target genes, we calculated the expression relative to the geometric mean of two housekeeping genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which demonstrated a considerable higher mRNA-expression profile compared to the other reference gene, TATA-box binding protein (TBP). After each PCR run, a melting curve analysis was carried out to control for production of primer dimers and/or unwanted PCR products. An RNA standard was also included in every PCR run to control for inter-assay variation. Gene specific primers from Eurogentec (Herstal, Belgium) and TIB Molbiol (Berlin, Germany) were: SRC-1 (sense, 5′-aggcccagagccagtttac-3′; anti-sense, 5′-caggatctccgatttgatggtta-3′), SRC-3/AIB1 (sense, 5′-gaccgcttttacttcaggcatt-3′; anti-sense, 5′-tgtgttaaccaggtcctcttgct-3′), PGC-1α (sense, 5′-cccatttgagaacaagactat-3′; anti-sense, 5′-ggttatcttggttggcttt-3′), NCoR (sense, 5′-gatctatactcgtctcatctccgt-3′; anti-sense, 5′-agcaggctgaaggacttcc-3′), LRH-1 (sense, 5′-gctctccagcaagcatcc-3′; anti-sense, 5′-tcatttggtcatcaaccttaa-3′), HER-2/neu (sense, 5′-ccagccttcgacaacctctatt-3′; anti-sense, 5′-tgccgtaggtgtccctttg-3′), GAPDH (sense, 5′-accacagtccatgccatcac-3′; anti-sense, 5′-tccaccaccctgttgctgta-3′) and TBP (sense, 5′-tgcacaggagccaagagtgaa-3′; anti-sense, 5′-cacatcacagctccccacca-3′).
Expression levels of mRNA were estimated using external standard curves with serially diluted plasmids with known concentration for each target gene, except for HER-2/neu where serially diluted cDNA from an HER-2/neu-positive patient sample were used. Fold change in mRNA expression during treatment was calculated using the crossing point (Cp) for each sample and the efficiency (Eff) of each transcript, using the formula Efftarget geneΔCp/Effhousekeeping geneΔCp. The fold change was estimated relative to both GAPDH and TBP, and thereafter calculated as the geometric mean of both (Pfaffl, 2001).
Statistical analysis
The relative values of mRNA expression for all genes analysed and tissue oestrogen levels were found to be log-normally distributed. Thus, all values are presented as their geometric mean with 95% confidence intervals. Comparison between response groups was carried out using the Mann–Whitney U-test. Changes in gene expression during treatment were analysed using Wilcoxon signed rank test, and correlation between parameters was analysed using the Spearman rank correlation coefficient. The Ki67, pS2 and percentage of apoptotic cells are given as their arithmetic means. To reduce the number of false positives, we set the threshold P-value for statistical significance to 0.01. All statistical analyses were carried out using SPSS software package, version 14.0.2 (SPSS, Chicago, IL, USA).
Results
A total of 31 patients were treated with anastrozole or letrozole as primary medical therapy for 13–16 weeks before surgery. One patient was excluded because of insufficient quantities of tissue available, leaving 30 tumour samples for gene expression analysis. Only ER-positive breast cancers were eligible for the study, but one tumour turned out to be ER negative at re-analysis with immunohistochemistry. The same tumour was the only HER-2/neu-amplified tumour in the study and was one out of six tumours that did not respond to therapy. Clinical data and tumour characteristics are summarised in Table 1.
Table 1. Treatment and tumour characteristics for the patients.
| Patient | Treatment | Hospital | Treatment period | ERa | PRa | Responseb | HER-2/neu |
|---|---|---|---|---|---|---|---|
| 1 | Letrozole | Bc | 16 | 100 | 30/40 | R | Neg. |
| 2 | Letrozole | B | 16 | >50 | >50 | R | Neg. |
| 3 | Letrozole | B | 16 | 70/80 | 10 | R | Neg. |
| 4 | Letrozole | B | 16 | 80 | <10 | R | NA |
| 5 | Letrozole | B | 16 | 100 | 100 | R | Neg. |
| 6 | Letrozole | B | 16 | 100 | 100 | R | Neg. |
| 7 | Letrozole | B | 16 | 100 | 100 | R | Neg. |
| 8 | Letrozole | B | 16 | >50 | >50 | R | Neg. |
| 9 | Letrozole | B | 16 | 100 | 100 | R | Neg. |
| 10 | Letrozole | B | 16 | 100 | 50 | R | Neg. |
| 11 | Letrozole | B | 16 | 80/100 | 0/20 | R | Neg. |
| 12 | Letrozole | B | 16 | >80 | >80 | R | Neg. |
| 13 | Letrozole | E | 13 | 5+3 | 1+2 | R | NA |
| 14 | Letrozole | E | 13 | 5+3 | 4+2 | R | NA |
| 15 | Letrozole | E | 13 | 5+3 | 3+3 | R | NA |
| 16 | Letrozole | E | 13 | 5+2 | 5+2 | R | NA |
| 17 | Anastrozole | B | 15 | 86 | 53 | R | Neg. |
| 18 | Anastrozole | B | 15 | 93 | 0 | R | Neg. |
| 19 | Anastrozole | B | 15 | 83 | 84 | R | Neg. |
| 20 | Letrozole | E | 13 | 5+3 | 5+3 | R | NA |
| 21 | Letrozole | E | 13 | 5+3 | 5+3 | R | NA |
| 22 | Anastrozole | B | 15 | 98 | 0 | R | Neg. |
| 23 | Anastrozole | B | 15 | 92 | 86 | R | Neg. |
| 24 | Anastrozole | B | 15 | 87 | 70 | R | Neg. |
| 25 | Letrozole | E | 13 | 5+3 | 5+3 | NR | NA |
| 26 | Anastrozole | B | 15 | 92 | 79 | NR | Neg. |
| 27 | Anastrozole | B | 15 | NA | NA | NR | Neg. |
| 28 | Anastrozole | B | 15 | 91 | 86 | NR | Neg. |
| 29 | Anastrozole | B | 15 | 2 | 0 | NR | Pos. |
| 30 | Anastrozole | B | 15 | 82 | 7.5 | NR | Neg. |
Abbreviations: NA=not available; Neg.=negative; Pos.=positive; R=responders; NR=non-responders.
Expressed as percentage of cells staining positively (IHC) or as an Allred score (Harvey et al, 1999).
Classification of treatment response was based on change in tumour size calculated as a product of the largest diameter and its perpendicular and subjects presented as R or NR.
Patients were treated at Haukeland University Hospital, Bergen (B) or Edinburgh Breast Unit Western General Hospital (E).
The mRNA expression levels of the co-activators SRC-1, SRC-3/AIB1, PGC-1α, co-repressor NCoR, the orphan nuclear receptor LRH-1 and the HER-2/neu growth factor receptor were examined in human breast cancer tissue before and during aromatase inhibition. Noteworthy, we did not observe any significant differences in mRNA expression between anastrozole- and letrozole-treated subjects or between the subgroups treated with letrozole for 13 and 16 weeks. Analysing all patients together, the mRNA levels of our target genes were as presented in Table 2.
Table 2. Influence of anastrozole and letrozole on SRC-1, SRC-3/AIB1, PGC-1α, NCoR, LRH-1 and HER-2/neu mRNA expression.
| Pre-treatment | On treatment | Fold change | P for changea | P between subgroupsb | |
|---|---|---|---|---|---|
| SRC-1 | |||||
| All patients | 0.2961 (0.2059–0.4260)c | 0.4034 (0.2583–0.6301)d | 1.40 (1.11–1.79) | 0.008 | 0.023 |
| Responders | 0.3576 (0.2496–0.5123) | 0.5585 (0.3888–0.8023) | 1.62 (1.29–2.04) | 0.002 | |
| Non-responders | 0.1391 (0.0419–0.4622) | 0.1098 (0.0220–0.5484) | 0.79 (0.38–1.65) | 0.463 | |
| SRC-3/AIB1 | |||||
| All patients | 0.3682 (0.3053–0.4440) | 0.4367 (0.3562–0.5354) | 1.32 (1.08–1.62) | 0.090 | 0.667 |
| Responders | 0.3482 (0.2832–0.4281) | 0.4223 (0.3344–0.5334) | 1.21 (0.97–1.52) | 0.078 | |
| Non-responders | 0.4562 (0.2606–0.7985) | 0.4969 (0.2803–0.8810) | 1.09 (0.56–2.10) | 0.753 | |
| PGC-1α | |||||
| All patients | 0.0023 (0.0014–0.0037)e | 0.0040 (0.0027–0.0058) | 1.91 (1.20–3.05) | 0.032 | 0.002 |
| Responders | 0.0017 (0.0011–0.0027) | 0.0041 (0.0028–0.0061) | 2.75 (1.74–4.34) | 0.006 | |
| Non-responders | 0.0072 (0.0021–0.0245) | 0.0037 (0.0009–0.0150) | 0.54 (0.22–1.31) | 0.116 | |
| NCoR | |||||
| All patients | 0.2028 (0.1447–0.2842) | 0.2342 (0.1756–0.2842) | 1.16 (0.92–1.46) | 0.245 | 0.437 |
| Responders | 0.1897 (0.1264–0.2848) | 0.2280 (0.1609–0.3231) | 1.20 (0.93–1.56) | 0.162 | |
| Non-responders | 0.2651 (0.1398–0.5026) | 0.2607 (0.1465–0.4639) | 0.98 (0.47–2.05) | 0.753 | |
| LRH-1 | |||||
| All patients | 0.0127 (0.0082–0.0196) | 0.0167 (0.0129–0.0216) | 1.31 (0.87–1.98) | 0.074 | 0.227 |
| Responders | 0.0117 (0.0067–0.0205) | 0.0162 (0.0120–0.0219) | 1.39 (0.81–2.38) | 0.091 | |
| Non-responders | 0.0169 (0.0093–0.0307) | 0.0184 (0.0088–0.0383) | 1.09 (0.62–1.90) | 0.753 | |
| HER-2/neu | |||||
| All patients | 0.5388 (0.3780–0.7681) | 0.8288 (0.5213–1.3180) | 1.35 (0.94–1.94) | 0.016 | 0.186 |
| Responders | 0.4777 (0.3525–0.6525) | 0.8659 (0.5721–1.3106) | 1.66 (1.27–2.18) | 0.008 | |
| Non-responders | 1.6320 (0.4482–5.9430) | 0.9298 (0.0702–12.3108) | 0.58 (0.08–4.13) | 0.893 | |
Pre-treatment levels were compared to on-treatment levels after 13–16 weeks using the Wilcoxon 2 group test.
Comparisons of mRNA-levels between responders and non-responders during treatment were performed using the Mann–Whitney U-test.
All the relative values are given as geometric means with 95% confidence intervals.
Exclusively for SRC-1, on-treatment levels are significant different between response groups P=0.009 (Mann–Whitey U-test).
Exclusively for PGC-1α, pre-treatment levels are in borderline significant different between response groups, P=0.012 (Mann–Whitey U-test).
We observed no correlations among pre-treatment mRNA levels of the co-activators SRC-1, SRC-3/AIB1, co-repressor NCoR and the orphan nuclear receptor LRH-1. However, PGC-1α mRNA expression pre-treatment level correlated to change in Ki67 expression.
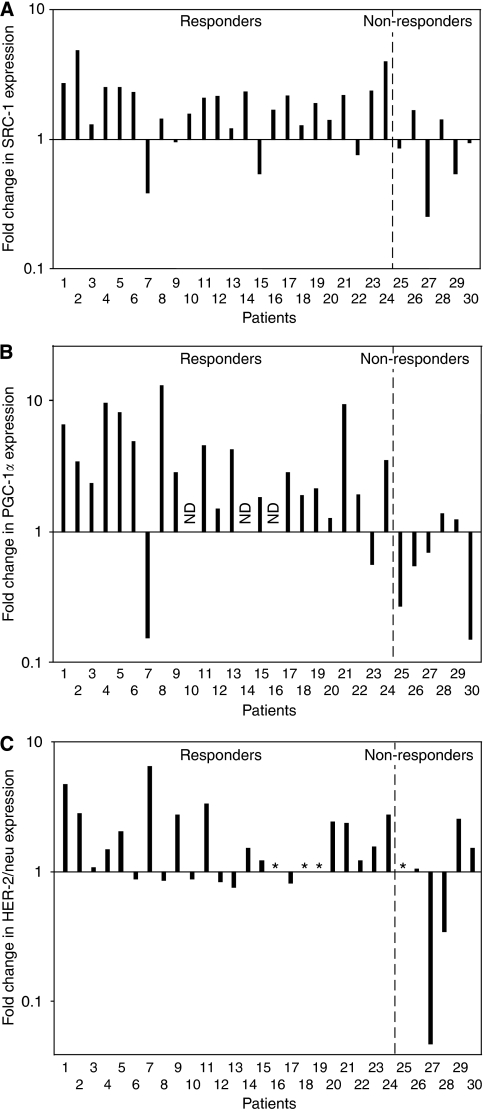
During treatment, the mRNA levels of SRC-1 were upregulated in 22 of 30 subjects (Figure 1A) with a mean fold change of 1.40 (P=0.008). A non-significant increase in SRC-3/AIB1 mRNA expression was also observed (mean change of 1.32; P=0.090). PGC-1α mRNA increased in 21 out of 27 tumours expressing detectable levels at baseline, but the observed change in the total patient group was not statistical significant (Figure 1B; mean change of 1.91; P=0.032). No significant change in expression of NCoR or LRH-1 was detected. HER-2/neu mRNA was upregulated in 18 out of 26 tumours (Figure 1C) with a mean fold change of 1.35 (borderline significance; P=0.016). The overall fold changes in SRC-1 mRNA expression correlated positively to expression of PGC-1α (R=0.565, P=0.002) as well as SRC-3/AIB1 (R=0.551, P=0.002).
Figure 1.
Individual fold changes in mRNA expression of SRC-1 (A), PGC-1α (B) and HER-2/neu (C) in patients during oestrogen deprivation. RNA was purified from the same breast tumour in the individual breast cancer patient before and after 13–16 weeks of treatment with either letrozole or anastrozole. Fold change in mRNA expression was estimated using real-time RT-PCR and presented relative to the housekeeping genes GAPDH and TBP. Calculations are based on the crossing point (Cp) for each sample and the efficiency (Eff) of each transcript, using the formula Efftarget geneΔCp/Effhousekeeping geneΔCp. Patients marked as not detected (n.d.) had Cp outside the detection limit. Patients marked by * are excluded due to insufficient tumour tissue left for analysis.
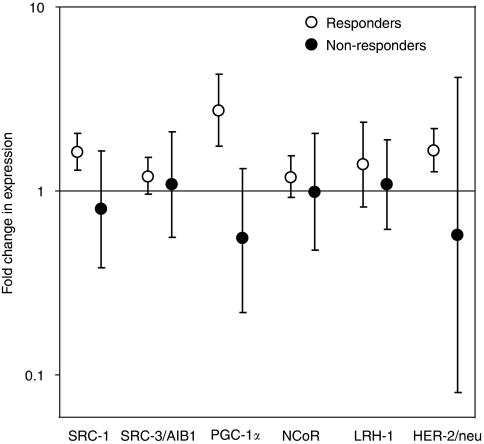
Among responders, SRC-1 was upregulated in 20 out of 24 tumours (Figure 1A, mean change of 1.62; P=0.002). This change during treatment appeared to be different compared to the mean change of 0.79 among six non-responders (P=0.023). Pre-treatment levels of PGC-1α mRNA were higher in tumours not responding compared to those responding to therapy (borderline significance; P=0.012). Notably, PGC-1α mRNA was upregulated in 19 out of 21 responders (mean change of 2.75; P=0.006). This increase was significantly different from the mean change of 0.54 in non-responders (P=0.002). HER-2/neu mRNA was upregulated in 15 out of 21 responders that were available for analysis (Figure 1C) with a significant mean fold change of 1.66 (P=0.008). This increase in responders appeared to be different compared to the mean change of 0.58 among non-responders (P=0.186). However, as for changes in mRNA expression of SRC-1, SRC-3/AIB1, NCoR and LRH-1, the difference between responders and non-responders was not significant (Figure 2).
Figure 2.
Fold change in mRNA expression of target genes during oestrogen deprivation. RNA was purified from tumours from breast cancer patients before and after 13–16 weeks of treatment with either letrozole or anastrozole. Patients were classified as responders (R) or non-responders (NR) based on clinical tumour measurements during treatment. SRC-1 (R, n=24; NR, n=6), SRC-3/AIB1 (R, n=23; NR, n=6), PGC-1α (R, n=21; NR, n=6), NCoR (R, n=24; NR, n=6), LRH-1 (R, n=19; NR, n=6) and HER-2/neu (R, n=21; NR, n=5) mRNA expression was estimated using real-time RT-PCR. Fold change in mRNA expression during treatment was calculated using the crossing point (Cp) for each sample and the efficiency (Eff) of each transcript, using the formula Efftarget geneΔCp/Effhousekeeping geneΔCp. The fold changes are presented as geometric mean with 95% confidence interval of the fold change calculated relative to the housekeeping genes GAPDH and TBP. Differences in fold changes between R and NR were analysed by the Mann–Whitney U-test: SRC-1; P=0.023, SRC-3/AIB1; P=0.667, PGC-1α; P=0.002, NCoR; P=0.437, LRH-1; P=0.227 and HER-2/neu; P=0.186.
Intra-tumoural concentrations of E1, E2 and E1S and biomarkers, including Ki67, pS2 and apoptotic cells, are presented in Table 3. As expected, PR, pS2 and Ki67 decreased significantly during treatment in both responders and non-responders, with a more profound decrease of Ki67 among responders. Except for a non-significant positive correlation between SRC-1 and LRH-1 mRNA expression and the expression of Ki67 during treatment (R=−0.502, R=−0.648 and P=0.040, P=0.017, respectively), no correlation between changes in mRNA levels and expression of pS2, Ki67 or degree of oestrogen suppression was recorded (data not shown).
Table 3. Change in biomarker and oestrogen levels during treatment with AIs.
| Pre-treatment | On treatmenta (%) | |
|---|---|---|
| E1b | 208.6 | 12.1 |
| E2b | 311.5 | 4.9 |
| E1Sb | 112.3 | 15.4 |
| Ki67c | 17.0 | 34.1 |
| pS2c | 45.3 | 28.0 |
| Apoptotic cellsc | 1.7 | 64.7 |
On treatment levels are presented as percentage of pre-treatment levels.
Intra-tumoural levels of E1, E2 and E1S (fmol/g) measured by an HPLC-RIA method and given as geometric means.
Ki67 and pS2 measured by immunohistochemistry and percentage of apoptotic cells analysed by the TUNEL method and results given as their arithmetic means.
Discussion
Changes in gene expression profiles in breast cancer tumours during endocrine treatment have been reported before (Kristensen et al, 2005; Mackay et al, 2007; Miller et al, 2007, 2009; Harvell et al, 2008). To our knowledge this is the first study evaluating expression of nuclear receptor co-regulators in breast cancer patients during oestrogen deprivation. The ER co-activators SRC-1 and SRC-3/AIB1 have been previously linked to endocrine sensitivity in breast cancer. High levels of SRC-1 and/or SRC-3/AIB1 are suggested to be associated with insensitivity to treatment with tamoxifen (Myers et al, 2004), and HER-2/neu over-expression confers an inferior response to treatment with tamoxifen as well as to AIs (Shin et al, 2006; Dowsett et al, 2008; Rasmussen et al, 2008).
We observed a significant increase in SRC-1 and PGC-1α mRNA expression in response to treatment with two third-generation non-steroidal AIs. SRC-1 and PGC-1α enhance ER activity, and increased levels may sensitise cells to oestrogens at lower concentrations (Lonard et al, 2007).
Several groups have reported MCF-7 cells exposed to E2 at low concentrations over time to develop a state of hypersensitivity, achieving growth stimulation at a hormone concentration 1 out of 1000 to 1 out of 10 000 the concentration required for wild-type cells (Lippman et al, 1976; Masamura et al, 1995; Martin et al, 2003). Although several mechanisms, including activation of the insulin-like growth factor receptor and mitogen-activated protein kinases, have been provided (Santen et al, 2005; Sabnis et al, 2007), some data indicate HER-2/neu may be important (Song et al, 2002; Martin et al, 2003, 2005). Still we lack scientific evidence showing that such mechanisms actually occur in vivo and can be related to therapy resistance. Although our data should be interpreted with care, to our knowledge they represent the first evidence of mechanisms that could possibly sensitise tumour cells to oestrogen stimulation in response to aromatase inhibition in vivo. The upregulation of co-activators and HER-2/neu was evident in treatment responsive tumours in contrast to no significant changes in the subgroup of non-responsive tumours. Our observations may suggest activation of regulatory mechanisms in response to E2 suppression in endocrine-sensitive cells that are absent or less active in tumours insensitive to hormonal manipulation. Thus, one possible hypothesis is that the increasing levels of SRC-1 and PGC-1α represent a cellular response to AIs and that the increase in co-activator levels may reflect the efficiency of endocrine therapy. At the same time, changes in gene expression that could potentially lead to increased oestrogen sensitivity could be one of several mechanisms contributing to acquired therapy resistance evolving over time. However, because the non-responders represent a small subgroup in this study, the data concerning the subgroups should be interpreted with caution.
Even though the levels of PGC-1α for all patients were more than a 100-fold lower compared to the other co-activators, pre-treatment PGC-1α expression did most clearly separate between the responding groups with a 4-fold higher geometric mean value among non-responders compared to responders. PGC-1α is known to interact with SRC-1 for full transcriptional activity (Puigserver et al, 1999; Bourdoncle et al, 2005), and it is an important regulator of LRH-1 and peroxisome-proliferator-activated receptor γ (Puigserver et al, 1999; Safi et al, 2005).
LRH-1 is suggested to exert oncogenic effects through effects on aromatase expression and cell-cycle regulators such as G1-phase cyclins (Clyne et al, 2002; Annicotte et al, 2005; Zhou et al, 2005). We observed no changes of LRH-1 mRNA during treatment, which would suggest that local aromatase upregulation by this transcriptional activator may not be a response to oestrogen deprivation. On the other hand, it is well known that the transcriptional activity of LRH-1 is stimulated through interaction with the SRCs (Xu et al, 2004). Thus, even though the LRH-1 mRNA expression is unaffected during oestrogen deprivation, this does not rule out that the transcriptional activity of LRH-1 is increased.
A significant increase in HER-2/neu expression was observed during therapy among tumours responsive to AIs. The serum level of HER-2/neu has been shown to be increased from baseline to time of progression in approximately 25% of patients treated with letrozole or tamoxifen (Lipton et al, 2005). In addition, in vitro studies have shown that oestrogen deprivation is associated with an increase in HER-2/neu expression (Dati et al, 1990; Read et al, 1990; Warri et al, 1991). Recently, it was reported that letrozole upregulates the protein level of HER-2/neu in MCF-7Ca xenografts in mice despite continued response to treatment, and it has been hypothesised that an inverse relationship exists between HER-2/neu and ERα (Sabnis et al, 2009). In line with this, it has been shown that HER-2/neu transcription can be repressed by oestrogen (Bates and Hurst, 1997). A recent report shows that the transcription factor-paired box 2 gene product (PAX2) and SRC-3/AIB1 compete for binding and regulation of HER-2/neu transcription in MCF-7 cells (Hurtado et al, 2008). High levels of SRC-3/AIB1 outcompete PAX2 leading to an increase in the HER-2/neu transcription after tamoxifen treatment (Hurtado et al, 2008). SRC-3/AIB1 has also been shown to be required for HER-2/neu oncogenic activity (Fereshteh et al, 2008). Even though we did not observe a correlation between changes in SRC-3/AIB1 and HER-2/neu mRNA expression in our study, the expression of both genes increased during oestrogen deprivation. It is possible that the enhanced level of HER-2/neu mRNA could be explained by loss of repression due to an increase in SRC-3/AIB1 or other ER co-activators. Interestingly, it has recently been reported that patients with ER-positive and HER-2/neu-negative breast cancer with a poor response to tamoxifen may obtain an increased time to progression by having the tyrosine kinase inhibitor lapatinib added to letrozole (Johnston et al, 2009). Thus, an increase in HER-2/neu expression may represent a circumvention of oestrogen deprivation.
In this study we have focused on accurate quantifications of mRNA expression, but it should be noted that changes in mRNA and protein expression are not always similar, and one should keep in mind that post-translational modifications and regulation of protein turnover rates may also affect the co-regulator protein levels (Greenbaum et al, 2003).
In conclusion, the results from this study with unique matched pre- and on-treatment samples suggest that co-activators and HER-2/neu are upregulated in tumours during AI therapy as an early response to effective oestrogen deprivation. Increasing levels of co-activator mRNA may represent a response by the cells to antagonise the oestrogen deprivation effect of anastrozole and letrozole.
Acknowledgments
This study was supported by grants from the Norwegian Cancer Society, Margareth Solbergs legat, Cancer Research UK, Foreningen for brystkreftopererte, Research Council of Norway. We appreciate the excellent technical assistance of Anne Merete Sellevold, Dagfinn Ekse and Ingebjørg Johnsen Hjetland. We also thank CK Glass and Robert Tjian for the supplied plasmids.
References
- Annicotte JS, Chavey C, Servant N, Teyssier J, Bardin A, Licznar A, Badia E, Pujol P, Vignon F, Maudelonde T, Lazennec G, Cavailles V, Fajas L (2005) The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene 24: 8167–8175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277: 965–968 [DOI] [PubMed] [Google Scholar]
- Bates NP, Hurst HC (1997) An intron 1 enhancer element mediates oestrogen-induced suppression of ERBB2 expression. Oncogene 15: 473–481 [DOI] [PubMed] [Google Scholar]
- Bautista S, Valles H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C (1998) In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res 4: 2925–2929 [PubMed] [Google Scholar]
- Berns EM, van Staveren IL, Klijn JG, Foekens JA (1998) Predictive value of SRC-1 for tamoxifen response of recurrent breast cancer. Breast Cancer Res Treat 48: 87–92 [DOI] [PubMed] [Google Scholar]
- Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92: 2247–2258 [DOI] [PubMed] [Google Scholar]
- Bouras T, Southey MC, Venter DJ (2001) Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res 61: 903–907 [PubMed] [Google Scholar]
- Bourdoncle A, Labesse G, Margueron R, Castet A, Cavailles V, Royer CA (2005) The nuclear receptor coactivator PGC-1alpha exhibits modes of interaction with the estrogen receptor distinct from those of SRC-1. J Mol Biol 347: 921–934 [DOI] [PubMed] [Google Scholar]
- Chan CM, Martin LA, Johnston SR, Ali S, Dowsett M (2002) Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J Steroid Biochem Mol Biol 81: 333–341 [DOI] [PubMed] [Google Scholar]
- Clyne CD, Speed CJ, Zhou J, Simpson ER (2002) Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem 277: 20591–20597 [DOI] [PubMed] [Google Scholar]
- Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369: 559–570 [DOI] [PubMed] [Google Scholar]
- Dati C, Antoniotti S, Taverna D, Perroteau I, De Bortoli M (1990) Inhibition of c-erbB-2 oncogene expression by estrogens in human breast cancer cells. Oncogene 5: 1001–1006 [PubMed] [Google Scholar]
- Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, Bishop H, Ellis I, Larsimont D, Sasano H, Carder P, Cussac AL, Knox F, Speirs V, Forbes J, Buzdar A (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, alone or in combination trial. J Clin Oncol 26: 1059–1065 [DOI] [PubMed] [Google Scholar]
- Fereshteh MP, Tilli MT, Kim SE, Xu J, O’Malley BW, Wellstein A, Furth PA, Riegel AT (2008) The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 68: 3697–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O’Higgins NJ, Hill AD, Young LS (2004) Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol 57: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler J, Berntsen H, Lonning PE (2000) A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and estrone sulphate levels in breast cancer tissue. J Steroid Biochem Mol Biol 72: 259–264 [DOI] [PubMed] [Google Scholar]
- Geisler J, Detre S, Berntsen H, Ottestad L, Lindtjorn B, Dowsett M, Einstein Lonning P (2001) Influence of neoadjuvant anastrozole (Arimidex) on intratumoral estrogen levels and proliferation markers in patients with locally advanced breast cancer. Clin Cancer Res 7: 1230–1236 [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20: 751–757 [DOI] [PubMed] [Google Scholar]
- Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, Aas T, Lonning PE (2008) Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res 14: 6330–6335 [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell DM, Spoelstra NS, Singh M, McManaman JL, Finlayson C, Phang T, Trapp S, Hunter L, Dye WW, Borges VF, Elias A, Horwitz KB, Richer JK (2008) Molecular signatures of neoadjuvant endocrine therapy for breast cancer: characteristics of response or intrinsic resistance. Breast Cancer Res Treat 112: 475–488 [DOI] [PubMed] [Google Scholar]
- Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17: 1474–1481 [DOI] [PubMed] [Google Scholar]
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365: 60–62 [DOI] [PubMed] [Google Scholar]
- Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF (2003) Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat 78: 193–204 [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS (2008) Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456: 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase H, Omoto Y, Toyama T, Yamashita H, Hara Y, Sugiura H, Zhang Z (2003) Clinical significance of AIB1 expression in human breast cancer. Breast Cancer Res Treat 80: 339–345 [DOI] [PubMed] [Google Scholar]
- Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366: 455–462 [DOI] [PubMed] [Google Scholar]
- Johnston S, Pegram M, Press M, Pippen J, Pivot X, Gomez H, Florance A, O’Rourke L, Maltzman J (2009) Lapatinib combined with letrozole vs. letrozole alone for front line postmenopausal hormone receptor positive (HR plus) metastatic breast cancer (MBC): first results from the EGF30008 Trial. Amer Assoc Cancer Research, pp 74S–75S
- Jordan VC, O’Malley BW (2007) Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol 25: 5815–5824 [DOI] [PubMed] [Google Scholar]
- Kristensen VN, Sorlie T, Geisler J, Yoshimura N, Linegjaerde OC, Glad I, Frigessi A, Harada N, Lonning PE, Borresen-Dale AL (2005) Effects of anastrozole on the intratumoral gene expression in locally advanced breast cancer. J Steroid Biochem Mol Biol 95: 105–111 [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Wang S, Medina D, O’Malley BW, Xu J (2005) Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res 65: 7993–8002 [DOI] [PubMed] [Google Scholar]
- Lippman M, Bolan G, Huff K (1976) The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res 36: 4595–4601 [PubMed] [Google Scholar]
- Lipton A, Leitzel K, Ali SM, Demers L, Harvey HA, Chaudri-Ross HA, Evans D, Lang R, Hackl W, Hamer P, Carney W (2005) Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer 104: 257–263 [DOI] [PubMed] [Google Scholar]
- List HJ, Reiter R, Singh B, Wellstein A, Riegel AT (2001) Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res Treat 68: 21–28 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O’Malley BW (2007) Nuclear receptor coregulators and human disease. Endocr Rev 28: 575–587 [DOI] [PubMed] [Google Scholar]
- Mackay A, Urruticoechea A, Dixon JM, Dexter T, Fenwick K, Ashworth A, Drury S, Larionov A, Young O, White S, Miller WR, Evans DB, Dowsett M (2007) Molecular response to aromatase inhibitor treatment in primary breast cancer. Breast Cancer Res 9: R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Farmer I, Johnston SR, Ali S, Dowsett M (2005) Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr Relat Cancer 12(Suppl 1): S75–S84 [DOI] [PubMed] [Google Scholar]
- Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278: 30458–30468 [DOI] [PubMed] [Google Scholar]
- Masamura S, Santner SJ, Heitjan DF, Santen RJ (1995) Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab 80: 2918–2925 [DOI] [PubMed] [Google Scholar]
- Miller WR, Larionov A, Renshaw L, Anderson TJ, Walker JR, Krause A, Sing T, Evans DB, Dixon JM (2009) Gene expression profiles differentiating between breast cancers clinically responsive or resistant to letrozole. J Clin Oncol 27: 1382–1387 [DOI] [PubMed] [Google Scholar]
- Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J, Murray E, Hampton G, Walker JR, Ho S, Krause A, Evans DB, Dixon JM (2007) Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genomics 17: 813–826 [DOI] [PubMed] [Google Scholar]
- Miller WR, White S, Dixon JM, Murray J, Renshaw L, Anderson TJ (2006) Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94: 1051–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19: 2596–2606 [DOI] [PubMed] [Google Scholar]
- Murphy LC, Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, Troup S, Adeyinka A, Watson PH (2000) Altered expression of estrogen receptor coregulators during human breast tumorigenesis. Cancer Res 60: 6266–6271 [PubMed] [Google Scholar]
- Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O’Higgins NJ, Hill AD, Young LS (2004) Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer 91: 1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E, Hill AD, Kelly G, McDermott EW, O’Higgins NJ, Buggy Y, Young LS (2005) Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res 11: 2111–2122 [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Qin J, Lanz RB (2008) Cracking the coregulator codes. Curr Opin Cell Biol 20: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R (2003) Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95: 353–361 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM (1999) Activation of PPARgamma coactivator-1 through transcription factor docking. Science 286: 1368–1371 [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, Mastropasqua MG, Price KN, Mery E, Lacroix-Triki M, Braye S, Altermatt HJ, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Gusterson BA, Thurlimann B, Coates AS, Viale G (2008) Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1-98 randomised trial. Lancet Oncol 9: 23–28 [DOI] [PubMed] [Google Scholar]
- Read LD, Keith Jr D, Slamon DJ, Katzenellenbogen BS (1990) Hormonal modulation of HER-2/neu protooncogene messenger ribonucleic acid and p185 protein expression in human breast cancer cell lines. Cancer Res 50: 3947–3951 [PubMed] [Google Scholar]
- Rowan BG, Garrison N, Weigel NL, O’Malley BW (2000) 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol 20: 8720–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A (2007) Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res 13: 2751–2757 [DOI] [PubMed] [Google Scholar]
- Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A (2009) Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res 69: 1416–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi R, Kovacic A, Gaillard S, Murata Y, Simpson ER, McDonnell DP, Clyne CD (2005) Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res 65: 11762–11770 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence Jr J, Berstein L, Yue W (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Suppl 1): S61–S73 [DOI] [PubMed] [Google Scholar]
- Shin I, Miller T, Arteaga CL (2006) ErbB receptor signaling and therapeutic resistance to aromatase inhibitors. Clin Cancer Res 12: 1008s–1012s [DOI] [PubMed] [Google Scholar]
- Song RX, Santen RJ, Kumar R, Adam L, Jeng MH, Masamura S, Yue W (2002) Adaptive mechanisms induced by long-term estrogen deprivation in breast cancer cells. Mol Cell Endocrinol 193: 29–42 [DOI] [PubMed] [Google Scholar]
- Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353: 2747–2757 [DOI] [PubMed] [Google Scholar]
- Wang LH, Yang XY, Zhang X, An P, Kim HJ, Huang J, Clarke R, Osborne CK, Inman JK, Appella E, Farrar WL (2006) Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell 10: 487–499 [DOI] [PubMed] [Google Scholar]
- Warri AM, Laine AM, Majasuo KE, Alitalo KK, Harkonen PL (1991) Estrogen suppression of erbB2 expression is associated with increased growth rate of ZR-75-1 human breast cancer cells in vitro and in nude mice. Int J Cancer 49: 616–623 [DOI] [PubMed] [Google Scholar]
- Wu RC, Feng Q, Lonard DM, O’Malley BW (2007) SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129: 1125–1140 [DOI] [PubMed] [Google Scholar]
- Xu PL, Liu YQ, Shan SF, Kong YY, Zhou Q, Li M, Ding JP, Xie YH, Wang Y (2004) Molecular mechanism for the potentiation of the transcriptional activity of human liver receptor homolog 1 by steroid receptor coactivator-1. Mol Endocrinol 18: 1887–1905 [DOI] [PubMed] [Google Scholar]
- Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, Moriya T, Simpson ER, Sasano H, Clyne CD (2005) Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res 65: 657–663 [PubMed] [Google Scholar]