Abstract
Background:
The von Hippel–Lindau gene (VHL) alteration, a common event in sporadic clear-cell renal-cell carcinoma (CCRCC), leads to highly vascularised tumours. Vascular endothelial growth factor (VEGF) is the major factor involved in angiogenesis, but the prognostic significance of both VHL inactivation and VEGF expression remain controversial. The aims of this study were to analyse the relationship between VHL genetic and epigenetic alterations, VHL expression and VEGF tumour or plasma expression, and to analyse their respective prognostic value in patients with CCRCC.
Methods:
A total of 102 patients with CCRCC were prospectively analysed. Alterations in VHL were determined by sequencing, Multiplex Ligation-dependent Probe Amplification (MLPA) and methylation-specific MLPA. Expression of pVHL and VEGF was determined by immunohistochemistry. Plasma VEGF was measured by enzyme-linked immunosorbent assay (ELISA).
Results:
VHL mutation, deletion and promoter methylation were identified in 70, 76 and 14 cases, respectively. Overall, at least one VHL-gene alteration occurred in 91 cases (89.2%). Both VEGF tumour and plasma expression appeared to be decreased in case of VHL alteration. Median progression-free survival and CCRCC-specific survival were significantly reduced in patients with wild-type VHL or altered VHL and high VEGF expression, which, therefore, represent two markers of tumour aggressiveness in CCRCC.
Conclusion:
Stratifying CCRCCs according to VHL and VEGF status may help tailor therapeutic strategy.
Keywords: renal-cell carcinoma, VHL, VEGF
Renal-cell carcinoma (RCC) accounts for 3% of all solid tumours. Seventy-five to 85% of RCCs are clear-cell carcinomas (clear-cell renal-cell carcinoma (CCRCC)), which are highly vascularised tumours. As much as 30% of patients do have metastases at the time of diagnosis and an additional 30–40% will develop metastases during follow-up, although a radical surgery has been initially performed (Rabinovitch et al, 1994). Significant progresses have recently been made in the medical treatment of metastatic RCC by targeting a number of growth factors, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) and their receptors (Motzer and Bukowski, 2006; Patard et al, 2008a). Indeed, median progression-free survival (PFS) time has been doubled either in first-line or in second-line therapy by targeting tumour angiogenesis (Motzer et al, 2007).
The von Hippel–Lindau (VHL) tumour suppressor has been isolated in 1993 (Latif et al, 1993). The protein encoded by the VHL gene (pVHL) is the substrate recognition component of a ubiquitin ligase complex that targets a transcription factor, hypoxia-inducible factor (HIF), for proteolysis. A biallelic VHL inactivation leads to HIF-1α accumulation and subsequent overexpression of genes, which are critical for tumour angiogenesis, cell proliferation and migration (Knebelmann et al, 1998; Cockman et al, 2000; Kim and Kaelin, 2004).
Although some studies have shown a positive relationship between genetic and epigenetic VHL gene alterations and VEGF tumour overexpression (Igarashi et al, 2002; Na et al, 2003), literature results are conflicting especially regarding the association between VHL mutation, usual prognostic parameters and survival (van Houwelingen et al, 2005; Schraml et al, 2002; Yao et al, 2002). In a recent study we have demonstrated that the absence of VHL mutation, associated with low Carbonic Anhydrase IX (CAIX) tumour expression, was associated to a particular aggressive CCRCC phenotype (Patard et al, 2008b). Both CAIX and VEGF are VHL/HIF downstream targets, but unlike CAIX, which is a surrogate indicator for VHL status, VEGF regulation is much more complex. Conflicting hypotheses also exist regarding its prognostic value in CCRCC (Jacobsen et al, 2002). Therefore, we aimed this prospective study for evaluating the association between VHL status (mutation, deletion, promoter methylation and pVHL expression), tumour VEGF expression, plasma VEGF levels and usual prognostic parameters in CCRCC. We subsequently analysed the respective prognostic value of these different biological tumour characteristics.
Materials and methods
Study population
A total of 102 patients operated for a sporadic CCRCC at the Department of Urology at the Rennes University Hospital between 2003 and 2004 were analysed. In case of distant metastases, patients were treated following surgery according to the standard of care of the period, which was immunotherapy. Surveillance following surgery included repeated clinical assessments, blood biochemistry tests, chest and abdominal CT scans. No patient was lost to follow-up. The study protocol was approved by the institutional ethics committee and informed consent for participating in this study was obtained in each case. The clinicopathological data of the patients are summarised in Table 1.
Table 1. Summary of the clinical and histopathological characteristics of the 102 patients with sporadic CCRCC.
| Variables | Value/number |
|---|---|
| Age (years) | 64.5 (21–83) |
| Sex (male/female) | 44 (43.1%)/58 (56.9%) |
| Tumour size (cm) | 7 (1.5–22) |
| Fuhrman grade | |
| 1 | 2 (1.9%) |
| 2 | 35 (34.3%) |
| 3 | 37 (36.3%) |
| 4 | 28 (27.5%) |
| Tumour stage | |
| 1 | 38 (37.3%) |
| 2 | 20 (19.6%) |
| 3 | 40 (39.2%) |
| 4 | 4 (3.9%) |
| Lymph node status | |
| 0 | 90 (88.3%) |
| 1 | 8 (7.8%) |
| 2 | 4 (3.9%) |
| Metastasis status | |
| 0 | 73 (71.6%) |
| 1 | 29 (28.4%) |
Abbreviation: CCRCC=clear-cell renal-cell carcinoma.
Pathological diagnosis is according to the Fuhrman grading system and UICC tumour-node-metastasis staging system. Values are presented as median (minimum−maximum) for continuous variables and number of patients (percent) for categorical variables.
Pathological analysis
Formalin-fixed paraffin sections were stained with hematoxylin and eosin–safran for light microscopy. The slides were reviewed by one pathologist (N Rioux-Leclercq). Only conventional CCRCCs were considered for analysis. Macroscopic and histological parameters, which were analysed included tumour size, tumour necrosis and nuclear Fuhrman grade (Fuhrman et al, 1982; Rioux-Leclercq et al, 2007b). Tumour stage was defined according to the TNM classification (Guinan et al, 1997).
Tissue sample management
All consecutive conventional CCRCCs and paired renal cortex samples from untreated patients undergoing partial or total nephrectomy were analysed. Immediately after macroscopic examination, small samples were collected from surgical specimens, frozen in liquid nitrogen and stored at –80°C. Genomic DNA was extracted using QIAamp DNA minikit (Qiagen, Courtaboeuf, France).
VEGF plasma measurement
Blood samples were collected before nephrectomy and processed as described (Rioux-Leclercq et al, 2007a). The plasma was stored at −80°C until analysed. Quantikine human VEGF Immunoassay (R&D Systems Europe, Lille, France) enzyme-linked immunosorbent assay (ELISA) was performed to quantitate VEGF levels (VEGF121 and VEGF165 free isoforms) in plasma. All samples were assessed in duplicate. The value of plasma VEGF (pVEGF) was expressed in pg/ml. The detection limit of the test was 9 pg−1ml and the upper limits of normal value used for VEGF in plasma was 62.5 pg ml−1 (Adams et al, 2000).
VEGF and pVHL tumour immunostaining
In each case, a representative slide of the tumour with the highest nuclear Fuhrman grade and the corresponding paraffin block were selected for immunohistochemistry. Tissue sections were deparaffinised in xylene using standard procedures.
For VEGF immunostaining, antigen retrieval was performed by immersing sections in a 0.01 M sodium citrate buffer (pH 6.0) and heated in a 600 W microwave four times for 2 min. The slides were then left to cool down for 30 min and rinsed in a phosphate-buffered saline solution. Slides were incubated at room temperature for 1 h with the polyclonal anti-VEGF antibody (sc-152, dilution 1/100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). A biotin–streptavidin detection system (Dako, Glostrup, Denmark) was then used with diaminobenzidine as the chromogen (Sigma-Aldrich, Lyon, France). Negative controls were performed by omitting the primary antibody and by incubating primary antibody with immunising VEGF peptide prior to slide treatment. VEGF immunoreactivity was expressed as the percentage of the VEGF-positive cells by scoring at least 1000 tumour cells at × 400 magnification. The immunohistochemical signals in the CCRCCs were evaluated as membranous and/or cytoplasmic. For prognostic analysis, a 30% cut-off was used according to previous reports (Jacobsen et al, 2004).
pVHL immunohistohemical staining of tissue sections was performed with a monoclonal anti-pVHL antibody (Ig33; Neomarkers, Fremont, CA, USA; Schraml et al, 2003; Valle et al, 2005), in an automated stainer using the ultraview DAB detection kit (Benchmark; Ventana, Tucson, AZ, USA) following antigen retrieval. Semi-quantitative assessment of the antibody staining was performed by a single uropathologist blinded to the clinicopathological variables (N Rioux-Leclercq). A negative control consisted of omitting the primary antibody. As control for loss of pVHL expression, CCRCC tissue from confirmed VHL genetic disease was used. The extent of staining was recorded as a percentage of the tumour tissue sample with positive pVHL expression. Tumours expressing pVHL were scored pVHL+, independently of the percentage of stained cells.
VHL mutational analysis
We amplified two overlapping fragments for exon 1 (1A and 1B) and one fragment for each of exons 2 and 3, covering part of the VHL 5′UTR, the entire coding sequence and exon–intron junctions (VHL GenBank accession no. AF010238). Primer pairs and PCR-sequencing conditions are described elsewhere (Patard et al, 2008b). Denaturing high-performance liquid chromatography (DHPLC) screening was carried out on a WAVE Nucleic Acid Fragment Analysis system (Transgenomic, Glasgow, UK) with a DNAsep column. Aberrant peaks were further analysed by direct sequencing using standard procedures. All mutations were confirmed in a second PCR and sequencing reaction.
VHL deletion analysis
Multiplex Ligation-dependent Probe Amplification (MLPA) analysis was used to detect deletions or duplications in the VHL gene (Schouten et al, 2002). The SALSA MLPA P016B VHL probe kit (MRC-Holland, Amsterdam, the Netherlands) was used. The kit contains eight probes to the VHL gene (four in exon 1, two in exon 2 and two in exon 3), additional probes to other genes on 3p and control probes to regions telomeric and centromeric from VHL. Detailed information on probe sequences, gene loci and chromosome locations can be found at www.mlpa.com.
Genomic DNA (50–200 ng) was denatured and the probes were allowed to hybridise (16 h at 60°C). PCR was performed on the samples in a volume of 50 μl containing 10 μl of the ligation reaction mixture using the PTC 200 thermal cycler (MJ Research, Waltham, MA, USA). Aliquots of 1.5 μl of the PCR reaction were combined with 0.3 μl ROX-labelled internal size standard (Applied Biosystems, Foster City, CA, USA) and 9 μl deionised formamide. Fragments were separated by electrophoresis on an Applied 3130XL capillary sequencer and quantified using the GeneMarker version 1.6 software (SoftGenetics). For copy-number detection, normal control DNA samples were included in each set of MLPA experiments. Interpretation was based on the comparison of peak heights between the control DNA and the tumour sample. Cut-off levels for loss of relative copy number were set at 0.75.
VHL promoter methylation analysis
Methylation-Specific-MLPA (MS-MLPA) was used to detect CpG island methylation (Jeuken et al, 2007). The probe design is similar to an ordinary MLPA probe, except that, for the methylation-specific probes, the sequences detected contain a methylation-sensitive restriction site (HhaI). The SALSA MS-MLPA kit ME001B Tumour suppressor-1 allows to detect aberrant methylation of CpG islands located in the promoter region of the VHL gene. DNA (50–200 ng) was denatured and the probes were allowed to hybridise (16 h at 60°C). The VHL probes used in this study for methylation quantification analysis contained one HhaI restriction site in the target recognition sequence. Following hybridisation, the samples were divided into two and one half of the samples was ligated, whereas for the other part of the sample ligation was combined with HhaI digestion enzyme. This digestion resulted in ligation of only the methylated sequences. PCR was performed on both parts of the samples and analysed by electrophoresis as described above. Reference unmethylated DNAs, isolated from blood from healthy volunteers, were included in each set of MLPA experiments. The unmethylated DNA will not generate a signal, and a normal probe signal will be detected if the site is methylated.
Statistical methods
χ2-test and Student's t-test were used for comparing qualitative and quantitative variables, respectively. Kaplan–Meier method and log-rank test were used for comparing survival in different groups. Endpoints for outcome assessment were PFS, which was defined as the time of surgery to the occurrence of local recurrence or distant metastases, and RCC-specific survival (RCC-SS), which was defined as death from RCC .All analyses were conducted with the SPSS 10.1 software, and P-value significance was fixed at 0.05.
Results
Patients and tumour characteristics
Sixty-eight (66.7%) and 51 patients (50%) had good performance status and no symptoms at diagnosis, respectively. Tumours were organ-confined (pT1-2) and low-grade (G1-2) in 58 (56.9%) and 37 cases (36.3%), respectively (Table 1). In 12 cases, nodal invasion was present at diagnosis (11.7%) and in 29 cases (28.4%) patients had synchronous distant metastases. During a median follow-up time of 31 months (1–62), 40 patients (39.2%) experienced disease progression and 27 subsequently died from cancer (26.5%).
Genetic and epigenetic VHL gene alteration – relationship with clinicopathological variables and outcome
A VHL gene mutation was found in 70 cases (68.6%). Mutations occurred in exon 1–3 in 30 (42.8%), 26 (37.2%) and 14 cases (20%), respectively. Stop, frameshift, missense, splice site and other mutation types were found in 10 (14.3%), 31 (45.7%), 19 (27.1%), six (8.6%) and four cases (4.3%), respectively (Supplementary Table S1). Although there was a trend for better PFS and RCC-SS in patients with VHL-mutated tumours, it did not achieve statistical significance (log-rank test, P=0.1 and 0.2, respectively). Mutation type and site of mutation did not impact either on PFS or on RCC-SS.
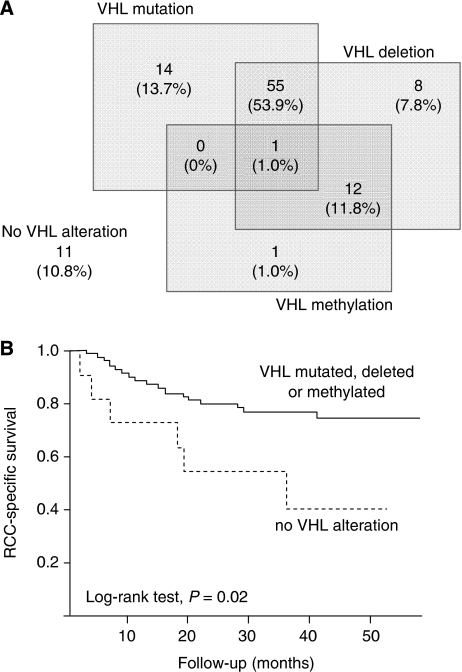
VHL deletion and promoter methylation occurred in 76 (74.5%) and 14 cases (13.7%), respectively (Figure 1A). Overall, VHL mutation, deletion or hypermethylation occurred in 91 cases (89.2%). When considering patients with no VHL mutation, deletion or hyper-methylation (n=11, 10.8%), it appeared that their outcome was significantly worse both for PFS and RCC-SS than patients having at least one VHL alteration (log-rank test, P=0.02; Figure 1B). This group was significantly associated with higher N stages (P=0.007) and Fuhrman grades (P=0.03).
Figure 1.
Alteration of the VHL gene in 102 CCRCC tumours. (A) Distribution of the different VHL alterations identified. (B) Cause-specific survival curve for patients with CCRCC based on VHL alterations (solid line, mutation, deletion or methylation of the VHL gene, n=91, 89.2%; broken line, no VHL alteration, n=11, 10.8%).
At least two alterations of the VHL gene, potentially leading to biallelic alteration, were found in 68 tumours (66.7%), whereas no or a single alteration of the VHL gene was found in 34 patients (33.3%). Comparison of these two groups yielded borderline prognostic significance for PFS (log-rank test, P=0.05) but not for RCC-SS (log-rank test, P=0.2). There was no relation with tumour size (P=0.6), T stage (P=0.2), N stage (P=0.4) or M stage (P=0.7).
pVHL pattern of expression and relationship with clinicopathological variables
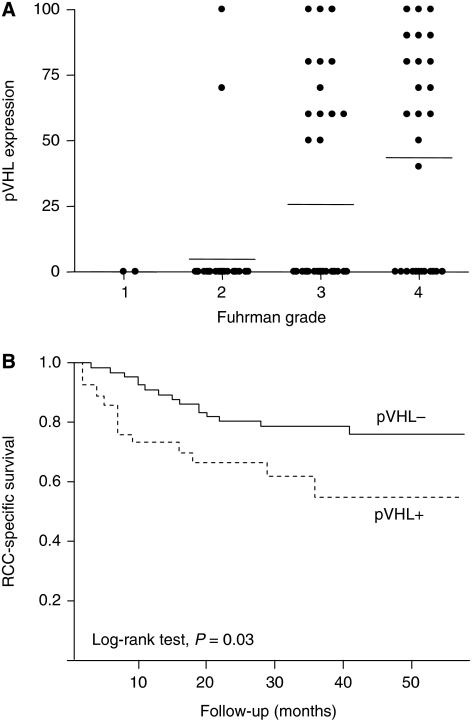
In normal kidney, a strong cytoplasmic staining was observed in tubular cells as well as a weak staining in mesangial cells. In tumours showing pVHL expression, the staining was strong, diffuse and cytoplasmic. pVHL tumour expression was not detected in 71 cases (69.6%). The absence of pVHL expression was associated with lower N stages (P=0.02), M stages (P=0.01), Fuhrman grades (P=0.001; Figure 2A) along with lower rates of tumour necrosis (P=0.005). Finally, absence of pVHL expression was associated with a more favourable outcome measured both by PFS (P=0.02) and RCC-SS (log-rank test, P=0.03; Figure 2B).
Figure 2.
pVHL expression in CCRCC. (A) Percentage of tumour tissue with positive pVHL expression according to Fuhrman grade. Horizontal lines represent mean values. (B) Cause-specific survival curve for patients based on pVHL expression (solid line, tumours not expressing pVHL, n=71, 69.6%; broken line, tumours expressing pVHL, n=31, 30.4%).
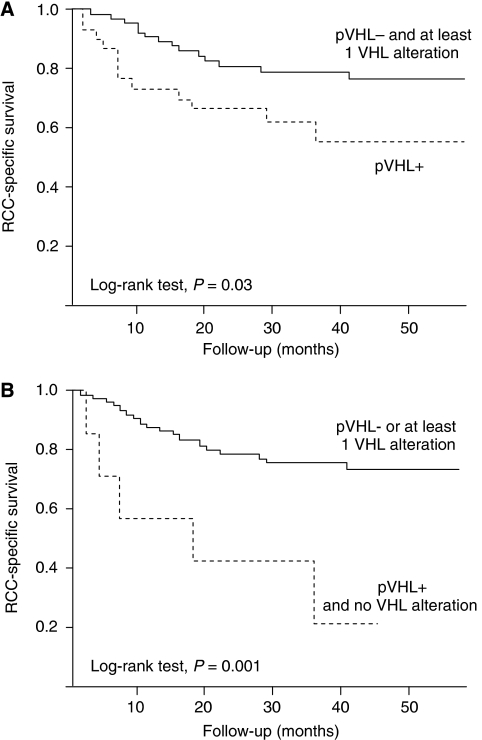
When selecting tumours with undetectable pVHL and at least one VHL alteration (n=67, 68.4%) and comparing this former group to tumours expressing pVHL (n=31, 31.6%), the first group exhibited better outcome both in terms of PFS (log-rank test, P=0.02) and in terms of RCC-SS (log-rank test P=0.03; Figure 3A). A small group of tumours (n=7, 6.9%) expressed pVHL and did not present any VHL alteration. This group had a median PFS of 7 months compared with 58 months for the rest of the population (log-rank test, P=0.004). The median RCC-SS time was 18 months for this particular group with no VHL abnormality, whereas it was not reached for the rest of the population (log-rank test P=0.001; Figure 3B).
Figure 3.
Cause-specific survival curves for patients with CCRCC based on VHL alterations and pVHL expression. (A) Survival curves for patients with tumours not expressing pVHL and containing at least one VHL alteration (solid line, n=67, 68.4%), and for patients with tumours expressing pVHL (broken line, n=31, 31.6%). (B) Survival curves for patients with tumours not expressing pVHL or containing at least 1 VHL alteration (solid line, n=95, 93.1%), and for patients with tumours expressing pVHL and presenting no VHL alteration (broken line, n=7, 6.9%).
VEGF tumour and plasma expression – relationship with clinicopathological variables and outcome
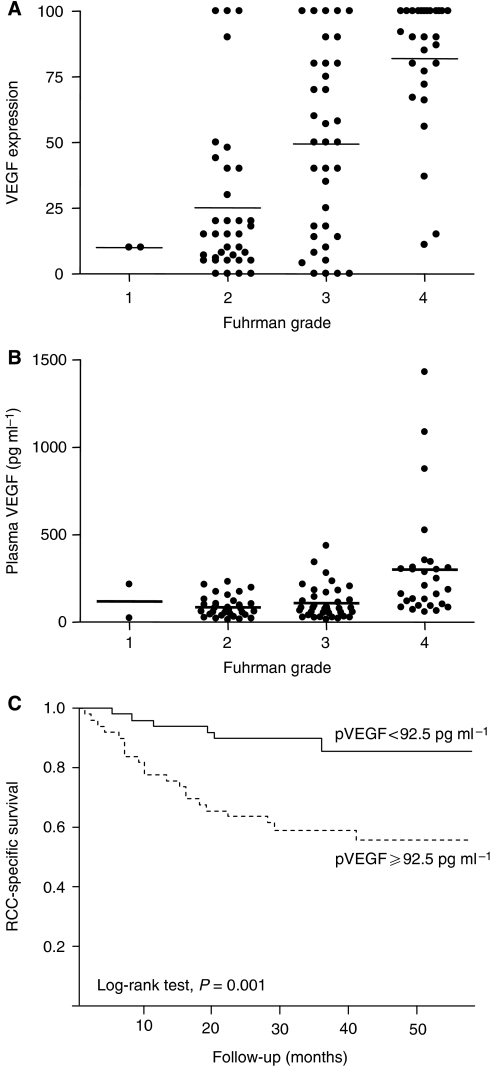
Positive staining for VEGF was defined as a membranous and/or cytoplasmic staining pattern of tumour cells. Weak staining was observed in podocytes and endothelial cells in non-tumoral kidney tissue. Median VEGF tumour expression was 49% (0–100). In 60 cases (58.8%) VEGF tumour expression was ⩾30%. The expression of VEGF in tumour was associated with T stage (P=0.03), M stage (P=0.03), Fuhrman grade (P=0.001; Figure 4A) but not with N stage (P=0.2) or ECOG performance status (P=0.4) (data not shown).
Figure 4.
VEGF expression in CCRCC. (A) Percentage of tumour tissue with positive VEGF expression according to Fuhrman grade. Horizontal lines represent mean values. (B) Concentration of VEGF in the plasma of CCRCC patients according to Fuhrman grade. Horizontal lines represent mean values. (C) Cause-specific survival curve for patients based on VEGF expression in plasma (solid line, patients with plasma VEGF<92.5 pg ml−1, n=56, 50%; broken line, patients with plasma VEGF ⩾92.5 pg ml−1, n=56, 50%).
Median VEGF plasma value was 92.5 pg ml−1 (13–1430), which is superior to what is reported for healthy donors (Adams et al, 2000). Plasma VEGF level was associated with T stage (P=0.001), Fuhrman grade (P=0.005; Figure 4B), ECOG performance Status (P=0.02), but not with N stage (P=0.07) or M stage (P=0.5; data not shown). Tumour and plasma VEGF were both predictors for PFS (log-rank test, P=0.01 and 0.002, respectively) and RCC-SS (log-rank test, P=0.05 and 0.001, respectively; Figure 4C). In summary, increased plasma or tumour VEGF expression was associated with more aggressive tumour pattern and poorer outcome.
Relationship between pVHL, VHL and VEGF
A strong relationship was observed between VEGF tumour expression, VEGF plasma level and pVHL detection (Table 2). However, unexpectedly, the absence of pVHL detection was associated with both lower VEGF tumour and plasma expression (P=0.0001 and 0.002, respectively). Similarly, tumours presenting biallelic inactivation of the VHL gene were associated with decreased VEGF tumour expression (P=0.0001) and plasma VEGF levels (P=0.005; Table 2). Consistently, when selecting tumours with no detectable pVHL and at least one VHL alteration, it appeared that this latter group exhibited decreased VEGF tumour expression (P=0.0001) and VEGF plasma levels (P=0.002) compared with tumours expressing pVHL. Finally, the group not harbouring any VHL abnormality (n=11, 10.8%) was also characterised by increased mean VEGF plasma (309.8±281.9 vs 183.4±139.9 pg ml−1, P=0.03) and mean VEGF tumour expression (77.4±32.4 vs 45.8±37.4, P=0.009) compared with tumours with either mutation, deletion or methylation of the VHL gene (n=91, 89.2%).
Table 2. Relationship between VHL gene inactivation and VEGF tumour or plasma expression.
| 2 or 3 VHL alteration (n=68) | 0 or 1 VHL alteration (n=34) | P-value | pVHL− (n=71) | pVHL+ (n=31) | P-value | |
|---|---|---|---|---|---|---|
| Mean VEGF tumour expression (%±s.d) | 40.3±35.5 | 68.9±36.5 | 0.0001 | 38.7±35.9 | 73.4±31.6 | 0.0001 |
| VEGF tumour expression ⩾30% | 35 (51.5%) | 25 (73.5%) | 0.007 | 33 (46.5%) | 27 (87.1%) | 0.0001 |
| Mean plasma VEGF level (pg/ml±s.d.) | 116.4±90.4 | 323.2±238.4 | 0.005 | 112.9±87.0 | 326.8±250.2 | 0.002 |
| VEGF plasma level ⩾92.5 pg/ml | 31 (45.6%) | 19 (55.9%) | 0.2 | 33 (46.5%) | 17 (54.8%) | 0.5 |
| Presence of tumour necrosis | 34 (50.0%) | 17 (50.0%) | 0.6 | 29 (40.8%) | 22 (71.0%) | 0.005 |
Abbreviations: pVHL=von Hippel-Lindau protein; s.d.=standard deviation; VEGF=vascular endothelial growth factor; VHL=the von Hippel–Lindau gene.
Values are presented as mean±s.d. for continuous variables and number of patients (percent) for categorical variables
When focusing on the population with at least one VHL alteration, it appeared that both VEGF tumour expression (cut-off 30%) and VEGF plasma level (cut-off, median value of 92.5 pg ml−1) were able to stratify two population subsets with distinct outcomes. Based on these finding, our total CCRCC population was, therefore, stratified into three distinct groups: Group 1, at least one VHL alteration and low VEGF expression; Group 2, at least one VHL alteration and high VEGF expression and Group 3, no VHL alteration regardless of VEGF expression. When stratifying with plasma VEGF level, the median PFS times for the three groups were 58, 27 and 17 months, respectively (log-rank test, P=0.005), whereas median RCC-SS time was not reached for the first two groups and was 36 months for the third group (log-rank test, P=0.003; Figure 5A). When stratifying the entire clear-cell population with VEGF tumour expression, the median PFS times for the three groups were not reached for the first group, was 58 months for the second group and 17 months for the third group (log-rank test, P=0.01). In the latest setting, median RCC-SS time was not reached for the first two groups and was 36 months for the third group (log-rank test, P=0.02; Figure 5B).
Figure 5.
Cause-specific survival curves for patients with CCRCC based on VHL alterations and VEGF expression. (A) Survival curves for patients based on VHL alterations and plasma VEGF (solid line, patients with at least one VHL alteration and plasma VEGF<92.5 pg ml−1, n=48, 47.1%; dotted line, patients with at least one VHL alteration and plasma VEGF ⩾92.5 pg ml−1, n=43, 42.1% and broken line, patients with a wild-type VHL, n=11, 10.8%). (B) Survival curves for patients based on VHL alterations and tumour VEGF expression (solid line, patients with at least one VHL alteration and less than 30% of cells expressing VEGF, n=40, 39.2%; dotted line, patients with at least one VHL alteration and more than 30% of cells expressing VEGF, n=51, 50.0% and broken line, patients with a wild-type VHL, n=11, 10.8%).
Multivariate analysis has been performed. Unfortunately, it did not reach significance, probably because of the limited number of tumours with no VHL alteration.
Discussion
The importance of tumour angiogenesis in CCRCC development and spreading has been recently strongly validated by the proven efficacy of antiangiogenic drugs that target mainly the VHL/VEGF pathway (Motzer et al, 2007; Figlin et al, 2008). Up till now, no prognostic factor has been validated in this setting and there is a growing need for defining biological tools for treatment monitoring (Golshayan et al, 2008). In this prospective series, we demonstrate that (1) VHL alterations appear to be associated with a favourable outcome, (2) VEGF instead of being overexpressed in VHL-inactivated tumours seems to be relatively low and (3) a small cohort of CCRCC with no VHL alteration exhibit a very unfavourable outcome.
Vascular endothelial growth factor upregulation mechanisms in CCRCC remain hypothetical. It has been demonstrated that VEGF is overexpressed both in tumour tissue and in blood samples in CCRCC (Jacobsen et al, 2002; Rioux-Leclercq et al, 2007a). It is generally believed that this overexpression is due to VHL inactivation that occurs with a high frequency in sporadic CCRCC (70–80%; Gnarra et al, 1994; Yao et al, 2002). Additionally, it is postulated that VHL inactivation leads to HIF constitutive expression and, therefore, induction of target genes including VEGF. However, VHL-independent mechanisms could explain VEGF upregulation and additional role have been described for VHL in CCRCC tumorigenesis (Kim and Kaelin, 2004). Therefore, there was a rationale for analysing in a cohort of sporadic CCRCC the relationship between VHL alterations and VEGF plasma and tumour expression.
In this series, we could confirm that plasma or tumour VEGF were elevated in the majority of patients and that both high plasma and tissue VEGF were associated with increased risk of dying from CCRCC. Unexpectedly, mean VEGF tumour expression and mean VEGF plasma levels were significantly increased in tumours with no VHL alteration as compared with tumours harbouring at least one VHL alteration. This trend was reproducible when examining both pVHL and VHL gene. Additionally, out of the 68 tumours with two or three VHL alterations, which potentially alter both alleles of the gene, 33 (48.5%) exhibited low VEGF tumour expression, and out of the 34 remaining tumours, 25 (73.5%) had high VEGF tissue expression. It clearly suggests that VHL-independent mechanisms are involved in VEGF upregulation in advanced CCRCC. Conversely, unexpected low VEGF levels were found in tumours with VHL alterations, indicating that VHL alteration does not necessarily trigger tumour progression.
Using a sensitive approach for mutation detection, we found a 68.6% VHL mutational rate along with 74.5 and 13.7% deletion and promoter methylation rates, respectively, which is consistent with what is generally reported in the literature (Banks et al, 2006; Nickerson et al, 2008). We also demonstrate that VHL abnormalities were associated with a favourable outcome. Our results are in accordance with the report of Yao et al (2002) who first presented a correlation between VHL mutation and better prognosis. Similarly, Parker et al (2005) demonstrated that absence of pVHL detected by immunohistochemistry was associated with improved cancer-specific survival. The present study is also in accordance with our previous analysis focusing on VHL and CAIX (Patard et al, 2008b) even though we failed to demonstrate here a clear association between VHL gene status and early TNM stages or grades.
Several other studies, however, were not able to correlate VHL status to clinicopathological parameters (Kondo et al, 2002) or prognosis (Brauch et al, 2000; Kim et al, 2005; Smits et al, 2008), and others linked VHL alteration with more advanced tumour stages (Schraml et al, 2002). These discrepancies may be partially explained by recruitment bias with an insufficient number of small incidental tumours, or the fact that TNM grouping was used and not separated in T, N and M stage. Methodological bias could also be involved. For example, in most of the studies VHL mutation analyses were performed on formalin-fixed, paraffin-embedded material (Schraml et al, 2002; Smits et al, 2008). It has already been shown for other genes that formalin fixation of archival specimens can induce sequence alterations (Williams et al, 1999; Marchetti et al, 2006). Finally, the way VHL is analysed is also of importance. In most series, tumours with biallelic inactivation of the VHL gene are compared with tumours presenting at least one functional allele. When using this approach, we did not find clear correlation with outcome. However, when separating tumours with no VHL alteration and tumours with at least one VHL hit, we were able to identify two groups non comparable in frequency but with very distinctive clinicopathological features and outcome.
The discrepancy between VHL studies in CCRCC could also be explained by the stage of cancer progression at which VHL status was evaluated. From this point of view, our classification of CCRCC into three different groups with different aggressiveness behaviours according to VHL and VEGF is of interest. The two VHL-altered groups with distinct prognostic profiles according to VEGF expression could be reflecting a progression process. VHL alteration, as it is now well admitted, occurs at an early stage of a disease, thus leading to a relative decrease of VEGF expression (Group 1 of our VHL/VEGF classification). These tumours are generally good prognostic tumours that are likely to be cured by surgery or that could be potentially good candidates for immunotherapy in case of metastases. Carbonic Anhydrase IX is generally overexpressed in such tumours and CAIX has been proven to be a good selection criteria for response to interleukin-2-based immunotherapy (Atkins et al, 2005; Patard et al, 2008b). Tumours with VHL alterations and high VEGF levels (Group 2) probably reflect additional molecular events following initial VHL alteration. These tumours with VEGF-driven aggressiveness would be good candidates for receiving tyrosine kinase inhibitors (TKIs) that target VEGF-R and PDGF-R. It is probably in this particular group that VEGF should be evaluated in the future as a predictive factor for response to TKIs. Two different studies suggested that tumours with loss of function of VHL were more likely to be sensitive to anti-VEGF drugs, thus suggesting that both VHL and VEGF status are potentially important for predicting response to TKIs (Rini et al, 2006; Choueiri et al, 2008b).
The third group with no VHL alteration could be seen either as a distinct molecular entity with no involvement of the VHL pathway. From this point of view it is interesting to note that the survival of patients with poor prognostic metastatic RCC according to the Memorial Sloan-Kettering Hospital Cancer Center (MSKCC) classification is increased by using mTOR inhibitors that do not target specifically the VHL/VEGF pathway (Hudes et al, 2007; Motzer et al, 2008). It is also interesting to note that non-clear-cell RCCs, which are never VHL-mutated, seem to be less sensitive to VEGF targeting drugs than their clear-cell counterpart, and that by contrast non-clear-cell RCCs seem to be sensitive to mTOR inhibitors (Choueiri et al, 2008a). Consistent with that vascular phenotype selection hypothesis, metastatic CCRCC after an initial phase of response to TKIs inevitably escape and progress.
In conclusion, classifying CCRCC according to VHL/VEGF status could potentially help physicians to choose the appropriate therapeutic strategy according to the molecular stage of the disease: surgery/immunotherapy, surgery/TKIs and mTOR inhibitors. We investigated a relatively small series of 102 patients with CCRCC. Consequently, subgroups are limited in size. A larger number of tumours with no VHL alteration would be needed to validate further our hypotheses through prospective clinical trials, and non-VHL molecular pathways that are involved in CCRCC should be better characterised.
Acknowledgments
We thank Marie-Claire Malinge for help with DHPLC analysis and fraction collection, and Stephane Dréano for technical support with MLPA experiments. This work was supported by grants from the ‘Ligue Départementale contre le Cancer’, ‘COmité de la REcherche Clinique du CHU de Rennes’ and the research program on kidney cancer from the ‘Institut National du Cancer’.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, Cairnduff F, Selby PJ, Perren TJ, Lansdown M, Banks RE (2000) Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 60: 2898–2905 [PubMed] [Google Scholar]
- Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, Febbo P, Upton M, Lechpammer M, Signoretti S (2005) Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res 11: 3714–3721 [DOI] [PubMed] [Google Scholar]
- Banks RE, Tirukonda P, Taylor C, Hornigold N, Astuti D, Cohen D, Maher ER, Stanley AJ, Harnden P, Joyce A, Knowles M, Selby PJ (2006) Genetic and epigenetic analysis of von Hippel–Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res 66: 2000–2011 [DOI] [PubMed] [Google Scholar]
- Brauch H, Weirich G, Brieger J, Glavac D, Rodl H, Eichinger M, Feurer M, Weidt E, Puranakanitstha C, Neuhaus C, Pomer S, Brenner W, Schirmacher P, Storkel S, Rotter M, Masera A, Gugeler N, Decker HJ (2000) VHL alterations in human clear cell renal cell carcinoma: association with advanced tumor stage and a novel hot spot mutation. Cancer Res 60: 1942–1948 [PubMed] [Google Scholar]
- Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B (2008a) Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 26: 127–131 [DOI] [PubMed] [Google Scholar]
- Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, Bhalla IP, Small EJ, Weinberg V, Sein N, Simko J, Golshayan AR, Sercia L, Zhou M, Waldman FM, Rini BI, Bukowski RM, Ganapathi R (2008b) von Hippel–Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180: 860–865 [DOI] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, Jaakkola P, Chang G-W, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH (2000) Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel–Lindau tumor suppressor protein. J Biol Chem 275: 25733–25741 [DOI] [PubMed] [Google Scholar]
- Figlin RA, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Negrier S, Huang X, Kim ST, Chen I, Motzer RJ (2008) Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). J Clin Oncol 26: 5024 [Google Scholar]
- Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6: 655–663 [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky I, Duan DR, Florence C, Pozzatti R, Walther MM, Bander NH, Grossman HB, Brauch H, Pomer S, Brooks JD, Isaacs WB, Lerman MI, Zbar B, Linehan WM (1994) Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7: 85–90 [DOI] [PubMed] [Google Scholar]
- Golshayan AR, Brick AJ, Choueiri TK (2008) Predicting outcome to VEGF-targeted therapy in metastatic clear-cell renal cell carcinoma: data from recent studies. Future Oncol 4: 85–92 [DOI] [PubMed] [Google Scholar]
- Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, Novick A (1997) TNM staging of renal cell carcinoma: workgroup no. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 80: 992–993 [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281 [DOI] [PubMed] [Google Scholar]
- Igarashi H, Esumi M, Ishida H, Okada K (2002) Vascular endothelial growth factor overexpression is correlated with von Hippel–Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer 95: 47–53 [DOI] [PubMed] [Google Scholar]
- Jacobsen J, Grankvist K, Rasmuson T, Bergh A, Landberg G, Ljungberg B (2004) Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int 93: 297–302 [DOI] [PubMed] [Google Scholar]
- Jacobsen J, Grankvist K, Rasmuson T, Ljungberg B (2002) Prognostic importance of serum vascular endothelial growth factor in relation to platelet and leukocyte counts in human renal cell carcinoma. Eur J Cancer Prev 11: 245–252 [DOI] [PubMed] [Google Scholar]
- Jeuken JW, Cornelissen SJ, Vriezen M, Dekkers MM, Errami A, Sijben A, Boots-Sprenger SH, Wesseling P (2007) MS-MLPA: an attractive alternative laboratory assay for robust, reliable, and semiquantitative detection of MGMT promoter hypermethylation in gliomas. Lab Invest 87: 1055–1065 [DOI] [PubMed] [Google Scholar]
- Kim JH, Jung CW, Cho YH, Lee J, Lee SH, Kim HY, Park J, Park JO, Kim K, Kim WS, Park YS, Im YH, Kang WK, Park K (2005) Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol Rep 13: 859–864 [PubMed] [Google Scholar]
- Kim WY, Kaelin WG (2004) Role of VHL gene mutation in human cancer. J Clin Oncol 22: 4991–5004 [DOI] [PubMed] [Google Scholar]
- Knebelmann B, Ananth S, Cohen HT, Sukhatme VP (1998) Transforming growth factor alpha is a target for the von Hippel–Lindau tumor suppressor. Cancer Res 58: 226–231 [PubMed] [Google Scholar]
- Kondo K, Yao M, Yoshida M, Kishida T, Shuin T, Miura T, Moriyama M, Kobayashi K, Sakai N, Kaneko S, Kawakami S, Baba M, Nakaigawa N, Nagashima Y, Nakatani Y, Hosaka M (2002) Comprehensive mutational analysis of the VHL gene in sporadic renal cell carcinoma: relationship to clinicopathological parameters. Genes Chromosomes Cancer 34: 58–68 [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L et al. (1993) Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260: 1317–1320 [DOI] [PubMed] [Google Scholar]
- Marchetti A, Felicioni L, Buttitta F (2006) Assessing EGFR mutations. N Engl J Med 354: 526–528 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bukowski RM (2006) Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol 24: 5601–5608 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372: 449–456 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124 [DOI] [PubMed] [Google Scholar]
- Na X, Wu G, Ryan CK, Schoen SR, di’Santagnese PA, Messing EM (2003) Overproduction of vascular endothelial growth factor related to von Hippel–Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 170: 588–592 [DOI] [PubMed] [Google Scholar]
- Nickerson ML, Jaeger E, Shi Y, Durocher JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Mukeria A, Holcatova I, Schmidt LS, Toro JR, Karami S, Hung R, Gerard GF, Linehan WM, Merino M, Zbar B, Boffetta P, Brennan P, Rothman N, Chow W-H, Waldman FM, Moore LE (2008) Improved identification of von Hippel–Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 14: 4726–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AS, Cheville JC, Lohse CM, Igel T, Leibovich BC, Blute ML (2005) Loss of expression of von Hippel–Lindau tumor suppressor protein associated with improved survival in patients with early-stage clear cell renal cell carcinoma. Urology 65: 1090–1095 [DOI] [PubMed] [Google Scholar]
- Patard J-J, Pouessel D, Bensalah K, Culine S (2008a) Targeted therapy in renal cell carcinoma. World J Urol 26: 135–140 [DOI] [PubMed] [Google Scholar]
- Patard JJ, Fergelot P, Karakiewicz PI, Klatte T, Trinh QD, Rioux-Leclercq N, Said JW, Belldegrun AS, Pantuck AJ (2008b) Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer 123: 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z (1994) Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol 12: 206–212 [DOI] [PubMed] [Google Scholar]
- Rini BI, Jaeger E, Weinberg V, Sein N, Chew K, Fong K, Simko J, Small EJ, Waldman FM (2006) Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and von Hippel–Lindau gene status. BJU Int 98: 756–762 [DOI] [PubMed] [Google Scholar]
- Rioux-Leclercq N, Fergelot P, Zerrouki S, Leray E, Jouan F, Bellaud P, Epstein JI, Patard JJ (2007a) Plasma level and tissue expression of vascular endothelial growth factor in renal cell carcinoma: a prospective study of 50 cases. Hum Pathol 38: 1489–1495 [DOI] [PubMed] [Google Scholar]
- Rioux-Leclercq N, Karakiewicz PI, Trinh QD, Ficarra V, Cindolo L, de la Taille A, Tostain J, Zigeuner R, Mejean A, Patard JJ (2007b) Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer 109: 868–874 [DOI] [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml P, Hergovitz A, Hatz F, Amin MB, Lim SD, Krek W, Mihatsch MJ, Moch H (2003) Relevance of nuclear and cytoplasmic von Hippel Lindau protein expression for renal carcinoma progression. Am J Pathol 163: 1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, Sauter G, Mihatsch MJ, Moch H (2002) VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol 196: 186–193 [DOI] [PubMed] [Google Scholar]
- Smits KM, Schouten LJ, van Dijk BA, Hulsbergen-van de Kaa CA, Wouters KA, Oosterwijk E, van Engeland M, van den Brandt PA (2008) Genetic and epigenetic alterations in the von Hippel-Lindau gene: the influence on renal cancer prognosis. Clin Cancer Res 14: 782–787 [DOI] [PubMed] [Google Scholar]
- Valle L, Cascon A, Melchor L, Otero I, Rodriguez-Perales S, Sanchez L, Cruz Cigudosa J, Robledo M, Weber B, Urioste M, Benitez J (2005) About the origin and development of hereditary conventional renal cell carcinoma in a four-generation t(3;8)(p14.1;q24.23) family. Eur J Hum Genet 13: 570–578 [DOI] [PubMed] [Google Scholar]
- van Houwelingen KP, van Dijk BA, Hulsbergen-van de Kaa CA, Schouten LJ, Gorissen HJ, Schalken JA, van den Brandt PA, Oosterwijk E (2005) Prevalence of von Hippel–Lindau gene mutations in sporadic renal cell carcinoma: results from the Netherlands cohort study. BMC Cancer 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Ponten F, Moberg C, Soderkvist P, Uhlen M, Ponten J, Sitbon G, Lundeberg J (1999) A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol 155: 1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y, Kubota Y, Kondo K-i (2002) VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 94: 1569–1575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.