Abstract
Background:
The tumour-host interaction at the invasive front of colorectal cancer, including the epithelial–mesenchymal transition and its hallmark ‘tumour budding’, is an important area of investigation in terms of prognosis. The aim of this study was to determine the prognostic impact of a ‘pro-/anti-tumour’ approach defined by an established ‘pro-tumour’ (tumour budding) and host-related ‘anti-tumour’ factor of the adaptive immunological microenvironment (CD8+ lymphocytes).
Methods:
Double immunostaining for CK22/CD8 on whole tissue sections (n=279; Cohort 1) and immunohistochemistry for CD8+ using tissue microarrays (n=191; Cohort 2) was carried out. Tumour buds, CD8+ and CD8+ T-lymphocytes : tumour buds indices were evaluated per high-power field.
Results:
In Cohort 1, a low-CD8+/ buds index was associated with lymph node metastasis (P<0.001), vascular invasion (P=0.009), worse survival in univariate (P<0.001) and multivariable (P<0.001) analysis, and furthermore in lymph node-negative patients (P=0.002). In Cohort 2, the CD8+/ buds index was associated with T stage (P<0.001), N stage (P=0.041), vascular invasion (P=0.005) and survival in patients with TNM stage II (P=0.019), stage III (P=0.004), and adjuvantly untreated (P=0.009) and treated patients (P<0.001).
Conclusion:
The CD8+ lymphocyte : tumour-budding index is an independent prognostic factor in colorectal cancer and a promising approach for a future prognostic score for patients with this disease.
Keywords: tumour budding, CD8+ lymphocytes, colorectal cancer, prognosis
During the Napoleonic Wars (1796–1815), one of the most effective methods to attack in battle was the deployment of heavy cavalry, which the opponent tried to repel by organizing the infantry into squares.
A similar picture can be observed at the invasion front of colorectal cancer. On one hand, the tumour mass invades the pericolic fat tissue, detaching clusters or single tumour cells (tumour buds) reflecting tumour progression; whereas, on the other hand the host attempts to confront this situation by building an ‘anti-tumour’ cytotoxic inflammatory response. This ‘pro-/anti-tumour’ model is supported by a range of studies proposing either independent tumour-related prognostic factors (pro-tumour), such as tumour grade, tumour border configuration, medullary subtype, CEA level, microsatellite instability, loss of heterozygosity (LOH) 18q status, p53 levels, TGFB1 type II receptor levels, VEGF expression, proliferation rate and metalloproteinase expression (Compton, 2006) or anti-tumour factors, such as CD3+, CD4+, CD8+, CD20+ lymphocytes, Granzyme B, FoxP3+ regulatory T cells (Tregs), CD16+ cells, and mast and dendritic cells (Dadabayev et al, 2004; Phillips et al, 2004; Sato et al, 2005; Chaput et al, 2009; Salama et al, 2009).
Consequently, several groups focus on the invasive front of colorectal cancer using the term epithelial–mesenchymal transition (EMT), which characterises tumour invasion by de-differentiated colorectal carcinoma cells (Brabletz et al, 2005). EMT and MET, the reverse transition from a mesenchymal to an epithelial phenotype, are crucial steps not only in embryonic development but also in tumour progression (Spaderna et al, 2007).
A histomorphological hallmark of EMT is the phenomenon of ‘tumour budding’ (Prall, 2007), which according to the third edition of ‘Prognostic Factors in Cancer’ published by the UICC in 2006, is considered an additional prognostic factor in colorectal cancer (Compton, 2006). A tumour bud is typically defined as a single tumour cell or tumour cell cluster of up to five cells at the invasive tumour front (Prall, 2007). Indeed, tumour budding has been shown to be associated with lymph node positivity, poorly differentiated tumours, presence of vascular and lymphatic invasion, local tumour recurrence and distant metastasis (Ueno et al, 2004a,2004b; Nakamura et al, 2005; Kazama et al, 2006; Ishikawa et al, 2008; Wang et al, 2009). In particular, patients with stage III disease have been reported to demonstrate a 5-year disease-free survival (DFS) of 62.1% in the absence of tumour budding and only a 35.1% DFS with this feature (Choi et al, 2007). Moreover, the presence of tumour budding has repeatedly been linked to poor clinical outcome, underlined by the adverse effect on overall survival independent of TNM stage (Hase et al, 1993; Ueno et al, 2004b).
Over the last 20 years, investigations on tumour immunity and host defence in colorectal cancer demonstrate promising results for immunotherapy. In most colorectal cancers, lymphocytic infiltration is composed predominantly of either CD4+ or CD8+ T cells and both cell types appear to be significantly increased in tumour as compared with normal tissue (Ropponen et al, 1997; Naito et al, 1998; Chiba et al, 2004; Koch et al, 2006). Several studies have shown that tumour infiltrating lymphocytes (TILs) within the stroma and around the tumour along the invasive margin are significantly related to overall- and disease-specific survival in both univariate and multivariable analysis (Ali et al, 2004; Canna et al, 2005; Pages et al, 2005). Galon et al (2006) evaluated by gene-expression profiling and immunohistochemistry, the type, density and location (whether at the invasive margin or the tumour centre) of TILs in a large number of cases. They evaluated CD3, CD8, granzyme B and memory CD45RO T cells, demonstrating a significant independent and positive effect of TILs on both recurrence and survival.
In colorectal cancer, mismatch-repair status (microsatellite stable (MSS) and microsatellite instability-high (MSI-H)) seems to relate highly to the number of CD8+ lymphocytes. Compared with MSS tumours, MSI-H cancers are characterised by prolonged survival time, significantly more frequent peritumoural lymphocytic infiltration at the invasive front and by an inherent abundance of intra-epithelial TILs (Jass et al, 1998; Michael-Robinson et al, 2001; Greenson et al, 2003; Jenkins et al, 2007). Nevertheless, many studies analyzing colorectal cancer samples stratified by mismatch-repair status also report a positive effect of CD8+ lymphocytes in mismatch-repair proficient colorectal cancers (Baker et al, 2007).
Although this wide range of possible additional prognostic factors in colorectal cancer is currently being investigated, still missing is an approach to include parameters reflecting the tumour dynamics. Such an approach has already been taken for the prognostication of breast cancer, namely the Bloom–Richardson–Elston (BRE) score, which encompasses information on mitoses, tubule differentiation and nuclear pleomorphism, and is used as an important prognostic feature additional to TNM staging.
Therefore, the aim of this study was to investigate on a potential ‘pro-/anti-tumour’ model and to test the prognostic impact of a ratio defined by an established pro-tumour (tumour budding) and anti-tumour (CD8+ lymphocytes) factor. To this end, two independent colorectal cancer patient cohorts from different centres were investigated using two different approaches, namely whole tissue sections (n=300) and the tissue microarray technique (n=221).
Materials and methods
Cohort 1-Whole tissue sections
Sample size determination
To reach 85% power with an expected risk ratio of 1.8 between prognostic groupings and potential loss of patient samples in 10% of cases the appropriate sample size for this study was determined to be 255 cases. Owing to the availability of material this number was increased to 300 cases.
Specimens: Paraffin-embedded tissue blocks of 300 resection specimens of patients treated between 1987 and 1996 at the University Hospital of Basel were retrieved from the archives of the Institute of Pathology, University Hospital of Basel, as well as at the Institute of Clinical Pathology, Basel, Switzerland. These 300 cases were randomly selected from a larger previously described cohort of 938 colorectal cancer patients with full clinico-pathological information (Zlobec et al, 2008). The use of material for this study was approved by the local research ethics committee.
Double immunostaining for CD8 and CK22
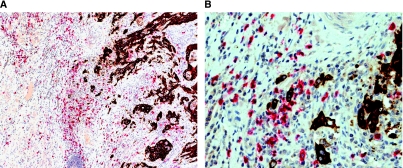
A double immunostaining procedure using anti-CD8 (for detection of CD8+ T-lymphocytes) and pan-cytokeratin (to facilitate visualization of tumour buds at the invasive front) was carried out on one representative slide cut at 4 μm from paraffin-embedded tumour blocks of all 300 colorectal cancer patients included in this study (Figure 1). Double staining was carried out using the BOND-MAX Automated Immunohistochemistry Vision Biosystem (Leica Microsystems GmbH, Wetzlar, Germany) according to the following protocol. First, tissues were deparaffinised and pre-treated with the Epitope Retrieval Solution 2 (EDTA-buffer pH8.8) at 100°C for 20 min. After wash steps, peroxidase blocking was carried out for 10 min using the Bond Polymer Refine Detection Kit DC9800 (Leica Microsystems GmbH). Tissues were again washed, then incubated with primary antibody against CK22 (Biomeda, Foster City, CA, USA, pan-CK22) for 30 min. Subsequently, tissues were incubated with polymer for 15 min and then with DAB-Chromogen for 10 min (Bond Polymer AP Red Detection Kit DS9305, Leica Microsystems GmbH). CK22 positive cells were therefore coloured in brown. After washing, incubation was carried out with anti-CD8 (DakoCytomation, Glostrup, Denmark, clone CD8/144B) for 30 min followed by application of AEC-substrate for 10 min and counterstaining with haematoxylin for 2 min.
Figure 1.
Double immunostaining for CD8 (red) and CK22 (brown): (A) Overview ( × 10) and (B) high power field ( × 40) of the invasive front of colorectal cancer showing CK22 positive tumour buds surrounded by CD8+ T-lymphocytes.
Evaluation of tumour buds and CD8+ T-lymphocytes
Tumour budding was defined as an isolated single cancer cell or a cluster of up to five cells at the invasive front of colorectal cancer (Prall, 2007). The tumour border was scanned at a × 100 magnification and the area of most intense budding was identified (Ueno et al, 2002). After selecting this field, the number of buds was counted using a 40 × objective lens to focus specifically on the presence of CD8+ T-cells most highly related to the microenvironment surrounding the tumour buds. Within this same field, all CD8+ lymphocytes were individually counted. In 10 cases, the abundance of CD8+ infiltrate led to cell counts exceeding 200 cells per field, and thus single cell counting was not feasible. These cases were assigned a CD8+ score of 200 cells. The ratio of CD8+ T-lymphocytes to the number of tumour buds (CD8+/ buds index) was obtained. In 13 cases when zero buds were identified, the count of CD8+ lymphocytes was not carried out.
Clinico-pathological characteristics
Of these 300 cases, 279 were evaluable for CD8 and CK22 protein expression, simultaneously. Hematoxylin and eosin (H&E) stained slides were reviewed and histomorphological data included histological subtype, pT stage, pN stage and tumour grade. The tumour border configuration and the presence of conspicuous peritumoural lymphocytic infiltration were defined according to Jass et al (1986). Clinical data were retrieved from patient records and included age at diagnosis, gender, tumour location and follow up. Clinical outcome of interest was disease-specific survival time, which was available for all 279 patients. Median follow-up time was 60 months. In all, 128 patients died of disease. Patient characteristics are listed in Table 1.
Table 1. Characteristics of patients in Cohort 1 (n=279).
| Clinico-pathological features | Frequency N (%) |
|---|---|
| Age (years) (n=279) | |
| Mean, range | 67.7, 37–96 |
| Tumour diameter (mm) (n=279) | |
| Mean, range | 54.0, 13–170 |
| Gender (n=279) | |
| Male | 122 (43.7) |
| Female | 157 (56.3) |
| Tumour location (n=279) | |
| Left-sided | 71 (25.4) |
| Right-sided | 111 (39.8) |
| Rectum | 97 (34.7) |
| Histological subtype (n=277) | |
| Adenocarcinoma | 252 (91.0) |
| Mucinous | 21 (7.6) |
| Other | 4 (1.4) |
| pT stage (n=274) | |
| pT1 | 6 (2.2) |
| pT2 | 35 (12.8) |
| pT3 | 187 (68.3) |
| pT4 | 46 (16.8) |
| pN stage (n=273) | |
| pN0 | 140 (51.3) |
| pN1 | 76 (27.8) |
| pN2 | 57 (20.9) |
| Tumour grade (n=274) | |
| G1 | 5 (1.8) |
| G2 | 247 (90.2) |
| G3 | 22 (8.0) |
| Vascular invasion (n=274) | |
| Absent | 206 (75.2) |
| Present | 68 (24.8) |
| Tumour border configuration (n=271) | |
| Infiltrating | 186 (68.6) |
| Pushing | 85 (31.4) |
| Peritumoural lymphocytic infiltration (n=274) | |
| Absent | 204 (74.5) |
| Present | 70 (25.6) |
| Microsatellite instability status (n=125) | |
| Stable/low | 95 (76.0) |
| Instable/high | 30 (24.0) |
| KRAS gene status (n=117) | |
| Wild-type | 82 (70.1) |
| Mutation | 35 (29.9) |
| BRAF gene status (n=106) | |
| Wild-type | 85 (80.2) |
| Mutation | 21 (19.8) |
| 5-year survival (%) (n=279) | |
| Rate (95%CI) | 60.3 (54–66) |
Continuous variables age and tumour diameter are described as the mean and range of values, whereas categorical variables are represented by the number of cases (% of cases). Survival time was obtained using the Kaplan–Meier method.
Microsatellite instability (MSI) status, KRAS and BRAF gene status
Genomic DNA was obtained from primary tumours using NucleoMag 96 Tissue Kit (Macherey Nagel, Oensingen, Switzerland) protocol and processed in the Xiril X-100 robot (Xiril, Hombrechtikon, Switzerland). Briefly, punched tissue was lysed in proteinase K. B-beads and MB2 buffer were added to the cleared lysate and shaken for 5 min at room temperature. The supernatant was removed and MB3 was added followed by shaking and supernatant removal. The genomic DNA was eluted with MB6 buffer. Genomic DNA was amplified by PCR using AmpliTaq Gold polymerase (Applied Biosystem, Foster City, CA, USA). KRAS (exon 2, codon 12 and 13) and BRAF (exon 15, codon 600) were amplified by a first and a nested PCR. Residual primers were removed using the EXOSAPit (Amersham, Otelfingen, Switzerland). Samples were then subjected to direct sequencing of single-stranded PCR products using the BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems) and the ABI Prism 3130 genetic analyser (Applied Biosystems). All products were sequenced bi-directionally. Analysis of MSI status was based on the multiplex amplification of the five microsatellites (BAT25, BAT26, D2S123, D5S346 and D17S250). An initial denaturation step at 95°C for 10 min was followed by 42 cycles at 95°C for 40 s, 54°C for 40 s and 72°C for 60 s. For the analysis, 1 μl of the DNA weight marker ROX 500 (Applied Biosystem) was added and 10 μl of deionised formamide in 3 μl of the PCR amplified solution. DNA was denaturated by incubation for 2 min at 95°C. The POP-7 polymer solution (Applied Biosystem) was used for the electrophoresis on the ABI Prism 3130 genetic analyser (Applied Biosystems). MSS and MSI-low (MSI-L) status were defined as instability at zero and one markers, respectively. MSI-H was characterised by the presence of instability in two or more markers (Umar et al, 2004).
Cohort 2-Tissue microarray
Colorectal cancer tissue microarray construction
A tissue microarray of 221 unselected, non-consecutive colorectal cancer patients treated at the Second Department of Pathology, University of Athens between the years 2004 and 2006 was constructed at the Institute for Pathology, University Hospital of Basel. The use of this material was approved by the local ethics committee of the University of Athens.
Each patient had multiple tissue punches taken from formalin-fixed, paraffin-embedded blocks using a tissue cylinder with a diameter of 0.6 mm, which were subsequently transferred into one recipient paraffin block (3 × 2.5 cm2) using a homemade semi-automated tissue arrayer. Tissues were obtained from the tumour centre, the invasive tumour front within the representative area of most intense tumour budding in all sections of the tumour, as determined from corresponding H&E slides, the normal adjacent mucosa (if available) and the transitional zone where tumour and normal adjacent mucosa first interact (if available). Each patient on average had 5.1 tissue punches included on this array. The final tissue microarray contained 1079 tissues, namely 437 tissues from the tumour centre, 430 from the invasive front, 90 from normal adjacent mucosa and 122 from the transitional zone. For the purposes of this study, only tissue punches from the invasive tumour front per patient were analysed.
Clinico-pathological features
H&E slides were reviewed and histomorphological data included histological subtype, pT stage, pN stage, pM stage, tumour grade, and vascular and lymphatic invasion. Clinical data were retrieved from patient records and included age at diagnosis, gender, tumour location and follow up. Information on adjuvant therapy was available for all patients. Patients with TNM stage IV disease were removed from this study, thus 191 patients with stage I–III disease constituted the final patient cohort. Clinical outcome of interest was disease-specific survival time, which was available for all 191 patients. Median follow-up time is 35 months and 44 patients died of the disease. Patient characteristics are listed in Table 2.
Table 2. Characteristics of patients in Cohort 2 (n=191).
| Frequency N (%) | |
|---|---|
| Age (years) (n=184) | |
| Mean, range | 67.9 (35–93) |
| Tumour diameter (mm) (n=189) | |
| Mean, range | 4.6 (1.0–12.0) |
| Gender (n=189) | |
| Male | 88 (46.6) |
| Female | 101 (53.4) |
| Tumour location (n=190) | |
| Left-sided | 113 (59.5) |
| Right-sided | 27 (14.2) |
| Rectum | 50 (26.3) |
| Histological subtype (n=189) | |
| Mucinous | 24 (12.7) |
| Other | 165 (87.3) |
| pT stage (n=189) | |
| pT1–2 | 52 (27.5) |
| pT3–4 | 137 (72.5) |
| pN stage (n=189) | |
| pN0 | 102 (54.0) |
| pN1–2 | 87 (46.0) |
| TNM stage (n=189) | |
| Stage I | 45 (23.8) |
| Stage II | 57 (30.2) |
| Stage III | 87 (46.0) |
| Tumour grade (n=189) | |
| G1–2 | 122 (64.6) |
| G3 | 67 (35.5) |
| Vascular or lymphatic invasion (n=191) | |
| Absent | 147 (91.1) |
| Present | 17 (8.9) |
| Adjuvant therapy (n=191) | |
| None | 69 (36.1) |
| Treated | 122 (63.9) |
| 5-year survival (%) (n=191) | |
| Rate (95%CI) | 67.5 (57–76) |
Continuous variables age and tumour diameter are described as the mean and range of values, whereas categorical variables are represented by the number of cases (% of cases). Survival time was obtained using the Kaplan–Meier method.
Immunohistochemistry
Immunohistochemistry was carried out using anti-CD8 antibody at the Second Department of Pathology, University of Athens. Briefly, 5-μm TMA sections were deparaffinised, and pre-treated with the Envision Flex Target Retrieval Solution pH8.8 (DM812, Dako) at 800°C for 6 min followed by incubation at room temperature for 10 min. After wash steps, peroxidase blocking was carried out for 10 min. Sections were again washed and then incubated with primary antibody against CD8 (DakoCytomation; clone CD8/144B) for 60 min. Subsequently, sections were washed with TBS and incubated with Real Envision Solution (Dako) for 30 min, then washed again in TBS and incubated with DAB-Chromogen for 10 min. After washing, sections were counterstained with haematoxylin for 2 min. Only peritumoural CD8+ T-lymphocytes in punches taken from the invasive tumour front were counted.
Cut-off score for low vs high budding, CD8+ and CD8+/ buds indices
All cut-off scores to classify patients as having a ‘low’ or ‘high’ index were obtained by receiver operating characteristic curve (ROC) analysis (Zlobec et al, 2007b). 50% of the data was randomly selected for this purpose. Using this method, a plot of the sensitivity and false positive rate (1-specificity) for discriminating between survivors and non-survivors was assessed. The most discriminating cut-off score was determined as the point on the ROC curve with the shortest distance to the coordinate (0, 1), namely with the maximum sensitivity and specificity for survival. The reliability of all cut-off scores was tested by re-sampling of the data (500 bootstrapped replications). The discriminatory ability of each feature for survival was also evaluated by analyzing the area under the ROC curve (AUC) and 95% confidence interval (CI).
Additional statistical analyses
The association of indices with categorical clinico-pathological features was obtained using the χ2 and Fisher's exact tests, where appropriate. Univariate survival analysis was carried out using the Kaplan–Meier and log-rank tests. Multivariable analysis was carried out using Cox's proportional hazards regression analysis after the verification of proportional hazards assumption. Hazard ratio (HR) and 95%CI were obtained to determine the prognostic effect of each index after adjustment. With logistic regression analysis, the odds ratios (OR) and 95%CI were obtained to determine the odds of death at 5 years with the use of different indices. All statistical analyses were carried out using SAS (V9, The SAS Institute, NC, Cary, USA).
Results
Cohort 1-Whole tissue sections
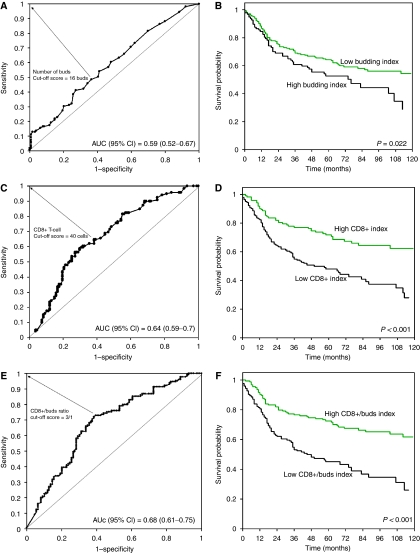
Tumour-budding index
A high tumour-budding index was defined, using ROC curve analysis, as at least 16 buds per × 40 field (Figure 2A). However, the ROC curve also highlights a relatively poor discrimination of survivors and non-survivors at 5-year follow up with an AUC value of 0.59 (95%CI: 0.52–0.67). A high-budding index was significantly associated with a more advanced pT stage (P=0.017), with the presence of lymph node metastasis (P=0.002), with the presence of vascular invasion (P=0.028) and with an infiltrating tumour border configuration (P=0.005) (Table 3). Patients with MSI-H cancers were significantly less prone to a high tumour-budding index (P=0.022), whereas no association was observed with KRAS or BRAF mutation. The odds of death from disease at 5 years in the group with a high-budding index was OR (95%CI)=1.89 (1.1–3.3) compared with those with a low index (P=0.022) (Table 4). In all, 5-year disease-specific survival rate of patients with high and low tumour-budding index was 53.0 (95%CI 42–63) and 63.9 (95%CI 57–70), suggesting a significantly worse prognosis in patients with high tumour-budding index (P=0.033) (Figure 2B). In patients with MSS/MSI-L tumours, a high-budding index was related to more advanced pT stage (P=0.019), pN stage (P=0.002), vascular invasion (P=0.004) and an infiltrating tumour border configuration (P=0.021) and worse survival in univariate analysis (P=0.02). In MSI-H patients, the high tumour-budding index was only related to an infiltrating tumour border configuration (P=0.015).
Figure 2.
Cohort 1. (A, C and E): Receiver operating characteristic (ROC) curves highlighting the ability of tumour budding (A), CD8+ lymphocytes (C) and CD8+/ buds index (E) to discriminate survivors and non-survivors. AUC=area under the ROC curve. Larger the AUC, more discriminating the feature. Arrows are drawn from the point on the curve used to classify patients into ‘low’ or ‘high’ indices based on their proximity to the coordinate (0,1). (B, D and F) corresponding Kaplan–Meier survival curves for ‘low’ and ‘high’ indices.
Table 3. Association of budding index, CD8+ index and CD8+/ buds index with clinico-pathological features – Cohort 1 (n=279).
| Budding index | CD8+ index | CD8+/buds index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinico-pathological feature | Low | High | P-value | Low | High | P-value | Low | High | P-value |
| Gender | |||||||||
| Female | 107 (56.9) | 50 (55.0) | 0.797 | 61 (54.0) | 87 (57.2) | 0.598 | 72 (54.1) | 85 (58.2) | 0.492 |
| Male | 81 (43.1) | 41 (45.1) | 52 (46.0) | 65 (42.8) | 61 (45.9) | 61 (41.8) | |||
| Tumour location | |||||||||
| Left-sided | 48 (25.5) | 23 (25.3) | 0.06 | 30 (26.5) | 37 (40.7) | 0.551 | 34 (25.6) | 37 (25.3) | 0.053 |
| Right-sided | 82 (43.6) | 29 (31.9) | 42 (37.2) | 62 (40.8) | 45 (33.8) | 66 (45.2) | |||
| Rectum | 58 (30.9) | 39 (42.9) | 41 (36.3) | 53 (34.9) | 54 (40.6) | 43 (29.5) | |||
| pT stage | |||||||||
| pT1–2 | 34 (18.6) | 7 (7.7) | 0.017 | 15 (13.5) | 20 (13.4) | 0.983 | 15 (11.4) | 26 (18.3) | 0.107 |
| pT3–4 | 149 (81.4) | 84 (92.3) | 96 (86.5) | 129 (86.6) | 117 (88.6) | 116 (81.7) | |||
| pN stage | |||||||||
| pN0 | 106 (57.9) | 34 (37.8) | 0.002 | 50 (45.5) | 81 (54.4) | 0.156 | 53 (40.5) | 87 (61.3) | < 0.001 |
| pN1–2 | 77 (42.1) | 56 (62.2) | 60 (54.6) | 68 (45.6) | 78 (59.5) | 55 (38.7) | |||
| Tumour grade | |||||||||
| G1–2 | 166 (90.7) | 86 (94.5) | 0.276 | 104 (63.7) | 135 (90.6) | 0.366 | 125 (94.7) | 127 (89.4) | 0.109 |
| G3 | 17 (9.3) | 5 (5.5) | 7 (6.3) | 14 (9.4) | 7 (5.3) | 15 (10.6) | |||
| Vascular invasion | |||||||||
| Absent | 145 (79.2) | 61 (67.0) | 0.028 | 79 (71.2) | 115 (77.2) | 0.271 | 90 (68.2) | 116 (81.7) | 0.009 |
| Present | 38 (20.8) | 30 (33.0) | 32 28.8) | 34 (22.8) | 42 (31.8) | 26 (18.3) | |||
| Tumour border configuration | |||||||||
| Infiltrating | 114 (63.0) | 72 (80.0) | 0.005 | 80 (73.4) | 102 (68.9) | 0.435 | 100 (76.9) | 86 (61.0) | 0.005 |
| Pushing | 67 (37.0) | 18 (20.0) | 29 (26.6) | 46 (31.1) | 30 (23.1) | 55 (39.0) | |||
| Peritumoural lymphocytic inflammation | |||||||||
| Absent | 137 (74.9) | 67 (73.6) | 0.825 | 93 (83.8) | 100 (67.1) | 0.002 | 108 (81.8) | 96 (67.6) | 0.007 |
| Present | 46 (25.1) | 24 (26.4) | 18 (16.2) | 49 (32.9) | 24 (18.2) | 46 (32.4) | |||
| KRAS gene status | |||||||||
| Wild-type | 55 (75.3) | 27 (61.4) | 0.11 | 31 (64.6) | 47 (74.6) | 0.253 | 38 (64.4) | 44 (75.9) | 0.176 |
| Mutation | 18 (24.7) | 17 (38.6) | 17 (65.4) | 16 (25.4) | 21 (35.6) | 14 (24.1) | |||
| BRAF gene status | |||||||||
| Wild-type | 54 (77.1) | 31 (86.1) | 0.273 | 36 (90.0) | 45 (75.0) | 0.063 | 41 (85.4) | 44 (75.9) | 0.219 |
| Mutation | 16 (22.9) | 5 (13.9) | 4 (10.0) | 15 (25.0) | 7 (14.6) | 14 (24.1) | |||
| Microsatellite instability status (MSS) | |||||||||
| MSS/MSI-L | 54 (69.2) | 41 (87.2) | 0.022 | 43 (86.0) | 47 (69.1) | 0.033 | 51 (83.6) | 44 (68.8) | 0.052 |
| MSI-H | 24 (30.8) | 6 (12.8) | 7 (14.0) | 21 (30.9) | 10 (16.4) | 20 (31.2) | |||
| Survival rate (%) (95% CI) | |||||||||
| 5-year | 63.9 (57–70) | 53.0 (42–63) | 0.033 | 47.2 (38–56) | 67.9 (60–75) | < 0.001 | 46.6 (38–55) | 72.8 (65–79) | < 0.001 |
Table 4. Comparison of the discriminatory ability of each feature for identifying survivors and non-survivors at 5-year follow up.
| Feature | Sensitivity | Specificity | Overall accuracy (95% CI) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Budding count (high vs low) | 0.413 | 0.737 | 0.59 (0.52–0.67) | 1.89 (1.1–3.3) | 0.022 |
| CD8+ count (low vs high) | 0.597 | 0.683 | 0.64 (0.59–0.7) | 3.18 (1.8–5.5) | <0.001 |
| CD8 : buds index (low vs high) | 0.721 | 0.61 | 0.68 (0.61–0.75) | 4.12 (2.4–7.1) | <0.001 |
Odds ratio (OR) indicate that the odds of disease-specific death at 5-years is 1.89 times higher in the high-budding count group compared with the low group; for CD8+ count, 3.18 times higher in the low compared with the high CD8+ count group and for the CD8 : buds index, is 4.12 times higher in those with a low compared with a high index.
CD8+ index
A high CD8+ index was characterised by the presence of at least 40 CD8+ cells per × 40 field (Figure 2C). The discriminatory ability of CD8+ as indicated by the AUC was 0.64 (95%CI 0.59–0.7), suggesting an improvement compared with the budding index alone. The odds of death from disease at 5 years was OR (95%CI)=3.18 (1.8–5.5) in patients with a low compared with a high CD8+ index. A high CD8+ index was associated with significantly more frequent peritumoural lymphocytic inflammation at the tumour front (P=0.002), with MSI-H status (P=0.033) and marginally with BRAF mutation. Patients with a high CD8+ index demonstrated a significantly prolonged survival time compared with those with a low CD8+ index (P<0.001) (Figure 2D). In MSS/MSI-L and MSI-H patients, the CD8+ index was not linked to any features of tumour progression although in MSS/MSI-L those with a high CD8+ index experienced a significant prolonged survival time (P=0.011).
CD8+/ buds index
A ratio of at least three times more CD8+ T-cells vs the number of tumour buds led to a strong discrimination of survivors and non-survivors of colorectal cancer, as indicated by an AUC value of 0.68 (95%CI 0.61–0. 75) (Figure 2E). The odds of death at 5 years in patients with a low CD8+/ buds index compared with those with a high index was OR (95%CI)=4.12 (2.4–7.1) indicating that on average the odds of death was 4.12 times greater in patients with a low compared with a high CD8+/ buds index (Table 4). Patients with a high CD8+/ buds index demonstrated more favourable features, such as early T stage (P<0.001), absence of vascular invasion (P=0.009), presence of a pushing/expanding tumour border configuration (P=0.005), presence of peritumoural lymphocytic inflammation at the invasive front (P=0.007) and a marginal association with MSI-H status (P=0.052). Most notably, a considerable difference in survival time between patients with low and high CD8+/ buds index was observed with patients with a low index demonstrating a highly adverse prognosis (P<0.001) (Figure 2F). This prognostic difference was also maintained in the group of MSS/MSI-L patients (P=0.001).
Independent prognostic effects of budding, CD8+ and CD8+/ buds indices
A comparison of the performance of each of these indices in multivariable survival analysis is outlined in Table 5. The effect of each of these factors was re-evaluated along with age, gender, pT stage, pN stage, tumour grade, vascular invasion and the tumour border configuration. Analysis was thus carried out only for patients with complete clinico-pathological information for all these features (n=226, 101 deaths). The budding index was not found to add independent prognostic information when analysed along with these additional features (P=0.85), whereas the CD8+ index (P<0.001) and the CD8+/ buds index (P<0.001) were highly relevant as prognostic factors in multivariable analysis, with similar relative risks of death suggesting a comparable performance of these two features as prognostic indices.
Table 5. Comparison of relative risks of buds, CD8+ and CD8+/ buds index in multivariable analysis with age, gender, pT stage, pN stage, tumour grade, vascular invasion and tumour border configuration in Cohort 1.
| Buds index only | CD8+ index only | CD8+/ buds index | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Index | ||||||
| Low | 1.0 | 0.85 | 1.0 | <0.001 | 1.0 | <0.001 |
| High | 1.04 (0.7–1.6) | 0.45 (0.3–0.7) | 0.44 (0.3–0.7) | |||
| Age (years) | ||||||
| Baseline | 1.0 | 0.041 | 1.0 | 0.026 | 1.0 | 0.021 |
| Increasing by year | 1.02 (1.0–1.1) | 1.02 (1.0–1.1) | 1.02 (1.0–1.1) | |||
| Gender | ||||||
| Male | 1.0 | 0.468 | 1.0 | 0.507 | 1.0 | 0.443 |
| Female | 0.86 (0.6–1.3) | 0.87 (0.6–1.3) | 0.86 (0.6–1.3) | |||
| pT stage | ||||||
| pT1–2 | 1.0 | 0.042 | 1.0 | 0.048 | 1.0 | 0.022 |
| pT3–4 | 2.41 (1.0–5.6) | 2.35 (1.0–5.5) | 2.69 (1.2–6.3) | |||
| pN stage | ||||||
| pN0 | 1.0 | <0.001 | 1.0 | <0.001 | 1.0 | 0.001 |
| pN1–2 | 2.27 (1.5–3.5) | 2.12 (1.4–3.3) | 2.06 (1.4–3.2) | |||
| Tumour grade | ||||||
| G1–2 | 1.0 | 0.205 | 1.0 | 0.738 | 1.0 | 0.873 |
| G3 | 0.63 (0.3–1.3) | 0.88 (0.4–1.9) | 0.94 (0.4–2.0) | |||
| Vascular invasion | ||||||
| Absent | 1.0 | <0.001 | 1.0 | 0.001 | 1.0 | 0.014 |
| Present | 2.2 (1.4–3.5) | 2.1 (1.3–3.3) | 1.79 (1.1–2.8) | |||
| Tumour border configuration | ||||||
| Pushing | 1.0 | 0.758 | 1.0 | 0.945 | 1.0 | 0.915 |
| Infiltrating | 1.09 (0.6–1.8) | 1.02 (0.6–1.8) | 1.03 (0.6–1.8) | |||
In order to prevent over-fitting of multivariable models, only the most relevant prognostic factors were included into sub-group analyses of lymph node-negative patients (n=140, 42 deaths), as well as of those with colon (n=176; 79 deaths) or rectal cancers (n=94; 44 deaths). In lymph node-negative patients, multivariable analysis with pT stage, vascular invasion and tumour border configuration revealed independent prognostic effects for both the CD8+ index (P=0.001) and the CD8+/ buds index (P=0.002), but not for the budding index. Moreover, in patients with colon or rectal cancer, the CD8+ index (P=0.015 and P=0.006, respectively) as well as the CD8+/ buds index (P=0.002 and P=0.016, respectively) maintained a significant independent prognostic effect after adjusting for pT stage, pN stage and vascular invasion.
Cohort 2-Tissue microarray
In Cohort 2, a high tumour-budding index in stages I–III patients was defined as at least six buds per punch and was related to a more advanced pT stage (P=0.029), more poorly differentiated tumours (P=0.003), presence of vascular invasion (P=0.045) and was highly related to a worse prognosis (P=0.003) (Table 6). The threshold value for a high CD8+ index was 13 cells per punch and was linked to lymph node negativity (P=0.053), early tumour grade (P=0.014), absence of vascular invasion (P=0.002) and a prolonged survival time compared with patients with a low CD8+ index (P=0.031).
Table 6. Association of budding index, CD8+ index and CD8+/ buds index with clinico-pathological features – Cohort 2 (n=191).
| Budding index | CD8+ index | CD8+/ buds index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinico-pathological feature | Low (n=131) | High (n=60) | P-value | Low (n=69) | High (n=121) | P-value | Low (n=68) | High (n=122) | P-value |
| Gender | |||||||||
| Male | 65 (50.4) | 23 (38.3) | 0.122 | 34 (49.3) | 54 (54.4) | 0.606 | 33 (48.5) | 55 (45.8) | 0.722 |
| Female | 64 (49.6) | 37 (61.7) | 35 (50.7) | 65 (54.6) | 35 (51.5) | 65 (54.2) | |||
| Tumour location | |||||||||
| Left-sided | 80 (61.5) | 33 (55.0) | 0.311 | 38 (55.1) | 74 (61.7) | 0.673 | 35 (51.5) | 77 (63.6) | 0.054 |
| Right-sided | 20 (15.4) | 7 (11.7) | 11 (15.9) | 16 (13.3) | 8 (11.8) | 19 (15.7) | |||
| Rectum | 30 (23.1) | 20 (33.3) | 20 (16.0) | 30 (25.0) | 25 (36.8) | 25 (20.7) | |||
| pT stage | |||||||||
| pT1–2 | 42 (32.3) | 10 (17.0) | 0.029 | 15 (21.7) | 37 (31.1) | 0.167 | 9 (13.4) | 43 (35.5) | 0.001 |
| pT3–4 | 88 (67.7) | 49 (83.0) | 54 (78.3) | 82 (68.9) | 58 (86.6) | 78 (64.5) | |||
| pN stage | |||||||||
| pN0 | 79 (71.2) | 23 (67.7) | 0.694 | 30 (60.0) | 71 (75.5) | 0.053 | 24 (58.5) | 77 (74.8) | 0.055 |
| pN1–2 | 32 (28.8) | 11 (32.4) | 20 (40.0) | 23 (24.5) | 17 (41.5) | 26 (25.2) | |||
| Tumour grade | |||||||||
| G1–2 | 93 (71.5) | 29 (49.2) | 0.003 | 37 (53.6) | 85 (71.4) | 0.014 | 29 (43.3) | 93 (76.9) | <0.001 |
| G3 | 37 (28.5) | 30 (50.8) | 32 (46.4) | 34 (28.6) | 38 (56.7) | 28 (23.1) | |||
| Vascular invasion | |||||||||
| Present | 8 (6.1) | 9 (15.0) | 0.045 | 12 (17.4) | 5 (4.1) | 0.002 | 12 (17.7) | 5 (4.1) | 0.002 |
| Absent | 123 (93.9) | 51 (85.0) | 57 (82.6) | 116 (95.9) | 56 (82.4) | 117 (95.9) | |||
| Survival rate (%) (95%CI) | |||||||||
| 5 years | 75.0 (62–84) | 52.9 (36–67) | 0.003 | 49.4 (30–66) | 77.1 (66–85) | 0.031 | 43.8 (26–60) | 81.5 (69–89) | <0.001 |
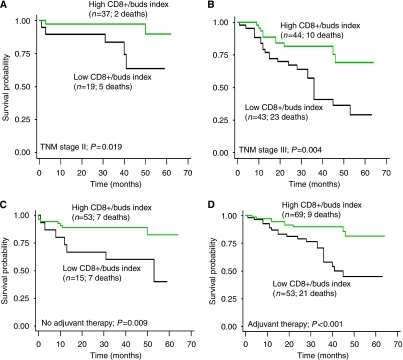
A ratio of three times more CD8+ lymphocytes to the number of tumour buds was identified as the most discriminating cut-off value to classify survivors and non-survivors. Patients with a high CD8+/ buds index had tumours that were more early pT stage (P=0.001), lymph node-negative (P=0.055), early tumour grade (P<0.001) and vascular invasion negative (P=0.002). Low CD8+/ buds index, led to a highly unfavourable prognosis compared with patients with a high CD8+/ buds index (P<0.001). This difference was also maintained in sub-group analysis of TNM stage II (P=0.019) and stage III (P=0.004) patients (Figure 3A and B). In particular, in both untreated and treated patients, a low CD8+/ buds index was linked to a shorter survival time (P=0.009 and P<0.001, respectively) (Figure 3B and C). Multivariable analysis for the budding index, CD8+ index and CD8+/ buds index was carried out accounting for pN stage, vascular and lymphatic invasion and treatment effect. Results outlined in Table 7 highlight once more the independent adverse prognostic effect of a low CD8+/ buds index after adjusting for these additional factors.
Figure 3.
Cohort 2. Kaplan–Meier survival curves illustrating the poorer outcome in patients with a low CD8+/ buds index in (A) TNM stage II patients, (B) TNM stage III patients, (C) patients not receiving adjuvant therapy and (D) patients treated with adjuvant therapy.
Table 7. Comparison of relative risks of buds, CD8+ and CD8+/ buds index in multivariable analysis with pN stage, vascular and lymphatic invasion and adjuvant therapy in Cohort 2.
| Buds index only | CD8+ index only | CD8+/ buds index | ||||
|---|---|---|---|---|---|---|
| Cohort 2 | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Index | ||||||
| Low | 1.0 | 0.029 | 1.0 | 0.473 | 1.0 | 0.005 |
| High | 1.96 (1.1–3.6) | 0.79 (0.4–1.5) | 0.39 (0.2–0.8) | |||
| pN stage | ||||||
| pN0 | 1.0 | <0.001 | 1.0 | <0.001 | 1.0 | 0.002 |
| pN1–2 | 4.11 (1.8–9.2) | 4.41 (2.0–9.7) | 3.58 (1.6–7.9) | |||
| Vascular/lymphatic invasion | ||||||
| Absent | 1.0 | <0.001 | 1.0 | 0.006 | 1.0 | 0.007 |
| Present | 3.41 (1.7–7.1) | 2.97 (1.4–6.4) | 2.76 (1.3–5.7) | |||
| Adjuvant therapy | ||||||
| Untreated | 1.0 | 0.057 | 1.0 | 0.068 | 1.0 | 0.076 |
| Treated | 0.5 (0.2–1.0) | 0.52 (0.3–1.1) | 0.53 (0.3–1.1) | |||
Discussion
The aim of this study was to investigate an alternative approach to reflect the dynamic at the tumour front of colorectal cancer using an index including tumour budding and CD8+ lymphocytes.
Briefly summarised, our results confirm the prognostic impact of the selected tumour and host-related factors. In both patient cohorts, a high tumour budding and a low CD8+ lymphocytes index were associated with tumour progression and worse survival. Indeed, tumour budding has previously been found to be associated with higher N stage, higher tumour grade and presence of vascular invasion, local tumour recurrence, distant metastasis as well as worse survival (Ueno et al, 2004a,2004b; Nakamura et al, 2005; Kazama et al, 2006; Prall, 2007; Ishikawa et al, 2008; Wang et al, 2009). There is also strong evidence for an association between a high number of CD8+ lymphocytes and absence of lymph node metastases, lower tumour grade, presence of peritumoural lymphocytes, mismatch repair-deficient status and better survival (Ropponen et al, 1997; Naito et al, 1998; Chiba et al, 2004; Galon et al, 2006; Koch et al, 2006; Baker et al, 2007).
The question, therefore, arises as to whether a CD8+ lymphocytes : budding index is more advantageous over the use of one out of the two parameters alone. With the exception of 5-year survival, a certain intra- and inter-group variability is observed when analysing the relationship of tumour budding or CD8+ lymphocytes with clinico-pathological parameters. In contrast, the use of a CD8+ lymphocytes : budding index better summarises these associations with key endpoints of tumour progression, namely presence of lymph node metastases, vascular invasion and worse survival, and moreover, in both cohorts. In addition, the CD8+/ buds index shows an increased discriminatory ability for identifying survivors and non-survivors at 5-year follow up compared with tumour budding or CD8+ lymphocytes count themselves. More specifically, patients with a low CD8+ lymphocyte : buds index have more than four-fold odds of death at 5 years compared with a three-fold or under two-fold risk for patients with low CD8+ counts or high number of tumour buds, respectively. In addition, the CD8+/ buds index maintained its significant impact on outcome in patients with stage II disease, in both colon and rectal cancer patients and also, independently of adjuvant therapy. Our previous work describes the absence of tumour budding in cases with marked peritumoural inflammatory reaction at the invasive margin of colorectal cancers and a strong correlation between this inflammation and the presence of TILs(Zlobec et al, 2007a). We hypothesised that an immune response to colorectal cancer could account for an improved prognosis in patients with abundant TIL count by specifically targeting budding cells, a so-called ‘nipping in the bud’ effect leading to improved prognosis. Although we cannot directly allude to the functional interaction of CD8+ lymphocytes and tumour budding in this study, our data nonetheless point to a strong link between favourable prognosis and a high CD8+ lymphocyte : tumour-budding index.
Several points in this study should still be considered. Although the impact of tumour budding on clinical outcome is validated by several groups, a standardised scoring system for this feature has not yet been established, although several similar methods have been proposed (Ueno et al, 2002; Shinto et al, 2005; Prall and Ostwald, 2007). This study highlights a new approach, which may be helpful in furthering the understanding of colorectal pathogenesis and prognosis, but requires validation by other research groups both in retrospective and prospective settings. Finally, our findings, particularly with regard to the test group may be somewhat influenced by the lack of clinical information regarding recurrence, metastasis and treatment although the majority of clinico-pathological features are adequately covered.
On the other hand, several factors strongly support the validity of our findings. The selection of parameters included in the proposed index is evidence based, as both tumour budding and the CD8+ lymphocytes have been tested and validated in colorectal cancer by different research groups. The index, which encompasses a tumour- and host-related feature, and therefore represents the more advantageous ‘multi-marker’ approach to prognosis, better reflects the tumour dynamic at the invasive front, compared with either feature alone. This study also benefits from the inclusion of two completely independent cohorts of colorectal cancer patients treated in Switzerland and Greece, respectively. In addition to whole tissue sections, we used the tissue microarray technique, a powerful tool for the investigation of novel or potential biomarkers, which has allowed us to evaluate hundreds of tissue cores for CD8+. Moreover, the evaluation of multiple tissue punches per tumour in this study exceeds the number of cores required for adequate representativity of tumour heterogeneity (Goethals et al, 2006). Although double staining was carried out for Cohort 1, only immunohistochemistry for CD8 was carried out on Cohort 2. These differences in methodology were chosen based on the ease of identification of buds. In whole tissue sections, budding can often be missed because of peritumoural inflammation, whereas tissue microarrays may aid the observer to focus on one specific area containing tumour buds. It is important to note that the independent prognostic effects of the CD8+/ buds index were found in both sub-groups despite the different laboratory circumstances and methodologies, thereby underlining the biological consistency of the CD8+/ buds index in colorectal cancer. Although we recognise that this particular index is unlikely to be a final solution for daily diagnostic practice, it may however serve as a basis that can be further extended to include other strong and validated ‘pro-tumour’ or ‘anti-tumour’. This approach could potentially lead to a new colorectal cancer prognostic score, which can be applied in addition to TNM staging, as is the case for the BRE score for breast cancers.
In conclusion, the CD8+ lymphocyte to tumour-budding index is an independent prognostic factor in colorectal cancer and represents biologically a ‘pro-/anti-tumour’ model that could be a promising approach for a future prognostic score in colorectal cancer.
Acknowledgments
This study is funded by the Krebsliga Beider Basel (IZ, LT and AL).
References
- Ali AA, McMillan DC, Matalka II, McNicol AM, McArdle CS (2004) Tumour T-lymphocyte subset infiltration and tumour recurrence following curative resection for colorectal cancer. Eur J Surg Oncol 30: 292–295 [DOI] [PubMed] [Google Scholar]
- Baker K, Zlobec I, Tornillo L, Terracciano L, Jass JR, Lugli A (2007) Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient vs proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer 43: 624–631 [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005) Opinion: migrating cancer stem cells–an integrated concept of malignant tumour progression. Nat Rev Cancer 5: 744–749 [DOI] [PubMed] [Google Scholar]
- Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92: 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J (2009) Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58: 520–529 [DOI] [PubMed] [Google Scholar]
- Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S (2004) Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer 91: 1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS (2007) Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis 22: 863–868 [DOI] [PubMed] [Google Scholar]
- Compton C (2006) Prognostic Factors in Cancer. John Wiley & Sons Inc: Hoboken, NJ, USA [Google Scholar]
- Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn C, Ensink NG, Tollenaar RA, van de Velde CJ, Kuppen PJ (2004) Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother 53: 978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964 [DOI] [PubMed] [Google Scholar]
- Goethals L, Perneel C, Debucquoy A, De Schutter H, Borghys D, Ectors N, Geboes K, McBride WH, Haustermans KM (2006) A new approach to the validation of tissue microarrays. J Pathol 208: 607–614 [DOI] [PubMed] [Google Scholar]
- Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, Trougouboff P, Tomsho LD, Kim E, Low M, Almog R, Rennert G, Gruber SB (2003) Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol 27: 563–570 [DOI] [PubMed] [Google Scholar]
- Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumor ‘budding’ in patients with colorectal cancer. Dis Colon Rectum 36: 627–635 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Yokoo T, Ishii T (2008) Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer 112: 924–933 [DOI] [PubMed] [Google Scholar]
- Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, Todd IP (1986) The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology 10: 437–459 [DOI] [PubMed] [Google Scholar]
- Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B (1998) Morphology of sporadic colorectal cancer with DNA replication errors. Gut 42: 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Hayashi S, O'Shea AM, Burgart LJ, Smyrk TC, Shimizu D, Waring PM, Ruszkiewicz AR, Pollett AF, Redston M, Barker MA, Baron JA, Casey GR, Dowty JG, Giles GG, Limburg P, Newcomb P, Young JP, Walsh MD, Thibodeau SN, Lindor NM, Lemarchand L, Gallinger S, Haile RW, Potter JD, Hopper JL, Jass JR (2007) Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 133: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H (2006) Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer 94: 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Beckhove P, Op den Winkel J, Autenrieth D, Wagner P, Nummer D, Specht S, Antolovic D, Galindo L, Schmitz-Winnenthal FH, Schirrmacher V, Buchler MW, Weitz J (2006) Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg 244: 986–992; discussion 992–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Robinson JM, Biemer-Huttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL (2001) Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 48: 360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58: 3491–3494 [PubMed] [Google Scholar]
- Nakamura T, Mitomi H, Kikuchi S, Ohtani Y, Sato K (2005) Evaluation of the usefulness of tumor budding on the prediction of metastasis to the lung and liver after curative excision of colorectal cancer. Hepatogastroenterology 52: 1432–1435 [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353: 2654–2666 [DOI] [PubMed] [Google Scholar]
- Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S (2004) Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg 91: 469–475 [DOI] [PubMed] [Google Scholar]
- Prall F (2007) Tumour budding in colorectal carcinoma. Histopathology 50: 151–162 [DOI] [PubMed] [Google Scholar]
- Prall F, Ostwald C (2007) High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol 38: 1696–1702 [DOI] [PubMed] [Google Scholar]
- Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM (1997) Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182: 318–324 [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27: 186–192 [DOI] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T-cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102: 18538–18543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR (2005) A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology 47: 25–31 [DOI] [PubMed] [Google Scholar]
- Spaderna S, Schmalhofer O, Hlubek F, Jung A, Kirchner T, Brabletz T (2007) Epithelial-mesenchymal and mesenchymal-epithelial transitions during cancer progression. Verh Dtsch Ges Pathol 91: 21–28 [PubMed] [Google Scholar]
- Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse K, Fujimoto H, Hase K (2004a) Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg 91: 327–333 [DOI] [PubMed] [Google Scholar]
- Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, Yoshimura K, Bekku S (2004b) Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127: 385–394 [DOI] [PubMed] [Google Scholar]
- Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC (2002) Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40: 127–132 [DOI] [PubMed] [Google Scholar]
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96: 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, Hyland J, O'Donoghue D, Sheahan K (2009) Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 33: 134–141 [DOI] [PubMed] [Google Scholar]
- Zlobec I, Baker K, Minoo P, Jass JR, Terracciano L, Lugli A (2008) Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein expression. Clin Cancer Res 14: 143–148 [DOI] [PubMed] [Google Scholar]
- Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR (2007a) Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol 212: 260–268 [DOI] [PubMed] [Google Scholar]
- Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A (2007b) Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 60: 1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]