Abstract
Purpose
To investigate delivery quality assurance (DQA) discrepancies observed for a subset of helical tomotherapy patients.
Methods and Materials
Six tomotherapy patient plans were selected for analysis. Three had passing DQA ion chamber (IC) measurements while three had measurements deviating from the expected dose by more than 3.0%. All plans utilized similar parameters, including: 2.5 cm field-width, 15 s gantry period, and pitch values ranging from 0.143–0.215. Preliminary analysis suggested discrepancies were associated with plans having predominantly small leaf open times (LOTs). To test this, patients with failing DQA measurements were replanned using an increased pitch of 0.287. New DQA plans were generated and IC measurements performed. Exit fluence data was also collected during DQA delivery for dose reconstruction purposes.
Results
Sinogram analysis showed increases in mean LOTs ranging from 29.8–83.1% for the increased pitch replans. IC measurements for these plans showed a reduction in dose discrepancies, bringing all measurements within ±3.0%. The replans were also more efficient to deliver, resulting in reduced treatment times. Dose reconstruction results were in excellent agreement with IC measurements, illustrating the impact of leaf-timing inaccuracies on plans having predominantly small LOTs.
Conclusions
The impact of leaf-timing inaccuracies on plans with small mean LOTs can be considerable. These inaccuracies result from deviations in MLC leaf latency from the linear approximation used by the treatment planning system and can be important for plans having a 15 s gantry period. The ability to reduce this effect while improving delivery efficiency by increasing the pitch is demonstrated.
Keywords: tomotherapy, treatment planning, delivery accuracy, patient throughput, leaf latency
1. Introduction
The incorporation of inverse planning and intensity modulation techniques into radiotherapy has resulted in the improved ability to conform planned dose distributions to clinically designated target volumes. This in turn allows for the delivery of therapeutic doses to target lesions with increased sparing of adjacent critical structures (1,2). While such techniques can offer significant advantages as compared with conventional delivery paradigms, their implementation requires the use of more complex planning and delivery systems.
The TomoTherapy Hi-Art II™ system utilizes a compact 6 MV linear accelerator (linac) placed on a CT ring gantry to rotationally deliver intensity modulated fan beams of radiation while the patient is translated through the gantry on a treatment couch (3,4). Intensity modulation is performed via a pneumatically powered, binary multi-leaf collimator (MLC). The Hi-Art II™ unit also contains a megavoltage CT (MVCT) detector array (5), located opposite the radiation source, which can be used for pre-treatment setup verification, delivery verification and dose reconstruction (6,7), as well as for machine commissioning and quality assurance purposes (8).
While numerous planning studies have demonstrated the dosimetric advantages of helical tomotherapy (9–11), the distinctive nature of the helical delivery pattern necessitates a different way of thinking about both treatment planning and quality assurance. From a treatment planning perspective, the ability to plan with 51 beam angles allows for tremendous flexibility when prescribing to complicated target volumes, often times surrounded by critical structures. However, the helical delivery pattern also requires the physicist or dosimetrist to designate non-conventional treatment parameters including the field width and pitch, which is the ratio of couch translation per rotation to the field width (12). Also, when optimizing, an upper limit on the modulation factor must be selected. Failure to choose judicious values for these parameters can compromise plan quality, needlessly increase the treatment time, as well as produce plans which are more difficult for the machine to accurately deliver.
From a quality assurance perspective, the dynamic properties of helical tomotherapy present new challenges, and a number of tests have been developed to assess the performance characteristics of the treatment machine (13,14). In addition, because every helical tomotherapy treatment is intensity modulated, patient specific delivery quality assurance (DQA) must be performed on every patient plan. Currently the procedure for DQA is largely the same as for other film-based IMRT QA procedures; using films and/or ion chamber measurements to verify the treatment in a solid-water or other tissue equivalent phantom (14,15). The problem with these methods is that when a given measurement fails to meet a specified criterion, it is often difficult to ascertain what went wrong. This problem is exacerbated in tomotherapy DQA because of the large number of beam angles used, and the inability to decouple and test individual projections.
This work arose out of a clinical situation where the authors were faced with the problem described above for patients at the University of Wisconsin planned for treatment on the TomoTherapy Hi-Art II™ system. For these patients, DQA ion chamber measurements were systematically found to be outside of the ±3% acceptance criteria. The goals of this study were to examine and compare various properties of the failing treatment plans against similar patient plans with passing DQAs in order to determine the underlying cause of the observed discrepancies and to improve the accuracy of delivery by using better choices of treatment planning parameters.
2. Materials and Methods
2.1 Observed DQA discrepancies
After a helical tomotherapy plan has been completed and approved by the attending physician(s), the treatment plan is verified by performing a patient specific DQA procedure. The DQA process consists of three basic components: planning, delivery, and analysis. In DQA planning, the TomoTherapy Hi-Art II™ treatment planning system (TPS) is used to calculate dose from the accepted treatment beams in a cylindrical solid-water phantom. The phantom position is adjusted prior to the calculation so that the target contour is in a region of the phantom where film, TLD, or ion chamber measurements can be made. After the dose calculation is complete, treatment procedures are generated and the DQA plan is delivered to the phantom.
Both film and ion chamber measurements are taken for each DQA plan in order to obtain both relative planar dose profiles and absolute point dose measurements. Analysis of these measurements is performed using the TomoTherapy TPS and consists of comparing the measured and calculated point doses, as well as looking at planar isodose and gamma distributions (16) computed between the measured film dose and the expected dose calculated by the TPS. At the University of Wisconsin (UW), the tolerances for a plan to be deemed acceptable are ±3% for measured point doses and a gamma value less than or equal to 1 for all points on the film lying within the mid to high dose regions (typically points contained by the 30% isodose line) using search criteria of 3% and 3 mm.
As mentioned previously, this work grew out of a clinical situation where a number of patients being planned for treatment on both tomotherapy units at UW had DQAs with point dose measurements falling outside of the specified ±3% tolerance. Relative dose distributions and gamma maps calculated from film measurements for these plans were deemed passing with respect to the stated criteria. Typical sources of DQA discrepancy such as phantom misalignment and machine output variation were eliminated from the list of primary causes by utilizing MVCT imaging for setup verification, and by alternating ion chamber measurements of failing DQA plans with passing plans having similar plan parameters; always seeing the same result with a near constant dose rate.
To diagnose this issue, six patients planned for treatment on Tomotherapy Machine #2 were selected for analysis. Three patients had plans with passing DQA measurements while three had plans with DQA ion-chamber measurements deviating from the expected dose values by more than 3%. All plans had similar planning parameters including a 2.5 cm field width and 15 second gantry period. Pitch values were also similar and ranged from 0.143–0.215. For each patient plan, normalized leaf timing sinograms - which contain information about the amount of time each leaf of the binary MLC is open relative to the total projection time, were obtained from the TPS and read into MATLAB™ (The MathWorks, Natick MA) for analysis. Knowing the gantry period and the number of projections per rotation, mean non-zero leaf open times were computed from these sinograms and are shown in Table 1 along with measured DQA point dose results.
Table 1.
Patient plan parameters and DQA measurement data
| Patient data |
Plan parameters |
DQA point dose |
||||
|---|---|---|---|---|---|---|
| Pt. No. | Disease site | Pitch | Mean LOT (ms) | Planned dose (Gy) | Measured dose (Gy) | Difference (%) |
| 1 | H & N | 0.143 | 59 | 1.589 | 1.66 | 4.47 |
| 2 | Thorax | 0.215 | 90 | 1.448 | 1.50 | 3.59 |
| 3 | Thyroid | 0.215 | 84 | 1.391 | 1.46 | 4.96 |
| 4 | Lung | 0.215 | 153 | 2.651 | 2.64 | −0.53 |
| 5 | Prostate | 0.172 | 132 | 2.877 | 2.86 | −0.48 |
| 6 | Pelvis | 0.215 | 139 | 2.487 | 2.48 | −0.16 |
All DQA point dose measurements were performed in a 30 cm diameter cylindrical solid water phantom using a calibrated A1SL ion chamber (Standard Imaging Inc., Middleton WI) having a collecting volume of 0.056 cm3. Measurements were repeated three times for each plan to reduce uncertainties caused by potential output fluctuations. In addition, to correct for day-today changes in the machine output, dose measurements were scaled to a monitor unit (MU) rate of 867 MU/min, which is the target MU rate for the machine in question based on output measurements and monitor chamber calibrations made at the time of commissioning. Mean values are reported for all ion-chamber dose measurements. For DQA results stated in Table 1, uncertainties calculated to the 95% confidence level were less than the precision of the measured quantities and so are not reported.
2.2 Replanning to increase mean leaf open time
As seen from Table 1, all plans with failing DQA measurements had considerably lower mean leaf open times. Based on this finding, it was hypothesized that increasing the mean leaf open time for Patients 1–3 in Table 1 would result in a reduction in delivery discrepancies seen in the DQA measurements. To test this hypothesis, Patients 1–3 were replanned using an increased pitch value. Increasing the pitch effectively increases the mean leaf open time by forcing the same prescription dose to be delivered in fewer gantry rotations.
At the UW, pitch values are typically chosen according to the rule:
| (1) |
This relation stems from a previous study (17) which showed that the use of these “good” pitch values tends to minimize thread effects seen in the dose distribution from helical field junctioning. While pitch values of 0.860 or 0.430 could have been used, conventional thinking suggests that increasing the pitch may result in a loss of longitudinal resolution in the dose distribution. For this study, a pitch value of 0.287 (N = 3) was chosen for all three replanned cases because it was the next highest pitch value in the series of good pitches relative to the original treatment plans.
Aside from changing the pitch, all three patients were replanned using the same field width and similar optimization parameters in an effort to reproduce similar dose distributions and DVHs. For Patients 1 and 2, this required that the planning modulation factor be increased from 2.0 to 2.5 (the planning modulation factor prevents unwanted high modulation by placing an upper limit on the actual modulation factor - which is defined as the ratio of maximum leaf open to the average of all non-zero leaf open times). After replanning, new DQA plans were generated and ion chamber dose measurements performed for both the original and replanned treatments. Measurements were performed three times for each DQA plan with each individual measurement scaled to correspond with a monitor unit rate of 867 MU/min.
2.3 Dose reconstruction
In addition to DQA ion chamber measurements, uncompressed fluence data was collected with the onboard MVCT detectors during delivery of both the original and replanned DQA procedures. Tools developed by TomoTherapy Inc. were used to reconstruct delivered fluence sinograms from MVCT detector data by looking at the signal profile of the individual detector channels taken over the time of each projection. By examining the width of these signal profiles, it is possible to determine the amount of time each leaf was actually open during the delivery. Based on this and assuming the same energy fluence rate used by the TomoTherapy TPS, delivered fluence sinograms were created and then used to recalculate the dose in the DQA phantom images for the six DQA plans.
After recalculation of the dose distributions, reconstructed dose values at the location of the ion chamber measurements were obtained for comparative purposes by noting the voxel locations of the ion-chamber collecting volume in the DQA phantom CT images and averaging the reconstructed dose values in these voxels. For consistency, all planned doses (quoted in Table 1 and Table 4) were obtained in the same fashion using the original planned DQA dose grids. Independent measurements were performed three times for each plan.
Table 4.
DQA point doses before and after replanning
| Original Plan |
Replan |
|||||
|---|---|---|---|---|---|---|
| Pt. No. | Planned dose (Gy) | Measured dose (Gy) | Difference (%) | Planned dose (Gy) | Measured dose (Gy) | Difference (%) |
| 1 | 1.589 | 1.66 | 4.47 | 1.589 | 1.59 | 0.06 |
| 2 | 1.448 | 1.50 | 3.59 | 1.446 | 1.47 | 1.66 |
| 3 | 1.391 | 1.46 | 4.96 | 1.388 | 1.41 | 1.59 |
3. Results
3.1 Increased pitch replanning
3.1.1 DVH analysis
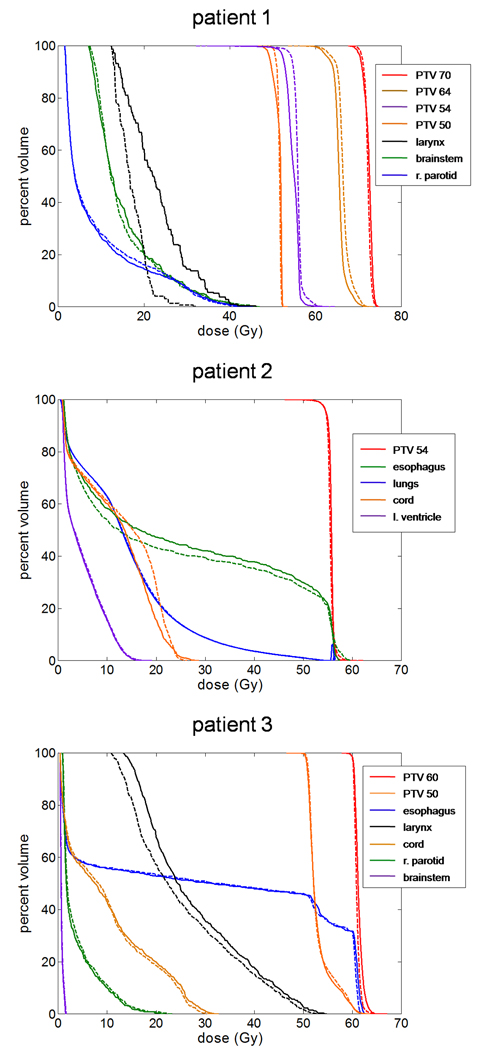
For each patient, DVHs corresponding to both the original and replanned treatments were plotted together and are shown in Figure 1. These plots illustrate that for each of the three cases, similar DVHs could be obtained when using an increased pitch. Physical dose distributions for the replanned treatments were also similar, and in all three cases were examined by the attending physician(s) and deemed to be clinically acceptable.
Figure 1.
Dose-volume histograms for three patients replanned using an increased pitch of 0.287. Solid curves represent the original plans while dashed curves indicate replans. For all three patients, similar DVHs were achieved using the increased pitch; though with Patients 1 and 2, this required an increase in the planning modulation factor from 2.0 to 2.5.
3.1.2 Leaf timing sinogram comparison
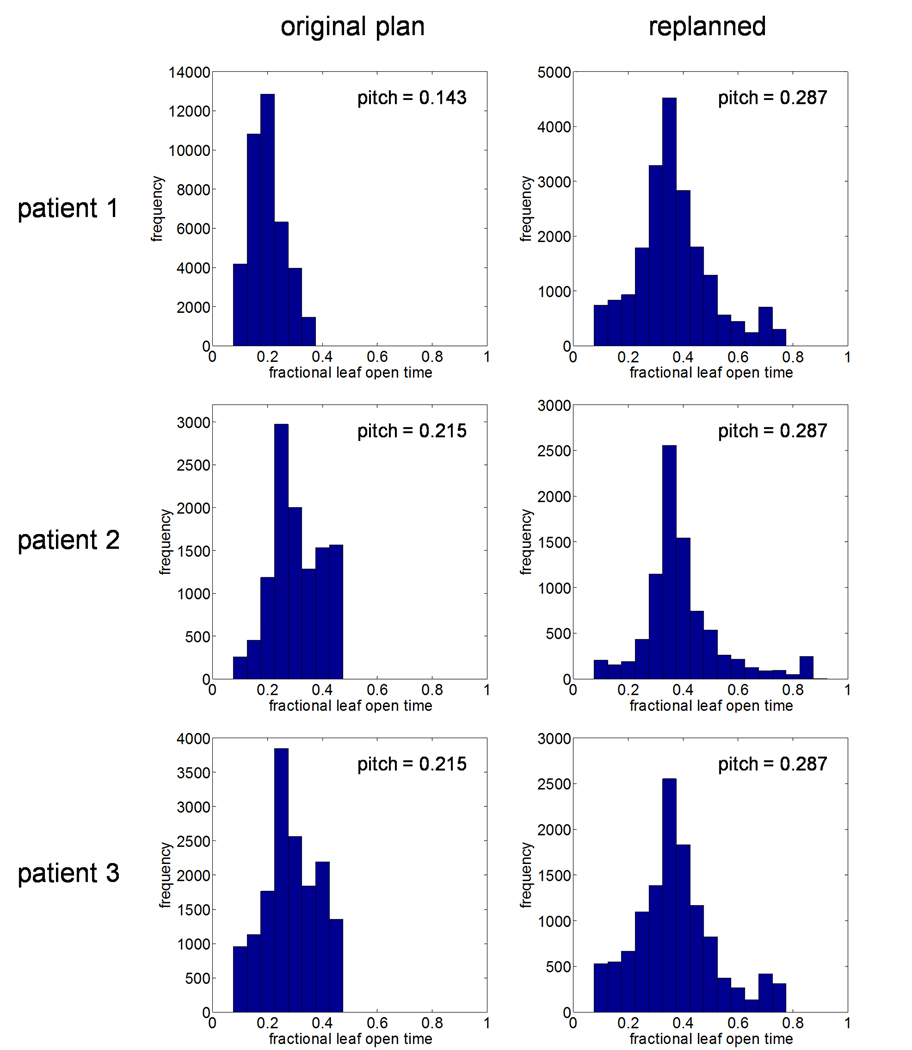
After replanning, leaf timing sinograms were read into MATLAB for analysis. Table 2 lists the mean non-zero leaf open times as well as the actual modulation factors for both the original and replanned treatments. In addition, frequency histograms of the non-zero leaf open times for the three sets of plans are shown in Figure 2. These results show that increasing the pitch resulted in increases in the mean leaf open time of 29.8–83.1% for the three cases studied. In addition, the actual modulation factor increased 21.8–54.5% relative to the original plans; indicating that to achieve similar plan quality, a wider range of intensity levels is required when using a larger pitch.
Table 2.
Mean non-zero leaf open times and actual modulation factors before and after replanning
| Mean leaf open times |
Modulation factor (actual) |
|||||
|---|---|---|---|---|---|---|
| Pt. No. | Original plan (ms) | Replan (ms) | Change (%) | Original plan | Replan | Change (%) |
| 1 | 59 | 108 | 83.1 | 1.65 | 2.01 | 21.8 |
| 2 | 90 | 116 | 28.8 | 1.45 | 2.24 | 54.5 |
| 3 | 84 | 109 | 29.8 | 1.54 | 1.98 | 28.6 |
Figure 2.
Frequency histograms of programmed leaf open times for both original and increased pitch plans taken from normalized leaf timing sinograms. Fractional leaf open times are relative to a projection interval of 294 ms. In all cases, increasing the pitch resulted in an increase in the mean leaf open time as well as an increase in the actual modulation factor.
3.1.3 Treatment time comparison
The effects of increasing the pitch on treatment time are shown in Table 3. These results indicate reductions in treatment time of 23.7–49.5% were achieved for the three patients replanned. For all replanned treatments, the gantry period remained unchanged at 15 s, thus these reductions are due solely to the use of fewer rotations to cover the treatment volume with the higher pitch plans.
Table 3.
Fraction treatment times before and after replanning
| Pt. No. | Original plan (s) | Replan (s) | Change (%) |
|---|---|---|---|
| 1 | 856.5 | 432.4 | −49.5 |
| 2 | 395.0 | 297.1 | −24.8 |
| 3 | 452.4 | 345.0 | −23.7 |
3.2 Replanned DQA dose measurements
Mean values from the DQA ion chamber dose measurements made before and after replanning are listed in Table 4. Uncertainties calculated to the 95% confidence level were less than the quoted precision of the measured values and so are not reported. These results show that delivery of the replanned treatments having increased mean leaf open times resulted in a reduction in measured point dose discrepancies so that DQA point dose measurements for all three cases were brought within the set acceptance criteria of ±3%.
3.3 Dose reconstruction measurements
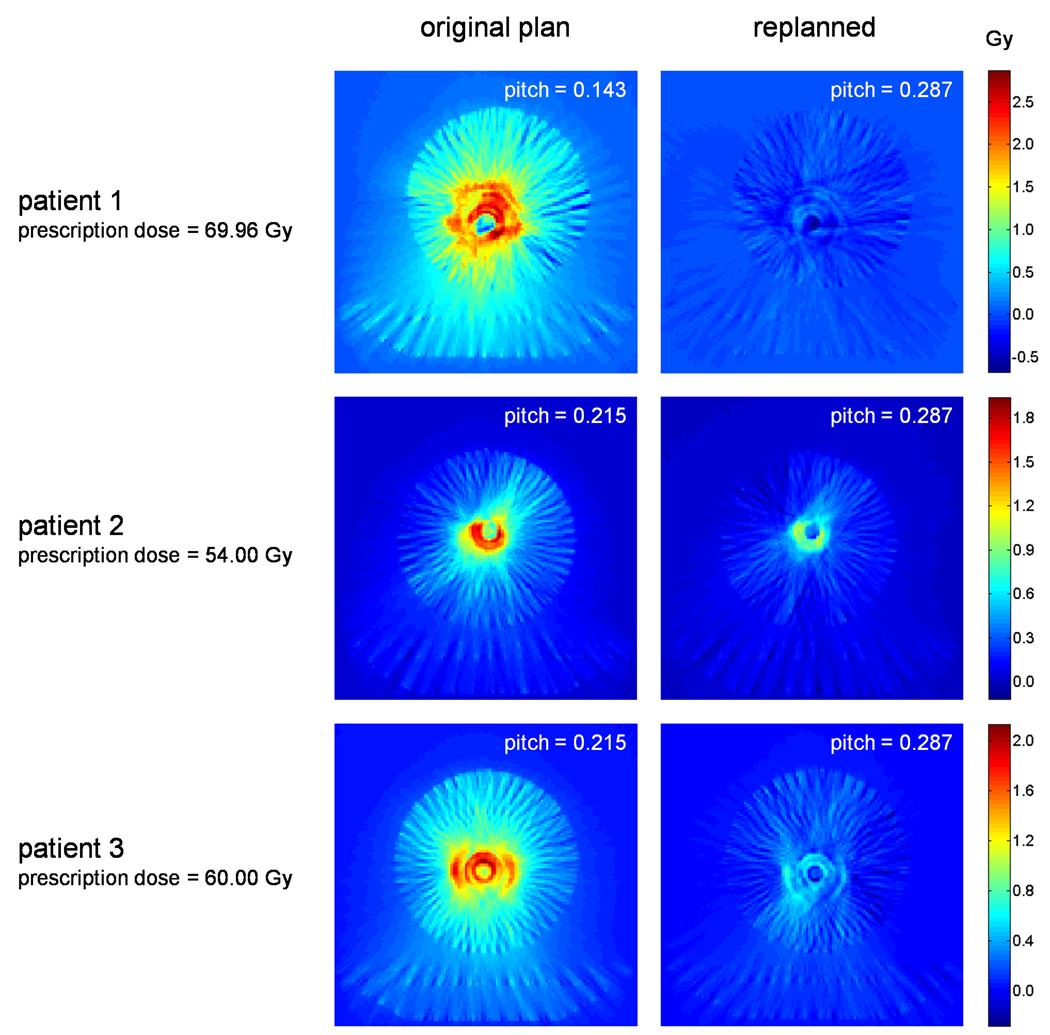
After reconstructing the dose for both the original and replanned DQA deliveries, both the planned and reconstructed dose grids were read into MATLAB for analysis. Differences were computed by subtracting the planned dose from the reconstructed dose on a voxel by voxel basis. Absolute dose difference maps, taken at the slice of the ion chamber collecting volume for each of the plans, are shown in Figure 3. In addition, Table 5 compares mean values of the reconstructed dose at the location of the DQA ion chamber measurements with the measured values previously listed in Table 4. Again, calculated uncertainties were less than the quoted precision of all measured dose values.
Figure 3.
Absolute dose difference maps taken between planned and reconstructed dose distributions for three patients both before and after replanning. Broad regions of high dose discrepancy are seen for original plans having mean leaf open times of approximately 60–100 ms. Discrepancies are considerably reduced for replans having increased mean leaf open times.
Table 5.
Comparison of reconstructed to measured point dose
| Original Plan |
Replan |
|||||
|---|---|---|---|---|---|---|
| Pt. No. | Reconstructed dose (Gy) | Measured dose (Gy) | Difference (%) | Reconstructed dose (Gy) | Measured dose (Gy) | Difference (%) |
| 1 | 1.66 | 1.66 | 0.00 | 1.58 | 1.59 | −0.63 |
| 2 | 1.50 | 1.50 | 0.00 | 1.48 | 1.47 | 0.68 |
| 3 | 1.45 | 1.46 | −0.68 | 1.41 | 1.41 | 0.00 |
Looking first at Figure 3, large regions of high dose difference are seen in the original plans for all three patients examined. In all cases, this discrepancy appears as a high dose wash with hot rings of dose standing out beyond the background. These dose differences are the result of MLC timing inaccuracies that occur during treatment delivery, with the ring artifacts being caused by one or more “slow” leaves. Dose differences were greatly reduced for the increased pitch plans, indicating a reduction in MLC inaccuracies for plans using larger leaf open times.
Focusing now on Table 5, results indicate excellent agreement between ion chamber measurements and reconstructed point dose values, with absolute dose differences less than or equal to 0.01 Gy (±0.68%) for all points measured.
4. Discussion
This work illustrates that the delivery of plans using predominantly small leaf open times can result in MLC inaccuracies that translate into dose discrepancies in excess of 3% percent. Furthermore, increasing the mean leaf open time by planning with a larger pitch can produce plans which are approximately equivalent from a dosimetric perspective, take less time to deliver, and are less susceptible to MLC delivery inaccuracies. To better understand the observed discrepancies, it is necessary to look at the behavior of the MLC leaves and the way in which this behavior is modeled in the TomoTherapy TPS.
There are two primary MLC effects that can impact the delivery of a planned dose distribution: tongue and groove/penumbra (TAG-P) effects and MLC leaf latencies (8). Both of these effects are accounted for in the TPS during the end-of-planning process; however, while TAG-P effects are accounted for on a leaf-by-leaf basis using leaf fluence output factors, leaf latencies are modeled assuming that on average, leaf behavior is linear and that all the leaves of a given MLC behave the same. In some cases these assumptions can be violated; resulting in MLC inaccuracies not unlike those observed in this work.
Leaf latencies describe differences between the programmed and actual leaf open times. These differences can arise for a variety of reasons including pneumatic and/or mechanical limitations on leaf motion, as well as signaling delays in the associated electronics that control the MLC. Leaf latencies are modeled in the TomoTherapy TPS by assuming the relationship between the actual and planned leaf open times is linear. The exact nature of this relationship is empirically determined by using the MVCT detectors to measure actual leaf open times as a function of programmed leaf open times for eight MLC leaves at projection intervals ranging from 200–1000 ms. The measured leaf open times for each programmed value is averaged over all eight leaves and a regression line is fit between programmed open times corresponding to 10–90% of the total projection interval. All leaves are assumed to obey the linear relationship equally and linear interpolation is employed to determine leaf latencies for projection times not actually measured (8).
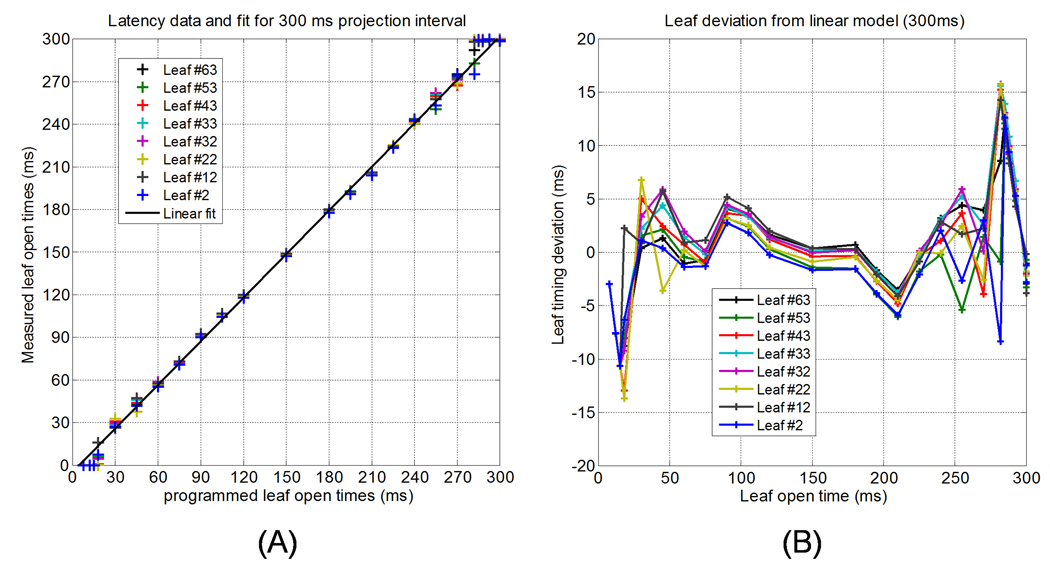
Figure 4 (a) shows leaf latency data measured at UW on Machine #2 for a 300 ms projection interval, while Figure 4 (b) shows the deviation of different leaves from the linear fit as a function of programmed leaf open time. Note that this projection time corresponds approximately to that of a 15 s gantry period. Looking at these figures, it is clear that the leaf latency becomes non-linear for leaf open times that are very small and for those approaching the full duration of the projection interval. For leaf open times below approximately 120 ms, deviations from the linear model on the order of 5 ms are observed for some leaves. While this may seem small, for programmed leaf open times of 50–60 ms, such deviations can result in the dose contributions from these leaf openings to be systematically off by 8–10%. For very long leaf openings (approaching the full projection interval), the magnitude of the observed leaf timing deviations is larger; however, relative to the longer programmed leaf open times, the impact of these deviations is markedly less (on the order of 2–5%).
Figure 4.
(a) Leaf latency data for Tomotherapy Machine #2 at UW using a 300 ms projection. Nonlinearities in the data are seen for programmed open times less than 100 ms. (b) Deviation of measured leaf open times from the linear approximation for the eight leaves used to model leaf latency.
The dose discrepancies seen in Figure 3 are likely due to a combination of effects. Small deviations of the collective leaf behavior from the linear latency approximation act to produce global dose inaccuracies (seen as the background high dose wash in the original plan difference maps of Figure 3) while variance in the individual leaf behavior results in outlier leaves with large deviations from the linear approximation. This produces the transverse ring artifacts seen in the dose difference maps of Figure 3. Increasing the programmed leaf open times by replanning with a higher pitch results in a reduction in both the magnitude as well as the impact of these leaf timing discrepancies. This is due to the fact that collectively, the leaves behave more like the linear approximation being made by the TPS station at longer leaf open times. In addition, the variance in leaf open times - though still present, constitutes a smaller fraction of the programmed leaf open time; reducing the impact of the observed deviations.
In TomoTherapy, the gantry period for a given plan is set by the maximum leaf open time as determined from the fluence sinogram after accounting for leaf latency and TAG-P effects. In cases where the required fluence calls for a gantry period beyond the allowable range of the machine, the gantry period is instead set by the system limit to either 15 s (min) or 60 s (max). In this study the gantry period for both the original and modified plans was 15 s, indicating that even with an increased pitch of 0.287, the maximum leaf open time was less than the projection interval of a 15 s gantry period. While the results of this work demonstrate that it is possible to deliver accurate dose distributions with a 15 s gantry period, because there is no way to ascertain whether plans with a 15 s gantry period are using predominantly short leaf open times, these plans must be carefully reviewed to determine if a larger pitch will enable a more accurate and/or efficient delivery.
One way to avoid this issue is to plan with a large enough pitch so as to force the gantry period to be greater than 15 s. The use of a larger gantry period increases the fidelity of leaf openings by increasing the range of leaf open times that fall in the linear region of the latency curve. In addition, for gantry periods greater than 15 s, the mean leaf open time can be approximated using:
| (2) |
where tmax is the maximum leaf open time, T is the gantry period, and MF is the actual modulation factor determined at the end of planning. The factor T /51 is the projection interval and is approximately equal to the maximum leaf open time when the gantry period is not set by a system constraint. From this relation, it is clear that with a gantry period greater than 15 s and a modulation factor less than 3.0, the mean leaf open time is always greater than 100 ms; which for the three cases examined in this work, was sufficient to bring the delivery accuracy within the specified DQA criteria for our institution.
In addition to increasing the mean leaf open time, the use of larger pitches can lead to a significant reduction in the per fraction treatment delivery time when the gantry period is being set to the system minimum. This in turn allows for increased patient throughput. Though conventional thinking suggests that the use of larger pitches may result in reduced longitudinal resolution of the dose distribution, this was not evident for the three patients replanned in this study. Instead, our results are consistent with those of Gutiérrez et al., who found the conformity of dose distributions to be relatively insensitive to changes in pitch (18).
5. Conclusion
In this study, DQA discrepancies observed for a number of patients planned for treatment on helical tomotherapy were investigated. An initial comparison of leaf timing sinograms for six patients indicated that failing DQAs were associated with plans having small mean leaf open times. Three patients with failing DQAs were replanned using an increased pitch and the corresponding mean leaf open times were increased 29.8–83.1% relative to the original plans. Dosimetric analyses showed nearly equivalent plans were achieved using the increased pitch values. After replanning, new DQA plans were created and ion chamber measurements showed a reduction in point dose discrepancies, bringing all measured doses within the set clinical tolerance of ±3%. In addition, MVCT detector data was used for dose reconstruction purposes and comparison between ion chamber measurements and reconstructed point doses showed excellent agreement for all plans.
Dose difference maps taken between planned and reconstructed dose distributions showed broad regions of high dose discrepancy, with transverse high dose ring artifacts standing out beyond the background for all three of the original plans with failing DQAs. Difference maps taken after replanning indicated these discrepancies were greatly reduced for plans with larger mean leaf open times and were in agreement with DQA results. Analysis of MLC leaf latency data for the machine being used suggested these discrepancies are due to a combination of effects, namely: collective nonlinearities in the leaf latency at leaf open times less than approximately 100 ms which are not accounted for in the TPS, as well as variance in the behavior of individual leaves, which is also unaccounted for.
To lessen the impact of MLC latency inaccuracies, it is recommended that the pitch value be chosen so that the gantry period is greater than the minimum value of 15 s. Doing this will not only improve the treatment accuracy by reducing the impact of leaf latency inaccuracies, but will also improve the efficiency of the treatment; thereby reducing the delivery time.
Acknowledgements
The authors would like to offer special thanks to Tim Chapman, Ed Chao, and Eric Schnarr at TomoTherapy Inc. for their invaluable help with this project. In addition, we would like to thank Wolfgang Tomé at the University of Wisconsin for his insightful comments and thoughtful discussions. This work was supported by NIH training grant T32-CA009206.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
A potential conflict of interest exists for Thomas R. Mackie, Ph.D., Gustavo Olivera, Ph.D., Quan Chen, Ph.D., and Emilie Soisson, M.Sc., who are employees of TomoTherapy, Inc., Madison, WI.
References
- 1.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int. J. Radiat. Oncol., Biol., Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 2.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int. J. Radiat. Oncol., Biol., Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 3.Mackie TR, Holmes TW, Swerdloff S, et al. Tomotherapy: a new concept in the delivery of dynamic conformal radiotherapy. Med. Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 4.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Sem. Rad. Onc. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 5.Ruchala KJ, Olivera GH, Schloesser EA, et al. Megavoltage CT on a tomotherapy system. Phys. Med. Biol. 1999;44:2597–2621. doi: 10.1088/0031-9155/44/10/316. [DOI] [PubMed] [Google Scholar]
- 6.Kapatoes JM, Olivera GH, Reckwerdt PJ, et al. Delivery verification in sequential and helical tomotherapy. Phys. Med. Biol. 1999;44:1815–1841. doi: 10.1088/0031-9155/44/7/318. [DOI] [PubMed] [Google Scholar]
- 7.Siebert RM, Ramsey CR, Garvey DR, et al. Verification of helical tomotherapy delivery using autoassociative kernel regression. Med. Phys. 2007;34:3249–3262. doi: 10.1118/1.2754059. [DOI] [PubMed] [Google Scholar]
- 8.Balog J, Olivera G, Kapatoes J. Clinical helical tomotherapy commissioning dosimetry. Med. Phys. 2003;30:3097–3106. doi: 10.1118/1.1625444. [DOI] [PubMed] [Google Scholar]
- 9.Scrimger RA, Tomé WA, Olivera GH, et al. Reduction in radiation dose to lung and other normal tissues using helical tomotherapy to treat lung cancer, in comparison to conventional field arrangements. Am. J. Clin. Oncol. 2003;26:70–78. doi: 10.1097/00000421-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Fiorno C, Dell’Oca I, Pierelli A, et al. Significant improvement in normal tissue sparing and target coverage for head and neck cancer by means of helical tomotherapy. Rad. Oncol. 2006;78:276–282. doi: 10.1016/j.radonc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama H, Westerly D, Mackie T, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int. J. Radiat. Oncol., Biol., Phys. 2006;64:962–967. doi: 10.1016/j.ijrobp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Yang JN, Mackie TR, Reckwerdt P, et al. An investigation of tomotherapy beam delivery. Med. Phys. 1997;24:425–436. doi: 10.1118/1.597909. [DOI] [PubMed] [Google Scholar]
- 13.Fenwick JD, Tomé WA, Jaradat HA, et al. Quality assurance of a helical tomotherapy machine. Phys. Med. Biol. 2004;49:2933–2953. doi: 10.1088/0031-9155/49/13/012. [DOI] [PubMed] [Google Scholar]
- 14.Balog J, Soisson ET. Helical tomotherapy quality assurance. Int. J. Radiat. Oncol., Biol., Phys. 2008;71:S113–S117. doi: 10.1016/j.ijrobp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Low DA. Quality Assurance of Intensity-Modulated Radiotherapy. Sem. Rad. Onc. 2002;12:219–228. doi: 10.1053/srao.2002.33700. [DOI] [PubMed] [Google Scholar]
- 16.Low DA, Harms WB, Mutic S, et al. A technique for the quantitative evaluation of dose distributions. Med. Phys. 1998;25:656–661. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 17.Kissick MW, Fenwick J, James JA, et al. The helical tomotherapy thread effect. Med. Phys. 2005;32:1414–1423. doi: 10.1118/1.1896453. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez AN, Westerly DC, Tomé WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: A planning study. Int. J. Radiat. Oncol., Biol., Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]