Abstract
Background
Sudden cardiac arrest (CA) is one of the leading causes of death worldwide. We sought to evaluate the impact of hydrogen sulfide (H2S) on the outcome after CA and cardiopulmonary resuscitation (CPR) in mouse.
Methods and Results
Mice were subjected to 8 min of normothermic CA and resuscitated with chest compression and mechanical ventilation. Seven minutes after the onset of CA, mice received sodium sulfide (Na2S, 0.55 mg/kg i.v.) or vehicle 1 min before CPR. There was no difference in the rate of return of spontaneous circulation (ROSC), CPR time to ROSC, and left ventricular (LV) function at ROSC between groups. Administration of Na2S 1 min before CPR markedly improved survival rate at 24h after CPR (15/15) compared to vehicle (10/26, P=0.0001 vs Na2S). Administration of Na2S prevented CA/CPR-induced oxidative stress and ameliorated LV and neurological dysfunction 24h after CPR. Delayed administration of Na2S at 10 min after CPR did not improve outcomes after CA/CPR. Cardioprotective effects of Na2S were confirmed in isolated-perfused mouse hearts subjected to global ischemia and reperfusion. Cardiomyocyte-specific overexpression of cystathionine γ-lyase (CGL, an enzyme that produces H2S) markedly improved outcomes of CA/CPR. Na2S increased phosphorylation of NOS3 in LV and brain cortex, increased serum nitrite/nitrate levels, and attenuated CA-induced mitochondrial injury and cell death. NOS3 deficiency abrogated the protective effects of Na2S on the outcome of CA/CPR.
Conclusions
These results suggest that administration of Na2S at the time of CPR improves outcome after cardiac arrest possibly via an NOS3-dependent signaling pathway.
Keywords: cardiopulmonary resuscitation, heart arrest, myocardial contraction, nitric oxide synthase, physiology
INTRODUCTION
Restoration of spontaneous circulation after prolonged whole body ischemia is an unnatural pathophysiological state created by successful cardiopulmonary resuscitation (CPR). According to recent epidemiological data, in-hospital mortality rate after cardiac arrest was 55–71 % in adults and children who regained any spontaneous circulation after CPR 1. Post-cardiac arrest syndrome, including neurological dysfunction, myocardial damage, and “sepsis-like” systemic inflammation is likely to contribute to the multi-system organ dysfunction and the ultimate demise of many victims 2. Although hypothermia has proven effective in clinical studies, no pharmacological agent yet tested has proven successful in improving outcome from CA/CPR.
Hydrogen sulfide (H2S) is a colorless gas with a characteristic rotten-egg odor found in various natural and industrial sources.3 In mammalian tissues, H2S is generated through the degradation of L-cysteine mainly by two enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL) 4. Both CBS and CGL are expressed at high levels in liver, kidney, and pancreas presumably for cysteine synthesis. CGL is also expressed in brain, heart, and smooth muscle.5, 6 While deficiency of CBS causes hyperhomocysteinemia, an established risk factor for cardiovascular disorders including sudden cardiac death, CGL deficiency causes cystathioninuria, an apparently benign condition albeit with an inconsistent and variable disease association.7
H2S exerts a host of biological effects on various targets, resulting in responses that range from cytotoxic to cytoprotective effects 5. While breathing high concentrations of H2S is toxic,5 breathing low concentrations of H2S reversibly reduces metabolism in rodents and improves survival after hemorrhagic shock in rats.8–10 Although administration of NaHS has been shown to worsen cerebral ischemia11 and lipopolysaccharide-induced systemic inflammation,12 recent studies showed that administration of NaHS or Na2S can attenuate ischemia-reperfusion (IR) injury in hearts,13–15 liver,16 and kidney.17 The protective effects of H2S appear to be mediated via anti-apoptotic and/or anti-inflammatory effects. However, the impact of Na2S on the outcome of CA/CPR, which is complicated by whole body IR injury and systemic inflammation, remains to be elucidated. Here, we report that administration of Na2S at the time of CPR markedly improves myocardial and neurological function and survival after CA/CPR in mice.
MATERIALS AND METHODS
Mouse
After approval by the Massachusetts General Hospital Subcommittee on Research Animal Care, we studied 2- to 3-month-old age and weight-matched male C57BL/6J wild-type and NOS3-deficient mice on a C57BL/6J background (NOS3−/−). We also studied 2- to 3-month-old male mice with cardiomyocyte-specific overexpression of CGL (CS-CGLtg)13 and their wild-type littermates (FVBN/J).
Material
Sodium sulfide (Na2S; IK1001) was a generous gift from Ikaria Inc. (Seattle, WA). Na2S was produced by using H2S gas (Matheson, Newark, CA) as a starting material and was formulated to pH neutrality and iso-osmolarity. Na2S was diluted in normal (0.9%) saline to the desired concentration immediately prior to administration.
Animal preparation
Mice were anesthetized with 100 µg/g of ketamine and 0.25 µg/g fentanyl delivered by intraperitoneal injection and mechanically ventilated (FiO2=0.21, mini-vent, Harvard Apparatus). Arterial blood pressure was measured via left femoral arterial line. A saline-filled microcatheter (PE-10, Becton Dickinson) was inserted into the left femoral vein for drug and fluid administration. Blood pressure and needle-probe ECG monitoring data were recorded and analyzed with the use of a PC-based data acquisition system.
Murine CPR model
Cardiac arrest was induced by administration of 0.08 mg/g potassium chloride through the femoral catheter and was confirmed by loss of arterial pressure and asystolic rhythm on ECG. After 8 min of cardiac arrest, chest compressions were delivered using a finger at a rate of 340∼360 beats per minute with resumption of mechanical ventilation (FiO2=1.0). Chest compressions were adjusted to provide a uniform rate seen on femoral artery pressure monitoring and a target femoral diastolic pressure of >20 mm Hg. Epinephrine was infused at 0.3 µg/min starting 30 seconds before CPR and continued until heart rate (HR) became higher than 300 bpm. Core body temperature was maintained at 37°C by a warming lamp. Return of spontaneous circulation (ROSC) was defined as the return of sinus rhythm with a mean arterial pressure (MAP) >40 mm Hg lasting at least 1 minute. Mice were weaned from mechanical ventilation at 1h after and extubated at 2h after CPR. In a subgroup of mice, at 24h after CPR, cardiac function was examined with a conductance pressure-volume catheter (SPR-839, Millar Instruments Inc.) under anesthesia. Mice subjected to sham surgery that were not subjected to cardiac arrest were used as controls. Study drug (Na2S) or vehicle was injected via the femoral venous line 1 minute before CPR. Based on pilot studies, we administered 0.55 mg/kg (7.0 µmol/kg) of Na2S. Effects of delayed administration of Na2S at 10 min after the start of CPR (post-CPR Na2S) were examined in a subgroup of mice.
Assessment of neurological function
Neurological function was assessed at 24h after CA/CPR or sham surgery using a previously-reported neurological function scoring system with minor modifications (Supplemental Methods) 18, 19.
Effects of Na2S on myocardial function in isolated-perfused mouse heart
Mice were heparinized and anesthetized using pentobarbital (60 mg/kg, i.p.). The heart was quickly removed and mounted on a 20 gauge cannula in ice-cold perfusion buffer (a modified Krebs-Henseleit buffer, containing [in mM] 118.5 NaCl, 24.9 NaHCO3, 11.2 glucose, 4 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2 sodium pyruvate, and 2.5 CaCl2). Retrograde perfusion was started using perfusion buffer continuously gassed with 95% O2 / 5% CO2 at 37°C. A constant hydrostatic coronary pressure of 65 mmHg was maintained throughout the experiment. The tip of an SPR-671 transducer (Millar Instruments, Houston, TX) was placed in a saline-filled PVC balloon that was inserted into the LV. The balloon was inflated to obtain a diastolic pressure of 5–10 mmHg, and LV pressure was monitored via the Millar transducer and continuously recorded. LV developed pressure (LVDevP), rate pressure product (RPP), maximum and minimum rate of LV pressure change (dP/dtmax and dP/dtmin), and the time constant of isovolumic LV relaxation (tau) were calculated. Hearts were electrically paced at 7 Hz using a Grass S88 stimulator (Grass Technologies, West Warwick, RI). Coronary flow rate was measured using an N1 in-line flowprobe and a T106 flowmeter (Transonic Systems, Ithaca, NY). After a 30-minute stabilization period, hearts were subjected to 20 min of ischemia followed by 60 min of reperfusion. Na2S (10 µM) or vehicle was administered into the perfusate starting immediately or 40 seconds after the start of reperfusion and continued for 5 min. Myocardial function was measured for the first 60 min after reperfusion.
Measurement of serum nitrate/nitrite
Concentrations of nitrite and nitrate were measured in serum samples obtained at 15 min after CA/CPR or sham surgery with a Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Mitochondrial permeability transition
Mouse heart mitochondria were isolated 15 min after CA/CPR or sham operation by differential centrifugation using the MITO-ISO1 kit (Sigma-Aldrich, Saint Louis, MO) following the manufacturer’s instructions. MPT was measured by monitoring the decrease in absorbance at 540 nm associated with mitochondrial swelling as previously described 20. Briefly, mitochondrial suspensions were diluted to a final concentration of 0.5 mg/ml in a buffer containing (in mM) 200 mannitol, 70 sucrose, 10 HEPES, and 5 succinate (pH 7.4). After 2 min of stabilization, 400 µM CaCl2 was added to induce MPT. The decrease in absorbance was monitored for 1000 seconds at room temperature with a spectrophotometer. The maximal rate of the absorbance decrease was obtained by linear regression over a 3-minute period.
Measurements of peroxide levels in serum
Concentrations of hydrogen peroxide were measured in serum samples obtained at 5 min after CA/CPR or sham surgery with a QuantiChrom Peroxide Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions.
Detailed description of reagents and protocol for Immunoblots and Histological studies are provided in Supplemental Methods.
Statistical Analysis
All data are expressed as mean±SEM. Normally distributed data were analyzed using unpaired t-test, two-way repeated measures ANOVA, or one-way ANOVA with a Holm-Sidak or Bonferroni post hoc test. Neurological function scores of CS-CGLtg versus FVBN/J wild-type mice were compared by Mann-Whitney Rank Sum test because the values are not normally distributed. Difference in survival rate was analyzed by Gehan-Breslow test. Sigmastat 3.01a (Systat Software Inc., San Jose, CA) and GraphPad Prism 5.0 (GraphPad Software Inc. La Jolla, CA) were used for statistical analyses.
RESULTS
Na2S improves survival rate after cardiac arrest
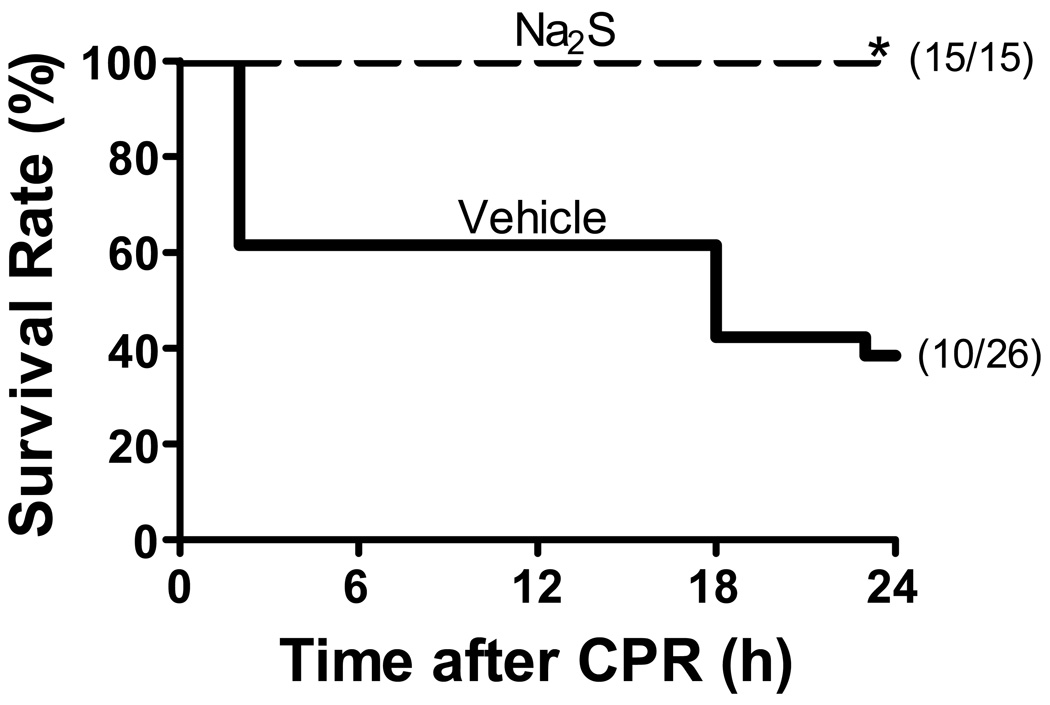
The rate of ROSC was greater than 95 % in mice treated with either vehicle or Na2S. There was no difference between treatment groups in the CPR time to ROSC or the total epinephrine dose (Supplemental Table 1). While only 10 out of 26 mice treated with vehicle survived 24 h after CPR, all 15 mice treated with Na2S 1 min before CPR survived 24 h after cardiac arrest and CPR (P=0.0001, Figure 1). In contrast, delayed administration of Na2S at 10 min after CPR did not improve the survival rate at 24 h after CPR (5 survived out of 11, P=0.9 vs vehicle). Although formal long term survival analysis was not done in the current study, in our pilot studies, more than 90% of mice treated with Na2S recovered completely by 72h after CPR and lived indefinitely. On the other hand, less than 20% of vehicle-treated mice survived 72h after CPR.
Figure 1.
Survival during the first 24h after cardiac arrest and CPR. Vehicle, mice subjected to CPR treated with vehicle. Na2S, mice treated with Na2S 1 min before CPR. *P=0.0001 vs Vehicle.
Since delayed administration of Na2S did not improve survival compared to vehicle, neurological and myocardial function and tissue injury were compared between groups of mice received vehicle or Na2S 1 min before CPR.
Na2S improved neurological function 24h after cardiac arrest and CPR
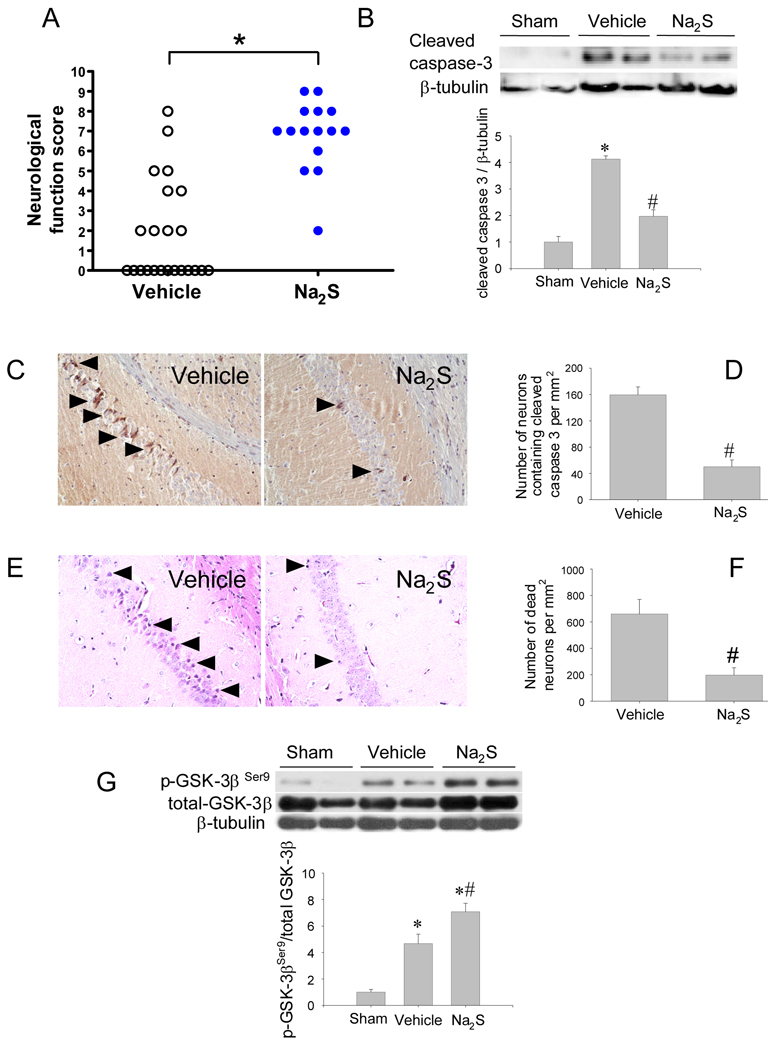
While neurological function was depressed in surviving mice 24h after CA/CPR, it was better in Na2S-treated mice than in vehicle-treated mice (P<0.0001, Figure 2A).
Figure 2.
Neuroprotective effects of Na2S. Panel A. Neurological function score in surviving mice at 24h after CA/CPR. Dead mice (indicated by score = 0) were excluded from the statistical analysis. *P<0.0001 vs Vehicle by unpaired t-test. Panel B. Representative immunoblots showing cleaved caspase 3 and β-tubulin protein expression in brain cortex of mice 15 min after sham operation or CA/CPR treated with vehicle or Na2S. Cleaved caspase 3 protein levels were determined by dividing cleaved caspase 3 immunoreactivity by β-tubulin immunoreactivity and normalized to values of sham-operated mice. *P<0.05 vs Sham. #P<0.05 vs Vehicle. Panel C. Representative photomicrographs of brain sections of mice treated with vehicle or Na2S showing cleaved caspase 3-immunoreactive neurons (indicated with black arrow heads) at 24h after CPR. Panel D. Summary of the number of neurons containing cleaved caspase 3 in the CA-1 region of the brain. #P<0.05 vs Vehicle. N=3 for each group. Panel E. Representative photomicrographs of brain sections of mice treated with vehicle or Na2S showing dead neurons (indicated with black arrow heads) at 72h after CPR in hippocampal CA-1 region. Panel F. Summary of the number of dead neurons in the hippocampal CA-1 region. #P<0.05 vs Vehicle. N=3 for each group. Panel G. Representative immunoblots showing phosphorylated and total GSK-3β at Ser9 in the brain cortex of mice 15 min after sham operation or CA/CPR treated with vehicle or Na2S. Relative phospho-GSK-3β levels were quantitated by dividing the phospho-GSK-3β immunoreactivity by total GSK-3β immunoreactivity and normalized to values of sham-operated mice. N=6–8 in each group. *P<0.05 vs Sham. #P<0.05 vs Vehicle.
Na2S prevents apoptosis in brain after cardiac arrest and CPR
Immunoblot analysis revealed that caspase 3 was activated at 15 min after CA/CPR in cerebral cortex of vehicle-treated mice (Figure 2B). The caspase 3 activation was markedly attenuated by Na2S. Histological studies revealed that the number of neurons containing cleaved caspase 3 in the CA1 region of the hippocampus was markedly increased at 24h after CA/CPR (Figure 2 C and D). Similarly, the number of dead neurons identified by morphological criteria21 in the CA1 region was markedly increased at 72h after CA/CPR (Figure 2 E and F). Administration of Na2S 1 min before CPR decreased the numbers of cleaved caspase 3-positive or dead neurons in the hippocampus. Since phosphorylation of GSK-3β has been suggested to inhibit apoptosis, we examined phosphorylation status of GSK-3β in the brain cortex extracts. The neuroprotective effects of Na2S were associated with enhanced GSK-3β phosphorylation at Ser9 (Figure 2G). These results suggest that administration of Na2S 1 min before CPR prevents apoptosis in the brain.
Na2S prevents myocardial dysfunction after cardiac arrest and CPR
There was no difference in HR and MAP between mice treated with vehicle or Na2S at ROSC or 60 min after CPR (Supplemental Table 1). Twenty-four hours after CA/CPR, HR, LV end-systolic pressure (LVESP), peak rate of LV pressure rise (dP/dtmax), end-systolic elastance (Ees), and preload-recruitable stroke work (PRSW) were depressed in vehicle-treated mice compared to sham but not in Na2S-treated mice (Table 1). Myocardial mechanical efficiency (estimated by the ratio of Ees to arterial elastance [Ea]) was impaired (decreased ratio) in vehicle-treated mice but not in Na2S-treated mice (Table 1). These results show that administration of Na2S at the time of CPR prevents post-CA myocardial dysfunction 24 h after CA/CPR.
Table 1.
Myocardial function 24 h after cardiac arrest and CPR
| Sham (n=5) |
Vehicle (n=6) |
Na2S (n=8) |
|
|---|---|---|---|
| HR, bpm | 627±8 | 559±24* | 575±19 |
| LVESP, mmHg | 118±8 | 78±11* | 105±6# |
| LVEDP, mmHg | 4±1 | 3±0 | 5±1 |
| dP/dtmax, mmHg/s | 17969±2290 | 10437±2275* | 15328±1417 |
| dP/dtmin, mmHg/s | −13404±1752 | −11919±2627 | −14559±734 |
| CO, ml/min | 11±1 | 10±1 | 13±1 |
| dP/dtmax/IP, s−1 | 203±9 | 191±10 | 197±9 |
| Ea, mmHg/µL | 7±0 | 4±1* | 5±1* |
| Ees, mmHg/µL | 27±7 | 3±0* | 11±2# |
| Ees/Ea | 4.0±1.1 | 0.8±0.1* | 2.4±0.5# |
| PRSW, mmHg | 158±22 | 41±8* | 118±13# |
| τ, milliseconds | 5.2±0.3 | 5.2±0.7 | 4.9±0.3 |
Values are mean±SEM. Sham, sham-operated mice; Vehicle, mice subjected to CPR treated with vehicle; Na2S, mice treated with Na2S 1 min before CPR; HR, heart rate; LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; dP/dtmax, maximum rate of developed left ventricular pressure; dP/dtmin, minimum rate of developed left ventricular pressure; CO, cardiac output; dP/dtmax/IP, dP/dtmax divided by instantaneous pressure; Ea, arterial elastance; Ees, left ventricular end-systolic ventricular elastance; PRSW, preload-recruitable stroke work; τ, time constant of isovolumic relaxation.
P<0.05 vs sham-operated mice.
P<0.05 vs Vehicle.
Na2S improves myocardial function in vitro ischemia-reperfusion
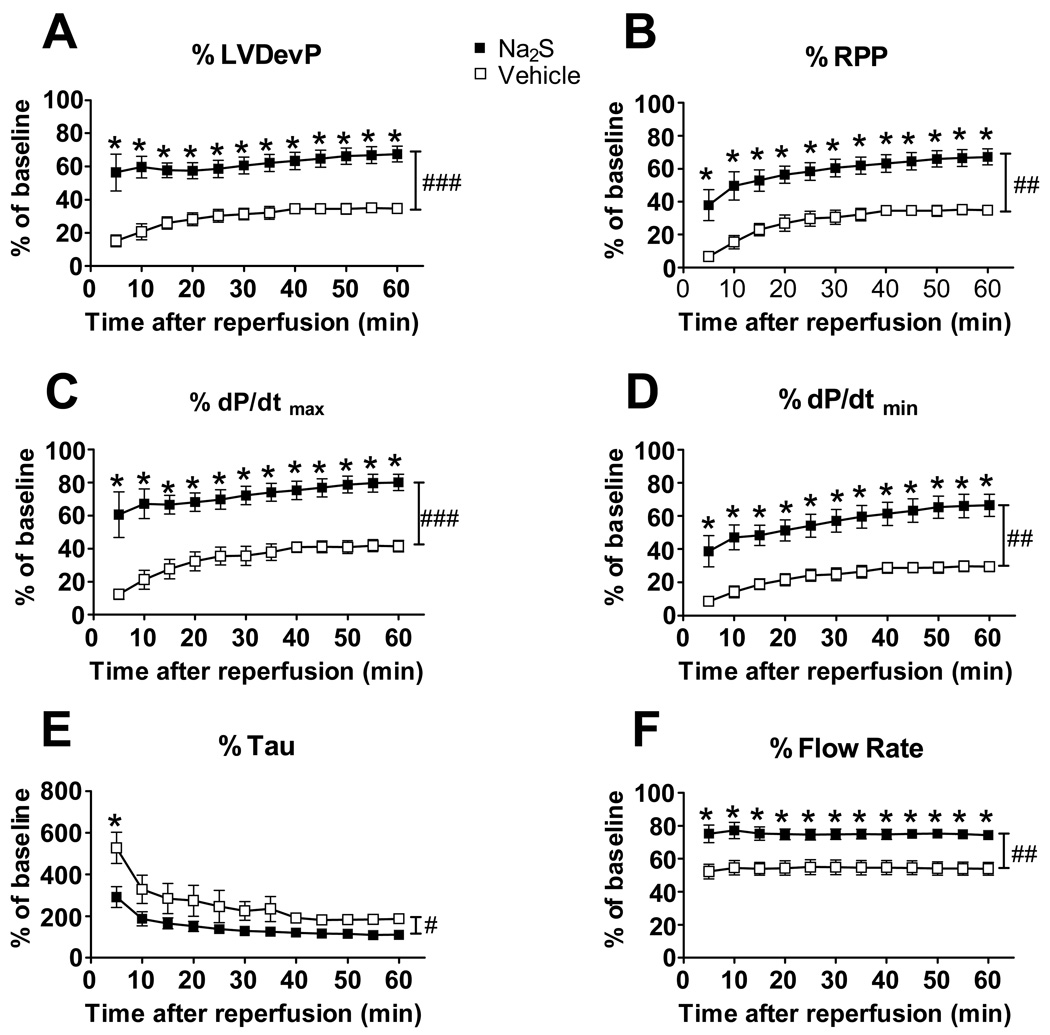
To examine the impact of Na2S on myocardial function after global IR, we studied the effects of Na2S in isolated-perfused mouse hearts. Myocardial function was depressed after 20 min of ischemia and reperfusion in vehicle-treated hearts. Administration of Na2S (10 µM) starting at the time of reperfusion after 20 min of ischemia markedly improved the recovery of myocardial function (Figure 3 A–F). Recovery of both systolic (dP/dtmax, LV developed pressure, and rate-pressure product) and diastolic (dP/dtmin and Tau) myocardial function was markedly augmented by Na2S compared to vehicle. Na2S also improved the recovery of coronary flow. Of note, delaying the start of administration of Na2S by 40 seconds after the start of reperfusion abolished the protective effects of Na2S on myocardial function after IR (Supplemental Figure 1). These results further support the concept that Na2S can preserve cardiac function after global myocardial IR when it is administered at the time of reperfusion.
Figure 3.
Effects of Na2S in global ischemia-reperfusion in isolated-perfused mouse hearts. Percentage of baseline values are shown for maximal LV developed pressure (Panel A. LVDevP), rate pressure product (Panel B. RPP), maximal rate of LV pressure rise (Panel C. dP/dtmax), maximal rate of LV pressure reduction (Panel D. dP/dtmin), the time constant of isovolumic LV relaxation (Panel E. Tau), and coronary flow rate (Panel F. Flow Rate). ###P<0.001, ##P<0.01, and #P<0.05 for comparisons of vehicle vs Na2S, as calculated by repeated measures two-way ANOVA. *P<0.01 vs vehicle of the same time point, as calculated by Bonferroni post-tests.
Na2S attenuates oxidative stress and protects mitochondria in the heart after CA/CPR
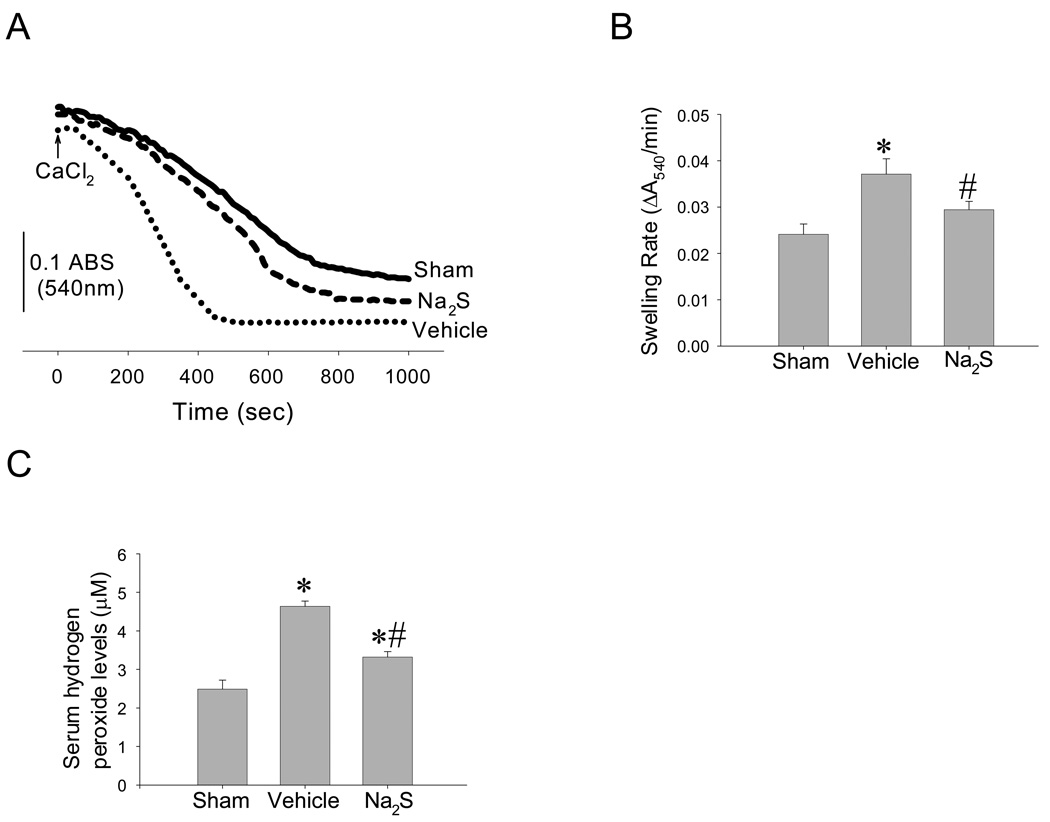
Cardiac arrest accelerated the rate of Ca2+-induced mitochondrial swelling, a measure of mitochondrial permeability transition pore opening, compared to the mitochondria of sham-operated mice. Na2S prevented the acceleration of Ca2+-induced mitochondrial swelling in cardiac mitochondria after CA/CPR (Figure 4 A and B). Na2S also prevented CA-induced increments in hydrogen peroxide levels in serum 5 min after CPR (Figure 4 C). These results suggest that Na2S attenuates oxidative stress early after CA/CPR and protects cardiac mitochondria.
Figure 4.
Effects of Na2S on mitochondrial permeability transition and oxidative stress. Panel A. Representative traces of calcium-induced mitochondrial swelling measured by spectrophotometry. Panel B. Quantification of the rate of Ca2+-induced mitochondrial swelling as a measure of mitochondrial permeability transition. *P<0.05 vs sham. #P<0.05 vs Vehicle. N=7 in each group. Panel C. Hydrogen peroxide levels in serum obtained at 5 min after CPR. N=3–6 in each group. *P<0.05 vs sham. #P<0.05 vs vehicle.
Cardiomyocyte-specific overexpression of CGL improves outcomes after CA/CPR
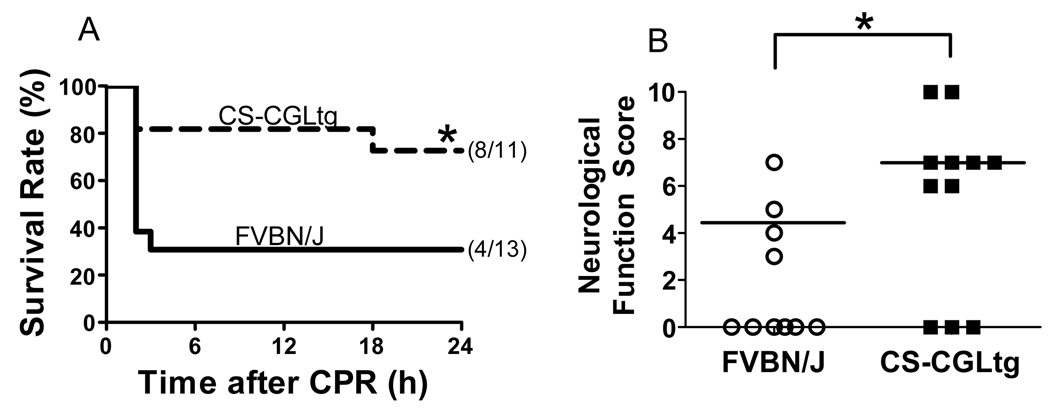
To examine the impact of endogenously produced H2S in the heart on the recovery from CA/CPR, we studied CS-CGLtg mice which have been extensively characterized and display increased H2S production in the heart.13 Compared to their wild-type littermates, CS-CGLtg mice had a markedly shorter CPR time to ROSC (516±62 vs 331±24 sec, P=0.016), required a smaller dose of epinephrine to achieve ROSC (1.5±0.2 vs 0.9±0.1 µg, P=0.015), and had a higher MAP at 60 min after CPR (28±2 vs 35±3 mmHg, P=0.046). Additionally, CS-CGLtg markedly improved neurological function (8±1 (median = 7) vs 5±1 (median = 4.5), P=0.048 by Mann-Whitney Rank Sum test) and survival rate (8 out of 11 vs 4 out of 13, P=0.03, Figure 5) 24h after CA/CPR. These results suggest that augmented cardiac H2S levels facilitated recovery after CA/CPR.
Figure 5.
Impact of endogenous H2S on the outcome of CA/CPR. Panel A. Survival during the first 24h after cardiac arrest and CPR in mice with cardiomyocyte-specific overexpression of CGL (CS-CGLtg, n=11) and their wild-type littermates (FVBN/J, n=13). *P=0.03 vs FVBN/J by Gehan-Breslow test. Panel B. Neurological function score in surviving mice at 24h after CA/CPR. Dead mice (indicated by score = 0) were excluded from the statistical analysis. Horizontal lines show median value of each group. *P=0.048 vs FVBN/J by Mann-Whitney Rank Sum test.
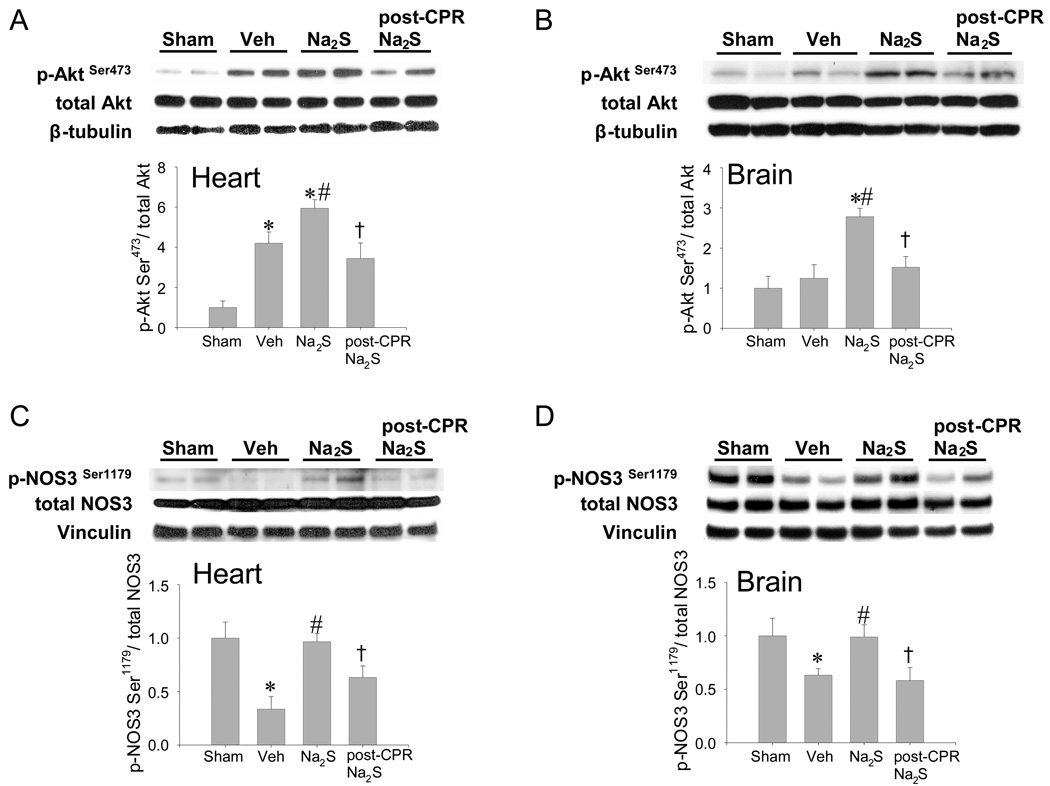
Na2S increases phosphorylation of Akt and AMPKα after cardiac arrest and CPR
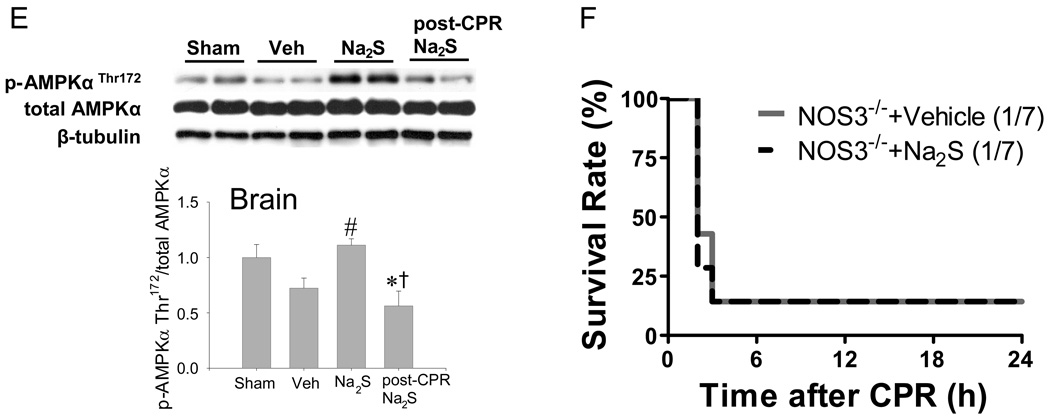
Since Akt- and AMPK-dependent signal contributes to cell survival in variety of pathological conditions,22, 23 we sought to investigate the role of AKT and AMPK in the protective effects of Na2S. Cardiac arrest and CPR increased Akt phosphorylation in LV compared with sham-operated mice (Figure 6A). Administration of Na2S 1 min before CPR increased Akt phosphorylation at Ser473 compared to vehicle both in the LV and brain after CA/CPR (Figure 6 A and B). Cardiac arrest and CPR decreased phosphorylation of NOS3 at Ser1179 in the LV and brain (Figure 6 C and D). Administration of Na2S prevented the de-phosphorylation of NOS3 at Ser1179 both in LV and brain after CA/CPR. Similarly, CA/CPR-induced de-phosphorylation of AMPKα at Thr172 was prevented by Na2S in the brain (Figure 6 E). Of note, delayed administration of Na2S at 10 min after CPR (post-CPR Na2S) failed to increase the phosphorylation of Akt, NOS3, and AMPKα compared to vehicle after CA/CPR. These observations suggest that protective effects of Na2S on organ function are associated with activation of Akt- and/or AMPKα-dependent pro-survival signals.
Figure 6.
Phosphorylated protein expression in brain cortex and left ventricle 15 min after sham operation or CA/CPR in mice treated with vehicle or Na2S 1 min before CPR (Veh and Na2S, respectively) or Na2S administered at 10 min after CPR (post-CPR Na2S). Relative phospho-AktSer473 levels were quantitated by dividing the phospho-Akt Ser473 immunoreactivity by total Akt immunoreactivity and normalized to values of sham-operated mice in the heart (Panel A) and brain (Panel B). Relative phospho-NOS3 Ser1179 levels were quantitated by dividing the phospho-NOS3 Ser1179 immunoreactivity by total NOS3 immunoreactivity and normalized to values of sham-operated mice in the heart (Panel C) and brain (Panel D). Relative phospho-AMPKα Thr172 levels were quantitated by dividing the phospho- AMPKα Thr172 immunoreactivity by total AMPKα immunoreactivity and normalized to values of sham-operated mice in the brain (Panel E). Two representative blots are shown out of 6–8 blots in each group for all proteins. Loading controls are shown to demonstrate equal sample loading. *P<0.05 vs sham. #P<0.05 vs vehicle, †P<0.05 vs Na2S. Panel F. Survival during the first 24h after cardiac arrest and CPR in NOS3−/− mice treated with vehicle (NOS3−/−+ Vehicle, n=7) and NOS3−/− mice treated with Na2S (NOS3−/−+ Na2S, n=7).
Na2S prevents reduction of nitrite/nitrate levels after CA/CPR
To examine whether or not the protective effects of Na2S were associated with augmented NO production, we measured serum nitrite and nitrate levels at 15 min after CA/CPR. Compared to sham-operated mice (4.7±0.8 µM), nitrite/nitrate levels were decreased in vehicle-treated mice (3.2±0.8 µM, P<0.01 vs sham) but not in Na2S-treated mice (4.3±0.2 µM, P=0.35 vs Sham, P<0.05 vs vehicle-treated mice) 15 min after CA/CPR.
Na2S does not improve outcome of CA/CPR in NOS3-deficient mice
To elucidate the role of NOS3/NO in the protective effects of Na2S, we examined whether or not Na2S improves outcomes of CA/CPR in NOS3−/− mice. Only 1 out of 7 NOS3−/− mice survived 24h after CA/CPR, and administration of Na2S 1 min before CPR did not improve the survival rate in NOS3−/− mice (1 out of 7 survived, Figure 6F). These results suggest that the protective effects of Na2S on the outcome of CA/CPR require NOS3.
DISCUSSION
The current study revealed that administration of Na2S at the time of CPR markedly improves myocardial and neurological function and survival after CA/CPR in mice. The neuroprotective effects of Na2S were associated with attenuated CA/CPR-induced caspase 3 activation in the brain. Global myocardial IR in isolated-perfused heart model confirmed the protective effects of Na2S. Administration of Na2S also prevented systemic oxidative stress and acceleration of mitochondrial permeability transition in the heart. Enhanced endogenous H2S production by cardiomyocyte-specific overexpression of CGL improved outcomes after CA/CPR. The salutary impact of Na2S treatment on the outcome of CA/CPR were associated with preserved serum nitrite and nitrate levels and enhanced phosphorylation of Akt and NOS3 in the brain and heart and AMPKα and GSK-3β in the brain. Finally, NOS3 deficiency abrogated the protective effects of Na2S on the outcome of CA/CPR. Taken together, these observations suggest that H2S confer organ protection and improve survival after CA/CPR at least in part via an NOS3-dependent mechanism.
The majority of research on cardiac arrest over the past half-century has focused on improving the rate of ROSC, and significant progress has been made. However, many patients succumb to the post-cardiac arrest syndrome after successful resuscitation. The current study was designed to investigate whether H2S could improve the outcome after “successful” resuscitation. While there was no difference in the rate of ROSC or CPR time to ROSC, mice that received Na2S immediately before CPR showed a markedly higher survival rate at 24h than did mice that received vehicle before CPR or Na2S 10 min after CPR. These observations suggest that administration of Na2S at the time of CPR improved outcomes at 24h after cardiac arrest by preventing the development of post-cardiac arrest organ injury and dysfunction after ROSC.
The current results suggest that Na2S can protect against the development of post-cardiac arrest neurological dysfunction and injury when administered at the time of CPR (see Figure 2A). Cardiac arrest and CPR activated caspase 3 and induced cell death in the brain of vehicle-treated mice. In contrast, the improved neurological function at 24h after CPR in mice treated with Na2S was associated with marked attenuation of caspase 3 activation and cell death in the brain after CPR. Previously, Qu and colleagues showed that administration of NaHS increased brain infarct volume after permanent middle cerebral artery occlusion 11. Differences in the models and the higher dose of NaHS (180 µmol/kg of NaHS, equivalent to ∼90 µmol/kg of Na2S 24) used in the Qu study compared to the current study (7 µmol/kg of Na2S) may explain the conflicting results of these studies.16 Pro-apoptotic effects of relatively high concentrations of H2S have also been reported in pancreas.25 Nonetheless, our results suggest that physiological level of H2S can have important protective effects on ischemic brain injury.
To examine the molecular mechanisms responsible for the anti-apoptotic effects of Na2S, we tested whether or not Na2S promotes Akt- or AMPK-dependent pro-survival signals in the brain. We found that Na2S increased phosphorylation of Akt and GSK-3β in the brain cortex. Akt-dependent phosphorylation of GSK-3β inhibits GSK-3β activity and thereby decreases apoptosis 26, 27. Similarly, administration of Na2S 1 min before CPR prevented the CA/CPR-induced dephosphorylation of AMPKα and NOS3. It has been reported that activation of cGMP-dependent protein kinase (PKG) may confer cardioprotection via GSK-3β.27 It is conceivable that Akt- and/or AMPKα-dependent NOS3 activation activates the PKG-dependent neuroprotective mechanism via GSK-3β phosphorylation in our model.
Similar to neurological function, myocardial function at 24h after CA/CPR was also improved by administration of Na2S (see Table 1). These results are reinforced by our findings that administration of Na2S starting immediately after reperfusion, but not when administered 40 seconds after reperfusion, markedly improved myocardial function in the isolated-perfused mouse heart subjected to global IR. These results are consistent with a previous report that NaHS was cardioprotective in isolated rat hearts 15. The relatively narrow temporal window of opportunity for Na2S to improve the outcome of CA/CPR is consistent with previous studies of ischemic postconditioning. For example, Cohen and colleagues recently reported that delay of the onset of postconditioning by only 1 minute aborts protection in rabbit hearts.28
MPT is a central mechanism of IR-induced death.20 Na2S prevented CA/CPR-induced acceleration of Ca2+-induced mitochondrial swelling, a measure of MPT, in LV mitochondria. The beneficial effect of Na2S was associated with attenuated oxidative stress indicated by decreased serum hydrogen peroxide levels (see Figure 4). Oxidative stress has been shown to trigger MPT.29 These observations suggest that the cardioprotective effects of Na2S may be mediated by inhibition of MPT.
Cardioprotective effects of endogenous H2S after IR have been suggested by studies showing deleterious effects of CGL inhibitors 30 and protective effects of CS-CGLtg 13 in myocardial IR. Our observation that CS-CGLtg mice had a markedly shorter CPR time to ROSC suggests endogenous H2S promotes the recovery of myocardial function after CA/CPR. Although CS-CGLtg mice also had improved neurological function and survival rate 24h after CA/CPR, these findings may be due to the shortened CPR time to ROSC, as well as possible neuroprotective effects of endogenously-produced H2S. Nonetheless, these observations support an important salutary effect of endogenous H2S production on the outcomes of CA/CPR.
Improved myocardial function in Na2S-treated mice was associated with increased phosphorylation of Akt and NOS3 in LV and elevated serum levels of nitrite plus nitrate combined compared to vehicle-treated mice 15 min after CA/CPR. In contrast, delayed administration of Na2S at 10 min after CPR did not increase the phosphorylation of Akt and NOS3 in LV and failed to improve the outcome of CA/CPR. Furthermore, NOS3 deficiency abrogated the protective effects of Na2S on the outcome of CA/CPR. In a previous study, we found that NOS3 deficiency markedly worsened outcomes of CA/CPR, suggesting an salutary role of NOS3 on the outcome of CA/CPR.19 Taken together, these results suggest a critical role of NOS3/NO in the mechanisms responsible for the protective effects of Na2S after CA/CPR.
In summary, the current study revealed robust protective effects of Na2S on the outcome of CA/CPR in mouse. Administration of Na2S 1 min before the start of CPR markedly improved myocardial and neurological function and survival 24h after normothermic CA/CPR. These observations, if extrapolated to human beings, may be highly clinically relevant because they suggest that Na2S administration can improve the outcome of cardiac arrest at the time of CPR when IV access is obtained. Further studies are warranted to elucidate the impact of exogenous and endogenous H2S on the longer term outcome of CA/CPR complicated by post-cardiac arrest syndrome.
Clinical Perspective.
Sudden cardiac arrest is one of the leading causes of death worldwide. Despite advances in cardiopulmonary resuscitation (CPR) methods, including the introduction of the automatic electrical defibrillator and therapeutic hypothermia, 60–80% of these arrests result in immediate death, and of the remaining, only about 5 percent are successfully resuscitated to the extent that they are returned to productive lives. Poor outcome from cardiac arrest is at least partly due to the lack of therapeutic possibilities. In this study, we examined effects of hydrogen sulfide, a gaseous molecule with multi-faceted protective properties, on the outcome of cardiac arrest and CPR in mice. We found that administration of sodium sulfide, a hydrogen sulfide donor, 1 min before CPR markedly improved neurological and myocardial function and survival after 8 min of cardiac arrest in mouse. These observations, if extrapolated to human beings, may be highly clinically relevant because they suggest that sodium sulfide administration can improve the outcome of sudden cardiac arrest at the time of CPR when intravenous access is obtained. Further studies are warranted to elucidate the impact of exogenous and endogenous hydrogen sulfide on the longer term outcome of cardiac arrest and CPR complicated by post-cardiac arrest syndrome.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ikaria Inc. for generously providing IK-1001 and Dr. Christophe Adrie for valuable advice.
FUNDING SOURCES
This work was supported by grants from NIH HL092141 (DLJ and JWE), HL092737 (JWE), HL70896 (KDB), HL71987 (FI), GM79360 (FI), and Gas-Enabled Medical Innovation Fund (FI).
Footnotes
CONFLICT OF INTEREST DISCLOSURES:
None.
References
- 1.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, Berg RA the National Registry of Cardiopulmonary Resuscitation Investigators. First Documented Rhythm and Clinical Outcome From In-Hospital Cardiac Arrest Among Children and Adults. JAMA: The Journal of the American Medical Association. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 2.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful Cardiopulmonary Resuscitation After Cardiac Arrest as a “Sepsis-Like” Syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 3.Kashiba M, Kajimura M, Goda N, Suematsu M. From O2 to H2S: a landscape view of gas biology. Keio J Med. 2002;51:1–10. doi: 10.2302/kjm.51.1. [DOI] [PubMed] [Google Scholar]
- 4.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;15(206):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 6.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Huff AM, Spence JD, Hegele RA. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin Genet. 2004;65:483–486. doi: 10.1111/j.1399-0004.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 8.Blackstone E, Morrison M, Roth MB. H2S Induces a Suspended Animation-Like State in Mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 9.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, Zapol WM. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison ML, Blackwood JE, Lockett SL, Iwata A, Winn RK, Roth MB. Surviving blood loss using hydrogen sulfide. J Trauma. 2008;65:183–188. doi: 10.1097/TA.0b013e3181507579. [DOI] [PubMed] [Google Scholar]
- 11.Qu K, Chen CPLH, Halliwell B, Moore PK, Wong PTH. Hydrogen Sulfide Is a Mediator of Cerebral Ischemic Damage. Stroke. 2006;37:889–893. doi: 10.1161/01.STR.0000204184.34946.41. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. AJP - Heart and Circulatory Physiology. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 16.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. AJP - Heart and Circulatory Physiology. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripatara P, SA PatelN, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine γ-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest. 2008;88:1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden HoekTL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 19.Nishida T, Yu JD, Minamishima S, Sips PY, Searles RJ, Buys ES, Janssens S, Brouckaert P, Bloch KD, Ichinose F. Protective effects of nitric oxide synthase 3 and soluble guanylate cyclase on the outcome of cardiac arrest and cardiopulmonary resuscitation in mice. Crit Care Med. 2009;37:256–262. doi: 10.1097/CCM.0b013e318192face. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 21.Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovascular Research. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Yang W, Wu L, Wang R. H2S, Endoplasmic Reticulum Stress, and Apoptosis of Insulin-secreting Beta Cells. Journal of Biological Chemistry. 2007;282:16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 26.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of Glycogen Synthase Kinase-3β During Preconditioning Through a Phosphatidylinositol-3-Kinase-Dependent Pathway Is Cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 27.Das A, Xi L, Kukreja RC. Protein Kinase G-dependent Cardioprotective Mechanism of Phosphodiesterase-5 Inhibition Involves Phosphorylation of ERK and GSK3β. Journal of Biological Chemistry. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MV, Yang XM, Downey JM. The pH Hypothesis of Postconditioning: Staccato Reperfusion Reintroduces Oxygen and Perpetuates Myocardial Acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 29.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 30.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.