Abstract
Recent studies have suggested the involvement of secretory phospholipase A2-IIA (sPLA2-IIA) in neuroinflammatory diseases. Although sPLA2-IIA is transcriptionally induced through the NF-κB pathway by pro-inflammatory cytokines, whether this induction pathway is affected by other intracellular signaling pathways has not been investigated in detail. In this study, we demonstrated the induction of sPLA2-IIA mRNA and protein expression in astrocytes by cytokines and detected the protein in the culture medium after stimulation. We further investigated the effects of oxidative pathways and botanical antioxidants on the induction pathway and observed that IL-1β-induced sPLA2-IIA mRNA expression in astrocytes is dependent on ERK1/2 and PI-3 kinase, but not p38 MAPK. In addition to apocynin, a known NADPH oxidase inhibitor, botanical antioxidants, such as resveratrol and epigallocatechin gallate, also inhibited IL-1β-induced sPLA2-IIA mRNA expression. These compounds also suppressed IL-1β-induced ERK1/2 activation and translocation of the NADPH oxidase subunit p67 phox from cytosol to membrane fraction. Taken together, these results support the involvement of reactive oxygen species from NADPH oxidase in cytokine induction of sPLA2-IIA in astrocytes and promote the use of botanical antioxidants as protective agents for inhibition of inflammatory responses in these cells.
Keywords: secretory phospholipase A2-IIA, astrocytes, NADPH oxidase, MAP kinases, apocynin, resveratrol, epigallocatechin gallate (EGCG)
1. INTRODUCTION
Phospholipases A2 (PLA2, EC3.1.1.4.) catalyze the hydrolysis of sn-2 fatty acids from phospholipids. There are more than 20 distinct mammalian isoforms of PLA2 belonging to the calcium-dependent cytosolic group IV PLA2 (cPLA2), the calcium-independent group VI PLA2 (iPLA2), or the small molecular weight group II secretory PLA2 (sPLA2) (Murakami et al., 1997; Sun et al., 2004; Sun et al., 2007; Burke and Dennis, 2008a, b). These enzymes are widely expressed across mammalian cell types and besides playing a role in maintaining integrity of phospholipids in the cell membrane, they are also involved in the production of arachidonic acid, a precursor for prostanoids.
Among more than 12 isoforms of sPLA2, considerable attention has been given to the sPLA2-IIA and inhibitors for this type of PLA2 (Boilard et al., 2006; Lambeau and Gelb, 2008; Oslund et al., 2008; Ibeas et al., 2009). This enzyme is a mediator connecting innate and adaptive immunity and is up-regulated in a number of coronary artery diseases, including atherosclerosis, sepsis, arthritis and infection (Leitinger et al., 1999; Tietge et al., 2005; Krijnen et al., 2006; Mallat et al., 2007; Kimura-Matsumoto et al., 2008; Ibeas et al., 2009). Up-regulation of sPLA2-IIA mRNA expression and immunoreactivity has been reported in rat brain after cerebral ischemia (Lin et al., 2004; Adibhatla and Hatcher, 2007) and in human Alzheimer’s disease brain (Moses et al., 2006). Furthermore, in vitro studies demonstrated the critical role of sPLA2-IIA in neuronal channels and activity (Kolko et al., 2002; Yagami et al., 2002; Mathisen et al., 2007).
Our earlier studies with cultured astrocytes provided evidence for the induction of sPLA2-IIA by inflammatory cytokines, such as interleukin 1-beta (IL-1β) and tumor necrosis factor alpha (TNFα) (Sun and Hu, 1995; Xu et al., 2003a). Subsequent studies indicated that sPLA2-IIA is transcriptionally induced by pro-inflammatory cytokines through the NF-κB pathway (Sun and Hu, 1995; Tong et al., 1999; Lappas et al., 2004; Jaulmes et al., 2005). Nevertheless, whether transcriptional synthesis of sPLA2-IIA is regulated by other signaling cascades has not been explored in sufficient detail.
Recent studies provided evidence that a phagocyte-like NADPH oxidase, capable of generating reactive oxygen species (ROS) in the form of superoxide, is functionally active in astrocytes (Noh and Koh, 2000; Abramov et al., 2005; Liu et al., 2005). This NADPH oxidase contains both membrane and cytoplasmic components, and its activation has been linked to a number of cell surface receptors and signaling cascades (Bedard and Krause, 2007). In this study, we examined the involvement of the NADPH oxidase and other oxidative signaling pathways in cytokine induction of sPLA2-IIA in astrocytes. In addition, we tested the effects of the botanical antioxidants resveratrol and epigallocatechin gallate (EGCG) on sPLA2-IIA expression.
2. MATERIALS AND METHODS
2.1. Materials
Apocynin, anti-β-actin antibody and BSA were purchased from Sigma (St. Louis, MO, USA). Cytokines were purchased from R & D Systems (Minneapolis, MN, USA). SB203580 and U0126 and LY294002 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Trizol, and Superscript III One Step RT-PCR kit were purchased from Invitrogen (Eugene, OR, USA). Oligonucleotide primers were obtained from Integrated DNA Technologies (Coralville, IA, USA). DITNC (immortalized rat astrocytes) were obtained from ATCC (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM), DMEM:F12 1:1, and TryPLE (trypsin) were purchased from GIBCO-BRL (Gaithersburg, MD, USA). Polyclonal antibodies for sPLA2-IIA were obtained from BioVendor (Candler, NC, USA). Anti-phospho-ERK1/2 and anti-ERK1/2 were obtained from Cell Signaling Technology (Danvers, MA, USA), and p67 phox antibodies were obtained from Upstate (Billerica, MA, USA). SuperSignal West Pico chemiluminescence was purchased from Pierce (Rockford, IL, USA).
2.2. Cell culture
The immortalized rat astrocyte cell line (DITNC) was maintained in DMEM with 10% FBS and 1% penicillin/streptomycin (P/S) at 37 °C with 5% CO2 and 95% humidity. Prior to experiments, cells were starved for 4 hours in DMEM medium, followed by treatments with different conditions as described. Polyphenols and inhibitors were added to the cell medium 1 h before treatment with cytokines for 18 hours.
2.3. Crude membrane preparation
In experiments requiring preparation of crude membrane fractions, astrocytes were pretreated with resveratrol or EGCG followed by exposing to IL-1β for 10 min. Cells were then suspended in 0.25 M sucrose, 5 mM MgCl2, 2 mM EGTA, 2 mM EDTA, 10 mM Tris (pH 7.5), 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml pepstatin, and 10 μg/ml aprotinin and were disrupted by brief sonication and centrifuged at 1000×g for 5 min at 4 °C to remove unbroken cells and nuclei. Supernatant was removed and centrifuged at 100,000×g for 1 h at 4 °C (SW40 rotor, Beckman ultracentrifuge). The membrane pellet was resuspended in buffer with 0.5% (v/v) Triton X-100 for 1 h at 4 °C (Min et al. 2004) and expression of p67 phox protein was analyzed by Western blot.
2.4. Western blot analysis
Astrocytes were cultured in 60 mm or 100 mm dishes until 90% confluent. After treatment, cells were washed with ice-cold PBS twice, followed by lysing with lysis buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 0.1% SDS, 1 mM PMSF, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 10 μg/ml aprotinin). Protein concentrations were determined by the Bradford assay (Bradford, 1976). Equivalent amounts of protein for each sample were resolved in a 15% SDS-Page and run for 60 minutes at 200 V. Proteins were subsequently transferred at 100 V for 1 h to nitrocellulose membranes. Membranes were incubated in Tris-buffered saline, pH 7.4, with 0.5% Tween 20 (TBS-T) containing 5% nonfat milk for 1 h at room temperature. Blots were reacted with the primary antibody (TBS-T with 5% milk) at 4 °C overnight, washed 3 times for 5 min in TBS-T, and then incubation with the secondary antibody (TBS-T with 5% milk) for 1 h at room temperature. After washing 3 times for 5 min with TBS-T, SuperSignal West Pico chemiluminescence reagents from Pierce were used to signal detection. Band density was measured using Quantity One software (Bio-Rad, Hercules, CA, USA). In some studies, the blots were striped using the standard protocol, washed and re-probed with anti-β-actin. The following antibody concentrations or dilutions were used: sPLA2-IIA (1: 1000), phospho-ERK1/2 (1:1000), ERK1/2 (1:2000), p67 phox (1:1000), β-actin (1:30000) secondary antibodies (1:5000).
2.5. Semi-quantitative RT-PCR
Cells were washed with PBS following treatment and RNA was isolated with Trizol (Invitrogen) according to manufacturer’s instructions. A SuperScript III One-Step kit (Invitrogen) was used according to manufacturer’s instructions. Briefly, one microgram of RNA was used in the one-step RT-PCR with 50 pmol of the following oligonucleotide primers designed from rat gene sequences for sPLA2-IIA: sense 5′-TGACTCATGACTGTTGTTACAACC-3′ and antisense 5′-TCTCAGGACTCTCTTAGGTACTA-3′ (amplifies a 493 bp fragment); and for β-actin: sense 5′-TGGAGAAGAGCTATGAGCTGCCTG-3′ and antisense 5′-GTGCCACCAGACAGCACTGTGTTG-3′ (amplifies a 289 bp fragment). Reverse transcription at 50°C for 30 min and inactivation of reverse transcriptase at 94 °C for 2 min were followed by 40 cycles of amplification for sPLA2-IIA cDNA (annealing at 55 °C for 1 min) or 30 cycles of amplification for β-actin cDNA (annealing at 55°C for 1 min). Each cycle included a 15 s denaturation step at 94 °C, an annealing step, and a 1 min extension step at 68 °C. A 5 min extension at 68 °C was carried out at the end of the final cycle. Amplified DNA was visualized on a 2% agarose gel with ethidium bromide and analyzed with Quantity One software.
2.6. Reverse transcription
Reverse transcription of RNA was carried out using the Taqman Reverse Transcription Kit following the manufacturer’s instructions (Ambion, Austin, TX, USA). Briefly, RNA concentration was determined with spectrophotometry at 260 nm and concentrations of samples were normalized to 30 μg/ml. Oligo d(T)16 was combined with other reaction components, and 6.15 μl of reaction mix was added to each tube containing 3.85 μl of RNA for a total volume of 10 μl. The samples were incubated at 25 °C for 10 min, followed by the reverse transcription step at 48 °C for 30 min, and reverse transcriptase inactivation at 95 °C for 5 min.
2.7. Quantitative real-time PCR
The ABI 7300 Taqman by Applied Biosystems was used for real-time PCR according to manufacturer’s instructions. A pre-designed primer and probe set was purchased for determination of expression of sPLA2-IIA (Assay ID Rn00580999) based on NCBI sequence NM 031598.1 with an amplicon length of 94 bp. A β-actin primer and probe was used as a control, with the following sequences: Forward 5′GCCCTGGCTCCTAGCACC-3′, Reverse 5′CCACCAATCCACACAGAGTACTTG-3′, and Probe 5′TGAAGATCAAGTCATTGCTCCTCCTGAGC-3′ with a FAM modification on the 5′ end, having an amplicon length of 73 bp. Briefly, 5 μl cDNA was combined with Taqman Universal PCR Master Mix, along with a FAM-MGB probe and primers, for a final concentration of 25 μl. The reaction plate was incubated as follows: 50 °C for 2 min, 95 °C for 10 min, and 50 cycles of denature (95 °C for 15 sec) and anneal/extend (60 °C for 1 min). The CT was determined and sPLA2-IIA transcript concentration was normalized against that of β-actin.
2.8. Statistical analysis
Data were analyzed by one-way ANOVA followed by Newman-Keuls multiple comparison test (V4.00, GraphPad Prism Software, Inc., San Diego, CA). Values of p<0.05 were accepted as significant.
3. RESULTS
3.1. Cytokine induction of sPLA2-IIA mRNA and protein expression in rat immortalized astrocytes (DITNC)
In the initial study, immortalized astrocytes (DITNC) were tested for induction of sPLA2-IIA mRNA expression by pro-inflammatory cytokines. The RT-PCR study indicated induction of sPLA2-IIA mRNA upon exposure to IL-1β and TNFα for 18 h (Fig 1A). Exposure of astrocytes with interferon gamma (IFNγ) alone did not cause the induction of sPLA2-IIA mRNA (data not shown).
Fig 1.
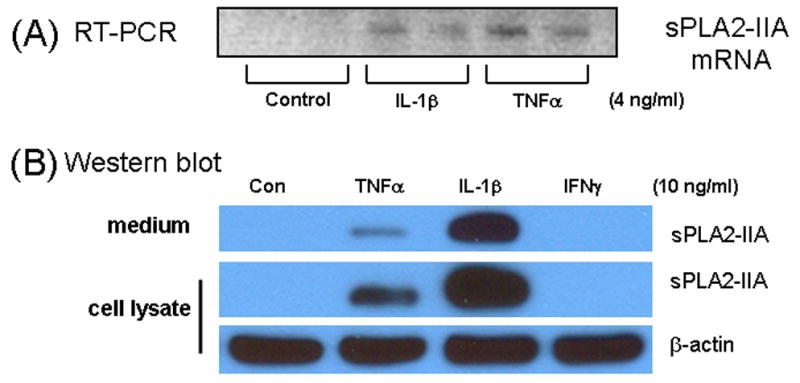
(A) Induction of sPLA2-IIA mRNA in immortalized rat astrocytes (DITNC) by IL-1β and TNFα. Astrocytes were treated with 4 ng/ml with IL-1β or TNFα for 18 h prior to measurement of sPLA2-IIA mRNA expression by RT-PCR. Results are representative of three independent experiments. (B) Induction of sPLA2-IIA protein in astrocytes. DITNC astrocytes were cultured in 60 mm dish and serum-starved for 4 h prior to treatment with TNFα, IL-1β and IFNγ (10 ng/ml) for 48 h. After treatment, culture medium was removed, cells washed with PBS and lysis buffer was added. Medium (40 μl) and cell lysate (15 μg protein) was used in Western blot as described in text. β-actin in cell lysate was used as control.
Western blot analysis was used to estimate sPLA2-IIA concentrations in the cell lysates and in the culture medium. As shown in Fig 1B, exposure of astrocytes to TNFα and IL-1β for 48 h induced visible sPLA2-IIA protein in both cell lysate and in the culture medium. Similar induction profile was observed in primary rat astrocytes (data not shown).
3.2. Involvement of protein kinases in the induction of sPLA2-IIA mRNA and protein by IL-1β
We tested the effects of several protein kinases, i.e., ERK1/2, p38 MAPK and PI-3 kinase, on induction of sPLA2-IIA by IL-1β. The MEK inhibitor, U0126, which inhibits MEK1/2, a direct activator of ERK1/2, was effective in inhibiting IL-1β-induced expression of sPLA2-IIA mRNA and protein (Fig 2). However, SB203580, an inhibitor for p38 MAPK, did not alter IL-1β induced sPLA2-IIA expression (data not shown). As shown in Figure 3, IL-1β induced sPLA2-IIA upregulation in DITNC cells was significantly attenuated by LY294002, a known inhibitor of PI-3 kinase.
Fig 2.
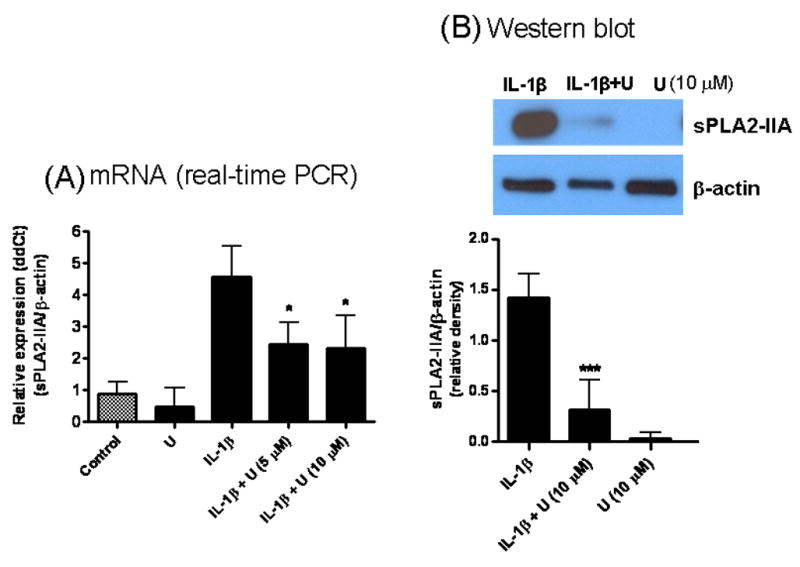
U0126 (U), a MEK inhibitor, inhibited IL-1β-induced sPLA2-IIA mRNA and protein expressions in astrocytes. (A) For mRNA expression, astrocytes were treated with U0126 (10 μM) alone, IL-1β (4 ng/ml) alone and IL-1β with U0126 (5 and 10 μM) for 18 h. U0126 was added to astrocytes 1 h before treating with IL-1β. sPLA2-IIA expression was measured by real-time PCR relative to β-actin expression as described in text. Results are mean ± SD from three experiments. (B) For protein expression, astrocytes were treated with U0126 (10 μM), IL-1β (10 ng/ml), and IL-1β + U0126 for 48 h. U0126 was added 1 h prior to adding IL-1β. After incubation, cell lysates were taken for Western blot analysis as described in text. β-actin was used as control. Results are mean ± SD from three experiments. *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β.
Fig 3.
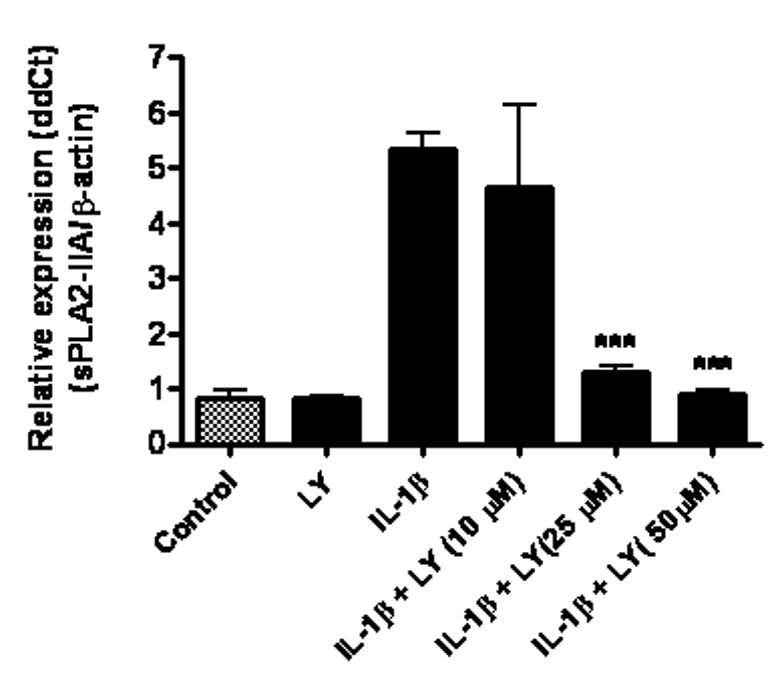
LY294002, a PI-3 kinase inhibitor, inhibited IL-1β-induced sPLA2-IIA mRNA expression in astrocytes. Astrocytes were treated with LY294002 (LY, 50 μM), IL-1β (4 ng/ml), and IL-1β with LY at 10, 25, and 50 μM for 18 h. LY was added to astrocytes 1 h prior to treatment with IL-1β. sPLA2-IIA mRNA expression was determined by real-time PCR with β-actin as control. Results are mean ± SD from three experiments. ***p<0.001 vs. IL-1β.
3.3. Inhibition of IL-1β-induced sPLA2-IIA mRNA expression by apocynin
We tested the possible involvement of NADPH oxidase in mediating IL-1β-induced sPLA2-IIA expression by treating astrocytes with apocynin, an inhibitor known to block the translocation of cytoplasmic subunits from docking with membrane subunits of NADPH oxidase (Stolk et al., 1994). As shown in Fig 4, apocynin at 500 μM effectively inhibited IL-1β-induced PLA2-IIA mRNA expression in astrocytes. Apocynin as well as the kinase inhibitors showed little to no effect on cell viability as demonstrated by MTT test (data not shown).
Fig 4.
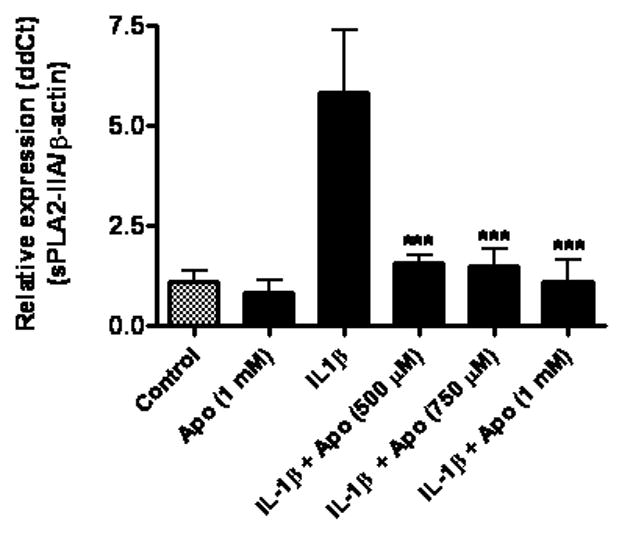
Apocynin, an NADPH oxidase inhibitor, inhibited IL-1β-induced sPLA2-IIA mRNA expression in astrocytes. Astrocytes were treated with apocynin (Apo, 1 mM), IL-1β (4 ng/ml), and IL-1β with apocynin at 500, 750 and 1000 μM for 18 h. Apocynin was added to astrocytes 1 h prior to treatment with IL-1β. sPLA2-IIA mRNA expression was determined using real-time PCR with β-actin as control. Results are mean ± SD from three experiments. ***p<0.001 vs. IL-1β.
3.4. Botanical polyphenols inhibited IL-1β-induced sPLA2-IIA mRNA through NADPH oxidase pathway
In this experiment, we tested the effects of resveratrol and EGCG on IL-1β-induced sPLA2-IIA in astrocytes. Our results show that pretreatment of astrocytes with resveratrol resulted in an inhibition of IL-1β-induced expression of sPLA2-IIA mRNA (Fig 5). Similarly, pretreatment of astrocytes with EGCG also resulted in a dose-dependent inhibition of IL-1β-induced sPLA2-IIA mRNA expression (Fig 6).
Fig 5.
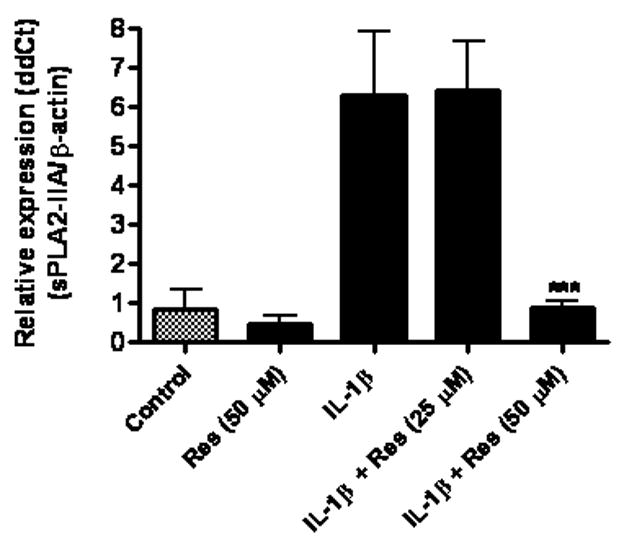
Resveratrol (Res) inhibited IL-1β-induced sPLA2-IIA mRNA expression in astrocytes. Astrocytes were treated with resveratrol (50 μM), IL-1β (4 ng/ml) and IL-1β with resveratrol at 25 or 50 μM for 18 h. Resveratrol was added to astrocytes 1 h prior to treatment with IL-1β. sPLA2-IIA mRNA expression was determined using real-time PCR with β-actin as control. Results are mean ± SD from three experiments. ***p<0.001 vs. IL-1β.
Fig 6.
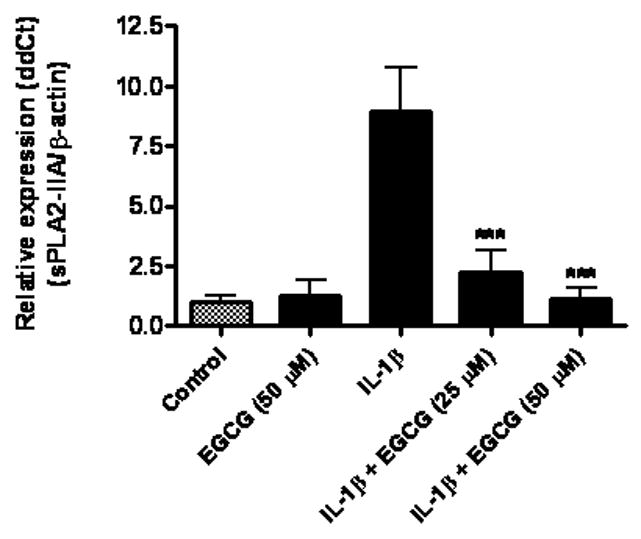
Epigallocatechin gallate (EGCG) inhibited IL-1β-induced sPLA2-IIA mRNA expression in astrocytes. Astrocytes were treated with EGCG (50 μM), IL-1β (4 ng/ml) and IL-1β with EGCG at 25 or 50 μM for 18 h. EGCG was added to astrocytes 1 h prior to treatment with IL-1β. sPLA2-IIA mRNA expression was determined using real-time PCR with β-actin as control. Results are mean ± SD from three experiments. ***p<0.001 vs. IL-1β.
With both resveratrol and EGCG inhibiting sPLA2-IIA mRNA expression, we further tested whether these compounds inhibit the translocation of the soluble NADPH oxidase subunit p67 phox to membranes. After pretreatment with resveratrol or EGCG, astrocytes were exposed to IL-1β for 10 min and expression of p67 phox protein in the membrane fraction was examined by Western blot analysis. As shown in Fig 7, IL-1β induced the translocation of p67 phox to the membrane fraction and resveratrol and EGCG each inhibited this translocation.
Fig 7.
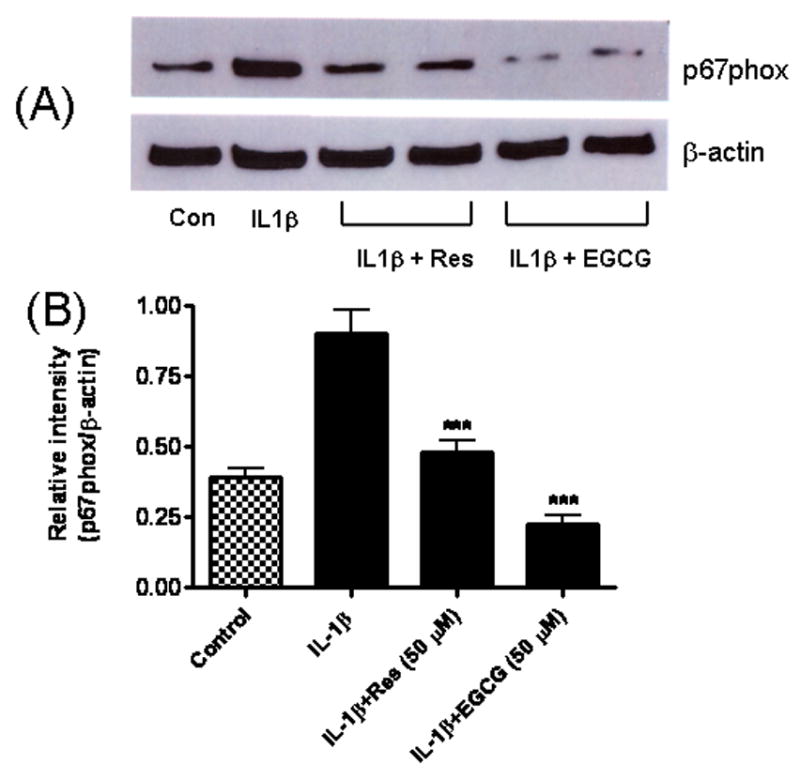
Resveratrol and EGCG inhibited IL-1β-induced translocation of p67 phox to the membrane fraction. Astrocytes were treated with IL-1β (4 ng/ml) or IL-1β plus Res (50 μM) and EGCG (50 μM) for 10 min. Cells were disrupted and cell cytosol and membrane fractions were separated by centrifugation as described in Method section. (A) Western blot analysis of p67 phox concentration in the membrane fraction compared to β-actin as control. (B) Relative intensity of p67 phox compared to β-actin. Results are mean ± SD from three independent experiments. ***p<0.001 vs. IL-1β.
3.5. Apocynin and botanical polyphenols inhibited IL-1β-induced ERK1/2 phosphorylation
Since ERK1/2 has been shown to be activated following NADPH oxidase activation in astrocytes (Pawate et al., 2004), we investigated the effects of apocynin, resveratrol, and EGCG on the phosphorylation of ERK1/2 after stimulation of astrocytes with IL-1β. As shown in Fig 8, all three compounds inhibited IL-1β-induced increase in phospho-p42 ERK.
Fig 8.
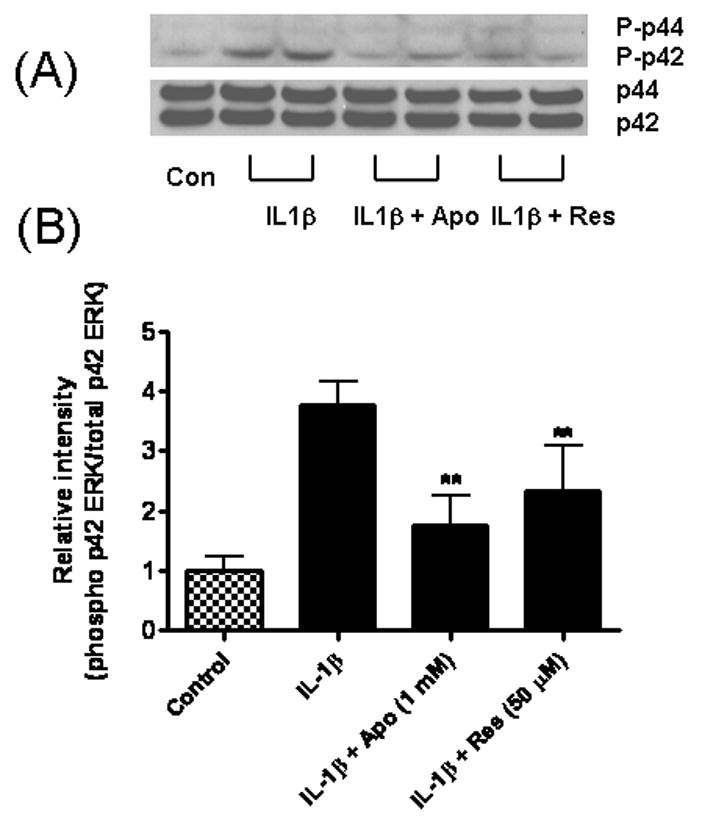
Apocynin and resveratrol inhibited IL-1β-induced ERK1/2 phosphorylation in astrocytes. Astrocytes were treated with IL-1β (4 ng/ml), or IL-1β plus apocynin (1 mM) and Res (50 μM) for 10 min. (A) Cell lysate was used for Western blot analysis for phospho-ERK1/2 and total ERK1/2. (B) Relative intensities of phospho-p42 ERK against total p42 ERK. Results are mean ± SD from three independent experiments. **p<0.01 vs. IL-1β.
4. DISCUSSION
The importance of sPLA2-IIA in neurodegenerative diseases, especially in association with inflammatory processes has started to emerge (Adibhatla and Hatcher, 2007; Sun et al., 2007). Studies linking this enzyme with transgenic mouse models have been hampered due to a missense mutation in the sPLA2-IIA gene in a number of mouse strains (Kennedy et al., 1995). However, studies with rat brain demonstrated the inflammatory properties of this enzyme and its upregulation in astrocytes by pro-inflammatory cytokines including IL-1β and TNFα (Oka and Arita, 1991; Tong et al., 1999; Rosenberger et al., 2004; Moses et al., 2006). In the present study, our Western blot analysis demonstrates for the first time that cytokine-induced sPLA2-IIA protein is secreted into the culture medium. This result is in agreement with our previous study showing active PLA2 activity in the culture media using radioactive phospholipids as substrate (Xu et al., 2003).
Although a number of studies have demonstrated the transcriptional induction of sPLA2-IIA through the NF-κB pathway (Andreani et al., 2000; Lappas et al., 2004), less is known about the roles of other signaling molecules in mediating the induction. In this study, we used real-time PCR to measure sPLA2-IIA mRNA expression in astrocytes, and demonstrated the involvement of ERK1/2 and PI-3 kinase (but not p38 MAPK) in sPLA2-IIA expression by IL-1β. In the CNS, ERK1/2 are important signaling molecules that integrate extracellular signals (Sweatt, 2001; Chu et al., 2004). Furthermore, ERK1/2 activation is implicated in a large number of intracellular factors, including Ca2+, PKC, nitric oxide, and PI-3 kinase (Andersen et al., 2003). In our recent studies with cortical neurons, ERK1/2 phosphorylation was stimulated by ROS produced by NADPH oxidase and in turn this signaling pathway led to activation of cPLA2 (Shelat et al., 2008). Other studies have also demonstrated that NADPH oxidase-generated ROS can activate the ERK1/2 pathway and vice versa, ERK1/2 activation can stimulate the phosphorylation of cytosolic subunits of NAPDH oxidase leading to an increased activity of the enzyme (Pawate et al., 2004; Miller et al., 2007; Yang et al., 2007a). In microglial cells, LPS-induced activation of PI-3 kinase and p38 MAPK (but not ERK1/2) pathways is dependent on ROS production by NADPH oxidase (Sun et al., 2008). These kinases are important in glial redox signaling and their inhibition can lead to the reduced production of inflammatory proteins (Bhat et al., 1998; Saha and Pahan, 2006; Yang et al., 2007b). Our results are in agreement with these findings. Interestingly, PKC activation of PI-3 kinase (Frey et al., 2006) can modulate sPLA2-IIA expression in IL-1β treated mesangial cells (Scholz et al., 1999). Apparently, depending on the cell types, cytokines may stimulate different kinase pathways and regulate the transcriptional events leading to sPLA2-IIA expression.
Although mechanism not well understood, ROS have been shown to play a role as second messengers in NF-κB activation (Schreck et al., 1992; Baeuerle and Henkel, 1994; Flohe et al., 1997). In this study, we provided evidence that NADPH oxidase-derived ROS is necessary for IL-1β induction of sPLA2-IIA. Our results are in accordance with other findings which demonstrated the involvement of NADPH oxidase in neuroinflammatory processes, especially glial activation (Brown, 2007). NADPH oxidase is considered an important non-mitochondrial source of oxidative stress in the brain and has been implicated in a number of neurodegenerative diseases including Alzheimer’s, Parkinson’s, HIV dementia, ischemic stroke, and multiple sclerosis (Bedard and Krause, 2007). The involvement of NADPH oxidase in IL-1β-mediated induction of sPLA2-IIA was demonstrated by inhibition with apocynin, a phenolic compound extracted from Picrorhiza kurroa, a creeping plant native to the mountains of India, Nepal, Tibet and Pakistan (Wang et al., 2008). Apocynin has been shown to block the activity of NADPH oxidase by interfering with the assembly of the cytosolic subunits with the membrane components (Stolk et al., 1994). The ability for apocynin to inhibit translocation of p67 phox to membranes is also indication for the involvement of NADPH oxidase in this process.
Due to their antioxidant properties, there is considerable interest to examine whether polyphenolic compounds including resveratrol from grapes and EGCG from green tea may offer protective effects and ameliorate progression of neurodegenerative diseases (Joseph et al. 2000, Son et al. 2008, Zaveri 2006, Mancuso et al. 2007, Cucciolla et al. 2007). Our earlier studies have demonstrated the abilities of resveratrol and also apocynin to protect against neuronal injury and glial activation due to cerebral ischemia-reperfusion (Wang et al., 2002; Wang et al., 2006). Other studies also demonstrated the ability of antioxidant botanical compounds to suppress the NF-κB pathway (Packer, 1998; Lavrovsky et al., 2000; Cindrova-Davies et al., 2007). Indeed, recent findings have indicated that the neuroprotective actions of these compounds arise from their modulation of important cellular signaling pathways (Mandel et al., 2004). In the current study, apocynin, resveratrol and EGCG were shown to inhibit IL-1β-induced sPLA2-IIA mRNA expression in astrocytes. The fact that these compounds could inhibit p67 phox translocation and IL-1β induced ERK activation further supports the notion that these antioxidants may act by targeting the NADPH oxidase pathway. Our results are in agreement with recent study indicating that both resveratrol and EGCG could inhibit NADPH oxidase activity in endothelial cells (Steffen et al., 2008).
In conclusion, this study provides evidence that protein kinases and oxidative pathways, most likely the NADPH oxidase pathway, promote the expression of sPLA2-IIA mRNA in astrocytes. This study further provides support for the down regulation of this inflammatory protein by inhibitors of NADPH oxidase and botanical antioxidants.
Acknowledgments
Supported by 2P01 AG018357 and 1R21 AT003859 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. Journal of Neuroscience. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Secretory phospholipase A2 IIA is up-regulated by TNF-alpha and IL-1alpha/beta after transient focal cerebral ischemia in rat. Brain Research. 2007;1134:199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Andersen JM, Myhre O, Fonnum F. Discussion of the role of the extracellular signal-regulated kinase-phospholipase A2 pathway in production of reactive oxygen species in Alzheimer’s disease. Neurochemical Research. 2003;28:319–326. doi: 10.1023/a:1022389503105. [DOI] [PubMed] [Google Scholar]
- Andreani M, Olivier JL, Berenbaum F, Raymondjean M, Bereziat G. Transcriptional regulation of inflammatory secreted phospholipases A(2) Biochimica et Biophysica Acta. 2000;1488:149–158. doi: 10.1016/s1388-1981(00)00117-7. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. Journal of Neuroscience. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boilard E, Rouault M, Surrel F, Le Calvez C, Bezzine S, Singer A, Gelb MH, Lambeau G. Secreted phospholipase A2 inhibitors are also potent blockers of binding to the M-type receptor. Biochemistry. 2006;45:13203–13218. doi: 10.1021/bi061376d. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochemical Society Transactions. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism and signaling. Journal of Lipid Research. 2008a doi: 10.1194/jlr.R800033-JLR200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A(2) Biochemistry. Cardiovascular Drugs and Therapy. 2008b doi: 10.1007/s10557-008-6132-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. European Journal of Biochemistry. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. American Journal of Pathology. 2007;170:1511–1520. doi: 10.2353/ajpath.2007.061035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6:2495–2510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radical Biology and Medicine. 1997;22:1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. Journal of Biological Chemistry. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- Ibeas E, Fuentes L, Martin R, Hernandez M, Nieto ML. Secreted phospholipase A2 type IIA as a mediator connecting innate and adaptive immunity: new role in atherosclerosis. Cardiovascular Research. 2009;81:54–63. doi: 10.1093/cvr/cvn234. [DOI] [PubMed] [Google Scholar]
- Jaulmes A, Janvier B, Andreani M, Raymondjean M. Autocrine and paracrine transcriptional regulation of type IIA secretory phospholipase A2 gene in vascular smooth muscle cells. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25:1161–1167. doi: 10.1161/01.ATV.0000164310.67356.a9. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Bielinski D, Fisher DR, Shukitt-Hale B. Oxidative stress protection and vulnerability in aging: putative nutritional implications for intervention. Mechanisms of Ageing and Development. 2000;116:141–153. doi: 10.1016/s0047-6374(00)00128-7. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. Journal of Biological Chemistry. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- Kimura-Matsumoto M, Ishikawa Y, Komiyama K, Tsuruta T, Murakami M, Masuda S, Akasaka Y, Ito K, Ishiguro S, Morita H, Sato S, Ishii T. Expression of secretory phospholipase A2s in human atherosclerosis development. Atherosclerosis. 2008;196:81–91. doi: 10.1016/j.atherosclerosis.2006.08.062. [DOI] [PubMed] [Google Scholar]
- Kolko M, de Turco EB, Diemer NH, Bazan NG. Secretory phospholipase A2-mediated neuronal cell death involves glutamate ionotropic receptors. Neuroreport. 2002;13:1963–1966. doi: 10.1097/00001756-200210280-00026. [DOI] [PubMed] [Google Scholar]
- Krijnen PA, Meischl C, Nijmeijer R, Visser CA, Hack CE, Niessen HW. Inhibition of sPLA2-IIA, C-reactive protein or complement: new therapy for patients with acute myocardial infarction? Cardiovascular and Hematological Disorders Drug Targets. 2006;6:113–123. doi: 10.2174/187152906777441830. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annual Review of Biochemistry. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Georgiou HM, Rice GE. Regulation of phospholipase isozymes by nuclear factor-kappaB in human gestational tissues in vitro. Journal of Clinical Endocrinology and Metabolism. 2004;89:2365–2372. doi: 10.1210/jc.2003-031385. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Experimental Gerontology. 2000;35:521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Leitinger N, Watson AD, Hama SY, Ivandic B, Qiao JH, Huber J, Faull KF, Grass DS, Navab M, Fogelman AM, de Beer FC, Lusis AJ, Berliner JA. Role of group II secretory phospholipase A2 in atherosclerosis: 2. Potential involvement of biologically active oxidized phospholipids. Arteriosclerosis Thrombosis and Vascular Biology. 1999;19:1291–1298. doi: 10.1161/01.atv.19.5.1291. [DOI] [PubMed] [Google Scholar]
- Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. Journal of Neurochemistry. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kang JH, Zheng RL. NADPH oxidase produces reactive oxygen species and maintains survival of rat astrocytes. Cell Biochemistry and Function. 2005;23:93–100. doi: 10.1002/cbf.1171. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Benessiano J, Simon T, Ederhy S, Sebella-Arguelles C, Cohen A, Huart V, Wareham NJ, Luben R, Khaw KT, Tedgui A, Boekholdt SM. Circulating secretory phospholipase A2 activity and risk of incident coronary events in healthy men and women: the EPIC-Norfolk study. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:1177–1183. doi: 10.1161/ATVBAHA.107.139352. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V. Natural antioxidants in Alzheimer’s disease. Expert Opinion on Investigational Drugs. 2007;16:1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. Journal of Neurochemistry. 2004;88:1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- Mathisen GH, Thorkildsen IH, Paulsen RE. Secretory PLA2-IIA and ROS generation in peripheral mitochondria are critical for neuronal death. Brain Research. 2007;1153:43–51. doi: 10.1016/j.brainres.2007.03.067. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Research. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: a new inflammatory factor for Alzheimer’s disease. Journal of Neuroinflammation. 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Critical Reviews in Immunology. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. Journal of Neuroscience. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Arita H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. Two distinct pathways of the gene expression. Journal of Biological Chemistry. 1991;266:9956–9960. [PubMed] [Google Scholar]
- Oslund RC, Cermak N, Gelb MH. Highly specific and broadly potent inhibitors of mammalian secreted phospholipases A2. Journal of Medical Chemistry. 2008;51:4708–4714. doi: 10.1021/jm800422v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L. alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metabolism Reviews. 1998;30:245–275. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. Journal of Neuroscience Research. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. Journal of Neurochemistry. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxidants & Redox Signaling. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz K, Vlachojannis GJ, Spitzer S, Schini-Kerth V, Van Den Bosch H, Kaszkin M, Pfeilschifter J. Modulation of cytokine-induced expression of secretory phospholipase A2-type IIA by protein kinase C in rat renal mesangial cells. Biochemical Pharmacology. 1999;58:1751–1758. doi: 10.1016/s0006-2952(99)00279-8. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. Journal of Experimental Medicine. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. Journal of Neurochemistry. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic Dietary Phytochemicals. Neuromolecular Medicine. 2008 doi: 10.1007/s12017-008-8037-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Archives of Biochemistry and Biophysics. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. American Journal of Respiratory Cell and Molecular Biology. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Sun GY, Hu ZY. Stimulation of phospholipase A2 expression in rat cultured astrocytes by LPS, TNF alpha and IL-1 beta. Progress in Brain Research. 1995;105:231–238. [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. Journal of Neurochemistry. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. Journal of Lipid Research. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun HN, Kim SU, Lee MS, Kim SK, Kim JM, Yim M, Yu DY, Lee DS. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent activation of phosphoinositide 3-kinase and p38 mitogen-activated protein kinase signal pathways is required for lipopolysaccharide-induced microglial phagocytosis. Biological & Pharmaceutical Bulletin. 2008;31:1711–1715. doi: 10.1248/bpb.31.1711. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tietge UJ, Pratico D, Ding T, Funk CD, Hildebrand RB, Van Berkel T, Van Eck M. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. Journal of Lipid Research. 2005;46:1604–1614. doi: 10.1194/jlr.M400469-JLR200. [DOI] [PubMed] [Google Scholar]
- Tong W, Shah D, Xu J, Diehl JA, Hans A, Hannink M, Sun GY. Involvement of lipid mediators on cytokine signaling and induction of secretory phospholipase A2 in immortalized astrocytes (DITNC) Journal of Molecular Neuroscience. 1999;12:89–99. doi: 10.1007/BF02736923. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Research. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Research. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY. Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 2008;15:496–503. doi: 10.1016/j.phymed.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Yagami T, Ueda K, Asakura K, Hata S, Kuroda T, Sakaeda T, Takasu N, Tanaka K, Gemba T, Hori Y. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Molecular Pharmacology. 2002;61:114–126. doi: 10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ, Shin DM, Lee YH, Lee DS, El-Benna J, Jo EK. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. Journal of Neuroinflammation. 2007a;4:27. doi: 10.1186/1742-2094-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MS, Min KJ, Joe E. Multiple mechanisms that prevent excessive brain inflammation. Journal of Neuroscience Research. 2007b;85:2298–2305. doi: 10.1002/jnr.21254. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sciences. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]


