Abstract
Lipogenesis is exquisitely regulated by nutritional/hormonal states. Transcription of fatty acid synthase (FAS), a central enzyme in lipogenesis, is low in fasting but increases drastically with feeding. In transcriptional activation of FAS by feeding/insulin, USF constitutively bound to the −65 E-box is required. Here, we show that USF functions as a molecular switch by recruiting various interacting proteins during the fasting/feeding transition. During feeding/insulin, USF-1 recruits and is phosphorylated by DNA-PK, which is dephosphorylated/activated by PP1. Phosphorylation of USF-1 allows recruitment of and acetylation by P/CAF, resulting in the FAS promoter activation. In fasting, USF-1 is deacetylated by HDAC9 causing the promoter inactivation. DNA break/repair components associated with USF also bring about transient DNA breaks during feeding-induced FAS activation. In DNA-PK deficient SCID mice, feeding induced USF-1 phosphorylation/acetylation, DNA-breaks, and FAS activation leading to lipogenesis are impaired, resulting in decreased liver and circulating triglyceride levels. Our study demonstrates that DNA-PK mediates the feeding/insulin-dependent lipogenic gene activation.
Introduction
To meet the constant energy requirement in the face of highly variable food supply, mammals employ intricate and precise mechanisms for energy storage. During feeding, excess carbohydrates are converted to fatty acids (de novo lipogenesis) for synthesis/storage of triacylglyerol, which then can be utilized during energy shortage, i.e., fasting. Lipogenesis is under tight nutritional and hormonal control (Sul, and Wang, 1998). Enzymes involved in fatty acid and triglyceride synthesis, such as Fatty Acid Synthase (FAS) (Paulauskis, and Sul, 1988; Paulauskis, and Sul, 1989) and mitochondrial glycerol-3-phosphate acyltransferase (mGPAT) (Dircks, and Sul, 1997; Jerkins, Liu, et al, 1995; Shin, Paulauskis, et al, 1991; Yet, Lee, et al, 1993; Yet, Moon, and Sul, 1995), are coordinately regulated during fasting/feeding. The expression of the lipogenic enzymes is very low in fasting, and is drastically upregulated during feeding accompanied by an increase in insulin secretion (Sul, Latasa, et al, 2000; Wang, and Sul, 1998). Thus, precise temporal changes in patterns of gene repression and activation are required for lipogenic gene regulation during fasting and feeding/insulin treatment.
By catalyzing 7 reactions in fatty acid synthesis, FAS is a central enzyme in lipogenesis. Regulation of FAS is mainly at the transcriptional level. We have been studying the FAS promoter as a model system to dissect the transcriptional activation by feeding/insulin. We mapped the insulin response sequence (IRS) of the FAS promoter in cultured cells at −65 E-box (Moustaid, Sakamoto, et al, 1993; Moustaid, Beyer, and Sul, 1994) where Upstream Stimulatory Factor (USF) −1/2 heterodimer binds (Moustaid, and Sul, 1991; Sawadogo, and Roeder, 1985; Wang, and Sul, 1995; Wang, and Sul, 1997). Functional analysis and Chromatin Immunoprecipitation (ChIP) in mice transgenic for various 5'-deletions and mutations of the FAS promoter-CAT reporter gene (Latasa, Moon, et al, 2000; Moon, Latasa, et al, 2000; Soncini, Yet, et al, 1995), however, showed that both USF binding to the E-box and sterol regulatory element-binding protein-1c (SREBP-1c) binding to the nearby sterol response element (SRE) are required for feeding/insulin mediated FAS promoter activation in vivo. Furthermore, although an increased expression of SREBP-1c (Shimomura, Bashmakov, et al, 1999) mainly through insulin activation of the PI3K pathway(Engelman, Luo, and Cantley, 2006; Taniguchi, Emanuelli, and Kahn, 2006) to bind the FAS promoter is critical for feeding/insulin response, SREBP-1c itself cannot bind its SRE without being recruited by USF which is constitutively bound to the −65 E-box (Griffin, Wong, et al, 2007; Latasa, Griffin, et al, 2003). Many of the lipogenic promoters contain closely spaced E-box and SRE at the proximal promoter region and we documented a similar mechanism for activation of FAS and mGPAT promoters (Griffin, Wong, et al, 2007). Thus, USF, along with SREBP-1c, plays a critical role in mediating the transcriptional activation of lipogenesis in response to feeding/insulin.
The requirement of USF (Sirito, Lin, et al, 1994) in induction of lipogenic genes, such as FAS, has been demonstrated in USF deficient mice (Casado, Vallet, et al, 1999). In humans, SNP studies have implicated USF-1 as a prime candidate of familial combined hyperlipidemia (FCHL) ( Pajukanta, Lilja, et al, 2004). How does USF regulate lipogenic gene transcription? USF levels do not change during fasting/feeding and it is constitutively bound to the FAS promoter in both conditions (Wang, and Sul, 1995). It is possible that posttranslational modification of USF underlies its function during fasting/feeding. Insulin regulates metabolism primarily through protein phosphorylation by the well characterized PI3K cascades (Engelman, Luo, and Cantley, 2006). Many of the metabolic effects of insulin are also mediated by protein dephosphorylation catalyzed mainly by protein phosphatase-1 (PPl) (Brady, and Saltiel, 2001). In this regard, USF has been previously reported to be phosphorylated by various kinases (Corre, and Galibert, 2005). However, the significance of USF phosphorylation in lipogenic gene transcription during feeding/insulin is not known. In addition, as with other transcription factors, USF may not independently function to regulate transcription but must recruit coactivators/corepressors. Such recruited factors may also include signaling molecules that transduce extracellular signals to bring about covalent modifications of USF. Thus, it can be postulated that USF and/or its potentially recruited cofactors need to be regulated by dynamic modifications such as phosphorylation/dephosphorylation in response to feeding/insulin. The identification of the coregulator(s) interacting with USF in a fasting/feeding dependent manner as well as the possible fasting/feeding dependent posttranslational modifications of USF that may occur in a stepwise or simultaneous fashion is required to understand how lipogenic gene promoters are activated by feeding/insulin. Recently, it has been reported that transient DNA break is required for regulated transcription (Ju, Lunyak, et al, 2006). Therefore, USF might also interact and recruit components involved in DNA break/repair in lipogenic gene promoter region for transcriptional activation in response to feeding.
Here, we report a novel mechanism for the sensing of nutritional/hormonal status by USF to regulate lipogenic gene transcription. We demonstrate that USF-1 phosphorylation by DNA-dependent protein kinase (DNA-PK), which is first dephosphorylated/activated by PP1, is an immediate response to feeding/insulin treatment. USF phosphorylation facilitates recruitment of SREBP to SRE. Phosphorylation of USF-1 also allows recruitment and acetylation by p300 associated factor (P/CAF) to result in FAS promoter activation. In contrast, during fasting, USF-1 association with histone deacetylase 9 (HDAC9) leads to USF-1 deacetylation and promoter inactivation. Thus, DNA-PK deficient SCID mice upon feeding show impaired USF-1 phosphorylation/acetylation, DNA-break, transcriptional activation of the FAS gene and lipogenesis, resulting in decreased liver and circulating triglyceride levels. Our present study, for the first time, shows DNA-PK to be critical to the feeding-dependent activation of lipogenic genes, linking DNA-PK in insulin signaling pathway.
Results
Identification of USF interacting proteins and their occupancy on lipogenic gene promoters during fasting/feeding
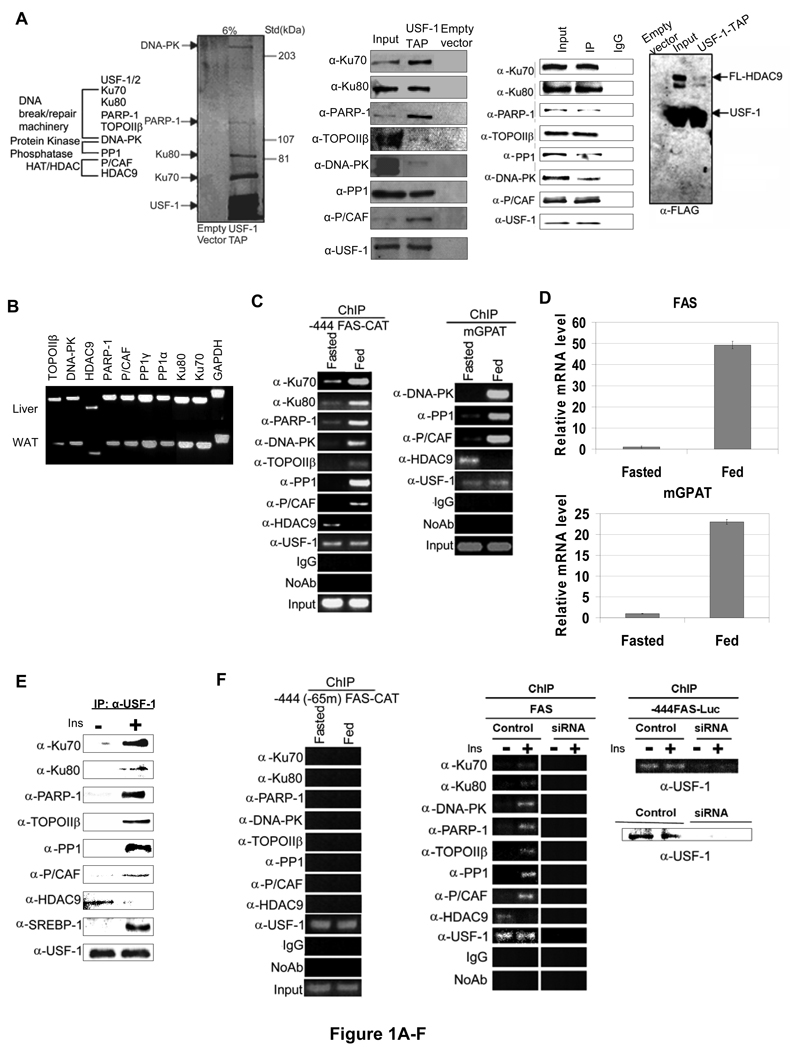
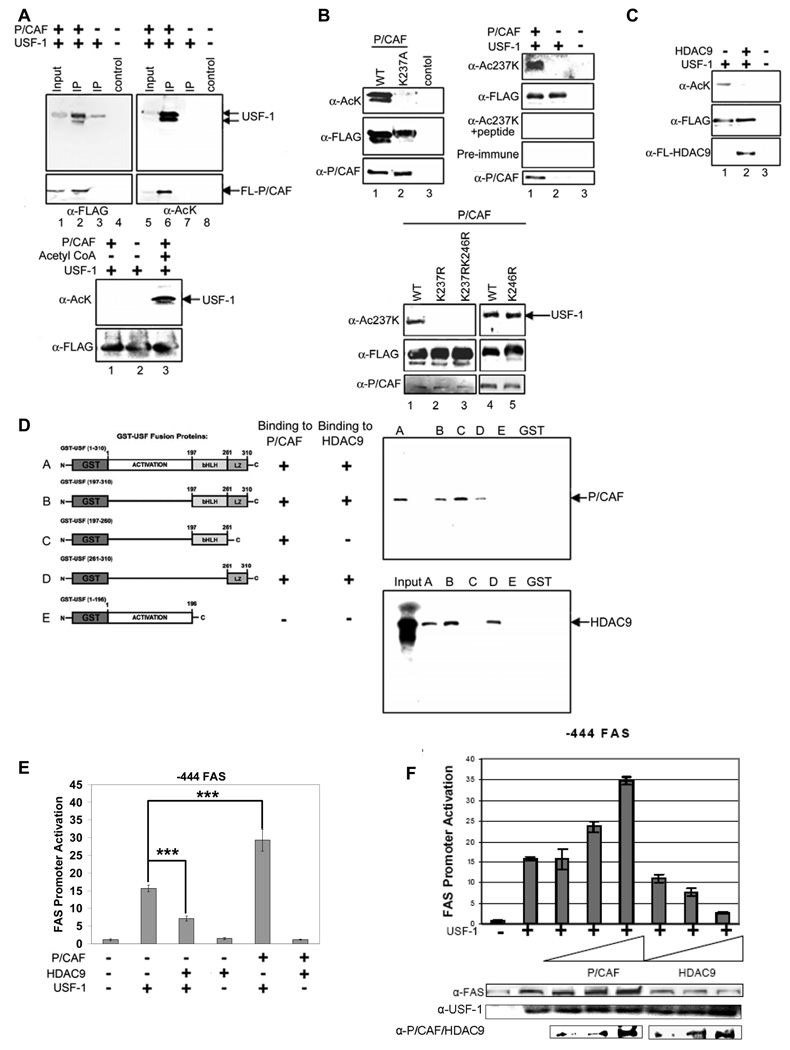
We have previously shown that USF is required for regulation of the FAS promoter activity in fasting/feeding (Wang, and Sul, 1995; Wang, and Sul, 1997). However, USF is constitutively bound to the FAS promoter in both fasted and fed states (Griffin, Wong, et al, 2007; Latasa, Griffin, et al, 2003). We postulated that USF may repress or activate the FAS promoter by recruiting distinct cofactors in fasted and fed conditions. In an attempt to identify those factors that are recruited by USF, we performed tandem affinity purification (TAP) and mass spectrometry (MS) analysis. The USF interacting proteins were purified from nuclear extracts prepared from 293 cells overexpressing USF-1 tagged with streptavidin and calmodulin binding peptides (TAP-tagged) as well as FLAG epitope at its carboxyl terminus. In addition to USF-1 and USF-2, we identified 7 polypeptides in the eluates by MS analysis (Fig. 1A, left panel and S. Table 2). These proteins fall into 3 categories, a) DNA break/repair components DNA-PK and its regulatory subunits, Ku70, Ku80, as well as poly(ADP-ribose) polymerase-1 (PARP-1), and Topoisomerase IIβ (TopoIIβ), b) protein phosphatase PP1, and c) P/CAF which belongs to the histone acetyltransferases (HAT) family. TAP using cells that were first cross-linked by DSP showed identical USF-1 interacting proteins (data not shown).
Figure 1. Purification of USF-1 interacting proteins.
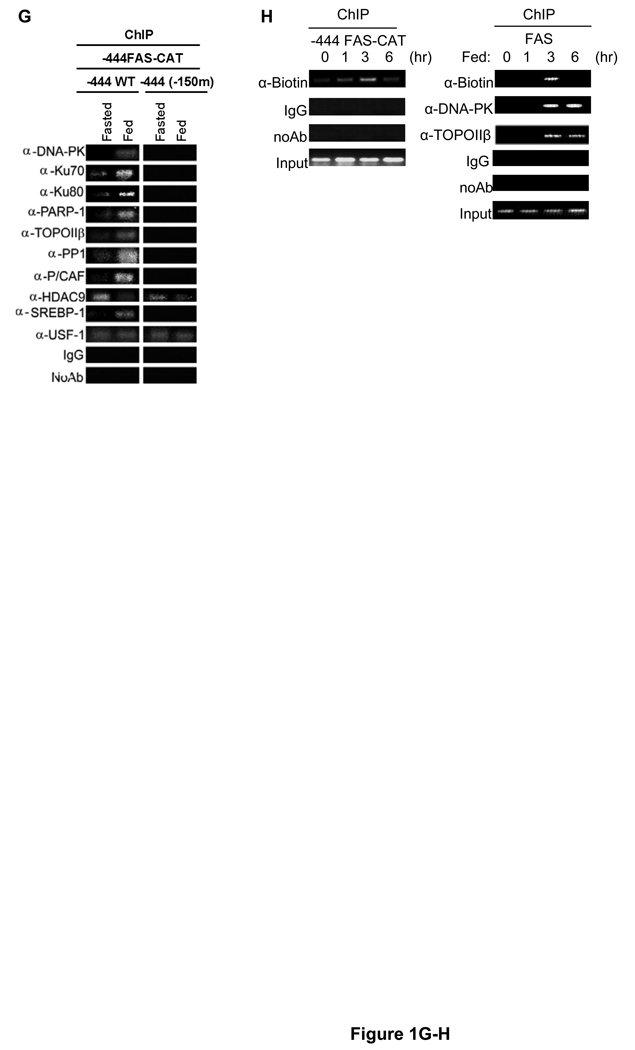
(A) The identities (left) of USF-1-associated polypeptides analyzed by MS are listed. Purified USF-1 interacting proteins were separated by SDS-PAGE for silver staining (2nd left). Immunoblotting of TAP eluates (middle) using antibodies against indicated proteins. Immunoprecipitated USF-1 (2nd left) from 293F cells with monoclonal anti-USF-1 antibodies was Western blotted with indicated antibodies. TAP eluates from 293F cells transfected with USF-1-TAP and HDAC9-FLAG were immunoblotted with indicated antibodies (right). (B) RNA from mouse liver and adipose tissue were used for RT-PCR. (C) ChIP for association of USF-1 interacting proteins to the −444 FAS-CAT promoter (left) or the mGPAT promoter (right) in livers from fasted or fed FAS-CAT transgenic mice (left). (D) FAS and mGPAT expression in liver determined by RT-qPCR. (E) IP of FLAG-tagged USF-1 from HepG2 cells treated with or without 100 nM insulin for 30 min. Immunoprecipitated USF-1 was immunoblotted with indicated antibodies. (F) ChIP for association of USF-1 interacting proteins to the −444(−65m) FAS-CAT (left) promoter in liver or the FAS promoter in HepG2 cells (right) transfected with control or USF-1 siRNA. USF-1 protein levels were analyzed by immunoblotting (bottom right). (G) ChIP for binding of USF-1 interacting proteins to the −444 FAS-CAT (left) and −444(−150m) FAS-CAT (right) promoter regions in mouse liver. (H) ChIP analysis for biotin incorporation into 3'-ends of DNA breaks and DNA-PK and TopoIIβ binding to the FAS-CAT (left) or the endogenous FAS promoter (right) in livers from fasted or fed FAS-CAT transgenic or wild type mice.
We detected at least five of the polypeptides having molecular weights corresponding to the above identified proteins by silver staining of the TAP-eluates separated by SDS-PAGE (Fig. 1A, 2nd left panel). Blue native (BN) gel electrophoresis of the TAP-eluates revealed the presence of a large USF-1 containing complex (S. 1B). Immunoblotting of the eluates using antibodies against each of the 7 polypeptides further confirmed the presence of all 7 polypeptides that were co-purified with TAP-tagged USF-1 (Fig. 1A, 3rd left panel). These identified proteins were specific to USF-1, because none of them were found with the control TAP-tag in silver staining or by immunoblotting. In confirming USF-1 interaction, coimmunoprecipitation followed by immunoblotting revealed the presence of all interacting proteins in endogenous USF-1 immunoprecipitates (Fig. 1A 2nd right panel). Furthermore, GST-pull down assay showed that DNA-PK and PARP-1, but not TopoIIβ, Ku70/Ku80 and PP1, can directly interact with USF-1 (S. 1A).
We also attempted to purify and identify USF interacting proteins by incubating liver nuclear extracts with bacterially expressed TAP-tagged USF immobilized on agarose beads. MS analysis identified an additional USF interacting protein HDAC9, a transcriptional corepressor that belongs to the class II HDAC family, which was co-purified with USF-1 when the nuclear extracts from fasted mice were used (data not shown). The interaction between HDAC9 and USF-1 was confirmed by detection of HDAC9 co-purified with USF-1 by TAP in cells overexpressing HDAC9 and USF-1 (Fig. 1A, right panel). Overall, except P/CAF which has been implicated to function with USF for histone modification in chromosomal silencing (West, Huang, et al, 2004), none of the above proteins have previously been shown to interact with USF.
We next examined expression of these USF interacting proteins in lipogenic tissues, e. g. liver and white adipose tissue (WAT). As shown in Figure 1B, all of the USF interacting proteins were expressed in lipogenic tissues. We then tested whether they occupy the FAS promoter in a fasting/feeding dependent manner. We performed ChIP in livers of fasted and fed transgenic mice expressing a CAT reporter gene driven by the −444 FAS promoter, a minimal FAS promoter sufficient for full response to fasting/feeding and diabetes/insulin treatments (Latasa, Moon, et al, 2000; Latasa, Griffin, et al, 2003; Moon, Latasa, et al, 2000). As shown before, we detected binding of USF in both fasted and fed conditions (Fig. 1C, left panel). In the fasted state, however, we detected the corepressor HDAC9 bound to the FAS promoter, but not other interacting proteins that we identified by TAP-MS. Upon feeding, HDAC9 was no longer bound to the promoter, but the FAS promoter was now occupied by the coactivator P/CAF, DNA break/repair components that include DNA-PK, Ku70/80, PARP-1, TopoIIβ, as well as PP1 (Fig. 1C, left panel). We next questioned whether these USF interacting proteins are also bound to lipogenic gene promoters that are regulated in the same manner as FAS during fasting/feeding. We performed ChIP analysis of the mGPAT promoter using antibodies against proteins that represent each of the 3 categories of the USF interacting proteins. Similar to what we observed with the FAS promoter, USF-1 was bound to the mGPAT promoter in both fasted and fed conditions (Fig. 1C, right panel). Furthermore, as seen with the FAS promoter, HDAC9 was bound to the mGPAT promoter only in fasting, whereas DNA-PK, PPI and P/CAF were bound only in the fed state. We also verified the regulated expression of FAS and mGPAT in these mice. As predicted, FAS and mGPAT mRNA levels were very low in livers of fasted mice, but upon feeding, they were induced drastically to approximately 50 and 25- fold, respectively (Fig. 1D). The similar binding pattern of USF interacting proteins suggests a common mechanism for lipogenic induction involving USF and its interacting proteins in response to feeding. Overall, USF-1 is constitutively bound to the FAS and other lipogenic promoters in both metabolic states, while USF interacting proteins are bound in a fasting/feeding dependent manner. We next investigated whether this is due to the differential interaction of USF with these proteins by employing insulin responsive HepG2 cells overexpressing USF-1. The levels of various USF interacting proteins in HepG2 cells were similar when cells were cultured in the presence or absence of insulin (S. 1D). We then compared the interaction of various proteins with USF-1 by coimmunoprecipitation. As shown in Figure 1E, in insulin treated cells, USF-1 preferentially coimmunoprecipitated with those proteins that were found to be bound to the lipogenic promoters in fed condition, whereas in the absence of insulin USF-1 preferentially interacted with HDAC9.
To further address whether the binding of the various interacting proteins to the FAS promoter is USF dependent, we performed ChIP in transgenic mice containing CAT driven by the −444 FAS promoter with a specific mutation at the USF binding site of −65 E-box (−444(−65m)). We previously have shown that, due to the loss of the critical −65 E-box where USF binds, the −444(−65m) FAS promoter does not have any activity although the promoter contains an additional USF binding site at −332 (Latasa, Griffin, et al, 2003). As shown in Figure 1F, left panel, we did not detect binding of any of the USF-1 interacting proteins to this FAS promoter containing the −65 E-box mutation, even though USF-1 was bound to the −332 E-box in both fasted and fed states. Furthermore, siRNA-mediated knock-down of USF-1 prevented recruitment of the USF-1 interacting proteins to the wild type FAS promoter (Fig. 1F, right panel). Overall, these data clearly demonstrate requirement of USF-1 binding to the −65 E-box for recruitment of various proteins to the FAS promoter.
We previously reported the presence of SRE nearby the E-box in the various lipogenic promoters that are coordinately induced by feeding/insulin (Griffin, Wong, et al, 2007). We also reported that USF binding to the E-box is necessary for SREBP binding to the SRE and that USF and SREBP-1 directly interact in the transcriptional activation of lipogenic genes (Latasa, Griffin, et al, 2003; Griffin, Wong, et al, 2007). We, therefore, examined whether the binding of the USF-1 interacting proteins to the FAS promoter is dependent on the SREBP-1 binding to SRE. We performed ChIP in transgenic mice containing CAT driven by the −444 FAS promoter with a specific mutation at the −150SRE (−444(−150m)). As shown in Figure 1G, we could not detect recruitment of the various interacting proteins to the FAS promoter containing the −150 SRE mutation during feeding. Similar results were observed in HepG2 cells transfected with −444(−150m) FAS-Luc or SREBP-1 siRNA. As a control, we examined the p53 promoter that has a proximal E-box but does not respond to feeding/insulin (S. 1C and S. 2D). upon insertion of an artificial SRE, however, the p53 promoter was activated by USF-1 recruiting various interacting proteins in response to insulin (S. 2D). These data demonstrate that nearby SRE is critical for USF-1 to recruit various interacting proteins.
As shown, the components of DNA break/repair machinery were recruited to the FAS promoter in fed state. In this regard, it has recently been reported that a transient DNA break is required for estrogen receptor regulated transcription (Ju, Lunyak, et al, 2006). We, therefore, investigated possible DNA breaks in the FAS promoter region by employing biotin-dUTP and terminal deoxynucleotidyl transferase (TdT) to tail the 3'-ends of DNA and subsequent ChIP analysis to detect DNA breaks using anti-biotin antibodies. We clearly detected DNA breaks in the −444 FAS-CAT as well as the endogenous FAS promoters after 3 hrs of feeding, a time point when binding of DNA-PK and TopoIIβ was detected (Fig. 1H). The observed DNA breaks in the FAS promoter region preceded the maximal FAS transcription that occurs 6 hrs after the start of feeding (Paulauskis, and Sul, 1989).
Feeding induced phosphorylation of USF-1
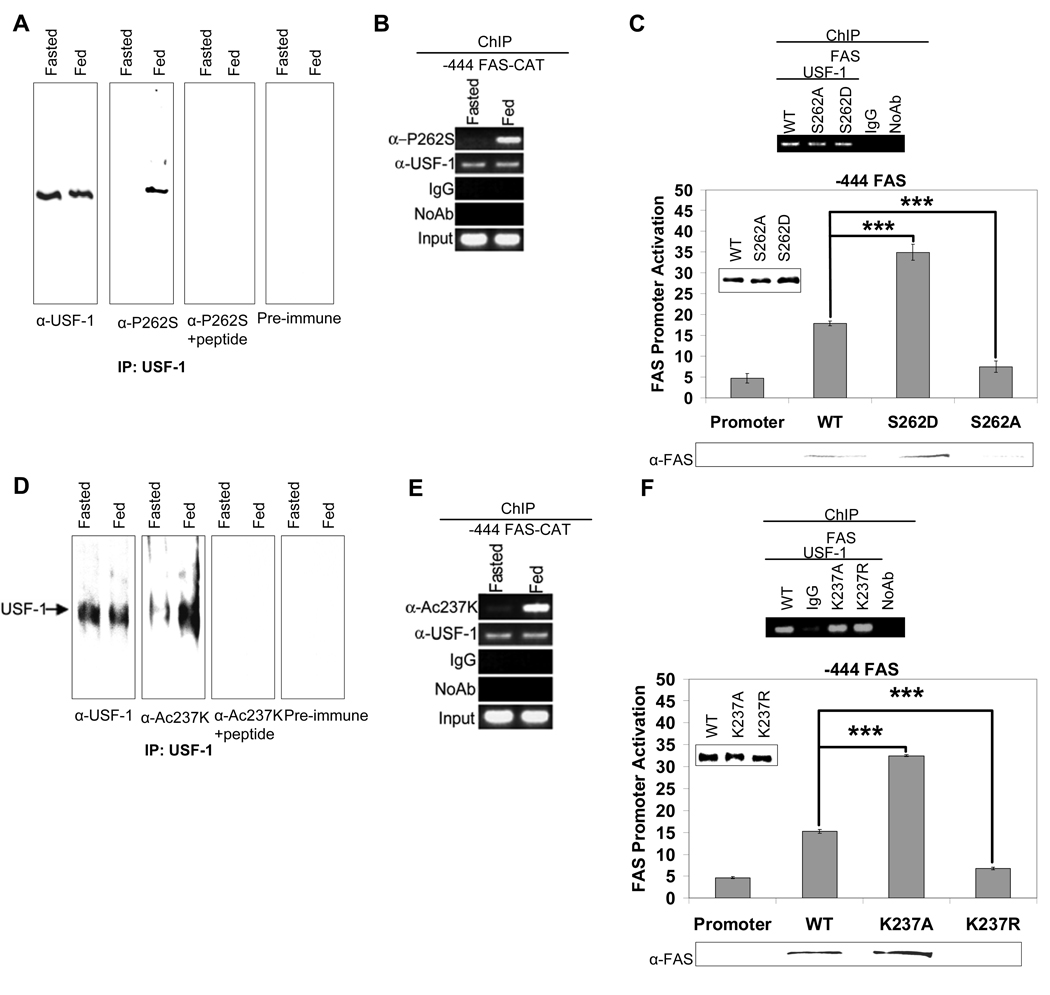
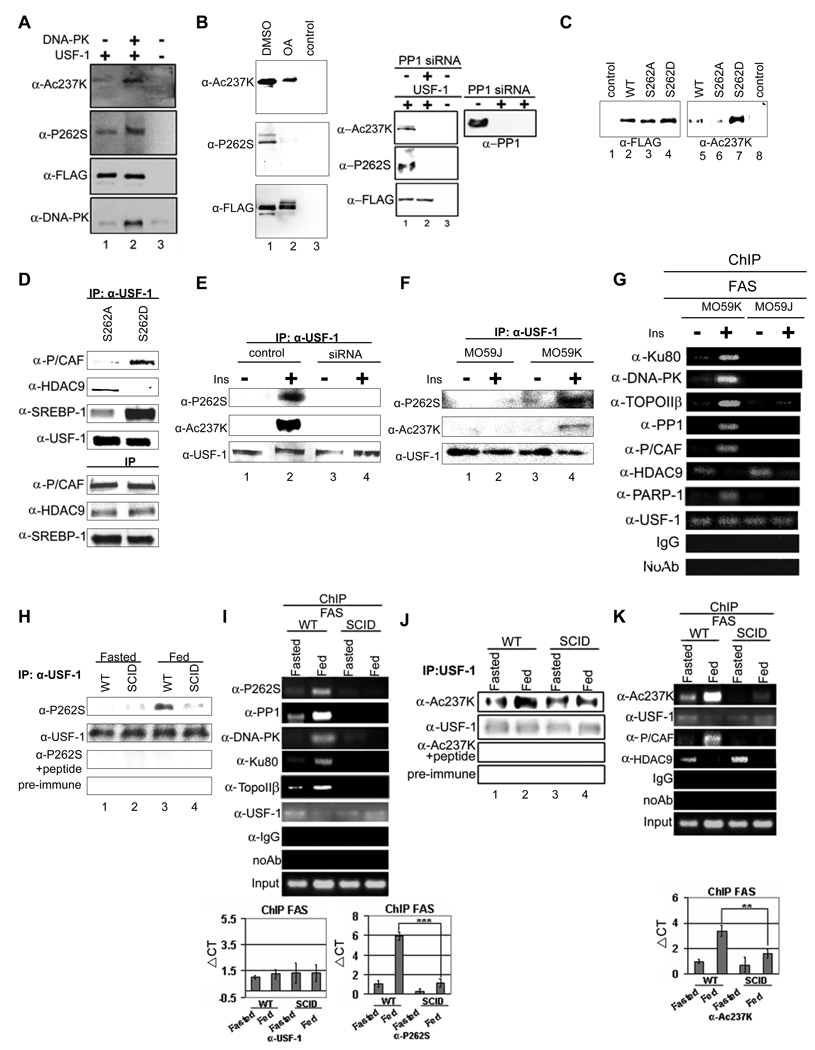
The fact that USF-1 remains bound to the FAS promoter irrespective of the metabolic state but USF-1 interacts with various proteins in a fasting/feeding dependent manner prompted us to investigate whether USF-1 is posttranslationally modified. To search for the existence of such site(s), we immunoprecipitated USF-1 from liver nuclear extracts of fasted or fed mice and performed MS analysis. Remarkably, we detected a phosphoserine residue at the S262 of USF-1 only in nuclear extracts from fed mice. We then raised antibodies against USF-1 peptide containing phosphorylated S262 (This antibody is referred as anti-P-USF-1) to examine S262 phosphorylation of USF-1. We detected higher S262 phosphorylation of USF-1 in the fed state than in the fasted state (Fig. 2A, panel 2). Pre-incubation of the anti-P-USF-1 with the phosphorylated peptide abolished the detection of S262 phosphorylation in fed state, an indication of the specificity of the antibodies (Fig. 2A, panel 3). We next asked whether USF-1 bound to the FAS promoter is phosphorylated at S262. ChIP analysis of FAS-CAT promoter using anti-P-USF-1 showed that this specific phosphoUSF-1 occupied the FAS promoter only in the fed state, even though USF-1 occupancy was detected in both fasted and fed conditions (Fig. 2B). Similarly, USF-1 bound to the mGPAT promoter was phosphorylated at S262 in fed state (S. 5D). Next, to test the functional significance of this S262 phosphorylation, we expressed FLAG-tagged-USF-1 containing a mutation at the S262 (S262D or S262A). We detected similar protein levels of transfected S262 mutants and wild type (WT) USF-1 (Fig. 2C, bottom panel). ChIP analysis of the FAS promoter using anti-FLAG antibodies showed no differences in the promoter occupancy between WT and FLAG-tagged USF-1 proteins harboring S262 mutation (Fig. 2C, top panel). However, the S262D mutant that mimics hyperphosphorylation activated the FAS promoter at a much higher level than WT USF-1, whereas the nonphosphorylatable S262A mutant could no longer activate the FAS promoter (Fig. 2C, bottom panel). By immunoblotting lysates from these cells, we also detected changes in FAS protein levels corresponding to the FAS promoter activity (Fig. 2C, bottom panel). Taken together, these data suggest that the feeding dependent phosphorylation of USF-1 at S262 is linked to FAS promoter activation.
Figure 2. Feeding-induced S262 phosphorylation and K237 acetylation of USF-1.
(A) USF-1 immunoprecipitates using monoclonal anti-USF-1 antibodies was Western blotted with polyclonal anti-USF-1 or anti-P-USF-1 antibodies. Immunoblotting with anti-P-USF-1 in the presence of peptide or with pre-immune serum are shown as controls. (B) ChIP for indicated proteins binding to the −444 FAS-CAT promoter in mouse liver. (C) ChIP using anti-FLAG antibodies (top) for WT USF-1 and S262 USF-1 mutant association to the FAS promoter in 293FT cells. The FAS promoter activity (bottom) in cells transfected with −444 FAS-Luc and WT USF-1 or S262 USF-1 mutants was monitored. Immunoblotting for protein levels of WT, S262 USF-1 mutants (insert) and FAS are shown. (D) Immunoprecipitated USF-1 was Western blotted with indicated antibodies. (E) ChIP for binding of indicated proteins to the −444 FAS-CAT promoter in mouse liver. (F) ChIP (top) for association of WT USF-1 and K237 USF-1 mutant to the FAS promoter in 293F cells. The promoter activity of the −444 FAS-Luc (bottom) in cells transfected with WT USF-1 or K237 USF-1 mutants was measured.
Feeding induced acetylation of USF-1
As shown in Fig. 1, USF-1 interacting proteins, HDAC9 and P/CAF, occupied the lipogenic gene promoters in fasted and fed states, respectively. This suggests that acetylation of USF-1 might be regulated in a fasting/feeding dependent manner. During the MS analysis of USF-1 for posttranslational modification(s), we identified two acetylated lysine residues at K237 and K246 of USF-1. However, when we performed MS analysis of immunoprecipitates from cells cotransfected with USF-1 and P/CAF that interacts with USF in the fed state, we detected acetylation of only K237, but not K246. We, therefore, raised antibodies against USF-1 peptide containing acetylated K237 (anti-Ac-USF-1) and used them to compare acetylation of USF-1 at K237 in fasted and fed states. Indeed, we detected higher K237 acetylation of USF-1 in the fed state (Fig. 2D, panel 2) compared to the fasted state. Pre-incubation of anti-Ac-USF-1 with the acetylated peptide abolished detection of K237 acetylation in the fed state indicating the specificity of the antibodies (Fig. 2D, panel 3). We next monitored the occupancy of the acetylated USF-1 on the FAS promoter. ChIP analysis of the FAS-CAT promoter using anti-Ac-USF-1 showed that the USF-1 bound to the FAS promoter was acetylated at K237 only in the fed state, even though USF-1 was bound to the FAS promoter in both fasted and fed states (Fig. 2E). These data indicate that K237 is likely to be a regulatory site of USF-1 during fasting/feeding and its acetylation might be catalyzed by P/CAF in the fed state.
To test the functional effects of this putative acetylation site, we expressed FLAG-tagged USF-1 with a mutation at the K237 (K237A or K237R) in 293 cells. ChIP analysis of the FAS promoter using anti-FLAG antibodies showed no difference in recruitment among WT USF-1, FLAG tagged USF-1 with the K237A mutation that mimics hyperacetylation, and the FLAG tagged USF-1 with nonacetylatable K237R mutation (Fig. 2F, top panel). However, in the FAS promoter-reporter assay, cotransfection of the K237A mutant activated the FAS promoter at a much higher level than WT USF-1, whereas 237R mutant could no longer activate the FAS promoter (Fig. 2F, bottom panel). These differences in promoter activation were reflected in the FAS protein levels upon immunoblotting of cell lysates (Fig. 2F, bottom panel). These data suggest that the feeding dependent acetylation of USF-1 is responsible for FAS promoter activation in the fed condition.
DNA-PK mediates feeding-dependent phosphorylation of USF-1
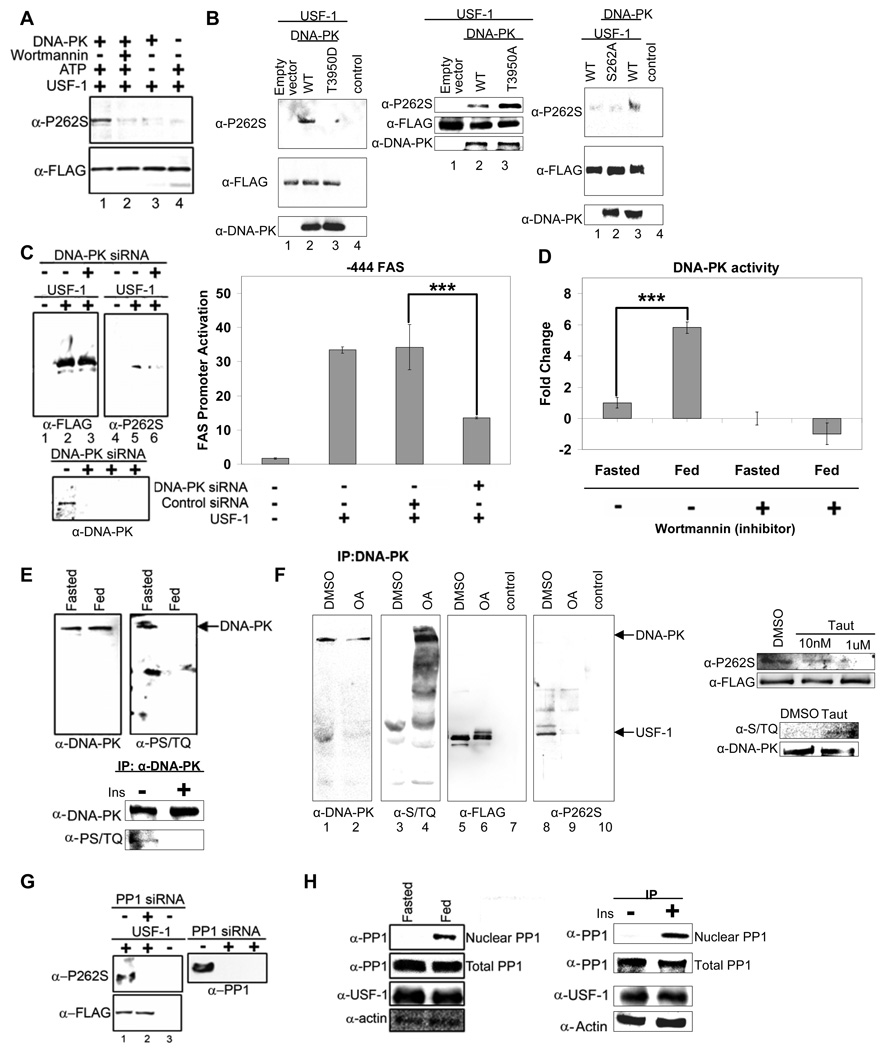
The first step in understanding how the feeding dependent phosphorylation of USF-1 activates the FAS promoter would be to identify the kinase that catalyzes this S262 phosphorylation. Search of numerous phosphoprotein databases predicted that a member of the PIKK family of kinases likely phosphorylates the S262 site. DNA-PK is a multimeric nuclear serine/threonine protein kinase, composed of the DNA-PK catalytic subunit and the Ku70/Ku80 regulatory subunits (Collis, DeWeese, et al, 2005). We found all of the DNA-PK subunits to be the USF-1 interacting proteins bound to the FAS promoter in the fed state. Therefore, to examine if S262 of USF-1 is a target of DNA-PK, we performed in vitro phosphorylation of bacterially expressed USF-1 by DNA-PK. Indeed, using anti-P-USF-1, we could easily detect S262 phosphorylation of USF-1 by DNA-PK (Fig. 3A, lane 1) in vitro. S262 phosphorylation was abolished when wortmannin was added at a concentration (Hashimoto, Rao, et al, 2003) that is effective to inhibit DNA-PK activity (Fig. 3A, lane 2). We also observed that S262 phosphorylation was dependent on DNA-PK concentrations (S. 3A). Based on these results and the fact that DNA-PK is associated with USF-1 in the fed state, we conclude that the S262 of USF-1 is a specific target of DNA-PK.
Figure 3. Feeding dependent S262 phosphorylation of USF-1 is mediated by DNA-PK that is dephosphorylated/activated in feeding.
(A) Bacterially expressed USF-1 was incubated with DNA-PK in the presence or absence of ATP or wortmannin (2 uM). (B) IP of cells overexpressing WT USF-1 and DNA-PK, T3950D DNA-PK (kinase dead) (left), T3950A DNA-PK or empty vector (middle) or overexpressing S262A USF-1 mutant with or without DNA-PK (right), using FLAG antibodies. DNA-PK protein levels were analyzed by immnoblotting. (C) IP (left) of cells cotransfected with USF-1, control siRNA or DNA-PK siRNA. The promoter activity of −444 FAS-Luc in cells transfected with WT USF-1 or siRNA was measured (right). (D) DNA-PK activity in liver nuclear extracts pretreated with or without wortmannin (2uM) was assayed using[ γ32P] ATP and biotinylated p53 peptide as substrate. (E) IP of liver nuclear extracts (top) or nuclear extracts from HepG2 cells (bottom) using anti-DNA-PK antibodies. (F) Total and phosphorylated DNA-PK levels were analyzed by westernblotting. IP of USF-1-FLAG from cells overexpressing USF-1 treated with either control DMSO or OA at 1uM (left) and Taut at indicated concentrations for 2 hrs (right). DNA-PK phosphorylation was detected from cells treated with Taut at 1uM for 2hrs. (G) IP of cells cotransfected with USF-1 and control or PP1 siRNA with anti-USF-1 antibodies. PP1 protein levels were analyzed by Western blotting as shown. (H) IP of nuclear extracts or total lysates from livers (left) or HepG2 cells (right) with anti-PP1 antibodies. USF-1 and β-actin protein levels were analyzed by Western blotting.
We next tested S262 phosphorylation of USF-1 by DNA-PK in cultured cells. We overexpressed USF-1 along with WT DNA-PK or kinase dead DNA-PK containing a T3950D mutation (this hyperphosphorylation mimicking mutation causes a reduction in its kinase activity (Douglas, Cui, et al, 2007)) or constitutive active DNA-PK containing a T3950A mutation that mimics dephosphorylation. We detected higher S262 phosphorylation of USF-1 immunoprecipitated from cells overexpressing WT DNA-PK (Fig. 3B, left panel, lane 2) but not from cells expressing DNA-PK with T3950D mutation (lane 3) or control cells (lane 1). Furthermore, we detected even higher S262 phosphorylation of USF-1 from cells expressing DNA-PK with T3950A mutation compared to WT DNA-PK expressing cells (Fig. 3B, middle panel, lane 3). Next, to investigate whether the DNA-PK mediated phosphorylation of USF-1 is S262 specific, we overexpressed WT USF-1 or the S262A mutant along with DNA-PK. WT USF-1 but not USF-1 containing S262A mutation was detected to have higher phosphorylation upon cotransfection with DNA-PK (Fig. 3B, right panel, lane 2 and 3). To further verify the role of DNA-PK in S262 phosphorylation, we performed siRNA-mediated knockdown of DNA-PK. Transfection of DNA-PK siRNA into 293 cells caused more than an 80% decrease in DNA-PK levels (Fig. 3C, bottom panel). We detected low but detectable S262 phosphorylation of USF-1, probably by the remaining DNA-PK in the control siRNA transfected cells (Fig. 3C, left panel, lane 5). But S262 phosphorylation was significantly reduced in the DNA-PK siRNA transfected cells (lane 6). Furthermore, FAS promoter activity in DNA-PK siRNA transfected cells was reduced by 65% compared to control siRNA transfected cells (Fig. 3C, right panel). The reduction in FAS promoter activity was similar to that observed upon transfection of nonphosphorylatable S262A USF-1 mutant (Fig. 2C). These results demonstrate that S262 phosphorylation of USF-1 is mediated by DNA-PK.
PP1 mediated dephosphorylation/activation of DNA-PK causes USF-1 phosphorylation upon feeding
We found that DNA-PK phosphorylates USF-1 at S262 and that S262 phosphorylation is lower in the fasted state but increases upon feeding. This prompted us to ask if changes in DNA-PK activity account for the differences in S262 phosphorylation during fasting/feeding. Using the specific DNA-PK substrate, a biotinylated p53 peptide, we compared DNA-PK activity in liver nuclear extracts of fasted or fed mice (Fig. 3D). While total DNA-PK protein levels remained the same (data now shown), DNA-PK activity in the fed state was 6-fold higher than in the fasted state. Wortmannin treatment drastically reduced DNA-PK activity when measured with the DNA-PK specific peptide as a substrate (Fig. 3D). This demonstrates that the kinase activity we detected is attributable to DNA-PK.
DNA-PK activity is known to be regulated by phosphorylation/dephosphorylation, independent of its activation by DNA (Douglas, Moorhead, et al, 2001). Thus, autophosphorylation of DNA-PK results in a decrease in its kinase activity, whereas dephosphorylation by PP1 activates DNA-PK (Douglas, Moorhead, et al, 2001; Douglas, Cui, et al, 2007). Among the PIKK family members, DNA-PK is the only kinase that is activated by dephosphorylation. To examine the involvement of DNA-PK in USF phosphorylation, we first examined the phosphorylation status of DNA-PK in fasted and fed states. DNA-PK phosphorylation was detected using phosphoserine/threonine antibodies that detect autophosphorylation at the S/TQ motifs of DNA-PK. As shown in Figure 3E, top panel, phosphorylation of DNA-PK was higher in the fasted state than in the fed state while DNA-PK protein levels did not change. In addition, we also found DNA-PK phosphorylation was not detectable in insulin treated HepG2 cells, whereas phosphorylation was easily detected in non-insulin treated cells (Fig. 3E, bottom panel).
During the examination of the occupancy of USF interacting proteins, we found that PP1 along with DNA-PK was bound to lipogenic gene promoters in the fed state (Fig. 1C) when lipogenesis is induced. In this regard, PP1 can mediate many of the metabolic effects of insulin. For example, dephosphorylation and activation of glycogen synthase by insulin is through recruitment of PP1 to the glycogen particle in the cytoplasm (Brady, and Saltiel, 2001). It is possible that PP1 which we found to be a USF interacting protein mediates the feeding/insulin signal by dephosphorylating DNA-PK. We therefore tested the S262 phosphorylation status of USF-1 upon treatment with okadaic acid (OA) which is known to prevent dephosphorylation of DNA-PK (Douglas, Moorhead, et al, 2001). As expected, phosphorylation of DNA-PK greatly increased in OA treated cells (Fig. 3F, left panel, lane 4), whereas DNA-PK autophosphorylation was reduced in cells overexpressing PP1γ (S. 3C). We next examined S262 phosphorylation in OA treated cells by Western blotting of immunoprecipitated USF-1 with anti-FLAG or anti-P-USF-1 antibodies. Compared to a single USF-1 band detected in control DMSO treated cells, several USF-1 bands were detected in OA treated cells, suggesting a multisite phosphorylation of USF-1 (Fig. 3F, lane 6). However, S262 phosphorylation of USF-1 that was easily detected in control cells was hardly detectable in OA treated cells (Fig. 3F, lane 9). To further test the specificity of PP1 on S262 phosphorylation status, we also used tautomycin (Taut) which is known to more selectively inhibit PP1. As expected, we easily detected phosphorylated DNA-PK in cells treated with Taut but not in control cells (Fig. 3F, right panel). On the other hand, S262 phosphorylation of USF-1 was detected in control cells as expected but was decreased in cells treated with Taut at 10 nM and was hardly detectable at 1 uM (Fig. 3F, right panel). We also tested the role of PP1 by using the siRNA approach. We transfected USF-1 along with PP1 or control siRNA. Transfection of PP1 siRNA caused more than an 80% decrease in PP1 levels (Fig. 3G). S262 phosphorylation of USF-1 did not increase, but rather, greatly decreased in PP1 knockdown cells (Fig. 3G, lane 2), indicating that PP1 does not directly dephosphorylate S262 phosphorylation. Furthermore, S262 phosphorylation could be restored upon cotransfection of constitutively active DNA-PK (S. 3D). This indicates that S262 phosphorylation is through DNA-PK that is first dephosphorylated/activated by PP1. We next compared the abundance of PP1 in liver nuclear extracts from fasted and fed mice. Indeed, We detected higher levels of PP1 in the nucleus in the fed state than in the fasted state, while PP1 protein levels in total cell lysates as well as PP1 gene expression levels did not change (Fig. 3H, left panel and S. 3E). Similarly, PP1 was not detected in nuclear extracts from control HepG2 cells but was increased upon insulin treatment (Fig. 3H, right panel). Overall, we conclude that the feeding-dependent S262 phosphorylation of USF-1 is mediated by DNA-PK. But, first, DNA-PK is dephosphorylated/activated by PP1 whose level in nucleus increases in response to feeding/insulin.
P/CAF mediated acetylation of USF-1 activates the FAS promoter, whereas HDAC9 mediated deacetylation causes promoter inactivation
Transcription factors recruit coregulators to efficiently and dynamically regulate transcription, and many of these coactivators and corepressors contain acetyltransferase (HAT) and deacetylase (HDAC) activities, respectively. HDAC9 and P/CAF are recruited by, and interact with, USF-1in a fasting/feeding dependent manner. Therefore, we next, examined if acetylation and deacetylation of USF-1 is through P/CAF and HDAC9, respectively. When we cotransfected USF-1 and P/CAF, by using pan-acetyl lysine antibodies, we detected higher acetylation of USF-1 (Fig. 4A, top panel, lane 6). We also performed in vitro acetylation using bacterially expressed USF-1 to investigate if this acetylation was the result of a direct action of P/CAF or an indirect action requiring other cofactors. Figure 4A bottom panel shows that USF-1 was acetylated in vitro by P/CAF (lane 3), while acetylation was not detected in the absence of P/CAF or acetyl CoA (lane 1 and 2). As shown in Figure 2, MS analysis of USF-1 in cells overexpressing P/CAF revealed a regulatory site at K237, the residue that was acetylated upon feeding. To examine if this site was a target of P/CAF, we overexpressed FLAG-tagged WT USF-1 or USF-1 mutated at K237 along with P/CAF. As detected by pan-acetyl lysine antibodies, only WT USF-1 was efficiently acetylated by P/CAF (Fig. 4B, top left panel, lane 1) but the K237A USF-1 mutant was not (lane 2). We next employed anti-Ac-USF-1 antibodies specific for USF-1 acetylated at K237 and detected higher K237 acetylation in cells overexpressing P/CAF (Fig. 4B, top right, lane 1). To further investigate whether P/CAF mediated acetylation of USF-1 is K237 specific, we overexpressed WT USF-1 and various (K237 and K246) USF-1 mutants along with P/CAF. WT and K246R (Fig. 4B, bottom panel, lane 1, 4 and 5) but not K237R or K237R/K246R (lane 2 and 3) of USF-1 were found to be acetylated upon cotransfection with P/CAF, demonstrating that acetylation of K237 but not K246, is mediated by P/CAF.
Figure 4. Acetylation of K237 of USF-1 by P/CAF and deacetylation by HDAC9.
(A) IP of cells overexpressing USF-1 and P/CAF with FLAG antibodies (top). Bacterially expressed USF-1 was in vitro acetylated with P/CAF (bottom). (B) IP of WT USF-1 or K237A USF-1 from cells transfected with P/CAF using FLAG antibodies (top left). P/CAF protein levels were analyzed by immunoblotting. IP of cells transfected with USF-1 and P/CAF (top right) or cells transfected with P/CAF along with WT USF-1 or USF-1 mutants (bottom). (C) IP of cells overexpressing USF-1 and P/CAF with HDAC9 using FLAG antibodies. (D) Bacterially expressed USF-1-GST was incubated with in vitro translated 35S-labeled P/CAF and HDAC9 before subjecting to GST-pull down. Purified USF-1 was subjected to autoradiography for labeled proteins. GST alone was used as a control. (E) The promoter activity of the -444 FAS-Luc upon cotransfection of USF-1 along with P/CAF or HDAC9. (F) The promoter activity of −444 FAS-Luc upon cotransfection of USF-1 along with increasing amount of P/CAF or HDAC9 was measured. Total cell lysates were immunoblotted for indicated proteins.
Since HDAC9 was recruited by USF-1 and bound to lipogenic gene promoters only in the fasted state, we speculated that HDAC9 would be an ideal candidate to remove the P/CAF mediated acetylation of USF-1 in the fed state. We transfected USF-1 and P/CAF along with HDAC9 or a control empty vector into 293 cells. We detected decrease in P/CAF catalyzed acetylation of USF-1 in cells cotransfected with HDAC9 (Fig. 4C, lane 2). Furthermore, we detected significant HDAC9 protein levels in liver nuclear extracts from fasted, but not fed, mice or in nuclear extracts of HepG2 cells cultured in the absence, but not in the presence, of insulin (S. 4A). These experiments indicate that, in the fasted state, nuclear HDAC9 is in higher abundance and is recruited to the FAS promoter to deacetylate USF-1.
We found by GST-pull down that USF-1 can directly interact with P/CAF and HDAC9 (S. 4C). We then dissected the domains of USF-1 required for interaction with P/CAF and HDAC9. As shown in Figure 4D, the bHLH domain of USF-1, the domain containing K237 that is acetylated by P/CAF, was sufficient for the interaction with P/CAF although the leucine-zipper (LZ) domain could weakly interact with P/CAF. On the other hand, for the USF-1 interaction with HDAC9, the LZ domain of USF-1 was sufficient for its interaction with HDAC9. Thus, the domains of USF-1 required for interaction are in proximity to K237, the residue modified by these HAT/HDAC.
Next, to address the functional significance of HDAC9 and P/CAF, we cotransfected the −444 FAS-Luc with USF-1 along with HDAC9 or P/CAF for FAS promoter-luciferase reporter assays. Cotransfection of USF-1 together with HDAC9 resulted in a 50% decrease in FAS promoter activity in a fashion similar to that we detected upon cotransfection of USF-1 containing a K237R mutation (Fig. 4E and Fig. 2F). In contrast, the expression of USF-1 with P/CAF resulted in a 2-fold higher promoter activity in a manner similar to that observed upon cotransfection of USF-1 containing the K237A mutation (Fig. 4E and Fig. 2F). Furthermore, cotransfection of P/CAF enhanced, while cotransfection of HDAC9 suppressed, USF-1 activation of the FAS promoter in a dose-dependent manner (Fig. 4F). We detected changes in FAS protein levels parallel to the FAS promoter activity. In addition, cotransfecting P/CAF or HDAC9 with USF-1 containing K237A or K237R mutation did not change the FAS promoter activity or FAS protein levels (S. 4E). These data indicate that acetylation and deacetylation of USF-1 catalyzed by P/CAF and HDAC9, respectively, function as a dynamic switch for the transition between fasting/feeding in the FAS promoter regulation.
Phosphorylation dependent acetylation of USF-1
Crosstalk between phosphorylation and acetylation has been previously recognized in the function of some transcription factors. Since USF-1 is both phosphorylated and acetylated at nearby sites and these posttranslational modifications are critical for USF-1 function in FAS promoter activation, we tested whether an increase in S262 phosphorylation of USF-1 could affect K237 acetylation. We cotransfected USF-1 and DNA-PK and examined S262 phosphorylation and K237 acetylation of USF-1. If S262 phosphorylation affects acetylation, cotransfection of DNA-PK would cause not only S262 phosphorylation of USF-1, but also K237 acetylation. Indeed, as shown in Figure 5A, S262 phosphorylation of USF-1 upon DNA-PK transfection strongly enhanced USF-1 acetylation at K237 (lane 2). Conversely, we examined the acetylation status of USF-1 upon OA treatment or siRNA mediated knockdown of PP1. We detected a significant level of K237 acetylation of USF-1 in control cells, which was reduced in OA treated cells (Fig. 5B, left panel, lane 2). Likewise, K237 acetylation of USF was high in control cells but was reduced to an undetectable level in PP1 siRNA transfected cells (right panel, lane 1). Inactivation of PP1 by OA treatment or siRNA mediated knockdown of PP1 caused phosphorylation/inactivation of DNA-PK resulting in reduced S262 phosphorylation of USF-1. This suggests that S262 phosphorylation brings about K237 acetylation. We then asked whether phosphorylation of USF-1 at S262 could affect USF-1 acetylation status by transfecting FLAG-tagged WT USF-1 or S262 mutants and examining the K237 acetylation status of the various USF-1 forms. We found that the S262A mutant had the lowest K237 acetylation among the three USF-1 forms (Fig. 5C, lane 6), whereas the S262D mutant displayed the highest acetylation, to a level significantly higher than WT USF-1 (Fig. 5C, lane 7). Overall these results demonstrate phosphorylation dependent acetylation of USF-1.
Figure 5. Feeding/insulin induced phosphorylation and acetylation of USF-1 are greatly reduced in DNA-PK deficiency.
(A) IP of FLAG-tagged USF-1 from cells transfected with DNA-PK using FLAG antibodies. Empty vector was used as control. (B) IP with FLAG antibodies of USF-1-FLAG from DMSO or OA treated cells overexpressing USF-1 (left) and from cells cotransfected with USF-1 and control or PP1 siRNA (right). (C) IP with FLAG antibodies of 293F cells transfected with FLAG-tagged WT USF-1 or S262 USF-1 mutants. Nuclear extracts from non-transfected cells were used as a control. (D) IP of USF-1-FLAG from HepG2 cells transfected with FLAG-tagged S262 USF-1 mutants. Immunoprecipitated USF-1 was immunoblotted with indicated antibodies (top). Total protein levels were also analyzed by immnnoblotting (bottom). (E) IP of nuclear extracts from HepG2 cells transfected with control or DNA-PK siRNA using monoclonal anti-USF-1 antibodies. (F) IP of nuclear extracts from M059J or M059K cells using anti-USF-1 antibodies. (G) ChIP for binding of indicated proteins to the FAS promoter in M059J or M059K cells. (H) IP of liver nuclear extracts from WT and SCID mice using monoclonal anti-USF-1 antibodies. (I) ChIP for indicated protein association to the FAS promoter in mouse liver. ChIP samples were analyzed by semi-quantitative PCR (top) or qPCR (bottom). (J) IP of liver nuclear extracts using anti-USF-1 antibodies. (K) ChIP for indicated protein association to the FAS promoter in liver.
We next attempted to examine the mechanism underlying S262 phosphorylation-dependent acetylation of USF-1. The simplest hypothesis would be that S262 phosphorylation/dephosphorylation affects recruitment of P/CAF and HDAC9 causing acetylation and deacetylation of K237 of USF-1, respectively. We tested this by examining the interaction of USF-1 with P/CAF and HDAC9 by coimmunoprecipitation and ChIP assays using S262 USF-1 mutants. Coimmunoprecipitation assay showed that the S262D mutant preferentially interacted with P/CAF in comparison to the S262A mutant (Fig. 5D). On the other hand, compared to the S262D mutant, the S262A mutant preferentially interacted with HDAC9, although the signal was low probably due to the low HDAC9 levels in the nucleus. We also examined whether S262 mutation of the USF-1 affects interaction of USF with SREBP-1 that we previously reported (Griffin, Wong, et al, 2007). Results showed that the S262D USF mutant preferentially interacted with SREBP-1 when compared to the S262A mutant. Taken together, these results show that the phosphorylation dependent acetylation of USF-1 functions as a sensitive molecular switch, detecting nutritional status during the transition of fasting/feeding.
Feeding/insulin-dependent phosphorylation/acetylation of USF-1 are diminished in DNA-PK deficiency
To further demonstrate the requirement of DNA-PK in mediating the feeding/insulin dependent phosphorylation/acetylation of USF-1, we transfected DNA-PK siRNA into HepG2 cells. Insulin treatment of these cells markedly increased S262 phosphorylation as well as K237 acetylation in control siRNA-transfected cells, while USF-1 levels remained the same (Fig. 5E, lane 1 and 2). In contrast, insulin mediated S262 phosphorylation/K237 acetylation of USF-1 in cells transfected with DNA-PK siRNA was markedly reduced and undetectable (Fig. 5E, lane 3 and 4). We next employed the human glioblastoma cell line, M059J, that lacks DNA-PKcs and DNA-PK activity, along with the related M059K cells containing WT DNA-PK (Feng, Park, et al, 2004) as a control. Treatment of M059K cells with insulin increased S262 phosphorylation and K237 acetylation of USF-1 (Fig. 5F, lane 3 and 4), whereas insulin treatment of M059J cells did not bring any significant increase in USF modifications (lane 1 and 2). These data demonstrate that DNA-PK is required not only for S262 phosphorylation but also for K237 acetylation of USF-1 upon insulin treatment.
By ChIP, we also tested whether recruitment of various proteins to FAS promoter by USF is dependent on DNA-PK (Fig. 5G). Those proteins that were found to be bound to the lipogenic gene promoters in the fed condition were recruited by USF in insulin treated M059K cells, but not in the DNA-PK deficient M059J cells. In the absence of insulin, HDAC9 was recruited by USF in both M059J and M059K cells, mostly likely because cytoplasmic export of HDAC9 was not affected by DNA-PK. Similarly, coimmunoprecipitation showed that USF-1 can interact better with various partners in insulin treated M059K but not in M059J cells (S. 5A). Furthermore, USF-1 interaction and recruitment of various proteins were abolished in 293 cells upon treatment with Taut that inhibits DNA-PK activity (S. 5B & 5C). Overall these results show that the recruitment of various proteins by USF-1 in feeding/insulin treatment is dependent on DNA-PK and DNA-PK mediated S262 USF-1 phosphorylation.
We next examined in vivo the DNA-PK mediated and feeding dependent S262 phosphorylation/K237 acetylation of USF-1, by employing DNA-PK deficient SCID (Severe Combined Immune Deficiency) mice. A spontaneous mutation in the DNA-PK gene causes a 90% reduction of the protein in SCID mice (Danska, Holland, et al, 1996), producing a phenotype highly reminiscent of DNA-PK null mice (Gao, Chaudhuri, et al, 1998). Indeed, feeding-induced phosphorylation of USF-1 at S262 was greatly reduced in SCID mice compared to that observed in WT mice (Fig. 5H, lane 4 and 3). ChIP analysis showed that the USF-1 detected on the FAS promoter in SCID mice in the fed state was not phosphorylated at S262 compared to the phosphoUSF-1 detected on the promoter in WT mice (Fig. 5I). Similarly, USF-1 bound to the mGPAT promoter was not phosphorylated at S262 in SCID mice in the fed state (S.5D). Furthermore, we could not detect occupancy by DNA-PK, Ku80, TopoIIβ and PP1 on the FAS promoter in SCID mice upon feeding (Fig. 5I). Since K237 acetylation of USF-1 is dependent on S262 phosphorylation as shown above, we investigated whether K237 acetylation was also reduced in SCID mice. We found that K237 acetylation upon feeding was greatly reduced in SCID mice compared to that detected in WT mice (Fig. 5J, lane 4 and 2). Furthermore, ChIP analysis of the FAS promoter in WT and SCID mice showed that the acetylated USF-1 bound to the FAS promoter in the fed state was greatly reduced in SCID mice (Fig. 5K) compared to WT mice. This decrease in acetylated USF-1 bound to the FAS promoter could be explained by the decreased recruitment of P/CAF by USF-1 (Fig. 5K). HDAC9 binding was not different between WT and SCID mice probably because cytoplasmic export of HDAC9 was not affected in SCID mice. Overall, these results show in vivo the requirement of DNA-PK for S262 phosphorylation of USF-1 and for P/CAF mediated K237 acetylation leading to transactivation of the FAS promoter.
Feeding-dependent activation of the FAS gene and de novo lipogenesis are diminished in DNA-PK-deficient SCID mice
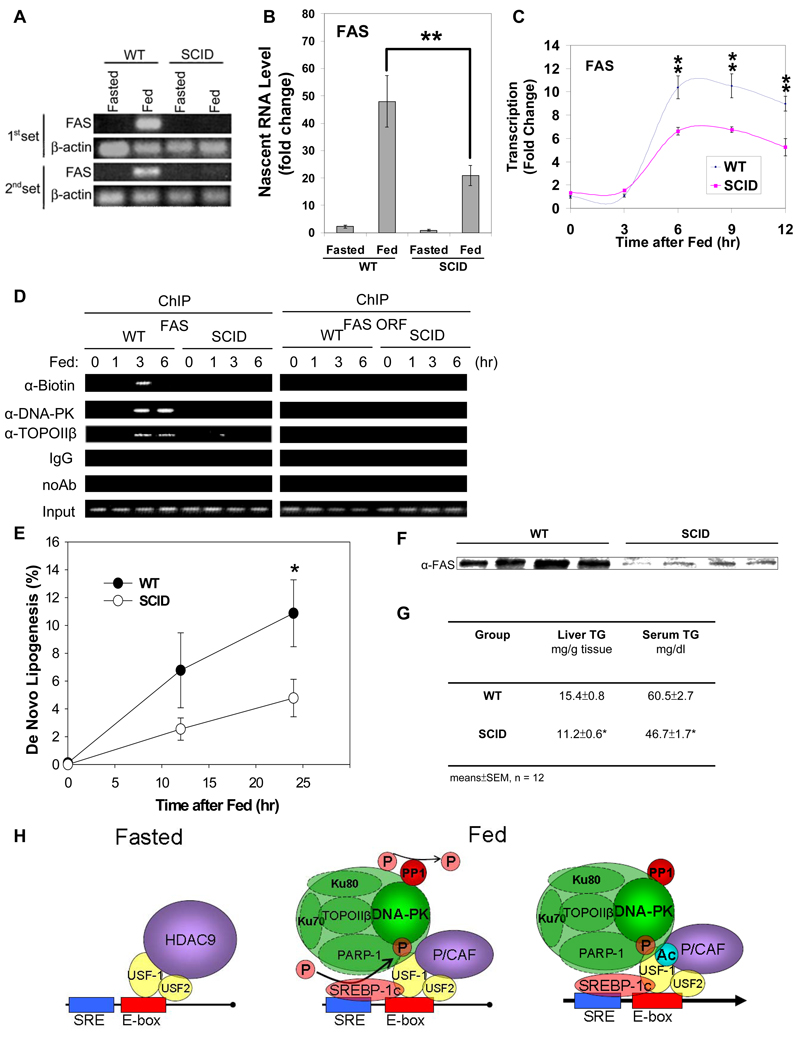
Since phosphorylation/acetylation of USF-1 for FAS promoter activation is through the PP1/DNA-PK mediated signaling pathway, we assessed the transcriptional activation of the FAS gene in DNA-PK deficient SCID mice during fasting/feeding. We first measured the nascent FAS RNA levels in liver nuclei from WT or SCID mice that were either fasted or fed (Fig. 6A) by RT-PCR. In WT mice, the FAS nascent RNA was not detectable in fasting but increased drastically upon feeding. On the other hand, the nascent FAS RNA was barely detectable in either fasted or fed SCID mice. RT-qPCR analysis indicated a 50-fold increase in FAS nascent transcript in WT mice upon feeding, while in SCID mice the increase was 20-fold, representing approximately a 50–60% decrease (Fig. 6B). We also investigated the effect of DNA-PK deficiency on FAS transcription by measuring the rate of transcription. Nuclei from WT and SCID mice upon feeding at various time points were used for run-on assays. The rate of transcription measured by RT-qPCR of the newly extended nascent transcripts increased up to 10-fold in WT mice 6 hrs after feeding, a result consistent with our previously published study (Paulauskis, and Sul, 1989). However, FAS transcription in SCID mice increased only by 6-fold, a 40% reduction in transcriptional activation compared to WT mice (Fig. 6C). Since we observed transient DNA breaks in the FAS promoter region that preceded transcriptional activation upon feeding (Fig. 1I), we next examined whether the DNA break occurs in the FAS promoter region in SCID mice, but could not detect transient DNA breaks, which we clearly detected in WT mice after 3 hrs of feeding (Fig. 6D). Furthermore, unlike in WT mice, ChIP analysis did not show binding of DNA-PK or TopoIIβ to the FAS promoter region in SCID mice. Since TopoIIβ catalyzes the DNA breaks, the absence of DNA breaks in the FAS promoter region in SCID mice can be attributed to the impaired TopoIIβ recruitment that is dependent on the DNA-PK catalyzed phosphorylation of USF-1 (Ju, Lunyak, et al, 2006). Thus, not only the diminished acetylation of USF-1, but also the impaired recruitment of the DNA break/repair components, which is dependent on USF-1 phosphorylation, probably contributed to the attenuated feeding-dependent transcriptional activation of the FAS gene in SCID mice. Overall, these results clearly show in vivo the critical role of DNA-PK in the activation of the FAS transcription by feeding.
Figure 6. Diminished FAS induction leading to blunted de novo lipogenesis and decreased triglyceride levels in liver and serum.
(A) Nascent RNA from liver nuclei were used for RT-PCR. (B) Nascent RNA from liver nuclei were used for RT-qPCR. Fold induction normalized by β-actin was shown. (C) Run-on of biotin labeled nascent transcripts from liver nuclei at different time points upon feeding were analyzed by RT-qPCR. (D) ChIP for biotin incorporation and indicated protein binding to the FAS promoter in liver. (E) Newly synthesized fatty acids during a 4 hr-period of body water labeling with 2H2O in livers from 9-wk old WT and SCID mice during feeding were measured. Values are means ± SEM, n = 12. (F) Equal amounts of Liver extracts from 9-wk old WT and SCID mice after 24 hrs of feeding were immunoblotted for total FAS protein levels. (G) Hepatic and serum triglyceride levels were measured in 9-wk old fed WT and SCID mice. (H) Schematic representation of USF-1 and its interacting partners and their effects on lipogenic gene transcription in fasting/feeding.
Since FAS plays a critical role in lipogenesis and is mainly regulated at the transcriptional level during fasting/feeding, the decrease in FAS transcription in SCID mice should result in a decrease in fatty acid biosynthesis. Thus, we examined in vivo hepatic de novo lipogenesis in WT and SCID mice using the stable isotope method. Fractional de novo lipogenesis was hardly detected in fasting but was increased drastically during a 24-hr period of feeding in WT mice (Fig. 6E). However, feeding induced fractional de novo lipogenesis was 60% lower in SCID mice after 24 hrs of feeding compared to WT mice. To confirm that the decrease in de novo lipogenesis in SCID mice was due to a decrease in FAS induction, we examined the FAS protein levels in livers of WT and SCID mice after 24 hrs of feeding. Indeed, FAS protein levels in SCID mice were significantly lower compared to WT mice (Fig. 6F). We next examined whether the blunted induction in de novo lipogenesis in SCID mice was reflected in lower hepatic and serum triglyceride levels. The hepatic triglyceride levels after 24 hrs feeding were approximately 30% lower in SCID mice compared to WT mice; and serum triglyceride levels were also significantly lower in SCID mice (Fig. 6G). Thus, impairment of feeding-dependent activation of FAS transcription in SCID mice leads to blunted induction in de novo lipogenesis resulting in lower hepatic and serum triglyceride levels. In this regard, SCID mice also had a lower adipose tissue mass, indicative of longterm defect in feeding induced lipogenesis (S. Table 1).
Discussion
FAS levels in the liver change drastically during varying nutritional states, correlating with circulating insulin/glucagon levels. During fasting, fatty acid synthesis is virtually absent. However, upon feeding, fatty acid synthesis is induced drastically. The induction of lipogenic enzymes during feeding has mainly been attributed to the increased insulin secretion. While many metabolic effects of insulin are mediated through protein phosphorylation by the activation of the well characterized PI3K cascade, insulin can also exert metabolic effects through dephosphorylation catalyzed mainly by PP1. A central issue in metabolic regulation is to define coordinated molecular strategies that underlie the transition from fasting to feeding, such as the transcriptional activation of lipogenesis along specific transduction pathways. Here, we report a novel pathway that underlies the feeding/insulin response, which is based on post-translational modifications of a key transcription factor, USF-1, by an atypical kinase, DNA-PK.
Differential binding of USF-1 interacting proteins to lipogenic gene promoters in fasted and fed states
To efficiently regulate transcription initiation, eukaryotic transcription factors recruit various coregulators. These coactivators/corepressors often have enzymatic activities to covalently modify transcription factors in response to extracellular stimuli. This study shows that USF recruits three different coregulator classes to lipogenic gene promoters. They are a) the DNA break/repair machinery, b) kinase/phosphatase, and c) HAT/HDAC family.
Here, we demonstrate that, for FAS promoter activation, USF interacting proteins are bound in a fasting/feeding dependent manner to the FAS promoter for the feeding response. The distinct binding pattern of USF interacting proteins on the FAS promoter in response to feeding/fasting is correlated with lipogenic gene activation/repression which involve molecular events that require the presence of specific coactivators/corepressors, respectively. We have previously shown that various lipogenic genes such as mGPAT are regulated coordinately with the FAS gene by feeding/insulin involving USF and SREBP-1c binding to the closely spaced E-box and SRE, respectively (Griffin, Wong, et al, 2007). Mice transgenic for the CAT gene driven by the FAS promoter containing various deletions and mutations allowed us to delineate the −65 E-box as the critical site for USF which recruits SREBP-1c that is induced upon feeding to bind the nearby SRE for FAS promoter activation by feeding/insulin (Latasa, Griffin, et al, 2003). Furthermore, we show that the USF-1 bound to the −65 E-box recruits various USF-1 interacting proteins as well as SREBP-1c to bind SRE. Herein, we address the molecular function of various USF-1 interacting proteins and USF-1 modifications required for FAS promoter activation. Furthermore, the same binding pattern of USF interacting proteins that we detected on the FAS and mGPAT promoter further demonstrates that differential recruitment of distinct USF interacting proteins might be a common key mechanism in the induction of lipogenic gene transcription in response to fasting/feeding.
Phosphorylation-dependent acetylation of USF-1 functions as a sensor for nutritional status
The recruitment of distinct interacting proteins by USF-1 to lipogenic gene promoters is critical for the fasting/feeding response. Yet, binding of USF-1 to the −65 E-box is unchanged in different metabolic states. Thus, the exact molecular mechanism linking USF-1 and fasting/feeding could not be explained. Since USF-1 levels and its binding to the E-box are unaltered between fasting/feeding, it can be predicted that USF-1 is regulated posttranslationally. Even though the changes in phosphorylation states of metabolic enzymes during the transition between fasting/feeding are common and well understood, the posttranslational modifications of transcription factors in these metabolic states are not well studied. We show here for the first time that S262 and the nearby K237 of USF-1 are modified in response to fasting/feeding. The S262 of USF-1 as well as nearby residues are conserved among mammalian species but is not found in USF-2 even though there is a 44% overall homology between USF-1 and USF-2 (Corre, and Galibert, 2005). Activation of the FAS gene by feeding has been shown to be impaired by 80% in either USF-1 or USF-2 knockout mice (Casado, Vallet, et al, 1999). Thus, USF functions as a heterodimer and both USF-1 and USF-2 were found to bind the FAS promoter (Wang, and Sul, 1995; Wang, and Sul, 1997). However, the unique S262 of USF-1 points towards its pivotal role as a sensor for lipogenic gene transcription.
There is increasing evidence for acetylation of some transcription factors in addition to the well-recognized histone acetylation (Gu, and Roeder, 1997) and reversible acetylation may be critical in regulation of transcription factor activity in response to different stimuli. However, USF acetylation has never been reported. Here, we have addressed USF-1 as a primary substrate for HAT/HDAC. K237 of USF-1 that we found to be acetylated and its nearby residues are conserved in various mammalian species. Acetylation of USF-1 at K237 is increased in the fed state when lipogenic gene transcription is induced. This is consistent with higher FAS promoter activity we observed upon transfection of the hyperacetylation mimicking USF-1 mutant. The functional significance of acetylation of transcription factors appears to be varied. In the case of p53 (Gu, and Roeder, 1997), acetylation results in stimulation of DNA binding, whereas acetylation of E2F may change protein stability (Martinez-Balbas, Bauer, et al, 2000). The fact that USF levels do not change during fasting/feeding and USF acetylation does not affect DNA binding but affects FAS promoter activation suggests transactivation results from USF acetylation. Further studies should clarify the exact functional consequence of USF acetylation.
Deacetylation is mainly mediated by HDACs which generally function as transcriptional repressors. HDAC9 that associates with USF-1 belongs to the class II HDAC. Since HDAC9 is localized in nucleus in fasting to deacetylate USF-1 and USF-1 is found to be deacetylated in fasting, we can conclude that HDAC9 is recruited to the FAS promoter in the fasted state to deacetylate USF-1 thereby repressing FAS promoter activity. Although HDAC9 has been shown to associate with transcription factors to repress transcription ( Mejat, Ramond, et al, 2005), to our knowledge, HDAC9 deacetylation of USF-1 that we report here, is the first non-histone substrate of HDAC9.
Cross talk between acetylation and phosphorylation is well recognized. In our present study, we show acetylation at K237 occurs on USF-1 that is already phosphorylated at S262 in cultured cells. Furthermore, S262 phosphorylation and K237 acetylation occur in coordination during fasting and feeding/insulin. We also show that S262 phosphorylation of USF-1 affects its interaction with P/CAF and HDAC9 and thus affects K237 acetylation. Together, these data provide firm evidence for the phosphorylation dependent acetylation of USF-1 and its function as a dynamic molecular switch in sensing the nutritional transition between fasting/feeding. Such a multi-step switch provides a way to fine tune the transcription of lipogenic genes in response to different nutritional states.
PP1 mediated dephosphorylation of DNA-PK is critical for feeding-dependent lipogenic gene transcription
It has been well established that PI3K pathway mainly mediates insulin signaling for metabolic regulation (Engelman, Luo, and Cantley, 2006). Our in vitro phosphorylation studies as well as the fact that S262 phosphorylation is abolished in DNA-PK deficient mice points to the notion that DNA-PK is the kinase for the S262 phosphorylation occurring in the fed condition. However, DNA-PK is not known to be a component in the PI3K pathway nor in the insulin signaling pathway. Although DNA-PK was previously implicated in phosphorylation of S473 of PKB/Akt (Feng, Park, et al, 2004), recent research indicates that mTORC2, another member of PIKK, is the authentic kinase that phosphorylates this critical site of PKB/Akt (Sarbassov, Guertin, et al, 2005). However, our present study suggests a link between DNA-PK and insulin signaling pathway.
Although the molecular mechanism is complex, the stimulation of PP1 by insulin has been well documented. For example, insulin inhibits breakdown and promotes synthesis of glycogen by activating primarily PP1. PP1 is compartmentalized in cells by discrete targeting subunits and several proteins called PTG (protein targeting to glycogen) can target PP1 to the glycogen particle where PP1 dephosphorylates enzymes in glycogen metabolism (Printen, Brady, and Saltiel, 1997). Recent studies indicate that PP1 can rapidly move between subcellular compartments with the aid of targeting units. PNUT, a PP1 associated cofactor, may act as a nuclear targeting subunit of PP1 (Allen, Kwon, et al, 1998). Since our results show that DNA-PK phosphorylation is lower and PP1 in nucleus is in higher abundance in feeding/insulin (Fig. 3H), we can postulate that feeding/insulin might regulate PNUT-mediated nuclear translocation of PP1 into the nucleus to activate DNA-PK. Here, the recruitment of PP1 to the FAS promoter during feeding is reduced in DNA-PK deficient mice indicating that DNA-PK might be recruited with PP1. Thus, we conclude that PP1 mediated dephosphorylation of DNA-PK is critical in transmitting the feeding/insulin signal to regulate lipogenic genes.
Among USF interacting proteins, DNA-PK along with Ku70/80, PARP-1 and TopoIIβ are identified. These proteins are known to function in double strand DNA break/repair and it has recently been shown that a transient double strand DNA-break is required for estrogen receptor dependent transcription (Ju, Lunyak, et al, 2006). Although Ku70, Ku80 and DNA-PK are in the same complex with PARP-1 and TopoIIβ, their function in DNA-break for transcriptional activation has not been reported. Here, we identified all components of DNA break/repair machinery for transcriptional activation of the FAS promoter by fasting/feeding and we observed transient DNA breaks that preceded transcriptional activation. This is similar to transient DNA breaks observed in the estrogen activated promoter. However, we show here a unique function of DNA-PK as a signaling molecule in response to feeding/insulin. Our interaction and promoter occupancy studies in DNA-PK deficient cells demonstrate the requirement of DNA-PK for USF-1 complex assembly and recruitment of its interacting proteins. Therefore, DNA-PK mediated USF-1 phosphorylation governs interaction between USF-1 and its partners. We have shown that SREBP-1 interacts more efficiently with the phosphorylated USF-1, which in turn enhances the interaction between USF-1 and DNA-PK, leading to USF-1 phosphorylation as well as subsequent recruitment of interacting proteins, an indication of positive feed-forward regulation. Thus, impaired transcriptional activation of lipogenic genes in DNA-PK deficient SCID mice is probably due to the dual effects of DNA-PK on USF-1 phosphorylation for feeding/insulin signaling and transient DNA break required for transcriptional activation. In SCID mice, we could not detect transient DNA breaks in the FAS promoter that we observed in WT mice upon feeding (Fig. 6D). This could be attributed to the impairment of feeding/insulin induced USF phosphorylation by DNA-PK in SCID mice, which results in a failure to recruit various USF-1 interacting proteins including those for transient DNA breaks such as TopoIIβ.
The SCID mice have not been previously reported to show defects in lipogenesis or insulin signaling. We show that phosphorylation of USF-1 catalyzed by DNA-PK is drastically reduced in SCID mice. Phosphorylation dependent acetylation of USF-1 is also decreased in SCID mice. Furthermore, such changes diminish feeding dependent transcriptional activation of lipogenic genes. Although it is unclear, the remaining transcriptional activation in SCID mice could be attributed to SREBP-1c induction in fed state. However, the recruitment of SREBP-1c to the lipogenic promoters is also affected by S262 phosphorylation of USF-1. Regardless, as the metabolic consequence, we detected defects in induction of hepatic de novo lipogenesis that normally occurs upon feeding of a high carbohydrate diet. The defects in lipogenic induction resulted in decreased not only hepatic triglyceride contents but serum triglyceride levels probably reflecting decreased VLDL secretion in SCID mice. These defects in turn were reflected in a decrease in adipose tissue mass.
Taken together, we propose the following model for the mechanism underlying USF function in the transcriptional regulation of lipogenic genes during fasting/feeding (Fig. 6H). In the fasted state, USF-1 recruits HDAC9 which deacetylates USF-1 to repress transcription despite its binding to the E-box (left panel). Upon feeding, DNA-PK, which is dephosphorylated/activated by PP1, phosphorylates USF-1 which then recruits SREBP-1 and other USF-1 interacting proteins. Thus, DNA-PK catalyzed phosphorylation of USF-1 allows P/CAF recruitment and subsequent acetylation of USF-1 (right panel). As a result, FAS transcription is activated by USF-1 in a reversible manner in response to nutritional status.
Experimental Procedures
Additional experimental procedures are available in the supplemental data.
Purification of USF-1 interacting proteins and preparation of nuclear extracts
TAP was performed as described previously (Griffin, Wong, et al, 2007). Purified protein mixture was subjected to mass spectrometry. Liver nuclear extracts were prepared by centrifugation through sucrose cushion in the presence of NaF (Griffin, Wong, et al, 2007).
Chromatin Immunoprecipitation
Livers from fasted or fed mice were fixed with DSG at 2 mM for 45 min at RT before formaldehyde cross-linking. ChIP was performed as described previously (Latasa, Griffin, et al, 2003).
In vitro phosphorylation, acetylation, and DNA-PK kinase assay
In vitro phosphorylation and acetylation were performed using recombinant/purified enzymes. DNA-PK kinase assay was performed with nuclear extracts pretreated with or without wortmannin using SignaTect DNA-PK assay system (Promega) and γ32P-ATP (Roche).
Nuclear run-on assay and preparation of nascent RNA
Nuclei were isolated as described previously (Paulauskis, and Sul, 1989) for nascent RNA and nuclear run-on assay (See the Supplemental Experimental Procedures for further details).
Measurement of de novo lipogenesis (DNL)
Fatty acids formed during a 4 hrs 2H2O body water labeling (See the Supplemental Experimental Procedures for further details).
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK81098 and DK75682 to H.S.S. We are grateful to Drs. Tony Kouzarides, Kathy Meek, Arthur Zelent, Angus Lamond, Moshe Oren and Jerry Shay for various HAT, DNA-PK, HDAC9, PP1, −0.7 p53-Luc and Kus constructs, respectively. We thank YongEun Lee, Shelley Ling, Nicole Cho, Eunice Kim, Philip Lee, JiHyun(Amy) Cho, Aabid Farukhi, Sue Lee and Helen Wong, for excellent technical assistance, Jenny Huang, Aditi Ananth, Melanie Ma, Fanny Yan and Nayoung Bae, for assistance, and Drs., Mary Ann Williams, Jen-Chywan (Wally) Wang, and Maryam Ahmadian for critical reading of the manuscript and Dr. Donald Scott for nuclear run-on protocol. We also thank Dr. Daniel P. Cashman for critical discussions of the data and review of the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PB, Kwon YG, Nairn AC, Greengard P. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J. Biol. Chem. 1998;273:4089–4095. doi: 10.1074/jbc.273.7.4089. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Saltiel AR. The role of protein phosphatase-1 in insulin action. Recent Prog. Horm. Res. 2001;56:157–173. doi: 10.1210/rp.56.1.157. [DOI] [PubMed] [Google Scholar]
- Casado M, Vallet VS, Kahn A, Vaulont S. Essential role in vivo of upstream stimulatory factors for a normal dietary response of the fatty acid synthase gene in the liver. J. Biol. Chem. 1999;274:2009–2013. doi: 10.1074/jbc.274.4.2009. [DOI] [PubMed] [Google Scholar]
- Collis SJ, DeWeese TL, Jeggo PA, Parker AR. The life and death of DNA-PK. Oncogene. 2005;24:949–961. doi: 10.1038/sj.onc.1208332. [DOI] [PubMed] [Google Scholar]
- Corre S, Galibert MD. Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Res. 2005;18:337–348. doi: 10.1111/j.1600-0749.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Danska JS, Holland DP, Mariathasan S, Williams KM, Guidos CJ. Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol. Cell. Biol. 1996;16:5507–5517. doi: 10.1128/mcb.16.10.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dircks LK, Sul HS. Mammalian mitochondrial glycerol-3-phosphate acyltransferase. Biochim. Biophys. Acta. 1997;1348:17–26. doi: 10.1016/s0005-2760(97)00106-9. [DOI] [PubMed] [Google Scholar]
- Douglas P, Cui X, Block WD, Yu Y, Gupta S, Ding Q, Ye R, Morrice N, Lees-Miller SP, Meek K. The DNA-dependent protein kinase catalytic subunit is phosphorylated in vivo on threonine 3950, a highly conserved amino acid in the protein kinase domain. Mol. Cell. Biol. 2007;27:1581–1591. doi: 10.1128/MCB.01962-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P, Moorhead GB, Ye R, Lees-Miller SP. Protein phosphatases regulate DNA-dependent protein kinase activity. J. Biol. Chem. 2001;276:18992–18998. doi: 10.1074/jbc.M011703200. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver DT, Alt FW. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- Griffin MJ, Wong RH, Pandya N, Sul HS. Direct interaction between USF and SREBP-1c mediates synergistic activation of the fatty-acid synthase promoter. J. Biol. Chem. 2007;282:5453–5467. doi: 10.1074/jbc.M610566200. [DOI] [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rao S, Tokuno O, Yamamoto K, Takata M, Takeda S, Utsumi H. DNA-PK: the major target for wortmannin-mediated radiosensitization by the inhibition of DSB repair via NHEJ pathway. J. Radiat. Res. (Tokyo) 2003;44:151–159. doi: 10.1269/jrr.44.151. [DOI] [PubMed] [Google Scholar]
- Jerkins AA, Liu WR, Lee S, Sul HS. Characterization of the murine mitochondrial glycerol-3-phosphate acyltransferase promoter. J. Biol. Chem. 1995;270:1416–1421. doi: 10.1074/jbc.270.3.1416. [DOI] [PubMed] [Google Scholar]
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- Latasa MJ, Griffin MJ, Moon YS, Kang C, Sul HS. Occupancy and function of the −150 sterol regulatory element and −65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell. Biol. 2003;23:5896–5907. doi: 10.1128/MCB.23.16.5896-5907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latasa MJ, Moon YS, Kim KH, Sul HS. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10619–10624. doi: 10.1073/pnas.180306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J. Clin. Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat. Neurosci. 2005;8:313–321. doi: 10.1038/nn1408. [DOI] [PubMed] [Google Scholar]
- Moon YS, Latasa MJ, Kim KH, Wang D, Sul HS. Two 5'-regions are required for nutritional and insulin regulation of the fatty-acid synthase promoter in transgenic mice. J. Biol. Chem. 2000;275:10121–10127. doi: 10.1074/jbc.275.14.10121. [DOI] [PubMed] [Google Scholar]
- Moustaid N, Beyer RS, Sul HS. Identification of an insulin response element in the fatty acid synthase promoter. J. Biol. Chem. 1994;269:5629–5634. [PubMed] [Google Scholar]
- Moustaid N, Sakamoto K, Clarke S, Beyer RS, Sul HS. Regulation of fatty acid synthase gene transcription. Sequences that confer a positive insulin effect and differentiation-dependent expression in 3T3-L1 preadipocytes are present in the 332 bp promoter. Biochem. J. 1993;292(Pt 3):767–772. doi: 10.1042/bj2920767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustaid N, Sul HS. Regulation of expression of the fatty acid synthase gene in 3T3-L1 cells by differentiation and triiodothyronine. J. Biol. Chem. 1991;266:18550–18554. [PubMed] [Google Scholar]
- Pajukanta P, Lilja HE, Sinsheimer JS, Cantor RM, Lusis AJ, Gentile M, Duan XJ, Soro-Paavonen A, Naukkarinen J, Saarela J, et al. Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1) Nat. Genet. 2004;36:371–376. doi: 10.1038/ng1320. [DOI] [PubMed] [Google Scholar]
- Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J. Biol. Chem. 1989;264:574–577. [PubMed] [Google Scholar]
- Paulauskis JD, Sul HS. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J. Biol. Chem. 1988;263:7049–7054. [PubMed] [Google Scholar]
- Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sawadogo M, Roeder RG. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985;43:165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Paulauskis JD, Moustaid N, Sul HS. Transcriptional regulation of p90 with sequence homology to Escherichia coli glycerol-3-phosphate acyltransferase. J. Biol. Chem. 1991;266:23834–23839. [PubMed] [Google Scholar]
- Soncini M, Yet SF, Moon Y, Chun JY, Sul HS. Hormonal and nutritional control of the fatty acid synthase promoter in transgenic mice. J. Biol. Chem. 1995;270:30339–30343. doi: 10.1074/jbc.270.51.30339. [DOI] [PubMed] [Google Scholar]
- Sul HS, Latasa MJ, Moon Y, Kim KH. Regulation of the fatty acid synthase promoter by insulin. J. Nutr. 2000;130:315S–320S. doi: 10.1093/jn/130.2.315S. [DOI] [PubMed] [Google Scholar]
- Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu. Rev. Nutr. 1998;18:331–351. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signaling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Wang D, Sul HS. Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway. Involvement of protein kinase B/Akt. J. Biol. Chem. 1998;273:25420–25426. doi: 10.1074/jbc.273.39.25420. [DOI] [PubMed] [Google Scholar]
- Wang D, Sul HS. Upstream stimulatory factor binding to the E-box at −65 is required for insulin regulation of the fatty acid synthase promoter. J. Biol. Chem. 1997;272:26367–26374. doi: 10.1074/jbc.272.42.26367. [DOI] [PubMed] [Google Scholar]
- Wang D, Sul HS. Upstream stimulatory factors bind to insulin response sequence of the fatty acid synthase promoter. USF1 is regulated. J. Biol. Chem. 1995;270:28716–28722. doi: 10.1074/jbc.270.48.28716. Correction: J. Biol. Chem. (1996) 271, 7873–7873. [DOI] [PubMed] [Google Scholar]
- West AG, Huang S, Gaszner M, Litt MD, Felsenfeld G. Recruitment of histone modifications by USF proteins at a vertebrate barrier element. Mol. Cell. 2004;16:453–463. doi: 10.1016/j.molcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Yet SF, Lee S, Hahm YT, Sul HS. Expression and identification of p90 as the murine mitochondrial glycerol-3-phosphate acyltransferase. Biochemistry. 1993;32:9486–9491. doi: 10.1021/bi00087a029. [DOI] [PubMed] [Google Scholar]
- Yet SF, Moon YK, Sul HS. Purification and reconstitution of murine mitochondrial glycerol-3-phosphate acyltransferase. Functional expression in baculovirus-infected insect cells. Biochemistry. 1995;34:7303–7310. doi: 10.1021/bi00022a003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.