Summary
To better characterize aging in mice, the Jackson Aging Center carried out a lifespan study of 31 genetically-diverse inbred mouse strains housed in a specific pathogen-free facility. We carried out clinical assessments every 6 months, measuring multiple age-related phenotypes including neuromuscular, kidney and heart function, body composition, bone density, hematology, hormonal levels, and immune system parameters. In a concurrent cross-sectional study of the same 31 strains at 6, 12, and 20 months, we carried out more invasive measurements followed by necropsy to assess apoptosis, DNA repair, chromosome fragility, and histopathology. In this report, which is the initial paper of a series, we describe the study design, median lifespans, and circulating IGF1 levels at 6, 12 and 18 months for the first cohort of 32 females and 32 males of each strain. Survival curves varied dramatically among strains with median lifespans ranging from 251 to 964 days. Plasma IGF1 levels, which also varied considerably at each time point, showed an inverse correlation with median lifespan at 6 months (R=−0.33, P=0.01). This correlation became stronger if the short-lived strains with a median lifespan<600 days were removed from the analysis (R=−0.53, P<0.01). These results support the hypothesis that the IGF1 pathway plays a key role in regulating longevity in mice and indicates that common genetic mechanisms may exist for regulating IGF1 levels and lifespan.
Keywords: genetics, mice, longevity, IGF1, aging
INTRODUCTION
Multiple studies in organisms ranging from flies and worms to humans have demonstrated that genetics plays an important role in determining lifespan (Kuningas et al. 2008). Furthermore, single gene manipulations can change the rate of aging. For example, mutations in genes of the insulin/IGF1 pathway, a well known evolutionarily conserved pathway, extend the lifespan in many taxa from worms to mammals (Kenyon 2005). In humans, polymorphisms of IGF1 pathway genes are significantly associated with longevity (Bonafe et al. 2003).
The mouse is an excellent model for aging research: mice and humans share ~99% of their genes (Boguski 2002), yet mice have a relatively short lifespan greatly facilitating aging studies. Furthermore, the outstanding genetic resources for the mouse include hundreds of inbred strains and mutants and sophisticated genetic engineering technology for manipulating its genome (Paigen 1995; Peters et al. 2007). Among the many insights into aging carried out in mouse models are retardation of aging caused by diet restriction (Walford et al. 1973; Harrison et al. 1984) and mutations, either spontaneous or genetically engineered, that increase lifespan such as pituitary hormone Pou1f1 and the growth hormone releasing hormone receptor, Ghrhr (Brown-Borg et al. 1996; Flurkey et al. 2001; Flurkey et al. 2002), insulin growth factor 1 receptor, Igf1r (Holzenberger et al. 2003), and the insulin receptor substrates Irs1 and Irs2 (Taguchi et al. 2007; Selman et al. 2008). Recently, the mouse has been used to test chemicals that may extend lifespan (Miller et al. 2007).
Quantitative trait loci (QTL) associated with aging in mice have been found (Klebanov et al. 2001; Miller et al. 2002), but these QTL studies utilized only a small number of inbred strains: C57BL/6J (B6), BALB/cByJ (cBy), 129S1/SvImJ (129S1), C3H/HeJ (C3), DBA/2J (D2), LP/J, NZW/LacJ (NZW), ST/bJ (ST), and MOLD/RkJ and CAST/EiJ. It has been suggested that aging studies in mice be carried out in F1 progeny or in F2 progeny derived from 4-way crosses (Miller 2006), but very little information about lifespan in multiple strains has been available to help researchers choose which strains to cross. To better utilize the genetic resources of the mouse in aging research, we initiated the Aging Phenome Project with the goal of characterizing aging-related phenotypes of multiple inbred mouse strains. This first report describes the design of our study, explains the rationale for many of our methods, and describes our findings on median lifespan, plasma IGF1, and the association of IGF1 with longevity.
RESULTS
We determined lifespan for the first cohort (32 females and 32 males/strain) of 32 inbred strains. The details of strain selection and experimental design are described in methods. However, we encountered a few problems with our selected strains. Two wild-derived strains, MOLF and WSB, were not at the same high health status as the other strains because they were housed in rooms that tested positive for the pathogens Helicobacter species, Pneumocystis carinii, and Pasteurella pneumotropica. Because we wished to keep the room and health status of all strains in the aging project consistent, we hysterectomy derived these two strains at the start of the study. As a consequence WSB was started later than the other strains, and since WSB is a long-lived strain, this has considerably delayed the completion of the study. MOLF, which is a poor breeder and required a long time for hysterectomy derivation and building up the colony, is not included in this paper, but MOLF data will be added to MPD. We also had to eliminate studies for some males due to excessive fighting. This report contains data for females of 31 strains (MOLF omitted) and males of 29 strains (MOLF, CAST, and P omitted). The numbers of mice of each strain are summarized in S. Table 1.
Types of death
Of the 1,913 mice entered into the longitudinal study, 366 (19.1%) were still living by the date of data collection (S. Table 1). Mice found dead or euthanized because they were moribund and about to die remained part of the dataset. Mice euthanized due to fighting (2.7% of deaths), mice that failed to recover from blood sampling, and mice that died due to laboratory error were excluded from the dataset. Together, these excluded mice accounted for 35 (4%) females and 46 (5%) males.
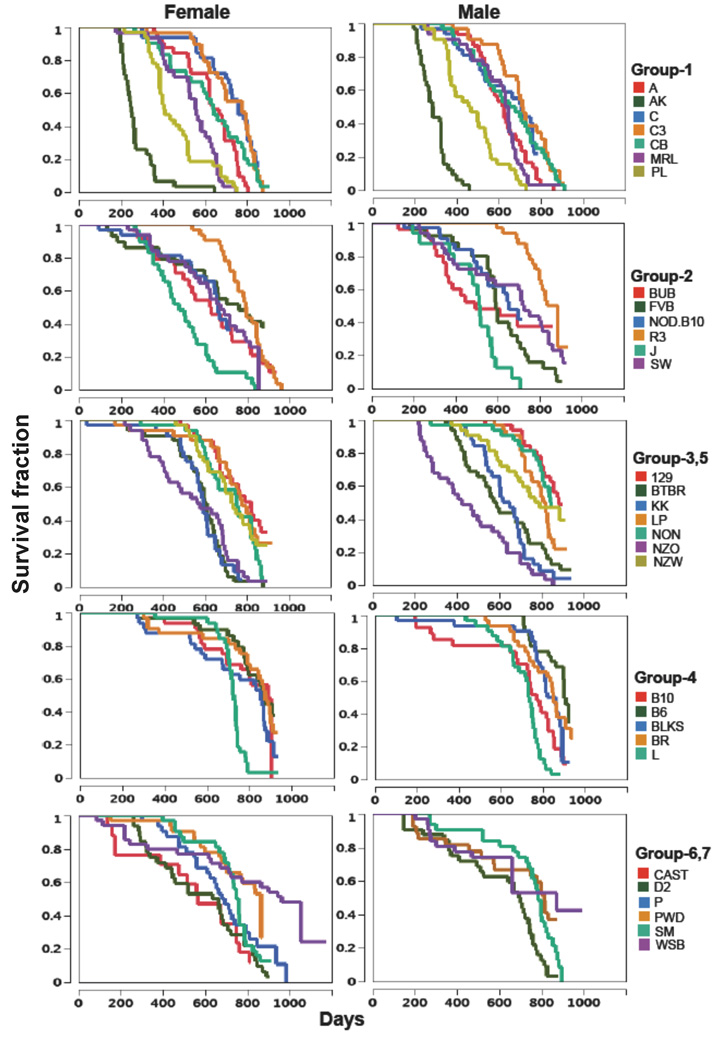
Survival curves
In most cases, survival curves for strains in the same genetic groups are presented on the same graph (males and females on separate graphs) except that groups 3 and 5 are combined in one graph and groups 6 and 7 in another (Figure 2). Survival curves varied widely within a group. In group 1, AK mice died early from lymphomas (Karpova et al. 2002; Sommer et al. 2007), and the premature deaths in this strain may not add much to our understanding of normal aging. In group 2, SJL mice died early, and previous studies reported a high incidence of reticulum cell sarcomas in this strain (Carswell et al. 1970; Stavnezer et al. 1989). Survival curves of group 4 strains, all members of the C57 family, were remarkably similar except for L, whose survival curve dropped sharply after 550 days.
Figure 2.
Survival curves.
Lifespans
The median lifespans with 95% CIs, calculated from the survival curves, are listed in Table 1 in order from the shortest female median lifespan to the longest. AK females and males had the shortest median lifespans (251 and 288 days respectively). PL males and females had the second shortest lifespans (476 and 514 days respectively). Median lifespans were more than 800 days for both sexes of strains 129S1, B6, BKS, BR, PWD and WSB and for one sex in strains B10, LP, NON, and R3.
Table 1.
Median lifespans in days and 95% confidence intervals
| Strain name | Female | Male | |||
|---|---|---|---|---|---|
| Full | Abbrevation | Median lifespan |
95% CI | Median lifespan |
95% CI |
| AKR/J | AK | 251 | 224~266 | 288 | 245~325 |
| PL/J | PL | 409 | 386~512 | 469 | 365~538 |
| SJL/J | SJL | 476 | 393~549 | 514 | 392~568 |
| MRL/J | MRL | 557 | 517~624 | 645 | 557~665 |
| CAST/EiJ | CAST | 567 | 173~748 | Not included | |
| NZO/H1LtJ | NZO | 576 | 405~682 | 423 | 286~568 |
| KK/H1J | KK | 593 | 564~614 | 650 | 548~693 |
| BTBR-T+tf/J | BTBR | 610 | 547~644 | 587 | 456~728 |
| BUB/BnJ | BUB | 628 | 457~726 | 493 | 354~NA |
| CBA/J | CBA | 644 | 532~756 | 679 | 539~750 |
| NOD.B10-H2b | NOD.B10 | 662 | 556~NA | 653 | 524~NA |
| DBA/2J | D2 | 665 | 413~707 | 701 | 492~745 |
| A/J | A | 669 | 610~694 | 623 | 555~681 |
| SWR/J | SWR | 672 | 548~785 | 726 | 498~841 |
| P/J | P | 693 | 560~792 | Not included | |
| C57L/J | L | 728 | 706~737 | 736 | 660~755 |
| NZW/LacJ | NZW | 732 | 617~817 | 790 | 642~NA |
| NON/LtJ | NON | 750 | 631~819 | 847 | 799~NA |
| BALB/cBy | CBy | 757 | 700~802 | 707 | 527~758 |
| SM/J | SM | 758 | 712~786 | 783 | 741~818 |
| FVB/NJ | FVB | 760 | 595~NA | 591 | 562~693 |
| C3H/HeJ | C3H | 777 | 659~813 | 714 | 630~802 |
| LP/J | LP | 778 | 700~835 | 822 | 723~834 |
| RIIIS/J | R3 | 793 | 735~842 | 886 | 799~NA |
| 129S1/SvImJ | 129S1 | 819 | 673~NA | 882 | 809~NA |
| C57BLKS/J | BKS | 853 | 672~873 | 860 | 784~881 |
| PWD/PhJ | PWD | 866 | 697~NA | 813 | 575~NA |
| C57BL/6J | B6 | 866 | 782~NA | 901 | 859~NA |
| C57BR/cdJ | BR | 877 | 792~912 | 849 | 754~907 |
| C57BL/10J | B10 | 888 | 692~NA | 770 | 677~825 |
| WSB/EiJ | WSB | 964 | 713~NA | 871 | 662~NA |
Median lifespans and 95% confidence intervals (CI) were calculated in JMP 6.0.4 using the survival reliability function. NA –the upper end of the 95% confidence interval was not available for some strains because insufficient numbers of mice have died during the time frame of this report.
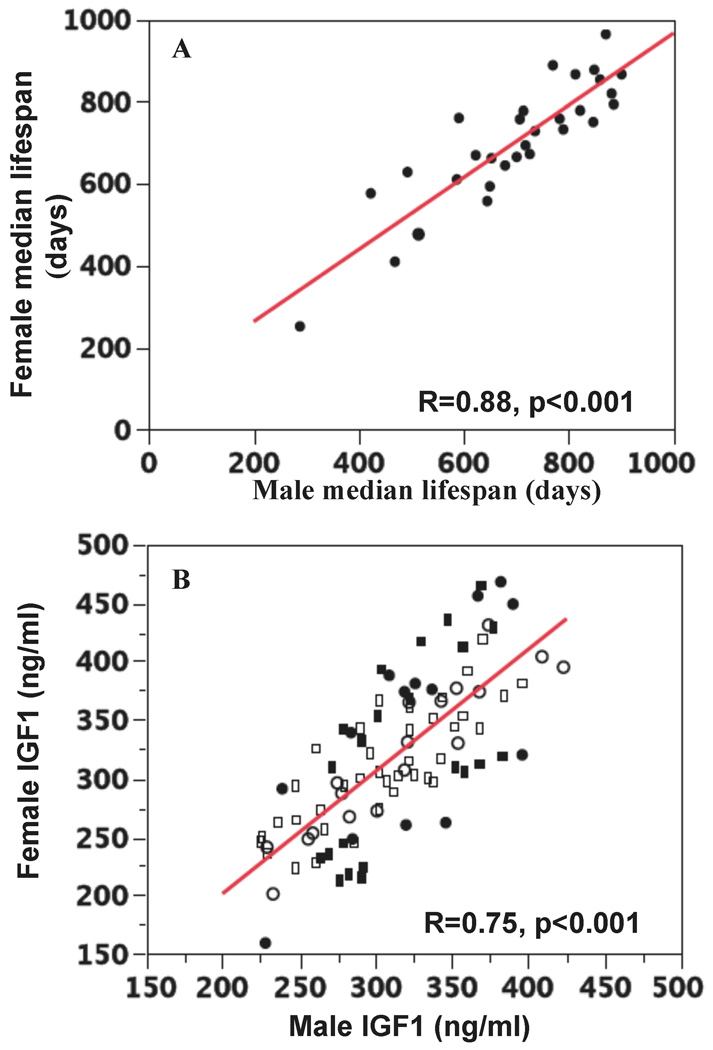
Within-strain median lifespans for males and females were significantly correlated with each other (R=0.88; P < 0.001; Figure 3A), and sex differences did not significantly affect lifespan (proportional hazard analysis, data not shown). In only one strain, NON, were the survival curves significantly different (log-rank test, p=0.013) between the sexes (males 750 days and females 847 days).
Figure 3.
A. Correlation of female and male median lifespans. B. Correlation of female and male IGF1 levels. IGF1 was measured at 6 (circles), 12 (squares) and 18 (rectangles) months; closed symbols indicate the IGF1 levels that were significantly different between female and male mice of the same strain.
All males from 6 strains and all females from 7 strains have died so far. The mean lifespans, the ages of 25% and 75% death, and the mean lifespans of the 20% longest-lived mice from these strains are presented in Table 2. Median and mean lifespans for these 13 groups were highly correlated with each other (R=0.97; P < 0.001). The median and mean lifespans of the top 20% of the longest-lived mice were also correlated (R2=0.70, P < 0.001).
Table 2.
Lifespans (days) for strains that are completed
| Age of | All mice | 20% longest lived mice | |||||
|---|---|---|---|---|---|---|---|
| Strai n |
Se x |
25% death |
75% death |
Mean | SEM | Mean | SEM |
| A | F | 534 | 750 | 631 | 23 | 776 | 9 |
| M | 533 | 708 | 613 | 24 | 785 | 18 | |
| AK | F | 214 | 321 | 275 | 17 | 421 | 48 |
| M | 244 | 329 | 293 | 12 | 394 | 19 | |
| BTB | |||||||
| R | F | 536 | 666 | 580 | 24 | 733 | 30 |
| C3 | F | 622 | 820 | 724 | 24 | 855 | 7 |
| CBA | M | 532 | 808 | 647 | 30 | 872 | 10 |
| MRL | F | 421 | 640 | 536 | 23 | 679 | 15 |
| NZO | M | 280 | 637 | 463 | 36 | 761 | 26 |
| PL | F | 386 | 520 | 462 | 24 | 696 | 17 |
| M | 365 | 558 | 471 | 23 | 674 | 19 | |
| SJL | F | 378 | 602 | 494 | 29 | 726 | 41 |
| M | 470 | 574 | 491 | 35 | 656 | 35 | |
Circulating IGF1
Comparison of IGF1 levels among inbred strains at the age of 6 months is shown in Table 3 with the males and females shown separately and with the strains arranged from the highest to lowest IGF1 level. Similar tables for IGF1 levels at 12 and 18 months of age are shown in S. Table 2A and 2B. Strain, sex, age, and interaction between sex and strains all had significant effects (p<0.01) on IGF1 levels as shown by ANOVA (data not shown).
Table 3.
IGF1 levels of inbred strains at 6 months
| Female | Male | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Mean (ng/ml) | SEM | N | Strain | Mean (ng/ml) | SEM | N | |||||||||||||||||||||||||
| NOD.B10 | A | 468 | 22 | 8 | MRL | A | 423 | 13 | 8 | |||||||||||||||||||||||
| BTBR | A | B | 456 | 16 | 8 | KK | A | B | 409 | 31 | 4 | |||||||||||||||||||||
| NZO | A | B | C | 449 | 11 | 8 | CAST | A | B | C | D | 396 | 11 | 4 | ||||||||||||||||||
| BUB | A | B | C | 431 | 33 | 8 | NZO | A | B | C | 390 | 10 | 7 | |||||||||||||||||||
| KK | A | B | C | D | 404 | 24 | 8 | NOD.B10 | A | B | C | D | 382 | 6 | 8 | |||||||||||||||||
| MRL | A | B | C | D | 395 | 10 | 8 | BUB | A | B | C | D | E | F | 374 | 15 | 4 | |||||||||||||||
| P | A | B | C | D | E | 388 | 28 | 8 | NZW | A | B | C | D | E | 368 | 11 | 8 | |||||||||||||||
| AK | A | B | C | D | E | F | G | H | 381 | 9 | 5 | BTBR | A | B | C | D | E | 367 | 13 | 8 | ||||||||||||
| SWR | B | C | D | E | F | 377 | 15 | 8 | cBy | B | C | D | E | F | 354 | 12 | 8 | |||||||||||||||
| BR | A | B | C | D | E | F | G | H | 376 | 7 | 5 | SWR | B | C | D | E | F | 353 | 20 | 8 | ||||||||||||
| NZW | B | C | D | E | F | G | H | 374 | 9 | 7 | LP | B | C | D | E | F | G | 346 | 12 | 8 | ||||||||||||
| L | B | C | D | E | F | G | 374 | 14 | 8 | C3H | B | C | D | E | F | G | 343 | 12 | 8 | |||||||||||||
| C3H | C | D | E | F | G | H | 366 | 17 | 8 | BR | B | C | D | E | F | G | H | I | 337 | 13 | 4 | |||||||||||
| NON | C | D | E | F | G | H | 365 | 16 | 8 | AK | C | D | E | F | G | H | 326 | 16 | 7 | |||||||||||||
| A | D | E | F | G | H | I | 339 | 9 | 7 | NON | C | D | E | F | G | H | I | 322 | 17 | 6 | ||||||||||||
| PWD | D | E | F | G | H | I | J | 331 | 22 | 8 | PWD | D | E | F | G | H | I | 321 | 18 | 8 | ||||||||||||
| cBy | D | E | F | G | H | I | J | 330 | 8 | 8 | 129S1 | D | E | F | G | H | I | 320 | 19 | 8 | ||||||||||||
| CAST | D | E | F | G | H | I | J | K | 320 | 12 | 7 | CBA | D | E | F | G | H | I | 319 | 4 | 8 | |||||||||||
| CBA | E | F | G | H | I | J | K | 307 | 7 | 8 | L | D | E | F | G | H | I | 319 | 6 | 7 | ||||||||||||
| R3 | F | G | H | I | J | K | 296 | 12 | 8 | P | E | F | G | H | I | J | 309 | 12 | 8 | |||||||||||||
| SJL | G | H | I | J | K | 291 | 15 | 8 | FVB | F | G | H | I | J | K | 301 | 16 | 8 | ||||||||||||||
| BKS | H | I | J | K | L | 287 | 10 | 8 | D2 | G | H | I | J | K | L | 285 | 10 | 8 | ||||||||||||||
| FVB | I | J | K | L | 272 | 12 | 8 | A | G | H | I | J | K | L | 284 | 9 | 8 | |||||||||||||||
| PL | I | J | K | L | 267 | 13 | 7 | PL | G | H | I | J | K | L | 283 | 6 | 8 | |||||||||||||||
| LP | I | J | K | L | 262 | 15 | 8 | BKS | H | I | J | K | L | 278 | 5 | 8 | ||||||||||||||||
| 129S1 | I | J | K | L | 260 | 16 | 8 | R3 | H | I | J | K | L | 275 | 7 | 8 | ||||||||||||||||
| B10 | K | L | 253 | 6 | 16 | B10 | I | J | K | L | 259 | 10 | 8 | |||||||||||||||||||
| D2 | J | K | L | 248 | 10 | 8 | B6 | I | J | K | L | 256 | 10 | 7 | ||||||||||||||||||
| B6 | J | K | L | 248 | 11 | 7 | SJL | J | K | L | 239 | 12 | 6 | |||||||||||||||||||
| MOLF | J | K | L | M | 241 | 20 | 6 | WSB | K | L | 233 | 5 | 6 | |||||||||||||||||||
| WSB | L | M | 201 | 22 | 7 | MOLF | L | 229 | 14 | 6 | ||||||||||||||||||||||
| SM | M | 159 | 17 | 8 | SM | L | 228 | 6 | 8 | |||||||||||||||||||||||
One way ANOVA analysis of IGF1 levels of inbred strains at 6-month was performed in JMP 7.0. Strains are not connected by the same letters are significantly different (p<0.05, adjusted by Tukey HSD method).
IGF1 levels for males and females of each strain were significantly correlated (R=0.74, p<0.001) as shown in Fig. 2B, which contains data for 6, 12, and 18 months. For some strains, female and male have significantly different levels of IGF1 as indicated by closed symbols in Figure 3B. Females of BTBR and L have significantly higher IGF1 levels at all three time points compared to males; in contrast, females of LP and WSB have significantly lower IGF1 than males at 2 time points.
The variation of IGF1 levels among these inbred strains is considerable (Table 3, S. Table 1A, 1B). At 6 months, female NOD.B10 mice have IGF1 levels that are 2.9-fold greater than SM (468± SEM 22 and 159±7 ng/ml), and male MRL mice have IGF1 levels that are 1.8-fold greater than SM (423±13 and 228±6 ng/ml). Similar differences are observed at the 12 and 18-month times (S Table 1A, 1B). The SM strain (SM is short for small) was selected for low body weight when young, and its low IGF1 levels may result from this selection.
Correlation of IGF1 levels with median lifespan
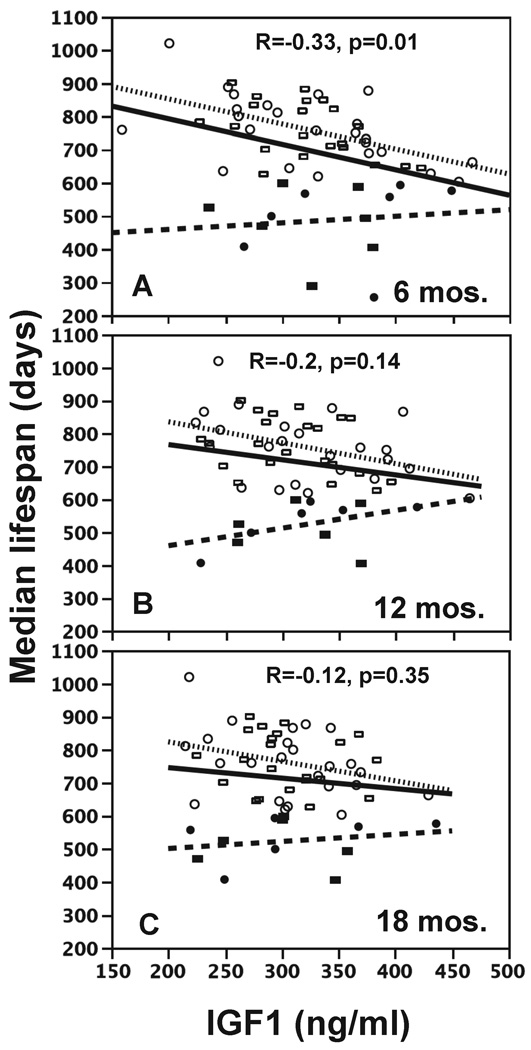
IGF1 levels at all three ages points are negatively correlated with median lifespan (solid lines in Figure 4) but only the correlation at 6 months was significant. However, some of strains died at a young age because of specific diseases. For example, most AK mice died of lymphomas, and the median lifespans were 251 days (females) and 288 days (males). Until the pathology is complete, we do not know which other strains died early from a specific disease. However, we separated the strains which died early (median lifespan <600 days) from the remainder and reanalyzed the correlations of IGF1 with aging for the two groups separately. In the short-lived strains (median lifespan <600 days), there was either no correlation or a modest, positive, but non-significant correlation of IGF1 and lifespan (showed as dashed lines in Fig. 3). For the longer lived strains, the negative correlation of IGF1 and lifespan became stronger and more significant (showed as dotted lines in Fig. 3): 6 months R=−0.53, p<0.01; 12 months R=−0.39, p<0.01; 18 months R=−0.3, p<0.05.
Figure 4.
Correlation of median lifespans with IGF1 levels. IGF1 was measured at 6-(A), 12- (B), or 18-(C) months. Females and males are indicated by circles and rectangles. Solid symbols are strains with median lifespans less than 600 days; open symbols are strains with median lifespan greater than 600 days. Correlations are given for all strains (solid line), strains with median lifespan less than 600 days (dashed line), or strains with lifespan greater than 600 days (dotted line).
DISCUSSION
Environmental and genetic effects on longevity
Human and murine aging are defined by signs of change in phenotypes, such as increased risk of frailty, disability, morbidity, and ultimately, mortality. In the past several decades, human lifespan has dramatically increased in developed countries because of significant improvements in environmental conditions and medical care (Oeppen & Vaupel 2002). This phenomenon is also seen in studies of aging mice. Comparing the current lifespan study, which is carried out in a specific pathogen free (SPF) environment, with a previous study conducted in 1966 where the environment was not specified (Storer 1966), the lifespans are considerably shorter in the 1966 study for most of the 22 inbred strains. For example, the mean lifespans of B6 female and male mice in the 1966 study are 692 and 676 days, considerably shorter than the 818 and 827 mean lifespans observed in a 1975 study in a clean environment (Storer 1966; Goodrick 1975) and shorter than the median lifespan of 866 and 901 days for female and male B6 in our study (the mean lifespan is not yet available).
Genetics also plays an important role in determining longevity. Studies of human twins and long-lived families estimate that 20~30% of the variation in human lifespan is determined by genetic factors (Herskind et al. 1996; Mitchell et al. 2001; Hjelmborg et al. 2006). Siblings of centenarians have a significantly higher chance of becoming centenarians compared to other members of their birth cohort, and offspring of long-lived siblings have a lower mortality risk already at middle age, whereas their spouses, with whom they share a common environment, do not show this survival benefit (Schoenmaker et al. 2006). In the current report, we observe dramatic strain-dependent variation in median lifespans. Median lifespans of the shortest-lived strain AK are less than one third of longest female and male median lifespans, found in WSB and B6.
Genetic regulation of IGF1 levels and its relationship with longevity
Epidemiological studies in normal human populations suggest that IGF1 levels are genetically regulated. In a cross sectional study of a healthy human population in northeast Germany, IGF1 levels varied from 30 to 460 ng/ml in 20~25 year old males (Friedrich et al. 2008). Human studies also reveal variations among different races. For example, among 503 nulligravid women between the ages of 17 and 35, black women had significantly higher IGF1 levels than white women (Jernstrom et al. 2001). Human twin studies further confirm the strong genetic regulation of IGF1 levels (Juul 2003). In the current study, our results showed considerable variation of IGF1 levels among inbred strains, confirming the importance of genetic influence on IGF1 levels. For example, at the age of 6 months, SM had IGF1 levels that were about one-third of the highest IGF1 levels found in NOD.B10 females and one-half those found in MRL males. These results suggest that genetic polymorphisms regulate IGF1 levels in both humans and mice.
Repressing IGF1 signaling can significantly increase longevity in many species from yeast to mammals (Longo & Finch 2003). In mice, the heterozygous knockout of Igf1r mice showed lower IGF1 signaling and the female lifespan was extended 33% (Holzenberger et al. 2003). In a human population study, one mutation in IGF1R, which was associated with reduced receptor activity, was over-represented in centenarians (Suh et al. 2008). However, whether circulating IGF1 levels are associated with longevity has not been fully investigated. In human studies, patients with mutations in growth hormone or growth hormone receptor have relatively low IGF1, but did not show extended longevity, presumably because of the metabolic diseases that accompanied the mutations (Laron 2001; Besson et al. 2003). In mouse studies, the Snell dwarf mouse, which has a mutation in Pou1f1, exhibited lower IGF1 and longer longevity (Flurkey et al. 2002). Harper et al. reported that IGF1 levels at the age of 15 months were associated with longevity in the progeny of a four way cross (Harper et al. 2003). However, these studies tested the relationship of IGF1 and longevity either in strains with pathological mutations, which would affect longevities by companion diseases, or in limited strains, which does not reflect broad genetic diversity of mice. The current study reports longitudinally measured IGF1 levels in multiple inbred strains. The correlation between IGF1 levels at 6, 12 and 18 months with median lifespan (Figure 3) strongly suggests that IGF1 level is involved in regulating longevity. This correlation also suggests that identifying the genetic regulators of IGF1 levels may provide insight for understanding the genetic regulation of longevity and clues for interventions that may extend longevity.
There are two potential underlying mechanisms for the negative correlation between IGF1 level and longevity. First, increased IGF1 levels may increase diseases; for example it has been suggested that elevated IGF1 increases the risk of cancer in human populations (Janssen & Lamberts 2004). When the pathology for these inbred strains in our cross sectional study is completed, the results may shed light on this issue. Second, decreased IGF1 levels may decrease the signaling of the IGF1 pathway, and consequently reduce the biological processes of aging and extend longevity (Longo & Finch 2003).
Selecting mouse strains for QTL crosses
QTL analysis is an unbiased, hypothesis-independent, genetic approach that has been used for more than 20 years to study the genetic regulation of quantitative traits. It identifies a particular region of the genome as associated with the trait being measured (Lander & Botstein 1989). The efficiency of a QTL analysis is determined by genetic diversity and phenotypic variation between parental strains (Sen et al. 2005). The data provided in this report allow researchers more choice in the selection of parents for QTL crosses for IGF1 or lifespan. This is true even if an investigator plans to carry out the aging study with controlled heterogeneity as has been suggested to improve robustness (Committee on Animal models for Research on Aging, 1981) and provide the genetic “buffering” that is lacking when all alleles are homozygous (Phelan & Austad 1994). Indeed, five longevity QTLs have been identified in two 4-way crosses (Klebanov et al. 2001; Miller et al. 2002).
Determining the right age for evaluating mice
To study the biological signs of aging, investigators have compared old mice with young mice. However, studies of biological aging should avoid both very young and very old mice, particularly mice in the last surviving third of a population because young mice may not be fully mature and old mice may have illnesses (Miller 2006). By characterizing age-related phenotypes of 31 inbred mouse strains at 6-month intervals throughout their lives and measuring their lifespan, we have accumulated baseline data that will help investigators choose mice of the appropriate age for their research.
Identifying new mouse models for age-related diseases
One of our objectives was to identify new models of age-related disease. We have already found models for muscular dystrophy, arthritis, renal disease, and abnormal electrocardiograms (reports are in preparation). This report, combined with those of the pathological analyses and genotyping using high density SNPs, will help identify the genetic determinants of age-related diseases. For example, this study includes five strains of the related C57 family originally derived from one mating in 1920s (B6, B10, BKS, BR, and L). Interestingly, although 4 of 5 strains have similar median lifespans (females: 853~888; males: 770~901 days), L has a much shorter median lifespan (females: 728 days; males: 736 days). Survival curves (Figure 2) show that survival in L mice dramatically decreased at the age of 18~19 months. Once the pathology has established the cause of death, the genetic determinants of that disease most likely will be contained in those small regions of the genome where L differs from the other 4 related strains.
This integrated aging project will provide the aging research community with a comprehensive survey of the health characteristics of many mouse strains at regular intervals throughout their lifespan. The mouse strains represent a broad diversity of genetic backgrounds, and the phenotypes range from molecular to cellular to organ function levels. The significant correlations of IGF1 levels with median lifespan strongly suggest that elucidating the genetic regulation of IGF1 will provide valuable insight into understanding of the genetic regulation of longevity. The IGF1 and lifespan strain survey data provide the research community with the information to optimize the design of genetic studies to understand the regulation of IGF1 and longevity.
EXPERIMENTAL PROCEDURES
Choice of strains
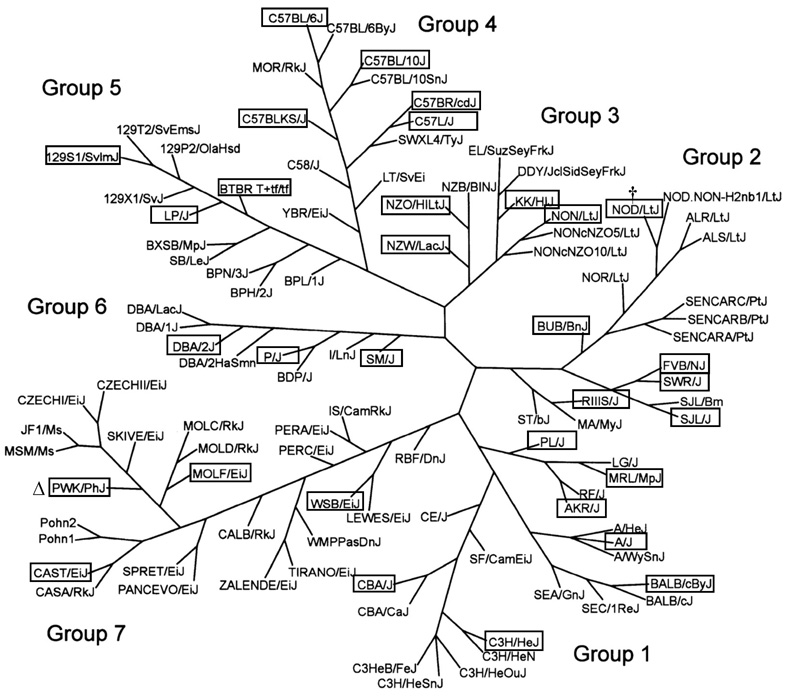
We selected the 32 strains because of their wide genetic diversity, which we estimated from the SNP genotyping used to separate 102 strains into 7 genetically related groups (Petkov et al. 2004). We chose the sequenced strain B6, the 15 strains resequenced by Perlegen Sciences (http://www.perlegen.com), and then added strains for diversity so that each of the 7 genetic groups was represented by 3–7 strains (Figure 1, Table 1). These strains included the 4 wild-derived strains representing the 4 major subspecies of laboratory mice: WSB/EiJ (WSB) for Mus mus domesticus, PWD/PhJ (PWD) for M. m. musculus, CAST for M. m. castaneus, and MOLF/RkJ (MOLF) for M. m. molossinus. NOD/LtJ (NOD), which is a resequenced strain, develops diabetes and dies early. Therefore, we substituted NOD. B10Sn-H2b/J (NOD.B10), a congenic strain with the NOD genetic background but a histocompatibility locus from a diabetes-resistant strain. We could not obtain sufficient quantities of P males so they were omitted from the study. We had to euthanize mice with severe fight-wounds from a few strains. In fact, so many CAST males were euthanized that we withdrew CAST males from the lifespan study. Males of strains SJL and BUB also fought. We replaced SJL males, and data on these replacements will appear in subsequent publications. However we could not obtain enough BUB male replacements. We had to euthanize several female BUB mice that attacked each other. Presumably, the fights were triggered by incompletely healed incisions made to implant the microchip identifiers. In the future, BUB mice implanted with a chip should be isolated until the skin heals completely. Furthermore, we were able to obtain only 25 instead of 32 CAST females.
Figure 1.
Strain selection. The mouse family tree was generated by Petkov et al. (2004). Strains involved in this study are framed. † NOD/LtJ (NOD) is susceptible to diabetes so we substituted the diabetes-resistant strain NOD.B10Sn-H2b/J (NOD.B10), which was generated by transferring the diabetes-resistant major histocompatibility locus H2b haplotype from the C57BL/10J (B10) to the NOD strain. Δ We used the PWD/PhJ (PWD) instead of the related PWK/PhJ strain; both were inbred from wild M. m. musculus pairs caught in the central Czech Republic.
Mice and husbandry
All mice were obtained from the Jackson Laboratory. After weaning, they were incorporated into the study in staggered cohorts over 3 months, except for WSB, which was started 6 months later. They were housed 4/pen in pressurized individually ventilated (PIV) polycarbonate cages measuring 31 ×31 × 21.4 cm divided into two pens supplied with high efficiency particulate air (HEPA) filtered air (Thoren Caging Systems Inc., Hazleton, Pennsylvania). These cages were located in the Genetic Resources Building, a barrier facility. Room entry procedures required personnel to don caps, facemasks, disposable gowns, shoe covers, and gloves. Personnel working in this facility are not allowed to have rodent pets. Mouse colonies in this facility are monitored four times a year for (and are free of) 15 viruses (mouse hepatitis virus, two mouse parvoviruses, reovirus, Theiler’s mouse encephalomyelitis virus, ectromelia virus, mouse rotavirus, thymic virus, pneumonia virus of mice, Sendai virus, murine cytomegalovirus, lactic dehydrogenase-elevating virus, K virus, mouse adenovirus, and polyoma virus), 17 bacterial species (including Helicobacter spp. and P pneumotropica), two Mycoplasma spp., external and internal parasites, and Encephalitozoon cuniculi. Autoclaved white pine shavings (Crobb Box Co. Ellsworth, Maine) were used as bedding. Cages and bedding were changed every two weeks. Mice had ad libitum access to acidified water (pH 2.8–3.1) and autoclaved pelleted diet with 6% fat (Lab diet 5K52, PMI Nutritional International, Bentwood, Mo). Temperature was maintained between 21–23 °C. To identify mice in the longitudinal group that were used for aging-related phenotype studies, microchips (Locus Technology, Inc., Manchester, MD 21102) were implanted subcutaneously.
Longitudinal study
Using pooled male and female unpublished lifespan data from a coauthor (DEH) and assuming that the standard deviations in this study would be similar, we calculated that 70 mice were required to detect a 10% increase in lifespan with a p value of 0.05 and a power of 0.8. We set up two cohorts for this longitudinal study. The first cohort of mice included 32 males and 32 females of each strain. With additional funding one year later, we added a second cohort of 32 females to give a total of 64 mice for one sex and 96 for pooled sexes. Every 6 months, 8 males and 8 females were tested by multiple clinical evaluations. We assessed neuromuscular function by forelimb grip strength and automated gait analysis, kidney function by blood urea nitrogen and urinary albumin and creatinine levels, liver function by alanine aminotransferase, albumin and total bilirubin levels, and immunological function by a fluorescent-activated cell sorting (FACS). Each 6-month evaluation included a complete hematological screen including complete differential blood count, hemoglobin, hematocrit, mean red blood cell volume and other 20 parameters, and routine clinical blood chemistries including blood urea nitrogen, albumin, total protein, lipase and other 18 parameters. In a 3-day test, we used comprehensive laboratory animal monitoring cages to assess food and water consumption, respiratory exchange ratios, metabolic heat production, rest/activity patterns, and sleep behavior. We also measured levels of hormones thought to be involved in the basic mechanisms of aging: insulin-like growth factor 1 (IGF1), insulin, leptin, and thyroxin.
Cross sectional studies
Fifteen females and fifteen males were euthanized at each of the three time points, 6-, 12- and 20-months old. We determined their body composition, bone mineral density, and heart function using an electrocardiogram. We necropsied them and preserved their tissues and organs (including tumors) for molecular, cellular, histological, and pathological analyses. To determine whether aging is associated with the accumulation of DNA damage, we carried out micronuclei tests, measured apoptosis, evaluated DNA repair ability, and measured chromosomal rearrangements by spectral karyotyping at the 12- and 20-month time points.
Estimating age of death (lifespan)
To determine the time and type of death, mice were inspected at least once daily. If older mice appeared to be too weak to obtain food, a mush of ground pellets and water was placed on the cage bottom so that they did not die from starvation. Moribund mice were euthanized if severely ill and it was judged they would not survive another 48 hours. A mouse was considered severely moribund if it exhibited more than one of the following six clinical signs: inability to eat or drink; abnormally low body temperature; severe lethargy (reluctance to move when gently prodded with forceps); severe balance or gait disturbance; rapid weight loss for a week or more; an ulcerated or bleeding tumor. The age at which a moribund mouse was euthanized was taken as the best available estimate of its natural lifespan.
Mice excluded from our analyses
Mice dying abnormally, such as those euthanized because of severe bites, those not recovering from blood sampling, and those dying as a result of laboratory error were excluded from our analyses. Mice were considered to have severe bites if their wounds covered over 20% of the skin or were bleeding or infected and did not significantly improve after a week of antibiotics. Fighting accounted for 2.7% of the deaths and was particularly troublesome for BUB and CAST females and BUB, FVB, and SJL males. We also excluded 7 females and 15 males that died within 24 hours after blood sampling at 18 months of age. Reasoning that death may have occurred from age-related intolerance to the stress of blood loss, we eliminated a few measurements for older mice, thus requiring a smaller blood sample (maximum 200 µl) and eliminating this cause of death. Five females and 1 male died as a result of laboratory error (escape, malfunctioning water bottle).
IGF1 assay
At the age of 6 months, 8 healthy mice of each strain and sex were selected from the longitudinal cohort for IGF1 measurements. At 12 and 18 months, if any of these mice were dead or obviously ill, they were replaced by other healthy mice. About 250 µl of blood was obtained by submandibular bleeding (Golde et al. 2005) using heparin-coated microhematocrit tubes. This method does not require anesthesia. Plasma was centrifuged at 14,000 rpm for 10 minutes at 4°C. Samples were saved at −70 °C prior to analysis. IGF1 levels were measured by a radioimmunoassay as previously described (Rosen et al. 2000).
Statistical methods
Before being analyzed, lifespan data were stratified by sex. We drew the survival curves using the Kaplan Meier method excluding censored mice. From these curves, we calculated median lifespans and 95% confidence intervals (CIs). We tested for differences between female and male longevity using the log-rank method. To correct for multiple testing, we used the Benjamini and Hochberg method (Smith et al.). Survival analyses were carried out using R language 2.40 (http://cran.r-project. org/bin/windows/base/). To determine the effects of genetic and sex differences on lifespan, we performed a proportional hazard fit study. Additional analyses were carried out using JMP 6.03 (The SAS institute, Cary, NC).
For IGF1, analyses were carried out using JMP 6.03. Z scores for each sample were calculated in each sex-strain- time point group. Outliers were defined as z score more than 2 and excluded from further analyses. ANOVA was used to compare IGF1 levels among inbred strains at different ages and between sexes. To correct for multiple testing, significance was adjusted by Turkey HSC method.
Because MOLF mice were started later than other strains, their IGF1 levels at 12 and 18 months have not been measured. Most AK mice died before 12 months, so that their IGF1 levels at 12 and 18 months are absent. Five outliers (z score > 2) of 1358 measurements were excluded from the analysis.
Public availability of data
The protocols for all studies are listed in www.agingmice.org. We are placing the raw data from this study in three publicly accessible databases, MPD (www.jax.org/phenome), the Mouse Tumor Biology Database (MTB, http://tumor.informatics.jax.org) and the Mouse Pathology Database (Pathbase, http://www.pathbase.net).
Supplementary Material
ACKNOWLEDGMENT
The authors thank Gerald McClearn, Steven Austad, James Nelson, and Richard Sprott, who constitute the External Advisory Committee of the Jackson Aging Center, for their advice and support during this project. We appreciate valuable comments and suggestions from Drs. Richard A. Miller and Dean H. Lang on the manuscripts. We thank Ray Lambert, Melissa Rockwood and Alicia Valenzuela for editing the paper. We also thank Nazira Bektassova, Chuck Donnelly, and Abigail Ames for programming support of this project in the computer program JaxTrack, Beth Sundberg for developing and managing the medical records database, and Steven Grubb for programming support of the Mouse Phenome Database. And finally this project could not have been successful without the outstanding technical help provided by Milly So, David Schultz, Dana Godfrey, and Trudy Radcliff.
This work was funded by a Nathan Shock Center grant AG 25707 from the National Institute of Aging, NIH, U.S. and by grants from the Ellison Medical Foundation to Beverly Paigen, Richard Woychik, John Sundberg, Kevin Mills, and Shirng-Wern Tsaih.
Contributor Information
Rong Yuan, Email: Rong.yuan@jax.org.
Shirng-Wern Tsaih, Email: sharon.tsaih@jax.org.
Stefka B. Petkova, Email: stefka.petkova@jax.org.
Caralina Marin de Evsikova, Email: caralina.evsikova@jax.org.
Shuqin Xing, Email: shuqin.xing@jax.org.
Michael A. Marion, Email: michael.marion@jax.org.
Molly A. Bogue, Email: molly.bogue@jax.org.
Kevin D. Mills, Email: kevin.mills@jax.org.
Luanne L. Peters, Email: luanne.peters@jax.org.
Carol J. Bult, Email: carol.bult@jax.org.
Clifford J. Rosen, Email: clifford.rosen@jax.org.
John P. Sundberg, Email: john.sundberg@jax.org.
David E. Harrison, Email: david.harrison@jax.org.
Gary A. Churchill, Email: gary.churchill@jax.org.
Beverly Paigen, Email: bev.paigen@jax.org.
Reference
- Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003;88:3664–3667. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
- Boguski MS. Comparative genomics: the mouse that roared. Nature. 2002;420:515–516. doi: 10.1038/420515a. [DOI] [PubMed] [Google Scholar]
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Wanebo HJ, Old LJ, Boyse EA. Immunogenic properties of reticulum cell sarcomas of SJL/J mice. J Natl Cancer Inst. 1970;44:1281–1288. [PubMed] [Google Scholar]
- Committee on Animal Models for Research on Aging. Mammalian Models for Research on Aging; National Academy Press; Washington, DC. 1981. [Google Scholar]
- Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich N, Alte D, Volzke H, Spilcke-Liss E, Ludemann J, Lerch MM, Kohlmann T, Nauck M, Wallaschofski H. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP) Growth Horm IGF Res. 2008;18:228–237. doi: 10.1016/j.ghir.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Harper JM, Wolf N, Galecki AT, Pinkosky SL, Miller RA. Hormone levels and cataract scores as sex-specific, mid-life predictors of longevity in genetically heterogeneous mice. Mech Ageing Dev. 2003;124:801–810. doi: 10.1016/s0047-6374(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW. Igf-I and longevity. Horm Res. 2004;62 Suppl 3:104–109. doi: 10.1159/000080508. [DOI] [PubMed] [Google Scholar]
- Jernstrom H, Chu W, Vesprini D, Tao Y, Majeed N, Deal C, Pollak M, Narod SA. Genetic factors related to racial variation in plasma levels of insulin-like growth factor-1: implications for premenopausal breast cancer risk. Mol Genet Metab. 2001;72:144–154. doi: 10.1006/mgme.2000.3130. [DOI] [PubMed] [Google Scholar]
- Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Karpova GV, Fomina TI, Abramova EV, Bel'skaya NV, Trofimova ES, Perel'muter VM. Hemopoietic and lymphoid organs in AKR/JY mice with thymic lymphoma. Bull Exp Biol Med. 2002;134:69–72. doi: 10.1023/a:1020669007938. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Klebanov S, Astle CM, Roderick TH, Flurkey K, Archer JR, Chen J, Harrison DE. Maximum life spans in mice are extended by wild strain alleles. Exp Biol Med (Maywood) 2001;226:854–859. doi: 10.1177/153537020122600908. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Mooijaart SP, van Heemst D, Zwaan BJ, Slagboom PE, Westendorp RG. Genes encoding longevity: from model organisms to humans. Aging Cell. 2008;7:270–280. doi: 10.1111/j.1474-9726.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Miller RA. Principles of Animal Use for Gerontological Research. London, UK: Elsevier Academic Press; 2006. [Google Scholar]
- Miller RA, Chrisp C, Jackson AU, Galecki AT, Burke DT. Coordinated genetic control of neoplastic and nonneoplastic diseases in mice. J Gerontol A Biol Sci Med Sci. 2002;57:B3–B8. doi: 10.1093/gerona/57.1.b3. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Hsueh WC, King TM, Pollin TI, Sorkin J, Agarwala R, Schaffer AA, Shuldiner AR. Heritability of life span in the Old Order Amish. Am J Med Genet. 2001;102:346–352. doi: 10.1002/ajmg.1483. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Paigen K. A miracle enough: the power of mice. Nat Med. 1995;1:215–220. doi: 10.1038/nm0395-215. [DOI] [PubMed] [Google Scholar]
- Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49:B1–B11. doi: 10.1093/geronj/49.1.b1. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Churchill GA, Donahue LR, Shultz KL, Burgess JK, Powell DR, Ackert C, Beamer WG. Mapping quantitative trait loci for serum insulin-like growth factor-1 levels in mice. Bone. 2000;27:521–528. doi: 10.1016/s8756-3282(00)00354-9. [DOI] [PubMed] [Google Scholar]
- Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, Slagboom PE, Westendorp RG. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet. 2006;14:79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Sen S, Satagopan JM, Churchill GA. Quantitative trait locus study design from an information perspective. Genetics. 2005;170:447–464. doi: 10.1534/genetics.104.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Bhasin JM, Baglione J, Settle M, Xu Y, Barnard J. Atherosclerosis susceptibility loci identified from a strain intercross of apolipoprotein E-deficient mice via a high-density genome scan. Arterioscler Thromb Vasc Biol. 2006;26:597–603. doi: 10.1161/01.ATV.0000201044.33220.5c. [DOI] [PubMed] [Google Scholar]
- Sommer AM, Bitz AK, Streckert J, Hansen VW, Lerchl A. Lymphoma development in mice chronically exposed to UMTS-modulated radiofrequency electromagnetic fields. Radiat Res. 2007;168:72–80. doi: 10.1667/RR0857.1. [DOI] [PubMed] [Google Scholar]
- Stavnezer J, Lasky JL, Ponzio NM, Scheid MP, Thorbecke GJ. Reticulum cell sarcomas of SJL mice have rearranged immunoglobulin heavy and light chain genes. Eur J Immunol. 1989;19:1063–1069. doi: 10.1002/eji.1830190616. [DOI] [PubMed] [Google Scholar]
- Storer JB. Longevity and gross pathology at death in 22 inbred mouse strains. J Gerontol. 1966;21:404–409. doi: 10.1093/geronj/21.3.404. [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Walford RL, Liu RK, Gerbase-Delima M, Mathies M, Smith GS. Longterm dietary restriction and immune function in mice: response to sheep red blood cells and to mitogenic agents. Mech Ageing Dev. 1973;2:447–454. doi: 10.1016/0047-6374(73)90035-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.