Abstract
In drosophila, fungal and Gram-positive bacterial molecular determinants are detected by circulating pattern recognition receptors (PRRs). Previous findings suggest that these PRRs activate yet unidentified serine protease cascades culminating in the cleavage of Spaetzle, the endogenous Toll receptor ligand, and triggering the immune response. We demonstrate here that the Grass protease defines a common activation cascade for PRR-mediated fungal and Gram-positive bacterial detection. The serine protease Persephone, previously shown to be specific for fungal detection in a cascade activated by secreted fungal proteases, was also required for sensing of proteases elicited by bacteria in the hemolymph. Hence, Persephone defines a parallel proteolytic cascade activated by danger signals such as abnormal proteolytic activities.
Introduction
Sensing of microbial infections induces innate immune responses in plants and in animals. Bacterial and fungal molecules such as cell wall components, which are common to most pathogens and often referred to as pathogen-associated molecular patterns (PAMPs), are detected by receptor proteins (or PRRs), either in circulation or on the cell membrane, in the endosome or in the cytosol1-4 of immune responsive cells. Activated receptors trigger signal transduction cascades and the transcription of effector genes. In plants, together with the direct detection of molecular patterns, defense mechanisms can also be activated indirectly by the sensing of host protein modifications induced by microbial activities. This indirect activation pathway of immune responses is called the guard system2,3.
In Drosophila melanogaster, the best-documented facet of the immune response involves the activation of two signal transduction cascades, the Toll and IMD pathways. These lead to the transcription by the fat body cells of genes encoding antimicrobial peptides (AMPs) and the subsequent secretion of the peptides into the hemolymph5-7. This defense mechanism discriminates between Gram-negative bacteria, which activate mostly the IMD pathway, and Gram-positive bacteria and fungi, which stimulate the Toll pathway. Both pathways culminate in the activation of NF-κB related transcription factors, Relish and Dorsal-related Immune Factor (DIF), respectively, and the transcription of hundreds of genes including those encoding the effector AMPs directed against the intruding microorganisms. The membrane spanning peptidoglycan recognition protein (PGRP)-LC, in association with PGRP-LE, senses directly diaminopimelic containing peptidoglycan (DAP-type PGN), common to most Gram-negative bacteria and to Gram-positive bacilli, and triggers the IMD intracellular signaling cascade8-12. In contrast, the transmembrane receptor Toll does not directly recognize microbial determinants but is activated by binding of an endogenous ligand, the proteolytically cleaved form of the cytokine-like protein Spaetzle (Spz)13. The detection of Gram-positive bacterial cell wall components such as lysine containing PGN (Lys-type PGN) requires a combination of different proteins, namely PGRP-SA, PGRP-SD and glucan binding protein 1 (GNBP1)14-16. Fungal determinants (mainly glucans) are detected by the circulating PRR GNBP3 (ref. 17). Binding of microbial ligands to these soluble PRRs induces, by an unknown mechanism, the activation of proteolytic cascades leading to the cleavage of Spz and subsequent Toll activation.
We are interested in deciphering the mechanisms that lead from recognition of microbial molecules in the hemolymph to the cleavage of Spz to its active ligand form. Toll and Spz were initially identified for their roles in dorso-ventral patterning during development. In the early embryo, the Toll pathway is activated by a cascade of four serine proteases. These proteases are activated upon cleavage of an N-terminal inhibitory domain, which is known as the clip domain for the two downstream proteases of the cascade, Snake and Easter18,19. Interestingly, genetic studies have indicated that these embryonic serine proteases are dispensable during the immune responses in larvae or adult flies20,21.
Several direct genetic screens aimed at identifying factors involved in the activation of the Toll pathway following fungal or Gram-positive bacterial infections. However these screens failed to identify the proteases of the cascades that culminate in the activation of Spz. The Persephone (Psh) protease was indirectly discovered in a screen for suppressors of the necrotic phenotype, characterized by a constitutive activation of the Toll pathway resulting from the absence of the serine protease inhibitor Necrotic22,23. It was subsequently shown that Psh is specifically required for the activation of the Toll pathway after fungal infection and for adequate resistance of flies to such infections23. However, Psh is not required downstream of GNBP3, the fungal PRR, but is activated by fungal virulence factors17. The Spz processing enzyme (SPE) that directly cleaves Spz during the immune response has been found by homology to a Bombyx mori serine protease24,25. Finally, an in vivo RNAi screen targeting drosophila serine proteases identified five candidate genes necessary for full activation of the Toll pathway after fungal and Gram-positive bacterial infections26. One of these proteases was shown to be specifically required for the detection of Gram-positive bacterial infections and named Grass (for Gram-positive specific serine protease). Together, these studies led to the current model in which three protease cascades separately activate the Toll pathway after recognition of Gram-positive bacterial determinants, fungal cell walls and fungal virulence factors, respectively.
However, even in absence of plain off-target effects, RNAi driven gene inactivation generally results in hypomorphic phenotypes, leading to incomplete characterization of gene function. Using a null mutation in grass we demonstrate here that Grass defines a common protease cascade downstream of both fungal and Gram-positive bacterial pattern detection that functions in parallel to a separate proteolytic cascade centered on Psh, sensing virulence factors, again from both bacteria and fungi, and guarding drosophila against abnormal proteolytic activity in the hemolymph.
Results
CG5896 overexpression activates the Toll pathway
We have previously shown that overexpression of full-length Psh protein induces the constitutive activation of the Toll pathway in the absence of any immune challenge23. We reasoned that expression in the hemolymph of full-length proteases acting upstream of Toll would give a similar phenotype as Psh. We therefore designed an overexpression screen (D. Rabel, L.E.C., V.L. and J.-M.R. in preparation). We expressed a series of protease genes using the UAS-GAL4 system and the heat-shock driver (hsp-GAL4) in transgenic flies and detected in the hemolymph by mass spectrometry the production of Drosomycin, a conventional readout of Toll activation. Of all the genes tested, CG5896 gave a clear phenotype and was analyzed further (Supplementary Fig.1a online).
CG5896 is required for fungal and bacterial detection
While our studies were in progress, Kambris et al.26 expressed double-stranded RNA directed against the CG5896 protease, which they named Grass. These authors observed that the transgenic flies were susceptible to Gram-positive bacterial infections and failed to respond to these infections by expression of the drosomycin gene. In contrast, the transgenic flies survived fungal infections and expressed drosomycin similarly to wild-type flies. Fly lines which we had constructed using double-stranded RNA to inactivate CG5896 also reduced the response to Gram-positive bacterial infection although in a variable manner (data not shown). We therefore decided to generate a null mutation of the CG5896 gene by mobilization of the P element XP11068 inserted 550 bp upstream of the CG5896 transcription start site. Flies carrying this insertion did not show any immune phenotype. Mobilizing the P element led to a small deletion starting at the insertion site to nucleotide 735 in the transcript, deleting the first 101 amino acids of the ORF. We noted that flies homozygous for this deficiency were fully viable and lacked any CG5896 gene product. We designated the null allele grassHerrade (Supplementary Fig. 1b online).
As expected, grassHerrade mutant flies were deficient in Toll pathway activation after a Gram-positive bacterial challenge and were highly susceptible to these bacteria (Fig. 1a,b). However, to our surprise, the flies were also deficient in their response to fungal infections by the entomopathogenic fungus Beauveria bassiana and the yeast Candida albicans (Fig. 1c,d and Supplementary Fig. 2 online). Thus, even if the reduction in drosomycin expression was less marked and more variable than that noted after a Gram-positive bacterial challenge, grass was clearly required for the detection of both Gram-positive bacterial and fungal infections. The RNAi-mediated inactivation, which resulted in a reduction of grass mRNA to 60% (26 and our unpublished data) was therefore not sufficient to reveal the involvement of Grass in fungal detection. Fungi have been reported to activate two different extracellular pathways upstream of Toll, one triggered by GNBP3-mediated recognition of fungal cell wall components, and the other by virulence factors, such as secreted proteases sensed by the circulating protease Psh. If grass were involved in only one of these two pathways, the consequence of the RNAi-mediated reduction of its activity would be masked by the activation of the other pathway, resulting in almost wild-type expression of drosomycin. A further reduction in Grass activity would then translate into a visible phenotype in grassHerrade null mutant flies.
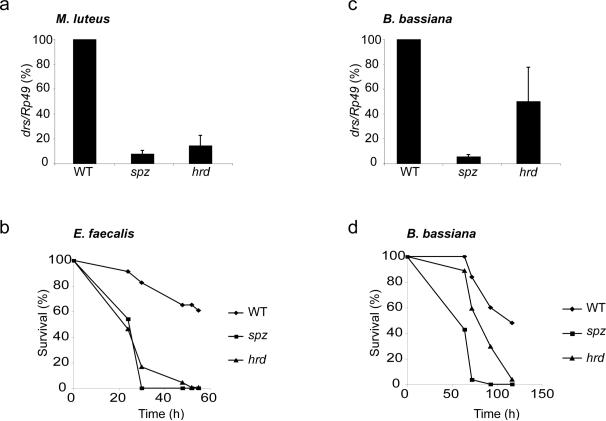
Figure 1. Grass is involved in sensing both fungal and Gram-positive bacterial infections.
(a) Quantification of RNA hybridization analysis of drosomycin (drs) gene expression 24 h after infection with the Gram-positive bacterium M. luteus. Ribosomal protein 49 (Rp49) messenger was used for normalization. drs mRNA expression (drs/Rp49) in wild-type (WT) flies was set to 100 as a control and values obtained with mutant flies were expressed as percentage of this value. Spaetzle (spz) mutant flies were used as Toll pathway mutant control. Each bar represents the mean of 4 independent experiments, error bars are SD. drs expression was reduced in grassHerrade mutant flies (hrd) (P = 1.3 × 10-9). (b) Survival of adult flies infected with E. faecalis. This result is representative of three independent experiments. (c) Quantification of RNA hybridization analysis of drs gene expression 48 h after infection with the fungus B. bassiana. Each bar represents the mean of 6 independent experiments, error bars are SD. drs expression is reduced in grassHerrade mutant flies (hrd) (P = 0.001). (d) Survival of adult flies infected with B. bassiana. This result is representative of three independent experiments.
Grass acts downstream of PRRs
We next undertook an epistatic analysis to clarify in which pathway Grass was required. We started by analyzing the relationship with SPE that has been shown, using RNAi-mediated knock-down experiments, to be the protease responsible for the cleavage of Spz24. During a loss of function screen for genes involved in resistance to fungal infections we identified a SPE mutant allele that we named Pasteur. SPEPasteur is a hypomorphic mutation, which affects the transcription of this gene (Fig. 2). We found that SPEPasteur homozygous flies were highly susceptible to fungal infections but that they showed only a slight reduction in Toll pathway activation. However, hemizygous mutant flies were, as expected, susceptible to both fungal and Gram-positive bacterial infections and showed a strong reduction in Toll pathway activation after either type of challenges. The SPEPasteur immune deficiency phenotypes could be rescued by expressing a wild-type copy of SPE in mutant flies (Fig. 2). The Toll pathway constitutive activation resulting from grass overexpression was abolished in SPEPasteur hemizygous mutant flies (Fig. 3). On the other hand, expression of an activated form of SPE triggered a strong activation of the Toll pathway that was not blocked in a grassHerrade mutant background (data not shown). Taken together, these data demonstrate that grass acts upstream of SPE.
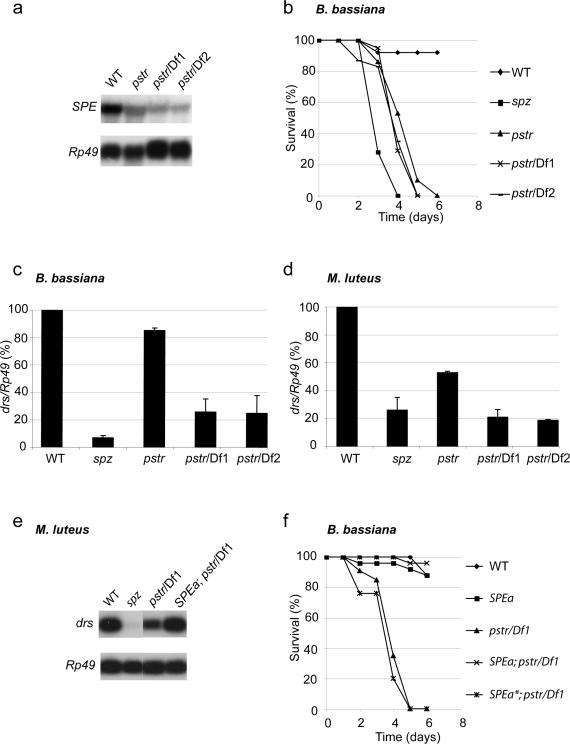
Figure 2. Isolation of the Spaetzle Processing Enzyme (SPE) mutant Pasteur.
(a) Representative RNA hybridization analysis of the SPE gene expression in wild-type (WT), SPEPasteur homozygous (pstr) and SPEPasteur over Df(3R) mbcR1 (pstr/Df1) or Df(3R) mbc30 (pstr/Df2) hemizygous mutant flies.
(b) Survival of adult flies infected by B. bassiana. Genotypes are as in (a).
(c,d) Quantification of RNA hybridization analysis of drosomycin (drs) gene expression, 48 and 24 hours after infection with (c) the fungus B. bassiana and (d) the Gram-positive bacterium M. luteus. Rp49 messenger was used for normalization. drs mRNA expression (drs/Rp49) in wild-type (WT) flies was set to 100 as a control and values obtained with mutant flies were expressed as percentage of this value. Each bar represents the mean of 3 independent experiments, error bars are SD. (c) drs expression is reduced in both SPEPasteur hemizygous mutant flies (P = 0.003 and P = 0,014). (d) drs expression is reduced in all combinations of SPEPasteur mutants (P = 2 × 10-4, P = 2 × 10-5 and P = 3 × 10-5).
(e,f) activated SPE (SPEa) or a catalytically inactive form of this gene (SPEa*) was expressed in SPEPasteur mutant flies using the UAS-GAL4 system under the control of an Actin-GAL4 driver. (e) Representative RNA hybridization analysis of drs gene expression 24 hours after infection with M. luteus. (f) Survival of adult flies infected by B. bassiana.
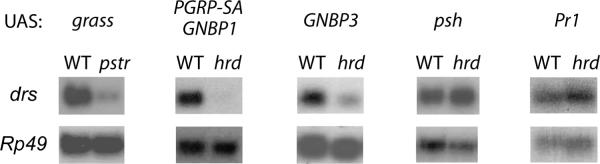
Figure 3. Grass acts downstream of the PRRs.
Several signal modifiers of the Toll pathway, grass, PGRP-SA and GNBP1, GNBP3, psh or PrI, were overexpressed in adult flies using the UAS-GAL4 system under the control of a heat-shock GAL4 driver. drs gene expression was measured by RNA hybridization in WT, SPEPasteur (pstr) or grassHerrade (hrd) mutant background.
Detection of Lys-type PGN requires both PGRP-SA and GNBP1 proteins. Overexpression of either PGRP-SA or GNBP1 alone has no effect on drosomycin expression, but the coexpression of both genes strongly activates the Toll pathway15. We noted that this activation was suppressed in grassHerrade flies, indicating that Grass acts downstream of a Gram-positive bacterial peptidoglycan recognition event (Fig. 3). GNBP3 is a PRR that senses fungal cell wall components. GNBP3 overexpression reproducibly induces a weak, but detectable, activation of the Toll pathway17 that was abolished in the grassHerrade mutant background (Fig. 3), demonstrating that Grass functions downstream of both fungal and bacterial PRR pathways.
While SPE was required for Toll pathway activation in flies overexpressing Psh (24 and our unpublished data), this activation was not affected in a grassHerrade mutant background (Fig. 3). We hypothesized that either Grass acts upstream of Psh or that the two proteins function in parallel pathways. The fungal protease Pr1 has been shown to activate the Toll pathway when expressed in the hemolymph of transgenic flies under the UAS-GAL4 system and the hsp-GAL4 driver. This activation was reported to require Psh but we noted that it was not abolished when we expressed Pr1 in a grassHerrade mutant background (17 and Fig. 3). Taken together, our data demonstrate that Grass functions downstream of the PRRs sensing either fungal or Gram-positive bacterial infections. Importantly however, Grass was not required downstream of the fungal protease Pr1 that activates Psh.
Psh senses microbial activities
Following a fungal infection, Toll pathway activation was always totally suppressed in spz mutant flies, whereas in psh or grassHerrade mutants drosomycin expression remained at 30-60% of that of wild-type with some variability between independent experiments (Figs. 1, 4a,b) possibly reflecting variations in the virulence state of fungi. Interestingly, in double mutant flies for psh and grassHerrade, Toll pathway activation was totally blocked and these flies were as susceptible as spz flies to fungal infections (Fig. 4a-c). However, compared to grassHerrade single mutant flies, Toll pathway activation was not further affected in double mutant flies for GNBP3Hades and grassHerrade (Fig. 4a,b). These data confirm that Grass and Psh cooperate in two parallel cascades activated downstream of PRRs and fungal proteases, respectively. These results prompted us to re-examine the involvement of Psh in the response to Gram-positive bacterial infections. A possible role for Psh in Gram-positive bacterial infections was suggested by the earlier observation that the signal-dependent cleavage of Necrotic, the Psh serine protease inhibitor, is abolished in a psh mutant background after both fungal and Gram-positive bacterial infections27. Flies challenged with Micrococcus luteus show a strong activation of the Toll pathway that we have shown to be greatly reduced in a grassHerrade mutant background. Interestingly, we also detected a weak but reproducible reduction in drosomycin expression in a psh mutant background. This reduction was more important in psh, grassHerrade double mutant than in grassHerrade single mutant flies (Fig. 4c,d) suggesting that Psh could also be involved in Gram-positive bacterial detection. We reasoned that if Psh were required for detection of Gram-positive bacterial proteases as it is for fungal proteases, then the Psh contribution to Toll pathway activation should be more important during infection by pathogenic bacteria. These bacteria may express more proteases or virulence factors than non-pathogenic bacteria. Indeed, M. luteus is not pathogenic for drosophila, which might explain the moderate requirement of Psh for the activation of the Toll pathway after infection by this bacterium. Enterococcus faecalis, a lethal pathogen for drosophila, also strongly activates the Toll pathway. We noted that this activation was moderately reduced in either grassHerrade, psh or the GNBP1Osiris null15 single mutants as well as in grassHerrade, GNBP1Osiris double mutants but was as reduced as in spz mutants in psh, grassHerrade double mutant flies (Fig. 4f,g). Correlatively, psh, grassHerrade double mutant flies were more susceptible to E. faecalis infection than grassHerrade single mutants as illustrated by survival tests (Fig. 4h). We concluded that Psh is also involved in the detection of Gram-positive bacteria.
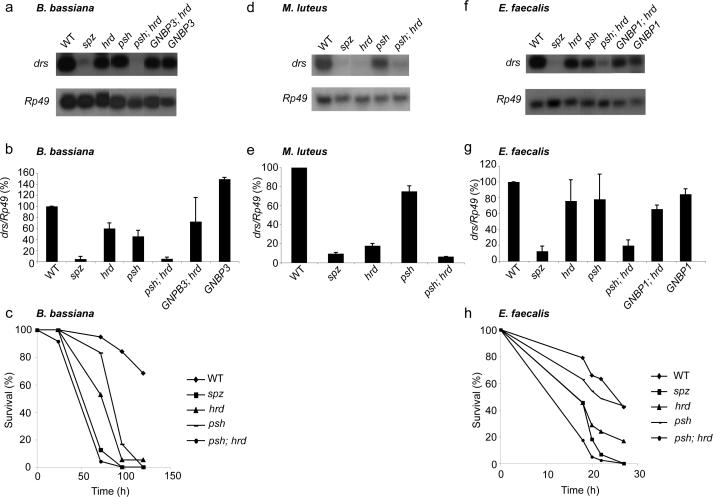
Figure 4. Grass and Psh define two parallel pathways that activate Toll cooperatively.
(a-f) Toll pathway activation was monitored by RNA hybridization analysis of drs messenger expression. Rp49 messenger was used as loading control and for normalization of drosomycin (drs) expression. drs mRNA abundance (drs/Rp49) in wild-type (WT) flies was set to 100 as a control and values obtained with mutant flies were expressed as percentage of this value. RNA hybridizations are representative of 3 independent experiments and histograms represent the mean of these results. Error bars are SD. (a,b) drs expression is reduced in grassHerrade (hrd) and psh (P = 0.002 and P = 9 × 10-4 respectively) 36 h after infection by the fungus B. bassiana. drs expression is reduced in psh, hrd double mutant flies compared to both single mutants (P = 7 × 10-4 and P = 0.003 respectively) and is similar to the expression in spz mutants (P = 0.9). drs expression is not significantly different in GNBP3Hades, hrd (GNBP3; hrd) double mutants compared to hrd single mutants (P = 0.65). (c) Survival curves of adult flies infected with B. bassiana. Results are representative of 3 independent experiments.
(d,e) drs expression 24 hours after infection by the Gram-positive bacterium M. luteus is significantly reduced in psh, hrd double mutant compared to hrd single mutant flies (p= 0.003) and is similar to that detected in spz mutants (P = 0.45). drs expression is significantly reduced in psh mutants (P = 0.002).
(f,g) drs expression 24 hours after infection by the Gram-positive bacterium E. faecalis is significantly reduced in psh, hrd double mutant flies (P = 1.5 10-7) and only slightly reduced in hrd, psh or GNBP1Osiris (GNBP1) single mutant flies (P = 0.18, P = 0.16 and P = 0.08 espectively). drs expression in psh, hrd double mutant is similar to that in spz mutants (P = 0.12) and not significantly different in GNBP1Osiris, hrd (GNBP1; hrd) double mutant compared to hrd single mutant (P = 0.6).
(h) Survival curves of adult flies infected with E. faecalis. Results are representative of 3 independent experiments.
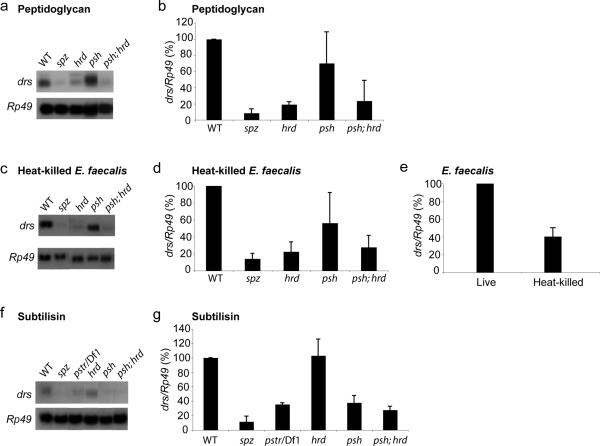
Toll pathway activation by coexpression of the PRR genes PGRP-SA and GNBP1 or injection of peptidoglycan from either M. luteus or E. faecalis (data not shown and Fig. 5a,b) did not require Psh, but was strictly dependent on Grass. Furthermore, when we challenged flies with heat-killed E. faecalis bacteria, the activation of Toll pathway was almost totally blocked in grassHerrade mutant flies and was not further reduced in double mutants for psh and grassHerrade (Fig. 5c,d). We also found that the level of activation by dead bacteria was low when compared to live E. faecalis infections (Fig. 5e), which indicates that factors expressed by live bacteria would be involved, together with structural determinants, in Toll pathway activation. Taken together, these results demonstrate that Psh is not implicated in signaling downstream of PRRs.
Figure 5. Psh is activated by bacterial proteolytic activities in the hemolymph.
Toll pathway activation was monitored in adult flies by RNA hybridization analysis of drs messenger expression. Rp49 messenger was used as loading control and for normalization of drs expression. drs mRNA abundance (drs/Rp49) in wild-type (WT) flies was set to 100 as a control and values obtained with mutant flies were expressed as percentage of this value. This result is representative of 3 independent experiments and histograms represent the mean of these results. Error bars are SD.
(a,b) drs expression 24 hours after injection of E. faecalis peptidoglycan is not significantly affected in psh mutant flies (P = 0.24) but is reduced in grassHerrade (hrd) (P = 2.3 × 10-6) or psh, hrd double mutant flies (P = 0.009) at a very comparable level (P = 0.45).
(c,d) drs expression 24 hours after injection of heat-killed E. faecalis is not significantly affected in psh mutant flies (P = 0.05) but is highly reduced in hrd single (P = 1.6 × 10-5) or psh, hrd double mutant flies (P = 6 × 10-5) at a very comparable level (P = 0.45).
(e) drs expression 24 hours after infection of WT flies with living or heat-killed E. faecalis is significantly reduced in flies challenged with dead bacteria (P = 2.7 × 10-5).
(f,g) drs expression 24 hours after injection of bacterial subtilisin is not affected in hrd mutant (P = 0.8) but is highly reduced in psh mutant flies (P = 1,9 × 10-8). The reduction in drs expression observed in psh is identical to that observed in the hemizygous SPEPasteur mutant (pstr/Df1) (P = 0.58) and is not further enhanced in psh, hrd double mutant flies (P = 0.1).
To test if Psh would activate the Toll pathway through sensing of bacterial proteolytic activities we injected exogenous proteases into the flies. Injection of sublethal doses of proteases from Bacillus subtilis or Aspergillus oryzea into the body cavity of flies strongly activated the Toll pathway in wild-type or grassHerrade flies. This activation was strongly reduced in psh mutant flies (Fig. 5f,g and Supplementary Fig. 3 online). Residual drosomycin expression observed in psh and SPEPasteur mutant flies might be explained by direct non-specific cleavage of Spz by the injected proteases. However, in these conditions 60% of drosomycin expression was specifically due to Psh-dependent SPE activation leading to Toll signaling (Fig. 5f,g and Supplementary Fig. 3 online). This result is in accordance with the observation that Psh, unlike most of the drosophila CLIP-domain containing serine proteases, is activated through cleavage following an histidine residue, which may be targeted by subtilisin-like proteases, instead of arginine or lysine residues, canonical substrates for the trypsin-like proteases such as Easter, Grass or SPE19,28.
Discussion
We report here the generation of a null mutation in the gene encoding the serine protease Grass. On the basis of RNA interference experiments this protease had been reported to play a role in Toll activation following Gram-positive infection26. We now show, through the analysis of the null mutant phenotypes, that Grass is indeed involved in activation of Toll following both Gram-positive bacterial and fungal infections. Grass acts downstream of the circulating pattern recognition receptors that detect Gram-positive bacterial and fungal conserved cell wall molecules (respectively peptidoglycan and β-glucan). Our data further suggest that the Psh protease might sense proteolytic activities elicited by both fungal and Gram-positive bacterial infections. The Toll pathway is activated by the expression of the fungal protease Pr1 (ref. 17) and, as shown here, by injection of proteases from B. subtilis or A. oryzea. This activation was strongly reduced in a psh mutant fly. In contrast, the Toll pathway was activated by peptidoglycans in a strictly Grass-dependent, Psh-independent manner. Toll activation in response to live microorganisms involved both Grass and Psh acting in parallel pathways, whereas heat-killed fungi or bacteria did not require Psh to activate the Toll pathway. Psh appears to sense the presence of abnormal proteolytic activities in the hemolymph. This notion is supported by the observation that a strong overexpression of Grass in transgenic flies resulted in a partially Psh-dependent Toll pathway activation (data not shown). This result suggests that Psh is able to sense the artificially high activity of the Grass protease in the hemolymph in the same way it detects pathogen-derived proteolytic activities. It implicates also that Grass is in a different configuration when artificially overexpressed in absence of signal or when normally activated by PRR-dependent microbial recognition. In the latter case, Grass would be associated with other serine proteases or serine protease homologs such as Spirit, Sphinx or Spheroid26 in a complex with PRRs directing Grass activity toward SPE. In contrast, overexpressed Grass would be detected as abnormal proteolytic activity by Psh resulting in downstream activation of SPE through Psh. We can correlate this finding with the phenotype of the necrotic mutation. Necrotic encodes a serine protease inhibitor whose inactivation leads to the deleterious and abnormal activation of several proteolytic activities resulting in early lethality of mutant flies22,29. One consequence of these abnormal proteolytic activities is a constitutive Toll pathway activation. The different phenotypes associated with the necrotic mutation are all strictly Psh dependent23. Indeed, Psh was isolated as a genetic suppressor of the necrotic mutation.
We propose a new model for Toll activation during the immune response of drosophila. The model proposed to date is based on two proteolytic cascades activated by circulating receptors capable of discriminating between bacterial and fungal infections and a third cascade required for sensing fungal proteases. Our results show that a first proteolytic cascade, comprising Grass, is activated by microbial cell wall components binding to PRRs. We further suggest that a second proteolytic cascade is activated by secreted proteases from microorganisms or abnormal proteolytic activities in the hemolymph, which are sensed by Psh in a way reminiscent of the guard system in plants. We refer to the first cascade as the PRR-dependent extracellular pathway. The molecular mechanism of this proteolytic cascade activation, probably involving the formation of a multiprotein complex containing PRRs is still unknown. We refer to the second cascade as the danger signal extracellular pathway. Both pathways are required for full activation of Toll dependent immune responses against pathogens.
One could speculate that during evolution a proteolytic cascade system upstream of the drosophila Toll receptor has provided a flexibility allowing appearance of new detection mechanisms. The components of a protease cascade downstream of GNBP1 were recently purified from Tenebrio molitor, a coleopteran insect30. However none of these components shows a strong homology to Grass, suggesting that the proteolytic cascades involved in immune defenses are subjected to divergent evolution. Psh may have evolved secondarily to add a new level of defense by sensing the activity of invading microorganisms. We suspect that the detection of bacteria and fungi was first based on specific recognition of molecular patterns. In the `arms race' between host and pathogens, and the probable emergence of escape mechanisms in pathogens masking their cell wall components or hampering detection, Psh provided a way of sensing microorganisms indirectly by their activity. Being aware of proteolytic activities allows the detection of microorganisms since many bacteria and fungi excrete proteases during the invasion process. The proteases they secrete, such as Pr1, display early signs of infection to the host and their sensing by Psh enables a rapid response against invaders. Detection of microbial activity appears to be more flexible than recognition of conserved molecular patterns during host-pathogen interaction. Indeed, the observation that psh shows the highest polymorphism between drosophila species31 demonstrates that psh is under strong selection.
Several virulence factors of E. faecalis are proteases that probably target Psh as heat-killed bacteria do not require Psh for Toll pathway activation. E. faecalis is not a natural drosophila pathogen, as it has to be artificially introduced into the flies. Identification of proteases from Gram-positive entomopathogenic bacteria is crucial to the understanding of host-pathogen interaction. Gram-negative bacteria and bacilli strongly engage the IMD pathway but also moderately and transiently activate the Toll pathway. The possibility that some proteases secreted by Gram-negative bacteria could activate the Toll pathway via Psh is still open.
Pathogen sensing by a dual system comprising a first branch that recognizes molecules common to many classes of microbes (PAMPs) and a second branch that responds to virulence factors, either directly or indirectly through their effects on host targets (danger signal) is well described in the plant immune system2. Here we demonstrate that a similar dual system is at work in drosophila. Mammalian cells utilize pattern recognition receptors, such as extracellular sensing Toll-like receptors (TLRs) and cytosolic sensing Nod-like receptors (NLRs) families, to detect microorganisms. It has been suggested that danger signals such as virulence factors or endogenous proteins released by damaged cells may also be detected directly by TLRs or NLRs32,33. In addition some pathogens secrete proteases enabling them to degrade adherent junctions and penetrate the epithelial barrier. It was recently shown that some bacterial proteases are able to cleave protease-activated receptor 2 (PAR2) leading to secretion of antimicrobial peptides and inflammatory cytokines in epithelial cells (see 34 for review). PARs are G-protein coupled transmembrane receptors that are activated by cleavage of their own N-terminal domain, which acts as a tethered ligand. PAR activation by endogenous proteases of the thrombin and trypsin families leads to inflammatory responses via NF-κB, AP1 and c/EBP-β transcription factors. PAR cleavage by injury-activated thrombin or bacterial proteases appears to be a danger signal sensing mechanism very similar to Psh-dependent Toll pathway activation in flies. These results demonstrate that as in plants, danger signal sensing works together with PAMP detection in animals. Analysis of a dual system encompassing PARs or other yet unidentified sensors in parallel to PRRs, will undoubtly shed a new light on our picture of microbial sensing in mammals.
Methods
Fly stocks
The following fly stocks have been previously described: Psh4, GNBP1Osiris 15, GNBP3Hades 17, UAS-psh23, UAS-PGRP-SA16, UAS GNBP115, UAS GNBP317, UAS Pr117, spz20, UAS-aSPE24. Df(3R)mbc31, Df(3R)mbc30, XP11068, heat shock and Actin Gal4 drivers were from Bloomington stock center. The original XP11068 Bloomington stock carried a homozygous lethal unknown mutation, and we constructed a homozygous viable line by recombination with w1108 flies. The grassHerrade mutation was subsequently created by imprecise excision of the XP11068 element from the recombinant line. SPEPasteur mutant was isolated by EMS-induced mutagenesis.
RH61984 and GH28857 were cDNA clones from the BDGP EST project that matched the CG5896-grass gene and the CG16705-SPE gene respectively. For the inactive version of the CG16705-SPE encoded protease, the catalytic serine (Ser 346) was replaced by a glycine using PCR-directed mutagenesis, with the following oligonucleotide where the modified codon is indicated in bold: 5'-CATGCGGTGGCGATGGTGGCGGTCCCCTTAT-3'. The PCR products were cloned into pUAST predigested with the Bgl II and Xho I enzymes35.
Infections, RNA hybridization and survival analysis
Bacterial and fungal strains E. faecalis, M. luteus, Escherichia coli, Agrobacterium tumefaciens, Candida albicans, B. bassiana have been previously described17,36. For heat killing, bacterial cultures (OD600 = 0,1) were heated twice at 95 °C for 20 min separated by 20 min at 4 °C. Killing was controlled by plating 100 μl of the culture on LB agar plates.
Infections by pricking with a tungsten needle, RNA hybridization and survival tests were performed as previously decribed23. Each individual experiment was performed on a sample of 12 males and 12 females.
Proteases injections: 18,3nl of a dilution of 1 in 2000 of a commercial solution of proteases from B. subtilis (Sigma-Aldrich, product number P5985, more than 16U/g) or A. oryzea (Sigma-Aldrich, product number P6110, more than 500U/g) in PBS was injected into the fly body cavity. 1 in 2000 dilutions were chosen for the induction of drosomycin with a survival rate after 24 h of more than 80%. Higher concentrations were lethal for the flies and lower did not induce drosomycin expression.
Peptidoglycan injection: 9.2 nl of a suspension at 5 mg/ml of sonicated peptidoglycan from M. luteus (Sigma-Aldrich) or E. faecalis (a generous gift from L. Gutmann, Inserm U872 Paris) were injected in the fly body cavity.
Supplementary Material
Acknowledgements
We dedicate this work to A. Killinc who found the original pasteur mutation. We are grateful to L. Gutmann, Inserm U872 Paris for the gift of E. faecalis peptidoglycan and to A. Meunier, S. Ozkan and R. Walther for technical help. L. E.-C. is supported by an “allocation de recherche” from the French Research Ministry and a doctoral fellowship from Association pour la Recherche contre le Cancer. This work is supported financially by the Centre National de la Recherche Scientifique and a National Institute of Health program grant 5PO1-AI044220-09.
References
- 1.Hoebe K, et al. Genetic analysis of innate immunity. Adv Immunol. 2006;91:175–226. doi: 10.1016/S0065-2776(06)91005-0. [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol. 2006;7:1243–1249. doi: 10.1038/ni1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 5.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 6.Royet J. Infectious non-self recognition in invertebrates: lessons from Drosophila and other insect models. Mol Immunol. 2004;41:1063–1075. doi: 10.1016/j.molimm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Ligoxygakis P. Pathogen recognition and signalling in the Drosophila innate immune response. Immunobiology. 2006;211:251–261. doi: 10.1016/j.imbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:359–362. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- 9.Gottar M, et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- 10.Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416:644–648. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- 11.Takehana A, et al. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc Natl Acad Sci USA. 2002;99:13705–13710. doi: 10.1073/pnas.212301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takehana A, et al. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004;23:4690–4700. doi: 10.1038/sj.emboj.7600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber AN, et al. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff V, et al. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- 15.Gobert V, et al. Dual Activation of the Drosophila Toll Pathway by Two Pattern Recognition Receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- 16.Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- 17.Gottar M, et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piao S, et al. Crystal structure of a clip-domain serine protease and functional roles of the clip domains. EMBO J. 2005;24:4404–4414. doi: 10.1038/sj.emboj.7600891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- 20.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 21.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo--shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Levashina EA, et al. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- 23.Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- 24.Jang IH, et al. A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Mulinari S, Hacker U, Castillejo-Lopez C. Expression and regulation of Spatzle-processing enzyme in Drosophila. FEBS Lett. 2006;580:5406–5410. doi: 10.1016/j.febslet.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Kambris Z, et al. Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006;16:808–813. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Pelte N, et al. Immune challenge induces N-terminal cleavage of the Drosophila serpin Necrotic. Insect Biochem Mol Biol. 2006;36:37–46. doi: 10.1016/j.ibmb.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawlings ND, Morton FR, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2006;34:D270–272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green C, et al. The necrotic gene in Drosophila corresponds to one of a cluster of three serpin transcripts mapping at 43A1.2. Genetics. 2000;156:1117–1127. doi: 10.1093/genetics/156.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CH, et al. A Three-step Proteolytic Cascade Mediates the Activation of the Peptidoglycan-induced Toll Pathway in an Insect. J Biol Chem. 2008;283:7599–7607. doi: 10.1074/jbc.M710216200. [DOI] [PubMed] [Google Scholar]
- 31.Jiggins FM, Kim KW. A screen for immunity genes evolving under positive selection in Drosophila. J Evol Biol. 2007;20:965–970. doi: 10.1111/j.1420-9101.2007.01305.x. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 33.Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- 34.Shpacovitch V, Feld M, Bunnett NW, Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 2007;28:535–544. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 36.Leclerc V, et al. Prophenoloxidase activation is not required for survival to microbial infections in Drosophila. EMBO Rep. 2006;7:231–235. doi: 10.1038/sj.embor.7400592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.