Abstract
Using CIBMTR data we compared the transplant outcomes of patients with chronic myeloid leukemia (CML) who were non-smokers (NS) and past or current smokers (PCS). There were 2193 NS and 625 PCS who received matched sibling and unrelated donor allografts for CML in first chronic phase. We looked for dose effects and identified low and high dose smoking groups (≥10 pack years, >1 pack per day). Outcomes were adjusted for known prognostic variables including the EBMT risk score. In multivariate analyses of sibling allograft recipients, relapse risk was higher (RR 1.67, p=0.003) in smokers than NS but the dose effects were not consistent. High dose smokers experienced a 50% TRM vs. 28% in the NS group at 5 years on univariate analysis and the RR was 1.57 (p=0.005) on multivariate analysis. Overall survival at 5 years was 68% in NS vs. 62% in the low dose smoking group vs. 50% in the high dose smoking group (p<0.001). Smoking did not significantly affect outcomes in unrelated donor recipients but numbers were smaller. High dose smoking is associated with a reduction in overall survival in patients having sibling allografts for CML. A prospective study with detailed demographic, pulmonary function and quality of life data would improve our understanding of this issue.
Keywords: smoking effect, hematopoietic cell transplantation, outcomes, chronic myeloid leukemia, dose effect
INTRODUCTION
Allogeneic stem cell transplantation is widely used to cure patients with leukemia and other haematological conditions. Various biological factors influence the transplant outcome of patients with chronic myeloid leukemia (CML). These include patient age(1) (Center for International Blood and Marrow Transplant Research (CIBMTR), unpublished data), performance status at transplant(2) and body mass index(1). Pre-transplant pulmonary function may also affect overall transplant outcome and post-transplant respiratory complications(3, 4). One of the major causes of pre-transplant respiratory abnormalities is cigarette smoking. Depending on the population studied, between 20 and 50% of adult allogeneic transplant candidates have a current smoking history and many additional patients have a past smoking history. Smoking, as well as affecting pulmonary function, can influence the risk of coronary artery disease(5) and is an important cause of lung cancer (which may be increased after allogeneic transplantation).(6) Smokers are known to have different demographics to non-smokers. They are more likely to be male, of a lower socioeconomic status(7, 8) and have a higher alcohol intake(9). In studies of the effect of smoking on health outcomes it is possible that these associations of smoking may affect the outcomes.
No large-scale studies address the effect of smoking on transplant outcome. The CIBMTR database, which includes data on smoking history, is ideal for this purpose. We hypothesised that a smoking history would significantly reduce the chance of a successful transplant outcome by increasing treatment related mortality (TRM), primarily through pulmonary complications, including infection. Relapse incidence was also studied because physicians may have altered conditioning in patients who smoke. Smoking may affect the incidence of secondary malignancies but this study was not designed to address this issue.
We elected to study patients with CML in first chronic phase (CP1) because we hypothesised that examining the effect of smoking in a chemotherapy naïve population would ‘isolate’ the effect of smoking. Smoking might make pulmonary complications more likely after pre-transplant chemotherapy but we wished to study the effect of smoking on transplant alone. This focus on CML also eliminated a potential source of patient heterogeneity and the prognostic factors affecting the transplant outcome of CML patients are well described(10). We analysed sibling and unrelated donor transplants separately as the latter has a greater TRM and may have received higher doses of TBI.
There are numerous practical implications of performing this study. Transplant teams will be able to inform better patients who smoke about the chances of a successful outcome. The study may generate information that enables transplanters to modify conditioning regimens to increase the chance of a successful outcome. Finally, when the causes of treatment failure are determined, transplanters may be able to direct their supportive care efforts to preventing specific problems.
PATIENT SELECTION AND INCLUSION CRITERIA
Patient data for this study were obtained from the CIBMTR. More than five hundred participating centers register consecutive allogeneic transplants to CIBMTR. Detailed demographic and clinical data are collected on a sample of registered patients. Compliance is monitored by on-site audits. Computerized error checks, physician reviews of submitted data, and on-site audits of centers ensure the quality of data.
This study included all patients between 1990 and 2004 aged 18 and above who received HLA-identical sibling or matched unrelated donor (URD) allogeneic transplants for CML in CP1 for whom a smoking history was known. Patients received busulphan and cyclophosphamide or TBI and cyclophosphamide for conditioning. Graft type was restricted to bone marrow or peripheral blood. Graft versus host disease (GVHD) prophylaxis was restricted to cyclosporine and methotrexate, tacrolimus and methotrexate, T cell depletion or cyclosporine and other immunosuppressive agents. Patients who received low dose oral busulphan prior to transplant were excluded.
The number of patients with CML in CP1 aged >18 who had allografts reported to the CIBMTR between 1990 and 2004 was 5461. 5022 patients received a sibling or unrelated donor allograft of marrow or peripheral blood. We only included the 4409 receiving Cy/TBI or Bu/Cy conditioning and excluded the patients who had received prior low dose busulphan, leaving 3880 patients. We confined our study to 3793 patients with specific types of GVHD prophylaxis (defined above). Finally we had quantitative smoking information for 2818 of these patients.
Smoking Data
Patients were categorised as non-smoker or past or current smokers based on self-reported responses extracted from medical notes by data managers completing the CIBMTR forms. The questions asked about smoking history varied slightly in 1989, 1995 and 2002. However all questionnaire versions enquired about duration and number of cigarettes per day. The quantitative data regarding number of years smoked and amount per day (<1 pack, 1 pack and >1 pack) enabling us to compare the major outcomes in these groups and look for a dose effect. In this study past or current smokers are termed ‘smokers’. We divided smokers into 2 ‘doses’: high dose smokers had accumulated ≥ 10 pack years and smoked >1 pack per day and low dose smokers had <10 pack years or 1 ≤ pack per day.
Statistical methods
Patient-, disease-, and transplant-related variables for patients in the three smoking groups were compared using chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. P-values for pair-wise comparison were adjusted using Bonferroni correction.
The primary endpoints were relapse, TRM, disease free survival (DFS), and overall survival (OS). The event relapse was defined as occurrence of CML (clinical and/or cytogenetic) posttransplant. TRM was defined as death within 28 days posttransplant or death without CML relapse. Smoking may affect the incidence of fungal infection but because our data does not allow us to verify this diagnosis, this was not an endpoint of the study.
Probabilities of TRM and relapse were calculated using the cumulative incidence function method.(11) Treatment-related death and relapse were the competing events. Data on patients without either competing event were censored at last follow-up. For analyses of survival, death from any cause was considered an event and surviving patients were censored at last follow-up. For analyses of DFS, we considered relapse or death an event.
All P values were 2 sided, and a value of less than .05 was considered statistically significant.
Cox proportional hazards models were used to adjust for patient-related, disease-related, and transplant-related covariates. A main effect term for smoking was forced into the model. The remaining covariates were included using a stepwise forward selection technique with a P value ≤ 0.05 as the criterion for inclusion in the final models. Other variables considered in the models include: recipient age, gender, region of transplant center, performance score, WBC at diagnosis, body mass index prior to transplant, spleen size at diagnosis, pre-transplant use of hydroxyurea, interferon, or gleevec, interval from diagnosis to transplant, year of transplant, HLA matching, conditioning regimen, use of antithymocyte globulin (ATG) or alemtuzamab antibody therapy prior to transplant, use of lung shielding in radiation therapy, GVHD prophylaxis, donor age, donor-recipient gender match, source of graft, EBMT risk score, cytomegalovirus (CMV) status, and coexisting disease. The EBMT risk score is a scoring system designed by the European Group for Blood and Marrow Transplantation to predict the survival after allogeneic transplant for CML patient.(10) Higher score indicates a lower probability of survival. The CIBMTR does not collect sufficient data to calculate a Sokol score. Pulmonary function test data is not routinely collected by CIBMTR.
The proportional hazards assumption for each variable was examined using time-varying covariate and graphical approaches. Stratified proportional hazards models were used when variables with non-proportional hazards were identified. No significant interactions between smoking and other explanatory variables were found. There were no statistically significant center effects. In addition to the comparison of nonsmokers with past/current smokers, we also considered models with subgroups of past/current smokers based on years smoked and average packs per day. The cut point for years smoked (<10 years vs. >10 years) was selected based on plots of the Martingale residuals. Since age is related to duration of smoking, we tested for confounding by analyzing the subgroup of patients 30 years of age and older to determine consistency of effect relative to the group of all patients. Analyses were performed with the use of SAS software, version 9.1 (SAS Institute, Cary, NC).
Because data regarding smoking exposure was limited we considered 5 models in looking for an effect of smoking. First we simply compared smokers and non-smokers. Secondly past or current smokers were divided according to duration of smoking (<10 years and >10 years). Thirdly, the average number of packs per day was divided into <1 pack, 1 pack, >1 pack. Fourthly, we compared smokers with ≥ 10 pack years and ≤ 10 pack years. In the fifth model we combined models 2, 3 and 4 and compared low and high dose smokers as stated above. This results and discussion will be focused on the fifth model.
RESULTS
Patient characteristics in sibling allograft recipients
Table 1 shows the characteristics of patients >18 years with CML who had sibling donor transplants and compares individuals who have never smoked (NS) and those who are low or high dose smokers. We divided smokers into 2 ‘doses’. In the sibling allograft recipients, high dose smokers (n=94) had accumulated ≥ 10 pack years and smoked >1 pack per day and low dose smokers (n=370) had ≤ 10 pack years or ≤ 1 pack per day. Overall the median number of years of smoking was 15 and 22% smoked > 1 pack per day.
Table 1.
Characteristics of patients ≥ 18 year receiving HLA-identical sibling donor transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| Non Smokers | Smokers |
|||||
|---|---|---|---|---|---|---|
| Low dosea | High dosea | |||||
| Variables | N | N (%) | N | N (%) | N | N (%) |
| Number of patients | 1649 | 370 | 94 | |||
| Age at transplant, years, median (range) | 1649 | 37 (18–61) | 370 | 38 (18–66) | 94 | 45 (22–58) |
| Age at transplant, years | 1649 | 370 | 94 | |||
| 18 – 29 | 419 (25) | 67 (18) | 6 (6) | |||
| 30 – 39 | 580 (35) | 134 (36) | 22 (23) | |||
| 40 – 49 | 466 (28) | 115 (31) | 48 (51) | |||
| ≥50 | 184 (11) | 54 (15) | 18 (19) | |||
| Male | 1649 | 888 (54) | 370 | 262 (71) | 94 | 68 (72) |
| Region | 1648 | 370 | 94 | |||
| United States | 499 (30) | 126 (34) | 53 (56) | |||
| Canada | 60 (4) | 14 (4) | 8 (9) | |||
| Europe | 594 (36) | 141 (38) | 20 (21) | |||
| Asia | 143 (9) | 20 (5) | 4 (4) | |||
| Australia/New Zealand | 88 (5) | 13 (4) | 3 (3) | |||
| Mideast/Africa | 139 (8) | 12 (3) | 3 (3) | |||
| Central/South America | 125 (8) | 44 (12) | 3 (3) | |||
| Karnofsky score (< 90%) | 1637 | 171 (10) | 366 | 42 (11) | 92 | 15 (16) |
| Number of packs per day | 370 | 94 | ||||
| ≤1 | -- | 363 (98) | -- | |||
| > 1 | -- | 7 (2) | 94 (100) | |||
| Number of years smoked, median (range) | -- | 370 | 12 (1–43) | 94 | 20 (5–44) | |
| Smoking pack-year, median (range) | -- | 370 | 10 (<1–3) | 94 | 34 (12–140) | |
| Smoking pack-year, | 370 | 94 | ||||
| ≤10 pack-year | -- | 222 (60) | -- | |||
| > 10 pack-year | -- | 148 (40) | 94 (100) | |||
| Body mass index, kg/m2 | 1635 | 369 | 94 | |||
| ≤22 | 380 (23) | 69 (19) | 19 (20) | |||
| 22–30 | 1012 (62) | 238 (64) | 59 (63) | |||
| > 30 | 243 (15) | 62 (17) | 16 (17) | |||
| White cell count at diagnosis, 109/L, median (range) | 1529 | 145 (1–800) | 347 | 114 (7–650) | 89 | 96 (4–387) |
| White cell count at diagnosis, 109/L | 1529 | 347 | 89 | |||
| < 50 | 282 (18) | 91 (26) | 26 (29) | |||
| 50 – 100 | 290 (19) | 68 (20) | 24 (27) | |||
| > 100 | 957 (63) | 188 (54) | 39 (44) | |||
| Spleen size at diagnosis | 1477 | 342 | 81 | |||
| Normal | 467 (32) | 127 (37) | 31 (38) | |||
| Enlarged | 1010 (68) | 215 (63) | 50 (62) | |||
| Coexisting diseases | 1646 | 369 | 94 | |||
| Cardiac and Pulmonary | 9 (1) | 2 (1) | 4 (4) | |||
| Cardiac | 107 (7) | 32 (9) | 14 (15) | |||
| Pulmonary | 28 (2) | 12 (3) | 7 (7) | |||
| Other | 214 (13) | 60 (16) | 20 (21) | |||
| None | 1288 (78) | 263 (71) | 49 (52) | |||
| Pre-transplant therapy for CML | ||||||
| Hydroxyurea | 1634 | 1510 (92) | 368 | 333 (90) | 94 | 78 (83) |
| Interferon | 1205 | 578 (48) | 269 | 127 (47) | 75 | 33 (44) |
| Imatinib | 1648 | 50 (3) | 370 | 9 (2) | 94 | 4 (4) |
| Time from diagnosis to transplant, months, median (range) | 1649 | 8 (<1–127) | 370 | 9 (1–72) | 94 | 7 (2–99) |
| Time from diagnosis to transplant, months | 1649 | 370 | 94 | |||
| < 6 | 522 (32) | 108 (29) | 38 (40) | |||
| 6 – 11 | 591 (36) | 138 (37) | 31 (33) | |||
| 12 – 23 | 380 (23) | 90 (24) | 18 (19) | |||
| ≥24 | 156 (9) | 34 (9) | 7 (7) | |||
| EBMT Risk Score | 1647 | 370 | 94 | |||
| 0–1 | 572 (35) | 92 (25) | 16 (17) | |||
| 2 | 717 (44) | 171 (46) | 48 (51) | |||
| 3 | 322 (20) | 91 (25) | 26 (28) | |||
| 4 | 36 (2) | 16 (4) | 4 (4) | |||
| Year of transplant | 1649 | 370 | 94 | |||
| 1990 – 1994 | 746 (45) | 155 (42) | 44 (47) | |||
| 1995 – 1999 | 655 (40) | 173 (47) | 44 (47) | |||
| 2000 – 2004 | 248 (15) | 42 (11) | 6 (6) | |||
| Conditioning regimen | 1649 | 370 | 94 | |||
| TBI/Cy ± other | 591 (36) | 124 (34) | 36 (38) | |||
| Bu/Cy ± other (no TBI) | 1058 (64) | 246 (66) | 58 (62) | |||
| Dose of Cyb, mg/kg | 1432 | 320 | 76 | |||
| 120 | 1267 (88) | 279 (87) | 65 (86) | |||
| 200 | 165 (12) | 41 (13) | 11 (14) | |||
| Dose of Bu, mg/kg | 1627 | 366 | 93 | |||
| No Bu | 591 (36) | 124 (34) | 36 (39) | |||
| < 12 | 59 (4) | 6 (2) | 5 (5) | |||
| 12–16 | 304 (19) | 72 (20) | 23 (25) | |||
| 16–17 | 613 (38) | 157 (43) | 25 (27) | |||
| ≥17 | 60 (4) | 7 (2) | 4 (4) | |||
| Dose of TBI, cGy | 1603 | 352 | 86 | |||
| Non-TBI | 1058 (66) | 246 (70) | 58 (67) | |||
| <1300 | 421 (26) | 79 (22) | 18 (21) | |||
| ≥1300 | 124 (8) | 27 (8) | 10 (12) | |||
| GVHD prophylaxis | 1649 | 370 | 94 | |||
| T depl ± other | 102 (6) | 25 (7) | 9 (10) | |||
| FK506 ± other | 58 (4) | 10 (3) | 4 (4) | |||
| MTX + CsA ± other | 1324 (80) | 293 (79) | 66 (70) | |||
| CsA ± other (no MTX) | 165 (10) | 42 (11) | 15 (16) | |||
| Donor age | 1580 | 352 | 88 | |||
| ≤29 | 460 (29) | 73 (21) | 8 (9) | |||
| 30 – 39 | 534 (34) | 123 (35) | 25 (28) | |||
| 40 – 49 | 405 (26) | 98 (28) | 40 (45) | |||
| ≥50 | 181 (11) | 58 (16) | 15 (17) | |||
| Gender match | 1647 | 370 | 94 | |||
| Male into male | 523 (32) | 141 (38) | 43 (46) | |||
| Male into female | 405 (25) | 55 (15) | 16 (17) | |||
| Female into male | 365 (22) | 121 (33) | 25 (27) | |||
| Female into female | 354 (21) | 53 (14) | 10 (11) | |||
| Donor-Recipient CMV status | 1555 | 350 | 91 | |||
| −/− | 391 (25) | 89 (25) | 24 (26) | |||
| −/+ | 200 (13) | 45 (13) | 14 (15) | |||
| +/− | 183 (12) | 40 (11) | 10 (11) | |||
| +/+ | 781 (50) | 176 (50) | 43 (47) | |||
| Graft type | 1649 | 370 | 94 | |||
| BM | 1331 (81) | 301 (81) | 78 (83) | |||
| PB ± BM | 318 (19) | 69 (19) | 16 (17) | |||
| Use of ATG or Campath | 1627 | 15 (1) | 365 | 3 (1) | 94 | 4 (4) |
| Lung shielding in radiation therapy | 1587 | 262 (17) | 360 | 50 (14) | 92 | 11 (12) |
| Follow-up of surviving patients, month | 1649 | 91 (2–209) | 370 | 98 (1–199) | 94 | 115 (19–193) |
Abbreviations: CML = chronic myelogenous leukemia; TBI = total body irradiation; Cy = cyclophosphamide; Bu = busulfan; GVHD = graft-versus-host disease; MTX = methotrexate; CsA = cyclosporine; BM=Bone marrow; PB=Peripheral blood.
Low dose smokers=smoking ≤ 10 pack-years or > 10 pack-years with 1 ≤ pack/day; high dose smokers=smoking ≥ 10 pack-years with > 1 pack/day.
Cy dose range 100–150 mg/kg classified as 120 mg/kg; Cy dose ≥ 150 mg/kg classified as 200 mg/kg.
Duration of follow-up:
Non-smoker: ≥ 1 year = 73%; ≥ 3 year = 61%; ≥ 5 year = 50%.
Low dose smoker: ≥ 1 year = 69%; ≥ 3 year = 59%; ≥ 5 year = 49%.
High dose smoker: ≥ 1 year = 70%; ≥ 3 year = 46%; ≥ 5 year = 45%.
Overall in the sibling allograft group, high dose smokers compared to NS were slightly older, more were male (72% vs. 54%), had a lower diagnostic WCC, slightly more were female to male transplants (27% vs. 22%) and had a higher EBMT risk score (83% vs. 65% were 2–4, p<0.001). Fewer high dose smokers had no coexisting medical diseases (52% vs.78%, p<0.001).
There was no evidence that the transplants were performed differently in smokers; cytotoxic drug doses were similar in the 2 groups as was the dose of TBI and there was no difference in lung shielding.
Major outcomes on univariate analysis
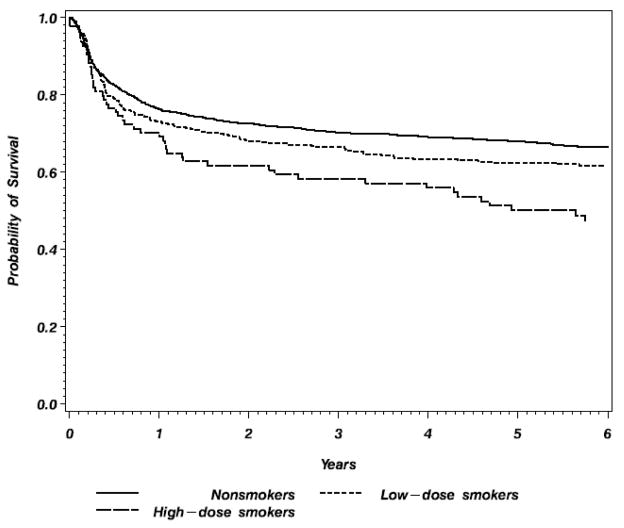
In the matched sibling donor group survival at 5 years was significantly lower in the high dose smoker group (50%) compared to the non-smoker and low dose smoker groups (68% and 62% respectively) (table 3 and figure 1). DFS was 20% lower in the high dose group than the non-smoker group (44% vs.64%, p<0.001). TRM at 5 years was similar in the non-smoker and low dose smoker groups (28% vs. 32%) but considerably higher in the high dose smoker group (50%, p<0.001). The absolute 5 year incidence of relapse is similar in the non-smoker and low and high dose smoker groups (8% vs. 10% vs. 6% respectively). There are no differences in the incidence of bronchopneumonia, interstitial pneumonitis and broncholitis obliterans among the 3 groups (table 3). There were no significant interactions between smoking and conditioning regimen (p=0.309 for TRM) or between smoking and GVHD prophylaxis (p= 0.310 for TRM).
Table 3.
Univariate outcome of patients ≥ 18 year receiving allogeneic transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| HLA-matched Siblings Donor |
Unrelated Donor |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoker Groupa | Never | Low Dose | High Dose | Never | Low dose | High Dose | ||||||||
| Outcomes | N | (95% CI) | N | (95% CI) | N | (95% CI) | P-value | N | (95% CI) | N | (95% CI) | N | (95% CI) | P-value |
| Relapse | 1565 | 347 | 88 | 514 | 119 | 30 | 0.837 | |||||||
| 100 days | 1 (0–1) | 1 (0–3) | 0 | 0.013 | 1 (0–2) | 1 (0–3) | 0 | |||||||
| 1 year | 3 (2–4) | 6 (4–9) | 3 (1–8) | 3 (2–5) | 3 (1–8) | 0 | ||||||||
| 3 years | 6 (5–8) | 9 (6–12) | 3 (1–8) | 6 (4–8) | 5 (2–10) | 0 | ||||||||
| 5 years | 8 (7–9) | 10 (7–14) | 6 (2–12) | 7 (5–9) | 5 (2–10) | 0b | ||||||||
| TRM | 1565 | 347 | 88 | <0.001 | 514 | 119 | 30 | 0.200 | ||||||
| 100 days | 12 (10–13) | 11 (8–15) | 17 (10–26) | 23 (19–26) | 19 (13–27) | 33 (18–51) | ||||||||
| 1 year | 22 (20–25) | 24 (20–29) | 28 (20–38) | 41 (37–46) | 42 (33–51) | 57 (39–74) | ||||||||
| 3 years | 27 (24–29) | 29 (24–34) | 41 (31–52) | 46 (42–51) | 48 (39–57) | 64 (46–80) | ||||||||
| 5 years | 28 (25–30) | 32 (27–37) | 50 (40–61) | 49 (44–53) | 50 (41–59) | 68 (50–83) | ||||||||
| DFS | 1565 | 347 | 88 | <0.001 | 514 | 119 | 30 | 0.293 | ||||||
| 100 days | 88 (86–89) | 87 (83–90) | 83 (74–90) | 76 (72–80) | 80 (72–86) | 67 (49–82) | ||||||||
| 1 year | 75 (73–77) | 70 (65–75) | 68 (58–77) | 56 (51–60) | 54 (45–63) | 43 (26–61) | ||||||||
| 3 years | 67 (65–69) | 63 (57–68) | 55 (45–66) | 48 (43–52) | 47 (37–56) | 36 (20–54) | ||||||||
| 5 years | 64 (62–67) | 58 (52–63) | 44 (33–54) | 44 (40–49) | 44 (35–54) | 32 (17–50) | ||||||||
| Bronchopneumonia | 1575 | 363 | 88 | 0.602 | 512 | 129 | 30 | 0.963 | ||||||
| 100 days | 10 (8–11) | 12 (9–16) | 11 (6–19) | 16 (13–19) | 15 (9–21) | 10 (2–23) | ||||||||
| 1 year | 18 (16–20) | 17 (14–21) | 19 (11–28) | 25 (21–29) | 26 (18–34) | 23 (10–40) | ||||||||
| 3 years | 23 (21–25) | 23 (19–28) | 26 (17–36) | 30 (26–35) | 29 (21–37) | 31 (16–48) | ||||||||
| 5 years | 25 (22–27) | 26 (21–31) | 26 (17–36) | 30 (26–35) | 31 (23–40) | 31 (16–48) | ||||||||
| IPN | 1634 | 359 | 94 | 0.018 | 534 | 129 | 29 | 0.671 | ||||||
| 100 days | 6 (5–7) | 8 (5–11) | 14 (8–22) | 13 (10–16) | 12 (7–19) | 14 (4–28) | ||||||||
| 1 year | 11 (10–13) | 12 (9–15) | 21 (13–30) | 20 (16–23) | 17 (11–25) | 21 (8–37) | ||||||||
| 3 years | 12 (11–14) | 14 (10–18) | 21 (13–30) | 21 (17–24) | 18 (12–26) | 25 (11–42) | ||||||||
| 5 years | 13 (11–15) | 15 (11–19) | 21 (13–30) | 21 (18–25) | 18 (12–26) | 25 (11–42) | ||||||||
| BO | 1320 | 298 | 78 | 0.731 | 444 | 104 | 25 | 0.473 | ||||||
| 100 days | 0 (0–1) | 0 | 1 (0–5) | 0 (0–1) | 0 (0–100) | 0 (0–100) | ||||||||
| 1 year | 2 (1–3) | 3 (1–5) | 3 (0–7) | 3 (2–5) | 2 (0–6) | 0 (0–100) | ||||||||
| 3 years | 4 (3–5) | 4 (2–7) | 4 (1–10) | 5 (3–7) | 2 (0–6) | 8 (0–27) | ||||||||
| 5 years | 4 (3–6) | 5 (2–8) | 6 (2–13) | 5 (3–8) | 2 (0–6) | 8 (0–27) | ||||||||
| Overall survival | 1649 | 370 | 88 | <0.001 | 512 | 119 | 30 | 0.278 | ||||||
| 100 days | 88 (86–89) | 88 (84–91) | 83 (74–90) | 76 (72–79) | 80 (71–86) | 67 (49–82) | ||||||||
| 1 year | 76 (74–78) | 73 (69–78) | 72 (62–80) | 56 (52–60) | 56 (47–65) | 43 (26–61) | ||||||||
| 3 years | 70 (68–73) | 66 (62–71) | 59 (48–69) | 50 (46–54) | 48 (39–57) | 36 (20–54) | ||||||||
| 5 years | 68 (66–70) | 62 (57–67) | 50 (40–61) | 46 (41–50) | 46 (37–55) | 32 (17–50) | ||||||||
Low dose smokers=smoking ≤ 10 pack-years or > 10 pack-years with 1 ≤ pack/day; high dose smokers=smoking ≥ 10 pack-years with > 1 pack/day.
No relapses were reported for the high dose smokers in the unrelated donor group, though small sample size and high TRM are important considerations. Confidence intervals are not relevant.
Abbreviations: TRM=Treatment related mortality, DFS=Disease free survival, IPN= interstitial pneumonitis, BO=Broncholitis obliterans, CI=Confidence interval.
Note: Comparing Non-smoker and low dose smoker vs. high dose smoker in the unrelated donor group:
Relapse: P-value=0.685
TRM: P-value=0.074
Overall survival: P-value=0.115
Figure 1.
Probability of overall survival of patients ≥18 year receiving HLA-identical siblings allogeneic transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
Although TRM was higher and DFS and OS were lower in the high dose recipients of unrelated donor grafts this was not significant (P-value=0.2, 0.3, and 0.3 respectively); this may relate to there being only 30 such patients.
Multivariate analysis of major outcomes in sibling allograft group Relapse
Smokers overall had a higher relative risk of relapse (RR 1.67, p=0.003). There was some evidence of a dose effect, although this was not consistent. More than 10 years smoking duration was associated with a higher RR of relapse however a higher number of packs smoked per day (data not shown) or high dose smoking overall were not associated with a higher chance of relapse. There was no difference in the incidence of acute and chronic GVHD in smokers and non-smokers (58% vs. 57% and 51% vs. 50% respectively, p=0.60 and 0.46 respectively).
Transplant related mortality
A multivariate analysis comparing TRM in sibling allograft recipients is shown in table 4. The relative risk of TRM is not different between nonsmokers and smokers overall. However high dose smoking was strongly associated with a higher TRM (RR 1.57, p=0.005). The effect of smoking on risk of TRM is significantly increased among 28 day survivors, (RR 1.65, p=0.002), and, importantly remains elevated for 100 day survivors (RR 1.81, p=0.002) and 1 year survivors (RR 3.29, p<0.001), suggesting a consistent effect over time.
Table 4.
Multivariate analysis comparing outcomes among patients ≥ 18 years old receiving transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| Variables | N | Relative Risk (95% CI) | P-value |
|---|---|---|---|
| HLA-identical sibling donor | |||
| Relapsea | |||
| Nonsmoker | 1563 | 1.00 | 0.008 |
| Past/current smokerb | |||
| Low dose | 347 | 1.75 (1.23–2.49) | 0.002 |
| High dose | 88 | 1.02 (0.44–2.36) | 0.960 |
| Treatment related mortalityc | |||
| Nonsmoker | 1563 | 1.00 | 0.008 |
| Past/current smoker | |||
| Low dose | 347 | 0.95 (0.77–1.88) | 0.657 |
| High dose | 88 | 1.57 (1.14–2.14) | 0.005 |
| Disease free survivald | |||
| Nonsmoker | 1563 | 1.00 | 0.012 |
| Past/current smoker | |||
| Low dose | 347 | 1.14 (0.95–1.37) | 0.162 |
| High dose | 88 | 1.52 (1.14–2.04) | 0.005 |
| Overall Survivale | |||
| Nonsmoker | 1563 | 1.00 | 0.049 |
| Past/current smoker | |||
| Low dose | 370 | 1.01 (0.84–1.22) | 0.910 |
| High dose | 94 | 1.44 (1.07–1.93) | 0.015 |
| Unrelated donor transplants | |||
| Relapsef | |||
| Nonsmoker | 514 | 1.00 | |
| Past/current smoker | 149 | 0.67 (0.28–1.56) | 0.351 |
| Treatment related mortalityg | |||
| Nonsmoker | 514 | 1.00 | |
| Past/current smoker | 149 | 1.02 (0.79–1.33) | 0.861 |
| Disease free survivalh | |||
| Nonsmoker | 514 | 1.00 | |
| Past/current smoker | 149 | 0.97 (0.76–1.25) | 0.834 |
| Overall Survivali | |||
| Nonsmoker | 544 | 1.00 | |
| Past/current smoker | 161 | 0.96 (0.75–1.21) | 0.708 |
Relapse model adjusted for recipient age, gender, region, spleen size at diagonosis, and GvHD prophylaxis.
Low dose smokers=smoking ≤ 10 pack-years or > 10 pack-years with 1 ≤ pack/day; high dose smokers=smoking ≥ 10 pack-years with > 1 pack/day.
TRM model adjusted for recipient age, gender, region, karnofsky score, GvHD prophylaxis, WBC count, EBMT risk score, and graft sources. Stratified on conditioning regimen/dose group.
DFS model adjusted for recipient age, gender, region, karnofsky score, GvHD prophylaxis, and time from diagnosis to transplant. Stratified on conditioning regimen/dose group.
Overall survival model adjusted for recipient age, gender, region, Karnofsky score, GvHD prophylaxis, EBMT risk score, and graft sources. Stratified on conditioning regimen/dose group.
Relapse model adjusted for recipient age, gender, and region.
TRM model adjusted for recipient age, gender, region, recipient CMV, GvHD prophylaxis, and EBMT risk score.
DFS adjusted for recipient age, gender, region, recipient CMV, GvHD prophylaxis, and EBMT risk score.
Overall survival model adjusted for recipient age, gender, region, recipient CMV, year of transplant, GvHD prophylaxis, and EBMT risk score.
Disease free and overall survival
DFS was shorter in smokers (RR 1.22, p=0.019, table 4). There were clear dose effects. High dose smokers had a significantly shorter DFS (RR 1.52, p=0.005).
However, OS was only affected by high dose smoking (RR 1.44, p=0.015) and this was confirmed by dose effects seen in models 2–4 (table 4). The distribution of causes of death, as reported by the HCT centers, was similar for the related and unrelated transplant recipients (table 5 and table 6).
Table 5.
Reported causes of death of patients ≥ 18 year receiving HLA-identical sibling donor transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| Non Smokers | Smokers |
||
|---|---|---|---|
| Low dose | High dose | ||
| Causes | N (%) | N (%) | N (%) |
| GVHD | 132 (24) | 32 (23) | 9 (18) |
| IPN | 95 (18) | 24 (17) | 9 (18) |
| Infection | 103 (19) | 31 (22) | 13 (25) |
| New malignancy | 5 (1) | 5 (4) | 1 (2) |
| Organ failure | 53 (10) | 14 (10) | 9 (18) |
| Other cause | 80 (15) | 20 (14) | 8 (16) |
| Primary disease | 73 (13) | 15 (11) | 2 (4) |
Table 6.
Reported causes of death of patients ≥ 18 year receiving unrelated donor transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| Non Smokers |
Smokers | ||
|---|---|---|---|
| Low dose | High dose | ||
| Causes | N (%) | N (%) | N (%) |
| GVHD | 60 (21) | 18 (24) | 7 (33) |
| IPN | 59 (20) | 9 (12) | 5 (24) |
| Infection | 79 (27) | 17 (23) | 2 (10) |
| New malignancy | 3 (1) | 1 (1) | 1 (5) |
| Organ failure | 27 (9) | 14 (19) | 2 (10) |
| Other cause | 43 (15) | 8 (11) | 3 (14) |
| Primary disease | 20 (7) | 8 (11) | 1 (5) |
We further analysed outcomes in the group of patients with a Karnofsky score <90 at transplant and found no differences between smokers and non-smokers (data not shown).
Unrelated donor transplant recipients
The clinical characteristics of UD recipients are shown in table 2 and univariate analysis of outcomes in table 3. For these analyses we compared non- and low-dose smokers (combined) with high dose smokers. TRM was lower in non- and low-dose smokers compared to high dose smokers (49% vs. 68%) but this was not significant (p=0.074). Survival at 5 years in the high dose group was 32% compared to 46% in the non- and low-dose smoker groups (p=0.115). In the multivariate analyses we compared non smokers with past and current smokers (table 4). There were no differences in the major outcomes (relapse, TRM, DFS or OS) between the two groups. Dose effects were also tested and no significant differences were found.
Table 2.
Characteristics of patients ≥ 18 years receiving matched unrelated donor transplants for CML in first chronic phase, reported to the CIBMTR, 1990–2004.
| Non Smokers | Smokers |
|||||
|---|---|---|---|---|---|---|
| Low dosea | High dosea | |||||
| Variables | N | N (%) | N | N (%) | N | N (%) |
| Number of patients | 544 | 131 | 30 | |||
| Age at transplant, years, median (range) | 544 | 34 (18–61) | 131 | 37 (19–58) | 30 | 43 (19–53) |
| Age at transplant, years | 544 | 131 | 30 | |||
| 18 – 29 | 165 (30) | 30 (23) | 2 (7) | |||
| 30 – 39 | 214 (39) | 46 (35) | 8 (27) | |||
| 40 – 49 | 145 (27) | 45 (34) | 15 (50) | |||
| ≥50 | 20 (4) | 10 (8) | 5 (17) | |||
| Male | 544 | 317 (58) | 131 | 89 (68) | 30 | 24 (80) |
| Region | 544 | 131 | 30 | |||
| United States | 173 (32) | 52 (40) | 19 (63) | |||
| Canada | 31 (6) | 8 (6) | 2 (7) | |||
| Europe | 245 (45) | 56 (43) | 7 (23) | |||
| Asia | 54 (10) | 13 (10) | 1 (3) | |||
| Australia/New Zealand | 20 (4) | 1 (1) | 0 (0) | |||
| Mideast/Africa | 8 (1) | 0 (0) | 0 (0) | |||
| Central/South America | 13 (2) | 1 (1) | 1 (3) | |||
| Karnofsky score (< 90%) | 535 | 49 (9) | 131 | 11 (8) | 30 | 5 (17) |
| Number of packs per day | 131 | 30 | ||||
| ≤1 | -- | 131 (100) | -- | |||
| > 1 | -- | -- | 30 (100) | |||
| Number of years smoked, median (range) | -- | 131 | 15 (2–35) | 30 | 20 (6–35) | |
| Smoking pack-year, median (range) | -- | 131 | 10 (1–35) | 30 | 35 (12–93) | |
| Smoking pack-year | 131 | 30 | ||||
| ≤10 pack-year | -- | 66 (50) | -- | |||
| > 10 pack-year | -- | 65 (50) | 30 (100) | |||
| Body mass index, kg/m2 | 535 | 126 | 30 | |||
| ≤22 | 116 (22) | 31 (25) | 5 (17) | |||
| 22–30 | 338 (63) | 73 (58) | 17 (57) | |||
| > 30 | 81 (15) | 22 (17) | 8 (27) | |||
| White cell count at diagnosis, 109/L, median (range) | 487 | 150 (4–790) | 115 | 126 (1–779) | 30 | 116 (19–334) |
| White cell count at diagnosis, 109/L | 487 | 115 | 30 | |||
| < 50 | 84 (17) | 34 (30) | 6 (20) | |||
| 50 – 100 | 83 (17) | 17 (15) | 7 (23) | |||
| > 100 | 320 (66) | 64 (56) | 17 (57) | |||
| Spleen size at diagnosis | 452 | 108 | 26 | |||
| Normal | 147 (33) | 53 (49) | 9 (35) | |||
| Enlarged | 305 (67) | 55 (51) | 17 (65) | |||
| Coexisting diseases | 543 | 131 | 30 | |||
| Cardiac and Pulmonary | 3 (1) | 0 (0) | 0 (0) | |||
| Cardiac | 26 (5) | 8 (6) | 5 (17) | |||
| Pulmonary | 9 (2) | 3 (2) | 3 (10) | |||
| Other | 78 (14) | 18 (14) | 5 (17) | |||
| None | 427 (79) | 102 (78) | 17 (57) | |||
| Pre-transplant therapy for CML | ||||||
| Hydroxyurea | 538 | 507 (94) | 130 | 114 (88) | 30 | 24 (80) |
| Interferon | 479 | 308 (64) | 118 | 86 (73) | 25 | 17 (68) |
| Imatinib | 543 | 48 (9) | 131 | 5 (4) | 30 | 0 (0) |
| Time from diagnosis to transplant, months, median (range) | 544 | 15 (1–111) | 131 | 16 (3–95) | 30 | 17 (6–39) |
| Time from diagnosis to transplant, months | 544 | 131 | 30 | |||
| < 6 | 50 (9) | 6 (5) | 0 (0) | |||
| 6 – 11 | 145 (27) | 38 (29) | 11 (37) | |||
| 12 – 23 | 180 (33) | 54 (41) | 14 (47) | |||
| ≥24 | 169 (31) | 33 (25) | 5 (17) | |||
| EBMT Risk Score | 526 | 122 | 28 | |||
| 0–1 | 10 (2) | 0 (0) | 1 (4) | |||
| 2 | 130 (25) | 21 (17) | 0 (0) | |||
| 3 | 226 (43) | 52 (43) | 12 (43) | |||
| 4 | 144 (27) | 42 (34) | 12 (43) | |||
| 5 | 16 (3) | 7 (6) | 3 (11) | |||
| Year of transplant | 544 | 131 | 30 | |||
| 1990 – 1994 | 192 (35) | 59 (45) | 17 (57) | |||
| 1995 – 1999 | 228 (42) | 57 (44) | 13 (43) | |||
| 2000 – 2004 | 124 (23) | 15 (11) | 0 (0) | |||
| Conditioning regimen | 544 | 131 | 30 | |||
| TBI/Cy ± other | 409 (75) | 100 (76) | 26 (87) | |||
| Bu/Cy ± other (no TBI) | 135 (25) | 31 (24) | 4 (13) | |||
| Degree of matching | 538 | 130 | 29 | |||
| Well Matched | 68 (13) | 19 (15) | 5 (17) | |||
| Partially matched | 162 (30) | 40 (31) | 13 (45) | |||
| Mismatched | 211 (39) | 57 (44) | 9 (31) | |||
| Unknown | 97 (18) | 14 (11) | 2 (7) | |||
| Dose of Cyb, mg/kg | 466 | 107 | 23 | |||
| 120 | 415 (89) | 93 (87) | 21 (91) | |||
| 200 | 51 (11) | 14 (13) | 2 (9) | |||
| Dose of Bu, mg/kg | 542 | 129 | 30 | |||
| No Bu | 409 (75) | 100 (78) | 26 (87) | |||
| < 12 | 13 (2) | 0 (0) | 0 (0) | |||
| 12–16 | 28 (5) | 6 (5) | 0 (0) | |||
| 16–17 | 85 (16) | 21 (16) | 3 (10) | |||
| ≥17 | 7 (1) | 2 (2) | 1 (3) | |||
| Dose of TBI, cGy | 523 | 124 | 29 | |||
| Non-TBI | 135 (26) | 31 (25) | 4 (14) | |||
| <1300 | 251 (48) | 65 (52) | 13 (45) | |||
| ≥1300 | 137 (26) | 28 (23) | 12 (41) | |||
| GVHD prophylaxis | 544 | 131 | 30 | |||
| T depl ± other | 117 (22) | 29 (22) | 7 (23) | |||
| FK506 ± other | 67 (12) | 10 (8) | 3 (10) | |||
| MTX + CsA ± other | 344 (63) | 87 (66) | 19 (63) | |||
| CsA ± other (no MTX) | 16 (3) | 5 (4) | 1 (3) | |||
| Donor age | 465 | 105 | 24 | |||
| ≤29 | 132 (28) | 20 (19) | 6 (25) | |||
| 30 – 39 | 180 (39) | 54 (51) | 10 (42) | |||
| 40 – 49 | 134 (29) | 26 (25) | 5 (21) | |||
| ≥50 | 19 (4) | 5 (5) | 3 (13) | |||
| Gender match | 532 | 124 | 28 | |||
| Male into male | 213 (40) | 57 (46) | 12 (43) | |||
| Male into female | 122 (23) | 30 (24) | 3 (11) | |||
| Female into male | 97 (18) | 26 (21) | 10 (36) | |||
| Female into female | 100 (19) | 11 (9) | 3 (11) | |||
| Donor-Recipient CMV status | 513 | 121 | 26 | |||
| −/− | 183 (36) | 39 (32) | 9 (35) | |||
| −/+ | 116 (23) | 40 (33) | 4 (15) | |||
| +/− | 81 (16) | 11 (9) | 4 (15) | |||
| +/+ | 133 (26) | 31 (26) | 9 (35) | |||
| Graft type | 544 | 131 | 30 | |||
| BM | 505 (93) | 127 (97) | 29 (97) | |||
| PB ± BM | 39 (7) | 4 (3) | 1 (3) | |||
| Use of ATG or Campath | 505 | 172 (34) | 121 | 33 (27) | 28 | 8 (29) |
| Lung shielding in radiation therapy | 505 | 170 (34) | 119 | 43 (36) | 29 | 13 (45) |
| Follow-up of surviving patients, months | 544 | 79 (4–194) | 131 | 90 (4–195) | 30 | 109 (13–157) |
Abbreviations: CML = chronic myelogenous leukemia; TBI = total body irradiation; Cy = cyclophosphamide; Bu = busulfan; GVHD = graft-versus-host disease; MTX = methotrexate; CsA = cyclosporine; BM=Bone marrow; PB=Peripheral blood.
Low dose smokers=smoking ≤ 10 pack-years or > 10 pack-years with 1 ≤ pack/day; high dose smokers=smoking ≥ 10 pack-years with > 1 pack/day.
Cy dose range 100–150 mg/kg classified as 120 mg/kg, Cy dose ≥ 150 mg/kg classified as 200 mg/kg.
Duration of follow-up:
Non-smoker: ≥ 1 year = 55%; ≥ 3 year = 43%; ≥ 5 year = 31%.
Low dose smoker: ≥ 1 year = 53%; ≥ 3 year = 41%; ≥ 5 year = 33%.
High dose smoker: ≥ 1 year = 54%; ≥ 3 year = 34%; ≥ 5 year = 27%.
DISCUSSION
Smoking has profound effects on health causing higher rates of malignancy, cardiac and pulmonary disease.(12) Nonetheless, a significant percentage of transplant candidates will be past or current smokers and physicians take smoking history as part of the pre-transplant evaluation. Some regard smokers as inferior transplant candidates and in borderline cases it may be a factor in the decision to proceed to transplant.
The major findings of this study are that in sibling allograft recipients high dose smoking (≥10 pack years and >1 pack/day, (20% of smokers)) was associated with clinically and statistically significantly reduced DFS and OS compared to non-smokers. The absolute magnitude of the reduction in survival of 18% is important and both transplanters and high dose smoking patients should be aware of these data. This effect is mediated by a higher TRM (50% vs. 28%) and although the relative risk of relapse was higher in smokers overall it was not increased in the high dose group. Analysis of univariate outcomes (table 3) suggested an effect on interstitial pneumonitis (p=0.018) but no effect on bronchopneumonia or bronchiolitis obliterans. The effects of smoking on TRM may not be just pulmonary as smoking has the potential to affect the function of other vital organs. Despite these findings we are not advocating that transplanters should withhold this therapy from this patient subset nor should it affect a patient’s health insurance status. Future research should focus on reducing the higher TRM in the high dose smoking group. Reduced intensity conditioning is one possible way of achieving this. We did not see significant effects on TRM and survival in the lower dose smoking group; this is biologically plausible but a prospective study would be of value in clarifying this finding. It is worth noting that there were not major differences in outcome in the recipients of unrelated donor transplants; it is possible that the higher TRM associated with unrelated transplantation masked a separate effect of smoking. Small numbers in the high dose group reduced the chance of demonstrating significant differences. Smoking may also have had an effect on relapse however this was only seen in low dose smokers (RR1.75) on multivariate analysis. The lack of an effect in high dose smokers may be due the higher TRM in this group. The apparent effect in low dose smokers was not due to less intense conditioning or via an effect on GVHD. Smoking may be immunomodulatory (inflammatory bowel disease is more common in smokers(13)); donor T cells may be rendered less able to mediate a graft versus leukemia effect. However we do not have data about smoking post transplant. A mouse model showed effects on dendritic cells and on T cell proliferation.(14) The smoking status of the donor might be of greater importance in this effect and there is a high incidence of smoking in the siblings of smokers.(15) This could explain the fact that there was no increase in relapse in unrelated donor recipients who tend to be healthy and smoke less. However the minority of smokers who continue to smoke post transplant may affect the donor T cells on a continuing basis. In a study from Boston(16) the risk of relapse appeared to be higher in smokers and increased with each pack year of exposure. In that study, 14 of 17 patients who had relapse smoked (p=0.01). The same group however found no effect of smoking on 1 year survival.(17)
Additionally, there may be effects on pulmonary function although reports vary. Twenty years ago the Seattle group(18) found that smoking was associated with a lower FEV1/FVC at 1 year post transplant (p=0.01); the effect on pulmonary function tests (particularly gas transfer) at 1 year was confirmed by a French group.(19) Gas transfer was impaired at baseline and during the first year post transplant in smokers, including in transplants with non-TBI conditioning.(20) Barrett and colleagues found that smoking increased TBI related pulmonary mortality 5 fold but that this effect could be reduced by giving a high CD34 dose.(3) However, effects on pulmonary outcomes were not seen after all studies. Ho and colleagues from Boston(4) found no increase in severe pulmonary complications post transplant.
This study has limitations that should influence data interpretation. First, the registry forms did not capture whether the smoking was current or past or if smoking was resumed after transplant. Secondly, we had limited ‘dose’ data and could not calculate pack years accurately in many cases which may explain the inconsistent dose-related findings. Thirdly, the self-reported smoking history may be inaccurate and there may be some under-reporting. Fourthly, knowledge of the demographic factors that are associated with smoking(21) would have improved our ability to make conclusions. Finally, in retrospect, it might have been informative to examine outcomes in other transplant eligible diseases as smoking may have more effect in patients who had substantial pre-transplant chemotherapy. In many countries fewer patients with early phase CML proceed to transplant now, however the EBMT risk score for CML has been validated for other diseases and it seems likely that the effect seen in CML patients would also be seen in patients with other haematological malignancies. Patients with diseases such as acute leukemia are exposed to recurrent episodes of neutropenia which has the potential to augment some of the organ related effects of smoking including pulmonary infection.
Further examination of this issue would require a prospective study; this would have several advantages. There would be more accurate correlation of past and current exposure of patients and their donors with outcome and this could be associated with regular pulmonary function tests. There would also be the opportunity to collect patient-reported outcomes and determine if there are effects on rehospitalisation, chest infections and reemployment. Furthermore, prospective demographic data could be collected, allowing the study to separate the effects of smoking from effects that the different demographic characteristics that smokers may have. Nonetheless, this study presents clinically important findings. It is the largest study ever that examines the impact of smoking on transplant outcome and contains data that patients and transplanters will be able to use in making clinical decisions.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 2.Marks DI, Cullis JO, Ward KN, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia using sibling and volunteer unrelated donors. A comparison of complications in the first 2 years. Ann Intern Med. 1993;119:207–214. doi: 10.7326/0003-4819-119-3-199308010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Savani BN, Montero A, Wu C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:223–230. doi: 10.1016/j.bbmt.2004.12.328. [DOI] [PubMed] [Google Scholar]
- 4.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:223–229. doi: 10.1053/bbmt.2001.v7.pm11349809. [DOI] [PubMed] [Google Scholar]
- 5.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 6.Lowe T, Bhatia S, Somlo G. Second malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1121–1134. doi: 10.1016/j.bbmt.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Office of population censuses and surveys. General household survey 1978: Cigarette smoking. OPCS monitor. 1978 Reference GHS 79/2. [Google Scholar]
- 8.Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 2006;368:367–370. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- 9.McKee SA, Harrison EL, O’Malley SS, et al. Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352:1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 11.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 12.Rigotti NA. Clinical practice. Treatment of tobacco use and dependence. N Engl J Med. 2002;346:506–512. doi: 10.1056/NEJMcp012279. [DOI] [PubMed] [Google Scholar]
- 13.Ekbom A, Brandt L, Granath F, Lofdahl CG, Egesten A. Increased Risk of Both Ulcerative Colitis and Crohn’s Disease in a Population Suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 14.Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J Immunol. 2008;180:6623–6628. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: a follow-up study of Montreal schoolchildren. CMAJ. 2005;173:377–379. doi: 10.1503/cmaj.1041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang G, Orav EJ, McNamara T, Tong MY, Antin JH. Depression, cigarette smoking, and hematopoietic stem cell transplantation outcome. Cancer. 2004;101:782–789. doi: 10.1002/cncr.20431. [DOI] [PubMed] [Google Scholar]
- 17.Chang G, Orav EJ, Tong MY, Antin JH. Predictors of 1-year survival assessed at the time of bone marrow transplantation. Psychosomatics. 2004;45:378–385. doi: 10.1176/appi.psy.45.5.378. [DOI] [PubMed] [Google Scholar]
- 18.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 19.Socie G, Mary JY, Esperou H, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2001;113:194–201. doi: 10.1046/j.1365-2141.2001.02678.x. [DOI] [PubMed] [Google Scholar]
- 20.Lund MB, Brinch L, Kongerud J, Boe J. Lung function 5 yrs after allogeneic bone marrow transplantation conditioned with busulphan and cyclophosphamide. Eur Respir J. 2004;23:901–905. doi: 10.1183/09031936.04.00084804. [DOI] [PubMed] [Google Scholar]
- 21.Marmot M. Smoking and inequalities. Lancet. 2006;368:341–342. doi: 10.1016/S0140-6736(06)68976-9. [DOI] [PubMed] [Google Scholar]