Abstract
High-level arsenic exposure is consistently associated with QT prolongation, a risk factor for arrhythmia and sudden cardiac death. Arsenic may act on QT by increasing cardiac calcium currents. The authors hypothesized that low-level arsenic exposure would be associated with QT duration and that this effect would be stronger among persons not using calcium channel blockers. They performed a cross-sectional analysis in elderly men from the Normative Aging Study to analyze associations between toenail arsenic and QT and heart rate-corrected QT (QTc) durations and to examine effect modification by calcium channel blocker use, using linear regression and adjusting for potential confounders. Participants were examined in Boston, Massachusetts, between 2000 and 2002 or in 2006. An interquartile range increase in arsenic concentration was associated with a 3.8-millisecond increase in QT (95% confidence interval: 0.82, 6.8) and a 2.5-millisecond increase in QTc (95% confidence interval: 0.11, 4.9). There was no evidence of effect modification by medication use for either QT (P = 0.93) or QTc (P = 0.58). The authors observed positive associations between a biomarker of arsenic exposure and QT duration but found no evidence of effect modification by calcium channel blocker use, possibly because of modest power.
Keywords: antioxidants; arrhythmias, cardiac; arsenic; calcium channel blockers; cardiovascular diseases; environmental health; long QT syndrome
Prolongation of the QT interval is a risk factor for arrhythmia and sudden cardiac death (1, 2). The QT interval is measured as the time elapsed between the beginning of the QRS complex and the end of the T wave in an electrocardiogram. It corresponds to the length of a ventricular electrical systole, covers the sustained calcium influx period of the heart cycle, and represents the duration of depolarization and repolarization. Because the duration of this interval is dependent on heart rate, it is usually adjusted for heart rate and expressed in a corrected form (QTc) in order to aid interpretation. Normal values for QT duration range between 300 and 440 milliseconds.
Arsenic prolongs the QT interval in animal studies and in cases of acute arsenic poisoning in humans (3–7). In addition, clinical studies demonstrate consistently that arsenic trioxide, used to treat acute promyelocytic leukemia, induces QT prolongation, torsades de pointes, and sudden death (8–10). Finally, 3 population-based epidemiologic studies found a positive association between high-level environmental arsenic exposure and QT interval duration (11–13). No studies have examined the relation between QT interval length and low-level arsenic exposure or have looked at the association between any level of arsenic exposure and QT by using a biomarker of dose. Instead, surrogates such as water concentration have been used.
We hypothesized that low-level environmental arsenic exposure would prolong QT interval duration in a general population study. We conducted a cross-sectional examination of the association between toenail arsenic concentration and QT and QTc interval lengths. Because arsenic exposure may prolong the QT interval by increasing cardiac calcium currents, which regulate the plateau phase of the cardiac action potential (3), we assessed for effect modification by current calcium channel blocker use. Further, because oxidative stress may mediate the association between arsenic and QT duration (14, 15), we examined effect modification by antioxidant intake.
MATERIALS AND METHODS
Study population
Our participants were from the Veterans Administration Normative Aging Study. This is an ongoing longitudinal study of aging established in 1963, details of which have been published previously (16). Briefly, the Normative Aging Study is a closed cohort of 2,280 male volunteers from the Greater Boston area aged 21–80 years at entry, who enrolled after an initial health screening determined that they were free of known chronic medical conditions. Participants were reevaluated every 3–5 years by using detailed on-site physical examinations and questionnaires.
All active participants were contacted prior to their study visit and asked to bring toenails when they presented for the visit between November 2000 and November 2002 (n = 512) or between July 2006 and December 2006 (n = 64). Nonparticipants failed to do so (n = 240), did not have acceptable QT measurements because of irregular electrocardiograms (n = 181), were missing information regarding C-reactive protein concentrations (n = 7), and/or had toenail arsenic concentrations below the detection limit (n = 2). Our analyses included 226 participants with information on all study variables who contributed toenail samples between November 2000 and November 2002 (n = 204) or between July 2006 and December 2006 (n = 22).
Physical parameters and medical history
Study center visits followed an overnight fast and abstention from smoking. Physical examinations included measurement of height and weight, and body mass index was calculated (weight (kg)/height (m)2). A physician measured blood pressure using a standard mercury sphygmomanometer with a 14-cm cuff while the participant was seated. Questionnaires evaluated smoking habits and medication use, with responses confirmed by an on-site physician. C-reactive protein concentrations were determined by using a high-sensitivity immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, Indiana) (17). Fasting serum glucose concentrations were measured by using the hexokinase method, with measurements performed in duplicate on an autoanalyzer (18).
Antioxidant and fish intakes were determined by using a food frequency questionnaire (19, 20). We calculated daily intakes of antioxidant nutrients by multiplying the frequency of consumption of each unit by the nutrient content of the specified portion and adding amounts from dietary supplements. Fish questions included canned tuna, shellfish, dark-meat fish, and other fish.
Toenail sample collection and arsenic analysis
Toenail samples from all 10 toes were collected. The whole sample was precleaned before analysis to remove extraneous contaminants by using the following procedure. Toenail samples were sonicated for 15 minutes in approximately 10 mL of 1% Triton X-100 solution (Dow Chemical Company, Midland, Michigan) in 15-mL plastic tubes. After sonication, samples were rinsed several times with distilled deionized water and dried at 60°C for 24 hours in a drying oven.
Toenail samples were weighed into a 15-mL plastic tube, digested with 1 mL of concentrated nitric acid for 24 hours, and then diluted to 5 mL with deionized water. Samples were further diluted as needed. Acid-digested samples were analyzed by an inductively coupled plasma mass spectrometer (Elan 6100; PerkinElmer, Inc., Waltham, Massachusetts). Analysis was performed by using an external calibration method with tellurium as the internal standard for arsenic.
Quality control measures included analysis of the initial calibration verification standard (standard reference material 1643e (trace elements in water); National Institute of Standards and Technology, Gaithersburg, Maryland), a 1-ng/mL standard arsenic solution, continuous calibration standards, and a procedural blank. Certified reference material GBW 07601 (human hair; Shanghai Institute of Nuclear Research, Chinese Academy of Sciences, Shanghai, China) was used as the quality control sample. We used a large preparation of GBW 07601 (2 g/L) to monitor daily variation.
The between-assay coefficient of variation for arsenic was 0.1. The detection limit for the analytical solution was 0.2 ng/mL. The detection limit for the sample itself varied according to sample weight and was equal to the detection limit for the analytical solution multiplied by the dilution factor. Because the weight of the sample varied from 0.002 g to 0.9 g, the detection limit varied from 0.001 μg/g to 0.42 μg/g. The average detection limit for this analysis was 0.02 μg/g. Two individuals had toenail arsenic concentrations below the detection limit and were excluded from the present study. Results were given as the average of 5 replicate measurements. Recovery of the analysis of the quality control standard by this procedure is 90%–110% with approximately 95% precision.
Electrocardiogram measurement and analysis
The electrocardiogram was measured with a 2-channel, 5-lead electrocardiogram monitor (Trillium 3000; Forest Medical, East Syracuse, New York) over approximately 5–10 minutes, by using a sampling rate of 256 Hz per channel. This procedure is described in detail elsewhere (21, 22). The electrocardiogram digital recordings were processed by using personal computer-based software (Trillium Platinum Holter Analysis Software for MS Windows; Forest Medical) to create a Mathcad (Parametric Technology Corporation, Needham, Massachusetts) file containing QT interval measurements. A Win32 console application (Microsoft Corporation, Redmond, Washington) was used to obtain QT and QTc values from the data. This application measured the QT interval from the beat onset to the end of the T wave only on normal or supraventricular beats and calculated the QTc value in milliseconds using Bazett's formula as described by Bednar et al. (23). The QT interval was not calculated if the T wave did not have sufficient amplitude, as determined by the program algorithm.
Statistical methods
We conducted a cross-sectional examination of the association between toenail arsenic concentration and QT duration measured at the same visit as toenail collection using multivariate linear regression. We used a 2-sided P value of P < 0.05 as the level of statistical significance for both the main effect of arsenic and interaction terms.
The following covariates were selected as potential confounders on the basis of a thorough review of the relevant literature: age, body mass index, mean arterial pressure, fasting glucose, serum C-reactive protein, current cigarette smoking (smoker vs. nonsmoker), and pack-years of smoking. We chose to adjust for season and year of clinical visit a priori. All covariates were included in regression models regardless of statistical significance.
To test the dose-response relation of QT and QTc durations with toenail arsenic, we reexamined our model in R using a penalized spline for arsenic. The penalized spline fits a 12-df regression spline to the dose-response curve but penalizes the coefficients of the spline, effectively constraining the number of degrees of freedom used. The degree of penalty (and constraint) was chosen by using generalized cross-validation (The R-Project for Statistical Computing; available at http://www.r-project.org/).
We examined effect modification by calcium channel blocker use and by antioxidant intake using interaction terms and stratified analyses. To assess for modification by antioxidant intake, we constructed a score representing the combined intake of vitamin C, vitamin A, and total carotene. For each nutrient, we assigned to participants a score of 1–3, corresponding to their tertile of intake. The overall intake score was obtained by summing scores for individual dietary components and was partitioned into 3 categories: low (scores 3–4), intermediate (scores 5–7), or high (scores 8–9). This analysis was performed on a subset of participants (n = 198), because data on antioxidants were available only through 2005. Models examining effect modification by antioxidant nutrients were adjusted for total daily energy intake, as well as previously mentioned covariates.
We compared participants included in our analyses with nonparticipants presenting during the period of toenail collection using Student's t test and chi-square analysis and examined bivariate associations between arsenic and participants’ characteristics using Student's t test and the Spearman correlation. The use of log-transformed arsenic measures in our regression models did not significantly alter the results (data not shown). We therefore used untransformed metal measures in all analyses. We examined the correlation between QTc and heart rate using Spearman's r.
RESULTS
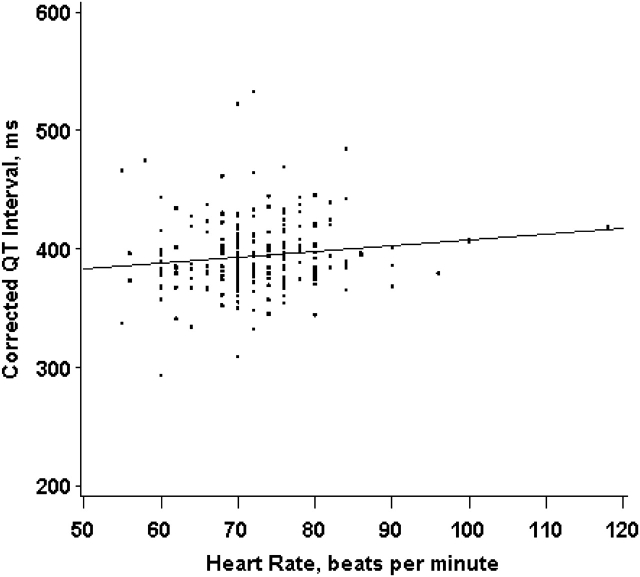
Our study population was composed of males with a mean age of 73 years and a mean body mass index of 28 kg/m2 (Table 1). The mean QT and QTc interval durations for this sample were 378 (standard deviation, 39) milliseconds and 395 (standard deviation, 30) milliseconds, respectively. Both QT and QTc interval durations were approximately normally distributed (data not shown). The median concentration of toenail arsenic was 0.069 μg/g (interquartile range, 0.052–0.11 μg/g). Participants and nonparticipants differed with respect to season and year of clinical visit (Table 1). Toenail arsenic concentrations were lower in the winter (P = 0.005) than during other seasons but were not associated with any other covariates or with dark-meat fish intake, other fish intake, shellfish intake, or intake of canned tuna (P > 0.05). We reported median toenail arsenic concentrations by characteristics of participants in Table 2. QTc interval duration was weakly correlated with heart rate (r = 0.13; P = 0.05) (Figure 1).
Table 1.
Characteristics by Participation Status, Normative Aging Study, Boston, Massachusetts, 2000–2002, 2006
| Characteristic | Participants (n = 226) |
Nonparticipants (n = 350) |
P Value (2 sided)a | ||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | ||
| Age, years | 73 (6) | 73 (7) | 0.38 | ||||
| Body mass index, kg/m2 | 28 (4) | 28 (4) | 0.11 | ||||
| Smoking status | |||||||
| Smoker | 10 | 4 | 16 | 5 | 0.92 | ||
| Nonsmoker | 216 | 96 | 332 | 95 | |||
| Pack-years of smoking | 33 (27) | 31 (26) | 0.52 | ||||
| Serum C-reactive protein, mg/L | 4 (7) | 3 (10) | 0.60 | ||||
| Fasting glucose, mg/dL | 107 (28) | 106 (26) | 0.58 | ||||
| Mean arterial pressure, mm Hg | 94 (10) | 94 (11) | 0.78 | ||||
| Season of visit | |||||||
| Spring | 63 | 28 | 74 | 21 | 0.01 | ||
| Summer | 49 | 22 | 99 | 28 | |||
| Fall | 63 | 28 | 123 | 35 | |||
| Winter | 51 | 23 | 54 | 15 | |||
| Year of visit | <0.001 | ||||||
| 2000 | 17 | 8 | 21 | 6 | |||
| 2001 | 135 | 60 | 141 | 40 | |||
| 2002 | 52 | 23 | 146 | 42 | |||
| 2006 | 22 | 10 | 42 | 12 | |||
Abbreviation: SD, standard deviation.
Using Student's t test and chi-square analysis, we compared participants included in our analyses with nonparticipants presenting during the period of toenail collection.
Table 2.
Toenail Arsenic Concentrations by Characteristics of Participants (n = 226), Normative Aging Study, Boston, Massachusetts, 2000–2002, 2006
| Characteristic | No. of Participants | Median Toenail Arsenic Concentration, μg/g | Interquartile Range, μg/g |
| Age, years | |||
| <73 | 106 | 0.08 | 0.06 |
| ≥73 | 120 | 0.06 | 0.05 |
| Body mass index, kg/m2 | |||
| <25 | 54 | 0.07 | 0.07 |
| 25–29 | 123 | 0.07 | 0.06 |
| ≥30 | 49 | 0.08 | 0.04 |
| Current smoking status | |||
| Smoker | 10 | 0.09 | 0.12 |
| Nonsmoker | 216 | 0.07 | 0.06 |
| Lifetime smoking, pack-years | |||
| <26 | 77 | 0.07 | 0.05 |
| ≥26 | 80 | 0.07 | 0.06 |
| Serum C-reactive protein, mg/L | |||
| <1.8 | 113 | 0.07 | 0.06 |
| ≥1.8 | 113 | 0.07 | 0.05 |
| Fasting glucose, mg/dL | |||
| <100 | 109 | 0.07 | 0.05 |
| ≥100 | 117 | 0.07 | 0.06 |
| Mean arterial pressure, mm Hg | |||
| <95 | 112 | 0.08 | 0.06 |
| ≥95 | 114 | 0.07 | 0.06 |
| Dark-meat fish intake, servings | |||
| <1/month | 93 | 0.07 | 0.06 |
| 1/month–6/week | 105 | 0.07 | 0.05 |
| Other fish intake, servings | |||
| <1/month | 60 | 0.07 | 0.06 |
| 1/month–6/week | 138 | 0.07 | 0.05 |
| Shellfish intake, servings | |||
| <1/month | 84 | 0.06 | 0.06 |
| 1/month–6/week | 114 | 0.07 | 0.05 |
| Intake of canned tuna, servings | |||
| <1/month | 56 | 0.07 | 0.05 |
| 1/month–6/week | 142 | 0.07 | 0.05 |
| Season of clinical visit | |||
| Spring | 63 | 0.06 | 0.04 |
| Summer | 49 | 0.09 | 0.07 |
| Fall | 63 | 0.08 | 0.08 |
| Winter | 51 | 0.06 | 0.03 |
| Year of clinical visit | |||
| 2000 | 17 | 0.06 | 0.03 |
| 2001 | 135 | 0.07 | 0.06 |
| 2002 | 52 | 0.07 | 0.06 |
| 2006 | 22 | 0.09 | 0.07 |
Figure 1.
Correlation between QTc (heart rate-corrected QT) interval duration and heart rate among Normative Aging Study participants (n = 226), Boston, Massachusetts, 2000–2002, 2006. The QTc interval duration is weakly correlated with heart rate (Spearman's r = 0.13; 2-sided P = 0.05). ms, milliseconds.
We estimated the change in QT and QTc durations associated with toenail arsenic concentration (Table 3) and expressed the results as the change associated with an interquartile range (0.059 μg/g) increment in exposure to arsenic. We found that a 0.059-μg/g increase in toenail arsenic was associated with a 3.8-millisecond increase in the QT interval (95% confidence interval: 0.82, 6.8; P = 0.01) and a 2.5-millisecond increase in the QTc interval (95% confidence interval: 0.11, 4.9; P = 0.04).
Table 3.
Estimated Change in QT and QTc Interval Durations Associated With an Interquartile Rangea Increase in Toenail Arsenic Concentration Among Normative Aging Study Participants (n = 226), Boston, Massachusetts, 2000–2002, 2006b
| Change in QT Duration, ms | 95% Confidence Interval | |
| QT interval | 3.8* | 0.82, 6.8 |
| QTc interval | 2.5* | 0.11, 4.9 |
Abbreviations: ms, millisecond(s); QTc, heart rate-corrected QT.
P < 0.05 (2 sided).
Corresponding to a 0.059-μg/g increment of toenail arsenic concentration.
All multivariate linear regression models are adjusted for age, cigarette smoking (smoker vs. nonsmoker), pack-years of smoking, season of clinical visit, body mass index, serum C-reactive protein, serum fasting glucose, year of clinical visit, and mean arterial pressure.
We tested the dose-response relation for arsenic and QT duration using penalized splines. For QT, generalized cross-validation found that a linear curve was the best fit, while for QTc a 1.26-df curve fit best. Hence, the dose-response curve appeared to be essentially linear for both of these associations.
We also examined effect modification by calcium channel blocker use because of information from prior research findings (3). Thirty-five participants reported using calcium channel blockers at the time of data collection. Use of these medications was not independently associated with QT or QTc duration. We found no evidence of statistical interaction between toenail arsenic and current calcium channel blocker use for either QT (P = 0.93) or QTc (P = 0.58) (data not shown).
Finally, we examined effect modification by antioxidant intake in a subset of participants (Table 4). Intake of antioxidant nutrients was not associated with either QT or QTc duration. We found no evidence of statistical interaction between toenail arsenic and antioxidant score for either QT (P = 0.28) or QTc (P = 0.95). However, among persons with low antioxidant intake, an interquartile range increment in arsenic exposure was associated with an 11-millisecond increase in QT duration (95% confidence interval: 0.078, 22; P = 0.05). Associations between arsenic and QT duration were much smaller and not significant among participants with intermediate or high antioxidant intake. We did not observe a similar pattern in stratified analyses for QTc duration.
Table 4.
Effect Modification of the Association Between an Interquartile Rangea Increase in Toenail Arsenic Concentration and QT Duration by Antioxidant Nutrient Intake Among a Subset of Normative Aging Study Participants (n = 198), Boston, Massachusetts, 2000–2002b
| Antioxidant Intake Scorec | Change in QT, ms | 95% Confidence Interval | Change in QTc, ms | 95% Confidence Interval |
| Low intake (n = 55) | 11* | 0.078, 22 | 4.1 | −4.1, 12 |
| Intermediate intake (n = 83) | 2.7 | −4.2, 9.6 | 0.24 | −6.3, 6.8 |
| High intake (n = 60) | 3.2 | −1.9, 8.4 | 3.9 | −0.04, 7.9 |
| Pinteraction | 0.28 | 0.95 | ||
Abbreviations: ms, millisecond(s); QTc, heart rate-corrected QT.
P < 0.05 (2 sided).
Corresponding to a 0.059-μg/g increment of toenail arsenic concentration.
All multivariate linear regression models are adjusted for age, cigarette smoking (smoker vs. nonsmoker), pack-years of smoking, season of clinical visit, body mass index, serum C-reactive protein, serum fasting glucose, year of clinical visit, mean arterial pressure, and daily total energy intake.
To assess modification by antioxidant intake, we constructed a score representing the combined intake of vitamin C, vitamin A, and total carotene. For each nutrient, we assigned participants a score of 1–3, corresponding to their tertile of intake. The overall intake score was obtained by summing scores for individual dietary components and was partitioned into 3 categories: low (3–4), intermediate (5–7), or high (8–9).
DISCUSSION
Toenail arsenic concentration is positively associated with QT and QTc interval durations in this cohort of elderly men. We found no evidence of statistical interaction between toenail arsenic concentration and calcium channel blocker use, possibly because of modest power to evaluate effect modification. This is the first study to examine the association between low-level environmental arsenic exposure and QT duration or to evaluate the association between any level of arsenic exposure and QT using an internal biomarker of dose. Our findings of even a modest relation between toenail arsenic and QT interval length, if confirmed, may have important implications because of widespread exposure to low or moderate levels of this metal across the US population.
This study utilized an automated method to measure QT duration. Automated methods have greater potential to perform objectively than manual measurements, and a recent study testing the reproducibility of automated multichannel QT interval measurements found that the use of an automatic algorithm for QT analyses allowed for accurate and highly repeatable QT measurements (24).
Because arsenic has a high affinity for sulfhydryl groups, it accumulates in keratin-rich tissues such as toenails and hair. These tissues provide an integrated measure of arsenic dose from all routes of exposure. Toenails grow more slowly than hair and are more protected from external environmental contaminants. Further, they provide a well-validated measure of exposure to the metal from water, food, soil, dust, and air (25). Because toenails are estimated to reflect arsenic exposures from the preceding 12–18 months in the general population, they represent much longer-term arsenic exposures than blood or urine measurements (25, 26). Nails usually grow more slowly in the elderly than in younger individuals (25).
Toenail arsenic concentrations in our analysis were similar to those of other US-based study populations (27, 28). Previous studies have assessed primarily the effects of inorganic arsenic, which is more toxic than the organic form. The assay used to measure toenail arsenic concentration does not distinguish between organic and inorganic forms of arsenic. However, organic arsenic compounds are derived mainly from seafood consumption (25), and the toenail arsenic concentration was not associated with fish or shellfish intake in our study population.
Most of our participants were supplied with water, an important source of inorganic arsenic, by the Massachusetts Water Resources Authority (Boston, Massachusetts). Arsenic is consistently undetectable (<1.0 μg/L) in this water supply, and other sources are likely in our study population (23). Sources of inorganic arsenic exposure in the general population include air, soil, dust, and food, particularly vegetables and rice (25, 29). Occupational sources of inorganic arsenic exposure include mining, smelting operations, wood preservation, and electronics (29).
Three cross-sectional, population-based analyses have assessed the relation between high-level environmental arsenic exposure and QT duration. Studies conducted in Inner Mongolia and Bangladesh found positive associations between well water arsenic exposure and prevalence of abnormal QTc prolongation (11, 13). A study conducted in Turkey found positive associations between water arsenic exposure and both QT and QTc interval lengths (12).
In the study conducted in Bangladesh, the mean well water arsenic concentrations for the reference group and 2 exposed subgroups were <10 μg/L, 443 μg/L, and 493 μg/L, respectively (13). In the study conducted in Turkey, the mean water arsenic concentrations were 659 μg/L in the exposed subgroup and 0 μg/L in the unexposed subgroup (12). Finally, in the study by Mumford et al. (11) conducted in Inner Mongolia, the exposure categories were <21, 100–300, and 430–689 μg/L. The water arsenic concentrations assessed in the 3 studies were much greater than both the current (10 μg/L) and previous (50 μg/L) maximum contaminant level in the United States. These studies do not allow direct insight into the arrhythmogenic effects of the low level of arsenic exposure relevant to most US-based study populations.
The study conducted in Inner Mongolia measured the toenail arsenic concentration among study participants (11). The low, medium, and high water exposure subgroups had mean toenail arsenic concentrations of 1.21 μg/g, 9.79 μg/g, and 24.61 μg/g, respectively, with an overall mean of 11.8 μg/g. In contrast, the mean toenail arsenic concentration in our study was 0.10 μg/g (range, 0.02–0.86 μg/g). Our findings of a positive association between toenail arsenic concentration and QT duration are consistent with those from the existing literature but extend it to much lower exposure levels and the use of an internal biomarker of dose.
Arsenic may prolong QT interval duration by increasing cardiac calcium currents, which regulate the plateau phase of the cardiac action potential. The metal may also act by reducing surface expression of the cardiac potassium channel human ether-a go-go related gene product protein (hERG), which is essential for repolarization of cardiac myocytes (3). Arsenic interferes with hERG trafficking to the cell surface by inhibiting hERG-chaperone complexes. The mechanism by which arsenic increases cardiac calcium currents is unclear but may be the result of direct enzymatic modification of the calcium channel or its accessory proteins (3, 4). In addition to changes in cardiac ion channels, possible mechanisms of arsenic-induced QT prolongation include alterations in DNA repair and methylation, generation of reactive oxygen species, and induction of cardiomyocyte apoptosis (14, 15, 30).
The finding that arsenic may lengthen QT interval duration by increasing cardiac calcium currents prompted Ficker et al. (3) to suggest that administration of calcium channel blockers could ameliorate arsenic trioxide-induced QT prolongation during treatment of acute promyelocytic leukemia. We therefore examined effect modification by current calcium channel blocker use in our analysis. Our results show no evidence of statistical interaction between toenail arsenic and medication use. This study did not have sufficient power to assess lower levels of effect modification because of modest sample size, however, and tests for statistical interaction are hampered by low power in general.
Because reactive oxygen species may mediate the association between arsenic and QT duration (14, 15), we also examined effect modification by antioxidant nutrients using an intake score. Vitamin C, vitamin A, and total carotene are all powerful antioxidants that may play a role in prevention of cardiovascular disease (31, 32), and dietary antioxidants may be interactive in their effects on cardiovascular health (33). Few studies have computed antioxidant intake scores from dietary data, and the validity of our score needs to be evaluated in future analyses.
We did not find evidence of statistical interaction between toenail arsenic and antioxidant intake for either QT or QTc duration. We observed a strong association between arsenic and uncorrected QT duration in the low antioxidant intake group and not in the intermediate or high intake groups, which, given our limited power, supports further research examining this interaction. However, we did not find a similar pattern in stratified analyses for QTc, which is the more clinically relevant measure of QT duration.
The changes we are examining do not rise to the level of clinical QT prolongation requiring a medical response, and the relation between QT duration and sudden cardiac death is clear only at the extremes of QT interval length (34). However, variations in blood pressure and cholesterol levels within the normal range have been associated with cardiac events and deaths (35, 36). Hence, controllable disturbances that shift the distribution of cardiac risk factors are of public health—as opposed to individual or clinical—concern. Moreover, part of our goal is to identify mechanisms by which environmental exposures may be related to cardiovascular disease, and these results indicate that disturbance of normal cardiac repolarization may be part of that pathway. Hence, we believe the findings are of interest regardless of the direct public health consequences of the changes. This is particularly true given the very low arsenic levels found in this population. Higher doses are found in areas with arsenic in groundwater and may result in greater impacts.
One potential limitation of our study is its cross-sectional design, which restricts inferences about causality. In addition, our sample size, though comparable to previous analyses, is relatively modest and does not provide satisfactory power to examine effect modification. Further, suitable information on additional important antioxidants such as vitamin E was not available for our analysis. Our study population was homogenous, consisting entirely of elderly men, 98% of whom were white. However, our findings are consistent with research conducted in more diverse populations. The finding of associations at these exposure levels, if confirmed, suggests that current efforts to limit arsenic exposure in the general population may be inadequate.
More research using a prospective cohort design is needed to assess the relation between low-level arsenic exposure and QT duration among large, diverse study populations. Future studies should also evaluate the association between low-level environmental arsenic exposure and QT interval duration among women because of their increased susceptibility to arsenic's effects on QT prolongation (11). Potential effect modification by calcium channel blocker use and antioxidant intake should be investigated in high-powered analyses.
Our findings show a positive association between toenail arsenic concentration and QT and QTc interval durations. We found no evidence of effect modification by calcium channel blocker use, possibly because of modest power. These results provide new information to guide future research regarding the arrhythmogenic effects of environmental arsenic exposure.
Acknowledgments
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Irina Mordukhovich); Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Robert O. Wright, Emmanuel Baja, Andrea Baccarelli, Helen Suh, Joel Schwartz); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Robert O. Wright, Chitra Amarasiriwardena, Joel Schwartz); Department of Environmental and Occupational Health, Istituto di Ricovero e Cura a Carattere Scientifico, Maggiore Hospital, Mangiagalli and Regina Elena Foundation and University of Milan, Milan, Italy (Andrea Baccarelli); and VA Normative Aging Study, Veterans Affairs Boston Healthcare System and Department of Medicine, Boston University School of Medicine, Boston, Massachusetts (David Sparrow, Pantel Vokonas).
This work was supported by the National Institute of Environmental Health Sciences (grants ES0002, ES015172-01, ES014663); the US Environmental Protection Agency (grants R827353, R832416); and the National Cancer Institute (grant 2-T32-CA09330). The Veterans Administration Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the US Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Conflict of interest: none declared.
Glossary
Abbreviations
- hERG
human ether-a go-go related gene product protein
- QTc
heart rate-corrected QT
References
- 1.Scott JL, Walls RM. QT interval prolongation. J Emerg Med. 1985;3(3):221–225. doi: 10.1016/0736-4679(85)90076-9. [DOI] [PubMed] [Google Scholar]
- 2.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 3.Ficker E, Kuryshev YA, Dennis AT, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66(1):33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Sun HL, Chu WF, Dong DL, et al. Choline-modulated arsenic trioxide-induced prolongation of cardiac repolarization in guinea pig. Basic Clin Pharmacol Toxicol. 2006;98(4):381–388. doi: 10.1111/j.1742-7843.2006.pto_319.x. [DOI] [PubMed] [Google Scholar]
- 5.Hall JC, Harruff R. Fatal cardiac arrhythmia in a patient with interstitial myocarditis related to chronic arsenic poisoning. South Med J. 1989;82(12):1557–1560. doi: 10.1097/00007611-198912000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Little RE, Kay GN, Cavender JB, et al. Torsade de pointes and T-U wave alternans associated with arsenic poisoning. Pacing Clin Electrophysiol. 1990;13(2):164–170. doi: 10.1111/j.1540-8159.1990.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CE, Luk HN, Wang TM, et al. Prolongation of cardiac repolarization by arsenic trioxide. Blood. 2002;100(6):2249–2252. doi: 10.1182/blood-2002-02-0598. [DOI] [PubMed] [Google Scholar]
- 8.Westervelt P, Brown RA, Adkins DR, et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98(2):266–271. doi: 10.1182/blood.v98.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21(19):3609–3615. doi: 10.1200/JCO.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi K, Yoshida H, Shigeno K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2000;133(11):881–885. doi: 10.7326/0003-4819-133-11-200012050-00012. [DOI] [PubMed] [Google Scholar]
- 11.Mumford JL, Wu K, Xia Y, et al. Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environ Health Perspect. 2007;115(5):690–694. doi: 10.1289/ehp.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildiz A, Karaca M, Biceroglu S, et al. Effect of chronic arsenic exposure from drinking waters on the QT interval and transmural dispersion of repolarization. J Int Med Res. 2008;36(3):471–478. doi: 10.1177/147323000803600311. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad SA, Khatun F, Sayed MH, et al. Electrocardiographic abnormalities among arsenic-exposed persons through groundwater in Bangladesh. J Health Popul Nutr. 2006;24(2):221–227. [PubMed] [Google Scholar]
- 14.Yamazaki K, Terada H, Satoh H, et al. Arrhythmogenic effects of arsenic trioxide in patients with acute promyelocytic leukemia and an electrophysiological study in isolated guinea pig papillary muscles. Circ J. 2006;70(11):1407–1414. doi: 10.1253/circj.70.1407. [DOI] [PubMed] [Google Scholar]
- 15.Flora SJ, Bhadauria S, Kannan GM, et al. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol. 2007;28(2 suppl):333–347. [PubMed] [Google Scholar]
- 16.Bell B, Rose CL, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Hum Dev. 1972;3:5–17. [Google Scholar]
- 17.Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47:418–425. [PubMed] [Google Scholar]
- 18.Leon L, Chu D, Stasiw R. New, More Specific Methods for the SMA 12/60 Multichannel Biochemical Analyzer: Advances in Automated Analysis. Tarrytown, NY: Mediad; 1977. [Google Scholar]
- 19.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of health aging. Gerontologist. 1966;6(4):179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope CA, III, Eatough DJ, Gold DR, et al. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect. 2001;109(7):711–716. doi: 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis. 2001;43(5 suppl. 1):1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 24.Hekkala AM, Väänänen H, Swan H, et al. Reproducibility of computerized measurements of QT interval from multiple leads at rest and during exercise. Ann Noninvasive Electrocardiol. 2006;11(4):318–326. doi: 10.1111/j.1542-474X.2006.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: a review. Environ Res. 2006;102(1):125–139. doi: 10.1016/j.envres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. Atlanta, GA: US Department of Health and Human Services; 2007. [PubMed] [Google Scholar]
- 27.Karagas MR, Tosteson TD, Blum J, et al. Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am J Epidemiol. 2000;152(1):84–90. doi: 10.1093/aje/152.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Slotnick MJ, Meliker JR, AvRuskin GA, et al. Toenails as a biomarker of inorganic arsenic intake from drinking water and foods. J Toxicol Environ Health A. 2007;70(2):148–158. doi: 10.1080/15287390600755232. [DOI] [PubMed] [Google Scholar]
- 29.Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao XY, Li GY, Liu Y, et al. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J Pharmacol. 2008;154(1):105–113. doi: 10.1038/bjp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101(10A):75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Palace VP, Khaper N, Qin Q, et al. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999;26(5-6):746–761. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 33.Gey KF. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br Med Bull. 1993;49(3):679–699. doi: 10.1093/oxfordjournals.bmb.a072640. [DOI] [PubMed] [Google Scholar]
- 34.Newton-Cheh C, Shah R. Genetic determinants of QT interval variation and sudden cardiac death. Curr Opin Genet Dev. 2007;17(3):213–221. doi: 10.1016/j.gde.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 36.Huxley R, Lewington S, Clarke R. Cholesterol, coronary heart disease and stroke: a review of published evidence from observational studies and randomized controlled trials. Semin Vasc Med. 2002;2(3):315–323. doi: 10.1055/s-2002-35402. [DOI] [PubMed] [Google Scholar]