Abstract
Levels of prepulse inhibition (PPI) depend on the interval between the startling and prepulse stimuli. Brown Norway rats show less PPI of the acoustic startle response than Wistar-Kyoto (WKY) rats when the interval between the prepulse and startling stimulus is 100 msec. Central administration of corticotropin-releasing factor (CRF) decreases PPI at this inter-stimulus interval. Here, the effect of CRF on PPI over a range of inter-stimulus intervals was examined in WKY and BN rats, and in the F1 generation of a cross between them. Rats received an intracerebroventricular infusion of either saline or CRF 30 min prior to testing PPI. Test trials included startle stimulus alone trials, and trials on which a prepulse stimulus of either 6, 12, or 15 dB above background preceded the startling stimulus by either 20, 75, 100, 500 or 2000 msec. CRF decreased PPI in WKY rats at all inter-stimulus intervals and all prepulse intensities, while the effect of CRF on PPI in BN rats only occurred at intermediate intervals. BN and WKY rats showed different levels of PPI only at the intermediate intervals. Baseline PPI in the F1 rats resembled the WKY phenotype. The CRF-induced change in PPI in the F1 generation has some qualities of the effects in each of the progenitor strains. These results suggest that both the effect of rats strain and of CRF on PPI depend on the inter-stimulus interval, and that there is an interaction between prepulse stimulus intensity and the inter-stimulus interval.
Keywords: Acoustic Startle, Brown Norway, CRF, Heritability, Prepulse Inhibition, Startle Modulation, Wistar Kyoto
Introduction
Prepulse inhibition (PPI) of the startle response is the reduction in startle amplitude that results from brief presentation of a non-startling prepulse stimulus, prior to presentation of a startling stimulus [1] and it is a measure of sensori-motor gating. PPI has been shown to be diminished in a number of psychiatric and neuropsychiatric disorders including schizophrenia [2,3,4,5], obsessive compulsive disorder [6], and Huntington’s disease [7]. Further, combat veterans with post-traumatic stress disorder (PTSD), as well as combat-exposed veterans without PTSD show diminished PPI [8], suggesting that stress can reduce PPI irrespective of a resulting psychiatric diagnosis. Manipulation of a number of neurotransmitter systems can diminish PPI in rodent models. For example, administration of dopamine receptor agonists, particular serotonin releasers and receptor agonists, and NMDA receptor antagonists decrease PPI [9].
Over the range of intensities used in most studies, there is a positive relationship between the intensity of the prepulse stimulus and the amount of startle inhibition [10]. However, the relationship between the length of the inter-stimulus interval and startle modulation is complex. Inhibition is optimal when the inter-stimulus interval is 30–500 msec [11]. At very short intervals (10 msec) the prepulse stimulus can sometimes, but not always, facilitate the startle response [12,13,14]. Interestingly, prepulse facilitation (PPF) can also result if the inter-stimulus interval is quite a bit longer than the optimal intervals for causing inhibition. Both intermediate- interval inhibition and long-interval facilitation have been known for quite some time in both humans [15] and rodents [11]. Patients with schizophrenia have also been reported to show less PPF than control subjects [16,17]. However, schizophrenia patients do not show diminished PPI at all inter-stimulus intervals [19,20,21], suggesting, as do other data, that different processes are taking place, or are accessible at different intervals. Since men show greater PPI than women, while women show greater PPF than men, it may be that both processes are taking place at the same time, and the readout is the net effect of the two [22]. Studies in rats also suggest that the inhibition seen at the most commonly used intervals can mask the smaller facilitation that is also taking place at those intervals [23].
The 41-amino acid neuropeptide, corticotropin-releasing factor (CRF), mediates a number of behavioral and endocrine responses to stress, although behavioral effects of central administration of the CRF are achieved independently of pituitary-adrenal axis activation [24,25]. CRF cell bodies and fibers are found in a number of brain regions [26], and CRF receptors are expressed in brain regions which mediate PPI such as the cortex, the hippocampus, the nucleus accumbens, and the basolateral nucleus of the amygdala [27,28,29,30,31]. We, and others, have shown that exogenously administered CRF diminishes PPI in rats [32,33,34] and mice [35,36], and CRF over-expressing mice show reduced PPI [37,38]. Further, the effect of CRF on PPI is mediated by the CRF1 receptor, not by glucocorticoid receptors [39]. There is evidence of elevated levels of CRF in the CSF of patients with a number of psychiatric disorders in which PPI is lower than normal [40,41,42,43,44], although the amount of PPI seen in patients with major depression is normal [45,46], even though this disorder is also characterized by elevated levels of CRF in the CSF [47]. Interestingly, levels of mRNA for the CRF binding protein are lower in the amygdala of patients with schizophrenia than control subjects [48].
Different rat strains show different levels of PPI [49,50,51], and there is evidence that short-interval PPF (10 msec) is also rat strain-dependent [13]. Inbred Brown Norway (BN) rats show significantly less PPI than inbred Wistar-Kyoto (WKY) rats [32,34,52], or outbred Sprague Dawley rats [14] at most intermediate inter-stimulus intervals that have been examined to date. However, Swerdlow et al., [14] also found that BN rats show more PPI than Sprague Dawley rats if the inter-stimulus interval is only 10 msec. The effects of relatively long inter-stimulus intervals on PPI in BN rats have yet to be examined. Further, potential differences between WKY and BN rats at a long interval (i.e. 2000 msec), which might result in PPF, have not been examined. Here, we predict that plasticity will be less in the BN rats, and that PPF may not be revealed in this strain. We hypothesize that CRF will affect PPI similarly at each inter-stimulus interval (i.e., the same percentage decrease in inhibition will occur).
There are two studies in which PPI levels in an F1 generation resulting from a cross between BN rats and another rat strain were examined. Palmer et al., [53] found that the F1 generation displayed a WKY-like, rather than a BN-like phenotype. The WKY rats used to create the F1 generation in that study are from a breeding colony that is maintained at the University of California, San Diego, and are not the WKY rats that we purchase from Harlan. Swerdlow and colleagues [54] found that baseline startle in the F1 generation from a Sprague Dawley X BN cross resembles the BN phenotype, while PPI resembles the Sprague Dawley phenotype in males, and is intermediate in females. The results of both of these studies suggest that the WKY-like baseline PPI phenotype will be displayed in an F1 generation of a cross between BN rats and WKY rats purchased from Harlan.
There is a small literature which suggests that the effects of drugs on PPI can also depend on the prepulse-to-startle interval [13, 55, 56]. Here, we will examine the effect of CRF on PPI (and possibly PPF) across a range of prepulse-to-startle intervals. Interestingly, adult offspring of an F1 cross between Sprague Dawley and BN rats were more sensitive to the effect of apomorphine on PPI than either parental strain [54]. This suggests that the inheritance pattern of a drug-induced change in the PPI phenotype cannot be predicted from the baseline phenotype. It also suggests that the inheritance pattern of a drug response phenotype is more complex than that of the baseline phenotype. Whether this is true of all drugs/neurotransmitters that reduce PPI is not known. Here, we will also examine the effect of CRF on PPI/PPF in the F1 generation of a cross between WKY and BN rats to examine whether the apparent sensitivity of the BN rats to CRF is heritable, and whether the heritability pattern is consistent across a range of intervals. If the response to CRF is simply an “additive” effect of a heterozygous genotype, then it should be intermediate to the WKY and BN responses. However, it may be more complex than that, and the response may be WKY-like at some prepulse parameters, and BN-like at others. Although baseline PPI is lower in BN than in WKY rats, BN rats show a further reduction in response to low-dose (0.3 μg) of CRF, while WKY rats do not. Thus, it appears that low baseline PPI and does not reduce sensitivity to a CRF-induced decrease in PPI. Therefore, the low baseline PPI should not interfere with our ability to detect a BN-like phenotype in CRF-treated F1 rats. Characterization of the phenotypic differences between strains on a number of PPI parameters will be helpful for studies of genetic contributions to the endophenotypes of psychiatric disorders in which there are sensori-motor gating abnormalities.
Materials and Methods
Animals
Subjects in the first experiment were male rats weighing 250–275 g at the time of surgery to implant guide cannula into the lateral ventricle. Wistar-Kyoto rats were obtained from Charles River, and Brown Norway rats were obtained from Harlan, Sprague Dawley. Rats were maintained in our vivarium on a 12 hr light/dark cycle for one week following arrival from the vendor. Additionally, we examined PPI in male rats from the F1 generation of a cross between WKY and BN rats in order to assess the inheritance pattern of both the baseline and CRF phenotypes. A small number of female rats from this F1 generation were also tested, although we have yet to examine PPI in females from the progenitor strains. Laboratory chow and water were available ad libitum except during testing. The rats were housed 2/cage until undergoing surgery, and were single-housed thereafter for an additional 5–7 days until testing. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. (n = 8–11 BN and WKY/group; 6–7 F1 rats/group)
Surgery to Implant ICV Guide Cannula
Rats were anesthetized with a mixture of isoflurane-in-air (1.5%) and placed into a stereotaxic instrument equipped with blunt ear bars. A stainless-steel guide cannula (22 gauge) was aimed unilaterally at the lateral ventricle (1.0 mm posterior and 2.0 mm lateral to Bregma) for subsequent intracerebroventricular (ICV) infusion of either saline, or rat/human CRF. The cannula extended 4.0 mm below the surface of the skull. Two jewelers’ screws were placed into the skull, and the entire assembly was held in place with dental cement. A dummy cannula was then placed into the guide. For ICV infusions, rats were gently held while the dummy cannula was removed and an infusion cannula was lowered into the guide. The infusion cannula extended 0.5 mm below the guide, and was attached to a Hamilton syringe via PE20 tubing. The infusions were made using the manually held Hamilton syringe over the course of 1 min, and the infusion cannula remained in place for an additional 30 sec after the infusion was complete. Testing took place 5–8 days after surgery.
Testing Startle Amplitude and Prepulse Inhibition
Rats received an ICV infusion of either saline, or one of two doses of CRF (0.3 or 3.0 μg in 6.0 μl) 30 min prior to testing. Rats were tested in one of two identical startle chambers (San Diego Instruments). Rats were placed into a clear acrylic cylindrical chamber (9 cm diameter; 14 cm length) that is enclosed in a sound- and vibration-attenuating cabinet. The cabinet is equipped with a 5 W bulb, and a fan for ventilation. The chamber sits upon a base, under which a piezoelectric accelerometer detects whole body startle responses. Output signals from the accelerometer are collected as 100 sequential 1 msec measurements starting at the onset of the startling stimulus (120 dB, 40 msec). The signals were rectified, digitized, and stored on a computer by an SR-LAB program (San Diego Instruments). Chambers were calibrated each day, and matched for sound intensity. Delivery of white noise acoustic stimuli, through a horn tweeter (Radio Shack), was also controlled by the SR-LAB program.
Each rat was placed into a startle chamber for a five min acclimation period prior to the delivery of any stimulus. All stimuli were presented on a 70 dB background. On the first and last 6 trials, the 120 dB white noise startling stimulus was presented alone. The remaining trials included additional startle stimulus alone trials (used to calculate percent PPI), and trials in which a prepulse stimulus which was either 6, 12, or 15 dB above background (20 msec) preceded the startling stimulus by either 20, 75, 100, 500 or 2000 msec. There were 8 trials of each type, except for the type in which the 12 dB prepulse stimulus preceded the startling stimulus by 20 msec: Inadvertently, there were only 7 trials of this type. There were also 8 trials on which no stimulus was presented, but activity within the testing chamber was assessed. The inter-trial interval averaged 20 sec. Average startle amplitude during the 100 msec following the onset of each startling stimulus was recorded and stored on a computer. All testing took place between the hours of 10:00 am and 3:00 pm. We have examined whether the prepulses used in this experiment elicit a startle response themselves, and have found that they do not (data not shown).
Data Analysis
Percent PPI data were subjected to analyses of variance (ANOVA) with rat strain and CRF dose as between-subjects factors and prepulse intensity and inter-stimulus interval as within-subjects factors. Startle amplitude data were also subjected to ANOVA using the between–subjects factors. Tukey tests and simple ANOVAs were used for post-hoc analyses.
Peptides
The CRF used in these experiments was kindly provided by Dr. Jean Rivier (Salk Institute, La Jolla, CA).
Results
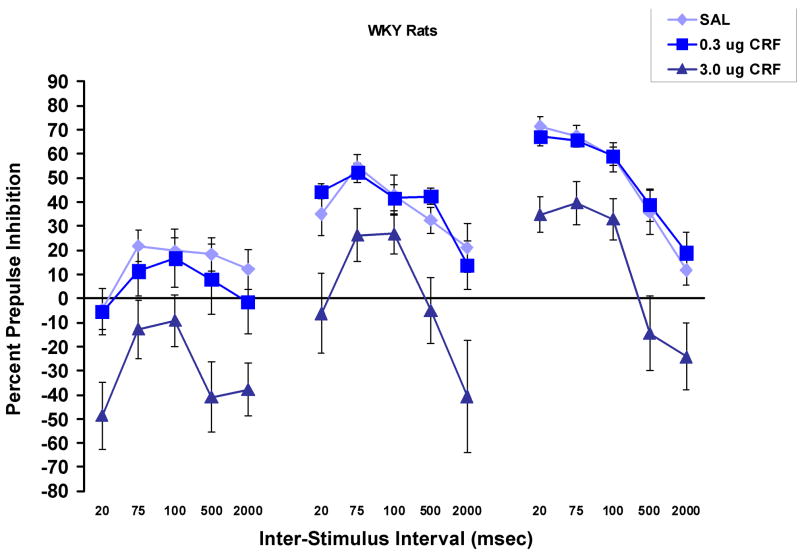
Figure 1 shows the percent prepulse inhibition at each inter-stimulus interval and each prepulse stimulus intensity in WKY (1a) and BN (1b) rats. There was an overall effect of CRF dose, F (2, 53) = 7.6, p = .001, and there were significant interactions involving CRF dose (described below and in Table 1). While there was no overall effect of rat strain, there was a strain × CRF dose interaction, F (2, 53) = 5.2, p < .01, that was due to the fact that overall, PPI was lower in BN than in WKY rats after treatment with saline or 0.3 μg of CRF, but was lower in WKY rats following 3.0 μg CRF. There was a significant interval × prepulse stimulus intensity interaction, F (8,424) = 22.3, p < .001, due to the interesting finding that the greatest amount of PPI was seen at the 20 msec interval when the prepulse stimulus was 15 dB, while at 6 dB, the greatest amount of PPI was found at the 75–100 msec interval. Additional main effects and interactions that are not reported in the text are shown in Table 1.
Figure 1.
Percent prepulse inhibition (mean ± SEM) at each inter-stimulus interval and each prepulse intensity in WKY (1a) and BN (1b) rats. A rat strain × CRF interaction was due to the fact that generally, PPI was lower in BN than in WKY rats after treatment with saline or 0.3 μg of CRF, but was lower in WKY rats following 3.0 μg CRF. The 3.0 μg dose of CRF decreased PPI in WKY to a greater extent at the 20, 500 and 2000 msec intervals than at the 75 or 100 msec intervals. CRF more effectively decreased PPI at the intermediate intervals in BN rats. Percent inhibition was more dependent on prepulse intensity at the 20 msec interval than at other intervals.
Table 1.
Significant Main Effects and Interaction not Reported in the Text
| Description | F (df) | p value |
|---|---|---|
| Experiment 1: WKY and BN Rats (Figure 1) | ||
| Prepulse Intensity | 178.0 (2,424) | < .001 |
| Prepulse Intensity × Rat Strain | 3.2 (4,424) | < .05 |
| Interval | 37.1 (4,424) | < .001 |
| CRF Dose × Interval (Overall) | 2.6 (8,424) | = .01 |
| CRF Dose × Interval (In BN Rats) | 2.47 (8,224) | < .02 |
| Rat Strain × Interval | 18.0 (4,424) | < .001 |
| Interval × CRF Dose × Rat Strain | 2.75 (8,424) | < .01 |
| Experiment 2: F1 Rats (Figure 6) | ||
| Prepulse Intensity | 69.4 (2,34) | < .001 |
| Interval | 35.7 (4,68) | < .001 |
| CRF Dose × Interval | 4.4 (8,136) | < .001 |
Figure 2 shows the data from WKY and BN rats that were saline-treated so that an evaluation of strain differences in PPI at different inter-stimulus intervals can be made. For these data, ANOVA revealed a significant rat strain × prepulse intensity × interval interaction, F (8, 136) = 5.46, p < .001. In light of this interaction, four t-tests, with Bonferroni corrections, were used to examine whether BN rats showed significantly less PPI at the 75 and 100 msec intervals at the 12 and 15 dB prepulse stimuli. The results of these t-tests follows: 12 dB prepulse at 75 msec, t (136) = 3.86, p < .001; 12 dB at 100 msec, t (136) = 2,7, p < .01; 15 dB at 75 msec, t (136) = q4.6, p < .001; 15 dB at 100 msec, t (136) = 4.2, p < .001. It is clear that PPI in the BN rats was not less than in the WKY at the extreme intervals (20 or 2000 msec).
Figure 2.
Percent prepulse inhibition in WKY and BN rats that were treated with saline. It can be seen that BN rats did not have lower PPI than WKY rats at the 20, 500 or 2000 msec interval, but did have lower PPI at the 75 and 100 msec intervals. T-tests were only performed on the data from trials using the 12 and 15 dB intensities;
* p < .01
** p < .001
Figure 3 shows the effect of rat strain and CRF on startle amplitude. There was a significant effect of rat strain, F (1, 53) = 18.26, p < .001, of CRF, F(2,53) = 4.00, p = .024, and a rat strain × CRF dose interaction, F (2,53) = 3.6, p = .034. All 3 significant effects were due to the fact that CRF dose-dependently increased startle amplitude in WKY rats only.
Figure 3.
Average Startle amplitude (mean ± SEM) on the trials used to calculate percent prepulse inhibition. CRF (3.0 μg) increased startle amplitude in only WKY rats.
* p < .05
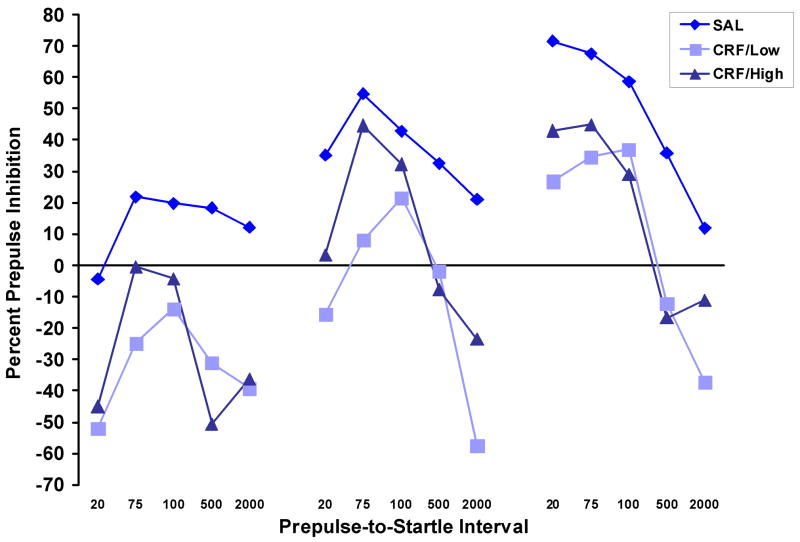
Since % PPI can be affected by a treatment that increases baseline startle amplitude, we subjected the startle data from the WKY rats that were treated with 3.0 μg CRF to a median split. Data from the half of the CRF-treated group with the lowest startle amplitude (CRF/Low) and the half with the highest amplitude (CRF/High) were then compared to the data from the saline-treated group. Figure 4a shows that while there was a significant effect of group, F (2,15) = 36.7, p < .001, only the CRF/High Startle group showed greater startle amplitude than the saline-treated group, p < .001. As shown in Figure 4b, there continued to be a significant effect of CRF on PPI, F (2,15) = 5.57, p < .02 and the CRF/Low Startle group showed significantly lower PPI than the saline-treated group, p < .02.
Figure 4.
Startle amplitude (a) and percent prepulse inhibition (b) in WKY rats that were treated with 3.0 μg CRF or saline. A median split was performed on the data from the CRF-treated group, and this resulted in a group of CRF-treated rats with low startle amplitude (CRF/Low) and a group with high startle amplitude (CRF/High). Startle amplitude was significantly greater in the CRF/High group than in the saline-treated group, but it was equivalent in the CRF/Low and saline-treated groups. Nevertheless, the CRF/Low group showed significantly less PPI than the saline treated group (b).
Figure 5 shows PPI in saline-treated F1 male rats and saline-treated male WKY and BN rats from the previous experiment. Since the F1 rats were tested in a separate experiment from the progenitors, statistical comparisons among the 3 groups could not be made. However, it can be seen that, in general, the PPI displayed by the F1 generation, more closely resembles that of the WKY than the BN rats. The exception is the PPI level at the 20 msec inter-stimulus interval. Here, the F1 rats show more PPI than either parental strain. Like the WKY rats, the F1 rats show more PPI than BN rats at the 75 and 100 msec inter-stimulus intervals when the 12 or 15 dB prepulse stimulus was used. At the 6 dB prepulse stimulus intensity, both WKY and F1 rats showed more PPI at the 100 msec interval than BN rats. Interesting, here, the F1 rats showed more PPI than WKY rats, although this result will have to be replicated in an experiment in which both strains are tested together before this claim can be made definitively.
Figure 5.
Percent PPI (mean ± SEM) in saline-treated male WKY and BN rats, and in the male F1 generation of a cross between these two inbred rat strains. The PPI phenotypes in the F1 generation resembled those seen in the WKY, rather than the BN progenitors.
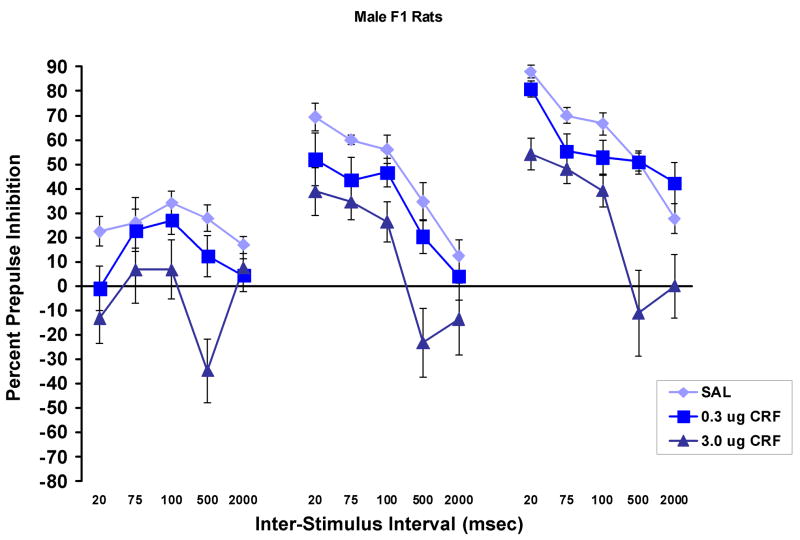
Figure 6 shows the effect of CRF on PPI in the male F1 rats (n = 6–7/group). There was a significant effect of CRF, F (1,17) = 10.4, p = .001, with Tukey post-hoc tests showing that the 3.0 μg dose significantly decreased PPI compared to both saline (p = .001) and to the 0.3 μg dose of CRF (p < .02). There was no difference between the effects of saline and 0.3 μg of CRF. Again, in this experiment, there was a significant prepulse stimulus intensity × inter-stimulus interval interaction, F (8,136) = 10.5, p < .001. This interaction occurred because, at the 6 dB prepulse intensity, there was more PPI at the intermediate intervals than at the 20 msec interval, while at the 12 and 15 dB prepulse stimulus intensities, the greatest amount of PPI was found at the 20 msec inter-stimulus interval.
Figure 6.
The effects of CRF on percent PPI (mean ± SEM) in the male F1 generation of the WKY × BN cross. The 3.0 μg dose of CRF significantly decreased PPI in these rats, while the 0.3 μg dose had no effect at any prepulse stimulus intensity or inter-stimulus interval. This pattern is the same as that which was seen in the WKY, but not in the BN rats.
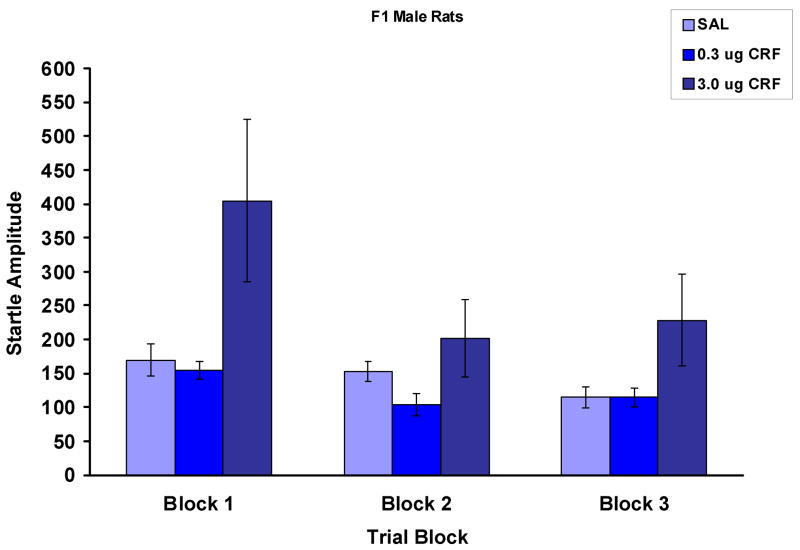
Figure 7 shows the effect of CRF on baseline startle amplitude on each of three trial blocks over the course of the testing session. Blocks 1 and 3 are the average of the first and last 6 trials, while block 2 is the average of the middle startle alone trials which were used to calculate percent PPI. Here, there was an overall trend towards an effect of CRF, F (2,17) = 3.3, p = .06. It appears that there was quite a robust effect of the 3.0 μg dose of CRF on startle amplitude in the first trial block, but that this was diminished during the block of trials (block 2) from which the data are used to calculate percent PPI.
Figure 7.
The effect of CRF on baseline startle amplitude (mean ± SEM) in the male rats from the F1 generation of the WKY × BN cross. Shown are the data from three trial blocks. Blocks 1 and 3 are the average of the first and last 6 trials. Block 2 is the average of the trials that were used to calculate percent PPI. Although CRF significantly increased startle amplitude on the first trial block, this effect had dissipated by the second block. This pattern of responsivity to CRF is unlike that shown by either of the progenitor strains. In WKY rats, the 3.0 μg dose increased startle amplitude over the entire course of the session, and in BN rats, CRF had no effect on startle amplitude.
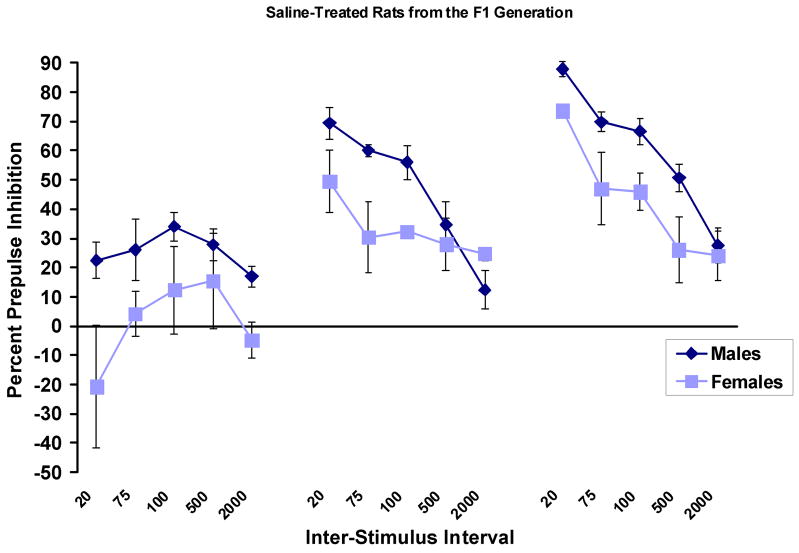
A limited number of female rats (3–4/group) from the F1 generation were also tested. Figure 8 shows PPI in saline-treated male and female rats from this generation. As expected, females showed significantly less PPI than males, F (1,7) = 6.2, p < .05.
Figure 8.
Percent PPI (mean ± SEM) in saline-treated male and female rats from the F1 generation of the cross between the WKY and BN rats. Female rats showed significantly less PPI than male rats.
CRF appeared to be less effective in reducing PPI in the female F1 rats than in the males. Although there was a significant over-all effect of CRF dose, F (2,7) = 4.9, p < .05, this was due to the fact that there was a difference between the effects of the low and high dose of CRF, and not due to a difference between the effect of CRF and saline (data not shown). It should be noted that these data are very preliminary, as there were only 3–4 females per dose. Further studies of females, which include progenitors, will have to be conducted before conclusions can be drawn.
Discussion
The results of this study reveal that BN rats show less PPI than WKY rats at intermediate inter-stimulus intervals (75 and 100 msec), but not at very short, or at very long intervals. Additionally, while CRF (0.3 μg) effectively diminished PPI even further in BN rats at the intermediate intervals, this effect was not apparent at the long and short intervals. In contrast, CRF (3.0μg) diminished PPI in WKY rats at each inter-stimulus interval, and resulted in PPF in this strain, particularly at the longest (2000 msec) interval. As in the past, these studies show that the effect of CRF on PPI is not due to changes in baseline startle amplitude. In this study, we also found PPF in CRF-treated WKY rats at the 200 msec inter-stimulus interval, and this effect was not seen in the BN rats. While we do not suggest that CRF caused PPF, we do suggest that it revealed a facilitation that is usually masked by inhibition, especially at intermediate inter-stimulus intervals and high-intensity prepulse stimuli. The fact that CRF did not reveal PPF in BN rats suggests that the strain may show less PPF as well as less inter-mediate interval PPI. Finally, the prepulse stimulus intensity required for maximal inhibition depends on the inter-stimulus interval.
In general, the baseline PPI phenotypes of the male F1 generation of the cross between the BN and WKY rats resembled that of the WKY progenitor. The exception was PPI at the 20 msec inter-stimulus interval, which was greater than that in either parental strain. However, because the F1 and progenitor strains were not tested in the same experiment, the results from the two generations could not be directly compared to each other and thus, caution must be exercised before concluding that the inheritance pattern of the baseline PPI phenotype is complex. The CRF-induced change in PPI in the F1 generation has some qualities of the effects in each of the progenitor strains. The 3.0 μg dose of CRF consistently decreased PPI, as it dies in the WKY strain (the exception was at the 15 dB prepulse stimulus at the 2000msec inter-stimulus interval). However, this dose of CRF did not result in appreciable PPF in the F1 generation, and this resembles a BN phenotype. Additionally, although there was no overall significant effect of the 0.3 μg dose of CRF on PPI in the F1 rats, a decrease at the intermediate intervals was seen. This is resembles the response of BN, but not WKY rats. Finally, CRF produced an effect on baseline startle amplitude which is not found in either progenitor strain: An initial increase which is not fully maintained across the session. This suggest that there is an, as yet, unidentified genetic contribution to the effect of CRF on startle, and that the startle and PPI responses to CRF are under the control of different mechanisms.
In this experiment, as in our past experiments, the 0.3 μg dose of CRF most effectively decreased PPI in BN rats, and the 3.0 μg dose most effectively decreased PPI in WKY rats. Although we do not yet know why this is so, we chose to use the most effective dose of CRF for each strain in these experiments. The apparent inverted u-shaped dose response to CRF seen in the BN rats is not without precedent. Risbrough et al., [35] found that, in mice, 0.3 μg but not 3.0 μg CRF decreases PPI. In their work, Risbrough et al., also found that the CRF1 and the CRF2 receptor appear to mediate opposing effects of CRF on PPI. Thus, one possibility is that the high dose acts at both receptors to affect PPI in the BN rats. What is not yet known, is why this does not seem to occur in the WKY rats. Perhaps such an effect would be seen were we to administer a higher dose of CRF to this strain.
The effect of CRF on baseline startle amplitude was also rat strain-dependent. While approximately half of the WKY rats showed a CRF-induced increase in startle amplitude, BN rats did not. The increase in startle amplitude in the WKY rats was not the cause of the CRF-induced decrease in percent PPI, as percent PPI was also significantly decreased in the WKY rats that did not show a CRF-induced increase in startle amplitude. Additionally, we have shown that ketanserin blocks the CRF-induced increase in startle amplitude in WKY rats without affecting the decrease in PPI [57]. The failure of the BN rats to show a CRF-induced increase in startle amplitude is unusual, but is in agreement with our previous results with this rat strain [33, 57]. This failure, as well as the relatively low levels of PPI seen in BN rats, suggests that the strain, in general, has relatively low startle plasticity. Additionally, in the present study, BN rats failed to show the CRF-induced PPF seen in the WKY rats, again suggesting diminished plasticity in the BN strain. However, we have shown that the indirect DA receptor agonist, methylphenidate does increase startle amplitude in BN rats and that the direct DA agonist, apomorphine causes considerable PPF in this rat strain [33]. Thus, it appears that the lack of a CRF-induced increase in startle and the lack of CRF-induced PPF is the result of a relative insensitivity to the effect of high-dose CRF on startle. Interestingly, this insensitivity to the 3.0 μg dose of CRF does not extend to a CRF-induced increase in activity which is robust in BN rats [57]. It should also be noted that CRF over-expressing mice show a smaller startle response than wild type mice in addition to diminished PPI [37].
Data from WKY rats suggest that PPI is strongest at the 75 and 100 msec interval, because although this PPI was disrupted by CRF, PPF was less evident than at other intervals. Reports from other laboratories show similar results. For example, Mansbach et al., [58] found that ketamine decreases PPI at intervals between 60 and 500 msec, and causes PPF at a 30 msec interval. These data, along with the data on the intensity × interval interaction, suggest that PPI and PPF are independent processes that may be occurring simultaneously, and that the behavioral “readout” is the net effect of the two processes.
The results of this study indicate that BN rats do not show less PPI than WKY rats at all inter-stimulus intervals. Indeed the BN rats had lower PPI than WKY rats at the commonly used 75 and 100 msec intervals, but not at the 20, 500, or 2000 msec intervals. This suggests that the processes governing very short- and very long-interval PPI are “normal” in BN rats, while intermediate-interval PPI, which is usually the most robust, is diminished in this rat strain. In general, the effect of interval length was more pronounced in WKY rats, and BN rats were relatively insensitive to the length of the interval. These results partially replicate those from a study by Swerdlow et al., [14] in which BN rats showed more PPI than Sprague Dawley rats at a 10 msec interval, equivalent PPI at a 20 msec inter-stimulus intervals, and less PPI at all higher intervals used in that study (maximum = 120 msec). Although the only interval that was common to both this study and the study by Swerdlow et al., [14] was the 20 msec interval, the pattern of the responses at increasing interval lengths was dissimilar in the two studies. At the 15 dB prepulse intensity, we found that the greatest amount of PPI at the 20 msec interval in both BN and WKY rats. Swerdlow and colleagues [14], found less PPI at the 20 msec interval than at intermediate intervals. The reasons for this difference are not clear.
While simple on its face, studies such as this reveal that PPI is a complex phenotype and that the amount of inhibition displayed depends on the parameters being employed. Thus, genetic differences, or differences in brain circuitry may only be reflected in differences with particular parameters. Our data, and those of Swerdlow et al., [13] suggest that neurobiological differences among strains may not affect PPI at all prepulse stimulus intensities or inter-stimulus intervals. If this is so, investigators will be able to ascertain whether particular neurobiological differences contribute to particular PPI phenotypes. Additionally, the differences between WKY and BN rats on both baseline PPI and CRF-induced changes in PPI are likely due to underlying differences in neurobiology and/or speed of synaptic conduction. We are beginning to explore these possibilities using measures of mRNA levels (for CRF and the CRF1 receptor, for example) and, in the future, electrophysiology.
Interestingly, we found a significant interval × intensity interaction that requires some consideration. In very few studies are both variables, prepulse intensity and inter-stimulus interval, manipulated in the same testing session. One exception is a study by Swerdlow et al., [59] in which such an interaction is not reported. In two reports in which mice were the subjects, intensity × interval interactions were found [60, 61]. Thus, it appears that conclusions about the effect of a particular interval on PPI at one prepulse intensity may not be generalizable to other intensities. Nevertheless, it is difficult to envision how processing speed would be affected by intensity. Thus, differences in PPI at different inter-stimulus intervals may not be due solely to the time, relative to the startle stimulus, that the information from the prepulse stimulus impinges on the startle circuit.
CRF did not increase PPI at any interval. This is noteworthy because dopamine receptor agonists can decrease PPI at some intervals and increase PPI at others [13, 55, 56, 59]. The present findings, along with those of Vinkers et al. [36], showing that CRF decreases PPI in both D1 and D2 receptor knockout mice, suggests that CRF does not decrease PPI via an indirect effect on dopamine. However, there are data that suggest otherwise. For example, haloperidol increases PPI in CRF over-expressing mice [38], and at least in WKY rats, haloperidol blocks the effect of exogenous CRF on PPI [32]. The role of DA in the effect of CRF on startle baseline is also unclear. For example, Meloni et al., [62] found that a selective D1 receptor antagonist blocks the CRF-induced increase in startle amplitude. However, this is not so in mice, and both D1 and D2 receptor knockout mice show a CRF-induced increase in startle amplitude [36]. We have found that the effect of CRF on startle, but not PPI, is blocked by ketanserin, a 5-HT2A/C antagonist [57]. The interaction between CRF and the monoamines to affect both baseline startle and PPI deserves further study as dysfunction of these interactions may be important in the development of some psychiatric disorders.
In humans with and without a psychiatric disorder, PPI levels are heritable [63, 64], and in rodents, there are genetic influences on PPI [65]. Responsivity to drug effects on PPI also shows heritable patterns, although these can be complex [54, 66]. The results of this experiment are in agreement with those of Palmer et al., [53], who found that the baseline PPI phenotype of an F1 generation of a WKY × BN cross resembles the WKY phenotype. These results, along with those of Swerdlow et al., [54] suggest that both Sprague Dawley and WKY rats have genes that are dominant over BN for the baseline PPI phenotypes. In the present study, this was so at each prepulse stimulus intensity and inter-stimulus interval with the exception of the 20 msec interval on which the F1 rats showed more PPI than either parental strain. Since the F1 rats and the parental strains were tested in two different experiments, this finding at the 20 msec inter-stimulus interval will have to be replicated with all rats being tested in the same experiment before anything can be said about a different heritability pattern for the 20 msec phenotype. The results of our preliminary experiments in which PPI was assessed in female F1 rats, reveal that the females showed less PPI than the males. This is in agreement with the results of a study by Swerdlow et al., [54].
Acknowledgments
This work was supported by MH065467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammond GR, McAdam DW, Ison JR. Effects of prestimulation on the electromyographic response associated with the acoustic startle reaction in rats. Physiol Behav. 1972;8:535–537. doi: 10.1016/0031-9384(72)90341-1. [DOI] [PubMed] [Google Scholar]
- 2.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 3.Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 4.Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naïve schizophrenia patients. Biol Psychiatry. 2002;52:863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- 5.Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 6.Schall U, Schon A, Zerbin D, Eggers C, Oades RD. Event-related potentials during an auditory discrimination with prepulse inhibition in patients with schizophrenia, obsessive-compulsive disorder and healthy subjects. Inter J Neurosci. 1996;84:15–33. doi: 10.3109/00207459608987247. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow NR, Paulsen J, Braff DL, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and specific threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 9.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psyciatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman HS, Wible BL. Role of weak signals in the acoustic startle. J Acoustic Soc Am. 1970;47:489–497. doi: 10.1121/1.1911919. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HS, Searle JL. Acoustic and temporal factors in the evocation of startle. J Acoustic Soc Am. 1968;43:269–282. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- 12.Ison JR, McAdam DW, Hammond GR. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiol Behav. 1973;10:1035–1039. doi: 10.1016/0031-9384(73)90185-6. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow NR, Shoemaker JM, Auerbach PP, Pitcher L, Goins J, Platten A. Heritable differences in the dopaminergic regulation of sensorimotor gating. II. Temporal, pharmacologic and generational analyses of apomorphine effects on prepulse inhibition. Psychopharmacology. 2004;174:452–462. doi: 10.1007/s00213-003-1480-4. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow NR, Talledo J, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal and “low gating” humans and rats. Neuropsychopharmacology. 2006;31:2001–2021. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- 15.Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 16.Hazlett EA, Romero MJ, Haznedar MM, New AS, Goldstein KE, Newmark RE, Siever LJ, Buchsbaum MS. Deficient attentional modulation of startle eyeblink is associated with symptom severity in the schizophrenia spectrum. Schizophrenia Res. 2007;93:288–295. doi: 10.1016/j.schres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 18.Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biol Psychiatry. 2004;55:518–523. doi: 10.1016/j.biopsych.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorders deficits. Am J Psychiatry. 2000;10:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 20.Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. Am J Psychiatry. 2007;164:61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: Effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- 22.Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological treatment studies. J Psychopharmacology. 2005;19:39–45. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- 23.Reijmers LG, Peeters BW. Acoustic prepulses can facilitate the startle reflex in rats: discrepancy between rat and human data. Brain Res Bull. 1994;35:337–338. doi: 10.1016/0361-9230(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 24.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Ann Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 25.Berridge CW, Dunn AJ. CRF and restraint stress decrease exploratory behavior in hypophysectomized rats. Pharmacol Biochem Behav. 1989;34:517–519. doi: 10.1016/0091-3057(89)90551-0. [DOI] [PubMed] [Google Scholar]
- 26.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 27.Bakshi VP, Geyer MA. (1999) Alpha-1-adrenergic receptors mediate sensorimotor gating deficits produced by dizocilpine administration in rats. Neuroscience. 1999;92:113–121. doi: 10.1016/s0306-4522(98)00752-0. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in the rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Souza EB. Corticotropin-releasing factor receptors in the rat central nervous system: Characterization and regional distribution. J Neurosci. 1987;7:88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 32.Conti LH. Characterization of the effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in Brown Norway and Wistar-Kyoto rats. Eur J Pharmacol. 2005;507:125–134. doi: 10.1016/j.ejphar.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 33.Conti LH, Berridge CW, Tayler JE. Both corticotropin-releasing factor and apomorphine reduce prepulse inhibition following repeated central infusion of corticotropin-releasing factor. Pharm Biochem Beh. 2006;85:261–272. doi: 10.1016/j.pbb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Conti LH, Murry JD, Ruiz MA, Printz MP. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- 35.Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on startle defensive behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinkers CH, Risbrough VB, Geyer MA, Caldwell S, Low MJ, Hauger RL. Role of dopamine D1 and D2 receptors in CRF-induced disruption of prepulse inhibition. Pharmacol Biochem Behav. 2007;86:550–558. doi: 10.1016/j.pbb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, Olivier Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry. 2002;51:583–590. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- 38.Dirks A, Groenink L, Westphal KG, Olivier JD, Verdouw PM, van der Gugten J, Geyer MA, Olivier B. Reversal of startle gating deficits in transgenic mice overexpressing corticotropin-releasing factor by antipsychotic drugs. Neuropsychopharmacology. 2003;28:1790–1798. doi: 10.1038/sj.npp.1300256. [DOI] [PubMed] [Google Scholar]
- 39.Groenink L, Dirks A, Verdouw PM, de Graaff M, Peeters BW, Millan MJ, Olivier B. CRF1 not glucocorticoid receptors mediate prepulse inhibition deficits in mice overexpressing CRF. Biol Psychiatry. 2008;63:360–368. doi: 10.1016/j.biopsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- 41.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CRF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chappell P, Leckman J, Goodman W, Bissette G, Pauls D, Anderson G, Riddle M, Scahill L, McDougle C, Cohen D. Elevated cerebrospinal fluid corticotropin-releasing factor in Tourette’s syndrome: comparison to obsessive compulsive disorder and normal controls. Biol Psychiatry. 1996;39:776–783. doi: 10.1016/0006-3223(95)00221-9. [DOI] [PubMed] [Google Scholar]
- 43.Forman SD, Bissette G, Yao J, Nemeroff CB, van Kammen DP. Cerebrospinal fluid corticotropin-releasing factor increases following haloperidol withdrawal in chronic schizophrenia. Schizophrenia Res. 1994;12:43–51. doi: 10.1016/0920-9964(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 44.Sautter FJ, Bissette G, Wiley J, Manquno-Mire G, Schoenbachler B, Meyers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 45.Ludewig S, Ludewig K. No prepulse inhibition deficits in patients with unipolar depression. Depress Anxiety. 2003;17:224–225. doi: 10.1002/da.10109. [DOI] [PubMed] [Google Scholar]
- 46.Perry W, Minassian A, Feifel D. Prepulse inhibition in patients with non-psychotic major depressive disorder. J Affect Disord. 2004;81:179–184. doi: 10.1016/S0165-0327(03)00157-5. [DOI] [PubMed] [Google Scholar]
- 47.Nemeroff CB. Psychopharmacology of affective disorders in the 21st century. Biol Psychiatry. 1998;44:517–525. doi: 10.1016/s0006-3223(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 48.Herringa RJ, Roseboom PH, Kalin NH. Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenia subjects. Neuropsychopharmacology. 2006;31:1822–1831. doi: 10.1038/sj.npp.1301038. [DOI] [PubMed] [Google Scholar]
- 49.Faraday MM, O’Donoghue VA, Grunberg NE. Effects of nicotine and stress on startle amplitude and sensory gating depend on rats strain and sex. Pharmacol Biochem Behav. 1999;62:273–284. doi: 10.1016/s0091-3057(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 50.Hall FS, Huang S, Fong G. Effects of isolation-rearing on acoustic startle and pre-pulse inhibition in Wistar and fawn hooded rats. Ann NY Acad Sci. 1997;821:542–544. doi: 10.1111/j.1749-6632.1997.tb48325.x. [DOI] [PubMed] [Google Scholar]
- 51.Varty GB, Geyer MA. Effects of isolation rearing on startle reactivity, habituation, and prepulse inhibition in male Lewis, Sprague Dawley, and FisherF344 rats. Behav Neurosci. 1998;112:1450–1457. doi: 10.1037//0735-7044.112.6.1450. [DOI] [PubMed] [Google Scholar]
- 52.Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse inhibition startle deficit in the Brown Norway rat: A potential genetic model. Behav Neurosci. 2000;114:374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- 53.Palmer AA, Breen LL, Flodman P, Conti LH, Spence MA, Printz MP. Identification of quantitative trait loci for prepulse inhibition. Psychopharmacology. 2003;165:270–279. doi: 10.1007/s00213-002-1258-0. [DOI] [PubMed] [Google Scholar]
- 54.Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008;88:280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swerdlow NR, Shoemaker JM, Pitcher L, Platten A, Kuczenski R, Eleey CC, Auerbach P. Genetic differences in startle gating – disruptive effects of apomorphine: Evidence for central mediation. Behav Neurosci. 2002;116:682–690. doi: 10.1037//0735-7044.116.4.682. [DOI] [PubMed] [Google Scholar]
- 56.Martin-Iverson MT, Else D. PHNO, a selective dopamine D2 receptor agonist, does not reduce prepulse inhibition of the startle reflex in rats. Psychopharmacology. 2000;151:38–48. doi: 10.1007/s002130000483. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland JE, Page ME, Conti LH. The effect of corticotropin-releasing factor on prepulse inhibition is independent of serotonin in Brown Norway and Wistar-Kyoto rats. Pharmacol Biochem Behav. 2008;89:324–337. doi: 10.1016/j.pbb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansbach RS, Geyer MA. Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine. Psychopharmacology. 1991;105:162–168. doi: 10.1007/BF02244303. [DOI] [PubMed] [Google Scholar]
- 59.Swerdlow NR, Platten A, Shoemaker J, Pitcher L, Auerbach P. Effects of pergolide on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 2001;158:230–240. doi: 10.1007/s002130100856. [DOI] [PubMed] [Google Scholar]
- 60.Plappert CF, Pilz PKD, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res. 2004;152:403–412. doi: 10.1016/j.bbr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 61.Varty GB, Walters N, Cohen-Williams M, Carey GJ. Comparison of apomorphine, amphetamine and dizocilpine disruptions of prepulse inhibition in inbred and outbred mice strains. Eur J Pharmacol. 2001;424:27–36. doi: 10.1016/s0014-2999(01)01115-3. [DOI] [PubMed] [Google Scholar]
- 62.Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of the startle reflex in humans. Neurosci Lett. 2003;353:45–48. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measure for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 66.Ellenbroek BA, Geyer MA, Cools AR. The behavior of APO-SUS rats in animal models with construct validity for schizophrenia. J Neuroscience. 1995;15:7604–7611. doi: 10.1523/JNEUROSCI.15-11-07604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]