1. Structure
The interleukin (IL)-1 family of cytokines includes the two agonists IL-1α (GeneID: 3552; accession # M28983) and IL-1β (GeneID: 3553; accession # AAC03536) coded by different genes (Arend, Palmer, and Gabay, 2008). Both cytokine agonists lack signal sequences for transport from the cell and, therefore, are released by cell injury or death. IL-1α (Fig. A) and IL-1β are synthesized as precursors that are cleaved by inflammatory caspase-1 during release from the cell. The IL-1 family also contains a specific inhibitor, IL-1 receptor antagonist that competes with both agonists for receptor binding and the balance between IL-1α/β and IL-1Ra is likely important in controlling the effects of IL-1 on epithelial and stromal cells in the cornea, as it is in many other organs (Arend, Palmer, and Gabay, 2008). Both IL-1α and IL-1β bind to the active type IL-1 receptor and inactive type II IL-1 receptor and employ the same interacting accessory protein (IL-1RAcP).
Fig.
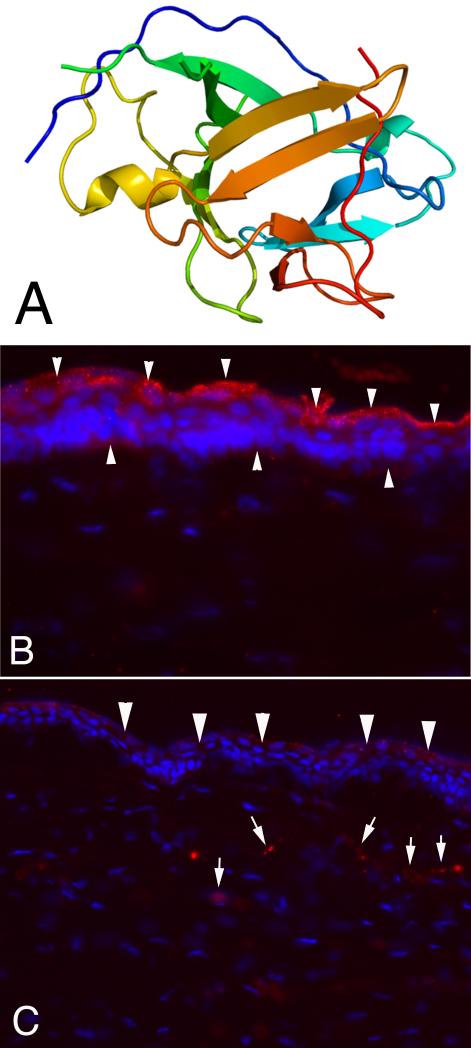
Model of structure of IL-1α and expression in unwounded and wounded rabbit corneas. A. Model structure of human interleukin-1 alpha. B. IL-1α is expressed constitutively throughout the corneal epithelium (arrowheads, red), but appears to be most highly expressed in apical epithelial cells. Note that little IL-1α is associated with keratocyte cells in the stroma of the unwounded cornea. Mag. 500X C. At one month after injury (in this case -9 diopter PRK that triggers haze and myofibroblast development) IL-1α is now detected in stromal cells (arrows, red), in addition to continued expression in the healed epithelium (arrowheads, red). Blue stain is DAPI staining of cell nuclei. Mag. 500X.
2. Function
2.1 Corneal expression
Both IL-1α and IL-1β are expressed by unwounded corneal epithelial cells (Fig. B) of humans and other species and are released into the tear film and corneal stroma after injury (Wilson, et al., 2001). Fini and coworkers demonstrated that corneal stromal cells, including corneal fibroblasts, produce IL-1 (Fig. C) in an autocrine loop after exogenous IL-1 binds to the receptors. IL-1 receptor antagonist is also produced by corneal epithelial cells, and to a lesser extent by corneal stromal cells. Factors that modulate the relative concentration of the agonists versus the antagonists are likely important in controlling downstream effects of IL-1 after the cytokine binds to active IL-1 receptors present on corneal cells.
2.2 Corneal functions
IL-1α and IL-1β modulate key functions of keratocytes, corneal fibroblasts and myofibroblasts during corneal wound healing (Wilson, et al., 2001). IL-1 has been shown to have an important role in modulating keratocyte apoptosis, likely via induction of Fas ligand. Since keratocytes constitutively express the Fas receptor, up-regulation of autocrine Fas ligand within the cells likely triggers autocrine suicide. Studies in mice have suggested that the resulting wave of anterior stromal keratocyte apoptosis may function as a protective firewall against the extension of viruses such as herpes simplex that infect the corneal epithelium and may otherwise spread to deeper corneal cells and even inside the eye.
Recent studies have demonstrated that IL-1, in coordination with transforming growth factor (TGF) β, also has an important role in modulating myofibroblast survival (Kaur, Chaurasia and Wilson, unpublished data, 2009). TGF β, derived primarily from corneal epithelium, penetrates into the stroma in the context of basement membrane defects and promotes the differentiation of myofibroblasts associated with corneal opacity in the anterior stroma. When stromal TGF β levels fall—presumably after the normal function of the basement membrane is restored—IL-1 triggers myofibroblast apoptosis. Death of the myofibroblasts and repopulation of the anterior stroma with keratocytes is likely a key event in clearing the extracellular matrix components that contribute to the corneal opacity (haze). IL-1 also modulates the production of collagenases, metalloproteinases, and other enzymes by keratocytes and corneal fibroblasts that directly mediate the breakdown and reabsorption of the extracellular materials excreted by myofibroblasts.
IL-1α and IL-1β are also important modulators of the inflammatory cell response to corneal injury (Dana, 2007; Stapleton, et al., 2008). IL-1 released from epithelial cells by injury is not only directly chemotactic to inflammatory cells, but the cytokines also bind to surviving keratocytes and profoundly up-regulate production of chemokines in these cells. Using gene array technology, many of the top twenty genes whose expression is up-regulated in corneal fibroblasts by IL-1α are chemokines, including monocyte chemotactic and activating factor (MCAF or MCP-1) and granulocyte stimulating factor (GSF). These chemokines are profoundly chemotactic to monocytes, granulocytes and other inflammatory cells—an observation that has been confirmed by direct microinjection of small amounts of MCAF and GSF into the mouse cornea. Recent studies in which topical IL-1RA profoundly decreased inflammatory cell infiltration into the cornea after epithelial scrape injury suggested that IL-1α and IL-1β are the primary regulators of this wound healing response (Stapleton, et al., 2008).
IL-1α and IL-1b are also important mediators of stromal-epithelial interactions that direct corneal epithelial healing after injury (Wilson, et al., 2001). Both keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) are produced at nearly undetectable levels in keratocytes in the normal unwounded cornea. After injury, however, these modulators are produced at high levels. They then bind to specific receptors expressed by corneal epithelial cells and modulate the proliferation, migration and differentiation of the epithelial cells, along with other growth factors such as epidermal growth factor and platelet-derived growth factor. In addition to regulating restorative functions of the corneal epithelium, IL-1 also stimulates the epithelial cells to produce molecules such as beta-defensin 2 that are involved in defending the tissue from microorganisms.
Thus, IL-1α and IL-1β regulate many of the most important responses to corneal injury—including mobilization of inflammatory cells and up-regulation of the production of molecules involved in the defense against pathogens, angiogenesis, stromal remodeling, elimination of wound healing cells, like myofibroblasts, after they are no longer needed, and orchestrating the production of growth factors that help restore epithelial structure and function. Clearly, the IL-1 cytokine-receptor system is a master regulator of the corneal response to injury.
IL-1 and IL-1 receptor antagonist are regulators of the angiogenesis response to corneal injury (Dana, 2007), including angiogenesis that may threaten transplanted corneal tissue. Thus, when IL-1β is implanted into the cornea, it is a potent stimulator of angiogenesis. Conversely, IL-1RA is an inhibitor of this response.
3. Disease Involvement
Since inflammation has a central role in many disease processes in the cornea, the IL-1/IL-1 receptor system is clearly a major contributor to the pathophysiology of a wide range of corneal and anterior segment maladies. These likely include dry eye disease, where the role of inflammation has become a dominant focus, infectious processes such as herpes simplex keratitis, microbial corneal ulcers and conjunctivitis, and myriad inflammatory disorders of the anterior segment of the eye, including diseases such as allergic conjunctivitis, marginal corneal ulcers, and Stevens-Johnson syndrome.
The other functions regulated by the IL-1/IL-1 receptor system are also important in a wide range of corneal diseases. Thus, IL-1 may be important in diseases where there is damage to the corneal stromal matrix, including stromal melting associated with rheumatologic, allergic, or infectious diseases. As was discussed in more detail earlier, if the hypothesized antagonistic functions of TGF β and IL-1 on myofibroblast viability are important, it follows that the IL-1/IL-1 receptor system likely plays a role in many disorders associated with changes in corneal transparency, including stromal scaring associated with infectious diseases of the cornea and stromal haze that occasionally complicates refractive surgical procedures such as photorefractive keratectomy (PRK).
4. Future Studies
Future studies to further refine our understanding of the interactions between the various members of the IL-1/IL-1 receptor family, and other cytokines such at TGF β, in the cornea and conjunctiva would likely provide important insights into the regulation of tissue interactions that contribute to corneal health, disease and wound healing.
Acknowledgements
Supported in part by US Public Health Service grants EY010056 and EY015638 from the National Eye Institute and Research to Prevent Blindness, New York, NY.
References
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival. Trans. Am. Ophthalmol. Soc. 2007;105:330–43. [PMC free article] [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia S, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008;86:753–7. doi: 10.1016/j.exer.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]