Abstract
T cell proliferation following activation is an essential aspect of the adaptive immune response. Multiple factors, such as TCR signaling, costimulation, and signals from cytokines each contribute to determine the magnitude of T cell expansion. In this report, we examine in detail the role of Jak3/γc-dependent cytokines in promoting cell cycle progression and proliferation of naïve T cells. Using naïve CD4+ T cells from Jak3-deficient mice and wild type CD4+ T cells treated with a small molecule inhibitor of Jak3, we find that these cytokine signals are not required for proliferation; instead they are important for the survival of activated T cells. In addition, we show that the percentage of cells entering the cell cycle and the percentage of cells in each round of cell division are comparable between Jak3-deficent and wild-type T cells. Further, cell cycle progression and the regulated expression of key cell cycle proteins are independent of Jak3/γc cytokine signals. These findings hold true over a wide range of TCR signal strengths. However, when CD28 costimulatory signals, but not TCR signals, are limiting, Jak3-dependent cytokine signals become necessary for the proliferation of naïve T cells. As CD28 signaling has been found to be dispensable for autoreactive T cell responses, these data suggest the potential for interfering with autoimmune T cell responses by inhibition of Jak3 signaling.
Introduction
T cell proliferation is essential for mounting an effective adaptive immune response. A key element of proliferation is the entry of cells into the cell cycle, a complex process that is tightly controlled by the ordered expression of cyclins, the activation of cyclin-dependent kinase (Cdk) enzymatic activity and the subsequent phosphorylation of relevant substrates. The first cyclin expressed during the G1 phase is a D-type cyclin, which is a rate-limiting factor for cell cycle progression from the G1 to the S phase. The induction of cyclin E occurs at the late G1 restriction point, and cyclin A is expressed at S phase entry (1). The activity of Cdks is stimulated by cyclins and inhibited by cyclin-dependent kinase inhibitors (CDKI), such as p27kip1. Cyclin/Cdk complexes phosphorylate the retinoblastoma (Rb) gene product, leading to the activation of the E2F transcription factor, which is required for the transcription of S phase genes.
T cell proliferation is induced following stimulation of the T cell receptor (TCR) and costimulatory molecules; in addition, cytokines such as IL-2 and IL-4, that signal through receptors sharing the common γ (γc) chain, have been shown to promote lymphocyte proliferation (2). Among these, IL-2 has long been recognized as the most potent T cell growth factor (3). In vitro studies have shown that IL-2 very efficiently promotes the growth of antigen-activated T cells (4, 5). Antigen- or mitogen-induced T cell proliferation in vitro can be substantially inhibited using monoclonal antibodies specific for IL-2 or the IL2R, suggesting that IL-2 is an essential element in T cell proliferation (6-8). In later studies, it was found that IL-2 promotes the transit of T cells through G1 to S phase of the cell cycle by up-regulating cyclin D2, cyclin D3, cyclin E and E2F, and down-regulating p27kip1 (9-12). Based on these findings, among others, the consensus view is that TCR and CD28 stimulation induce quiescent T cells to leave G0 and enter the G1 phase of the cell cycle (13); in addition, these signals induce the expression of the high-affinity IL-2 receptor and stabilize the IL-2 message, rendering the cells competent for IL-2-driven proliferation.
Recent studies performed in intact animals have challenged this view and demonstrated IL-2- or γc cytokine-independent T cell expansion in vivo. When adoptively-transferred, IL-2-deficient or IL-2R-deficient DO11.10 T cells challenged with OVA peptide underwent comparable expansion compared to wild type T cells (14, 15). Similarly, after correcting the autoimmune defect in IL-2Rβ-deficient mice by selective expression of IL-2Rβ in the thymus, IL-2R β-/- T cells also showed normal expansion during both primary and secondary immune responses (16). Finally, Di Santo and colleagues reported that naïve γc chain-deficient T cells proliferate robustly in response to antigenic stimulation in vivo (17). Together, these results indicate that γc cytokine signals are not absolutely required in vivo for T cell proliferation.
Several in vitro studies also suggest that T cell proliferation can occur in an IL-2-independent manner. For instance, except under conditions of suboptimal stimulation, IL-2 or IL-2R antibody blockade cannot completely inhibit T cell proliferation (18, 19). Further, IL-2- or IL-2R-deficient T cells can be induced to proliferate in response to specific antigens or mitogens, although the proliferation is generally reduced compared with that of control T cells (20-23). Finally, several studies have suggested that TCR plus CD28 stimulation controls cell cycle progression independently of IL-2. Using IL-2 or IL-2R blocking antibodies, or IL-2-deficient cells, these studies indicated that TCR/CD28 engagement could promote T cell proliferation by inducing the expression of cyclin D and cyclin E, enhancing the transcriptional activity of E2F, and down-regulating the inhibitory function of p27kip1 (24-30).
However, there are a number of caveats with these studies that have hampered the general acceptance of the view that T cell proliferation does not require IL-2 or other γc cytokine signals. First, the failure to completely block T cell proliferation with IL-2 or IL-2R antibodies may reflect the lower affinity of these interactions relative to the affinity of IL-2 for its receptor. Second, several of these studies were performed using tumor cell lines or anergic T cells, which may not reflect the requirements of primary naive T cells. A similar concern applies to the studies using IL-2- or IL-2R-deficient T cells, as these cells are also not naïve T cells (14). Finally, these in vitro studies did not rule out the possibility that T cell proliferation was being induced by γc cytokines other than IL-2; although IL-2 is the main γc cytokine that is secreted when T cells are initially activated in vitro, IL-4 and IL-21 are also produced by activated T cells and can promote T cell proliferation (31-33). Therefore, none of these studies conclusively demonstrated that naïve T cell proliferation was γc cytokine-independent.
In this report, we investigated the requirement of γc cytokines in the proliferation and cell cycle control of primary naïve T cells in vitro. We analyzed the proliferation of naïve CD4+ T cells from the mice lacking Janus kinase 3 (Jak3), a tyrosine kinase that is exclusively associated with γc chain and is essential for signaling via all γc cytokine receptors (34-39). We complemented these experiments with analysis of wild type naïve CD4+ T cells treated with a pharmacological inhibitor of Jak3. Together, these studies demonstrated that Jak3-dependent γc cytokine signals are not required for naïve primary CD4+ T cell proliferation and cell cycle regulation in vitro. We also show that when CD28 costimulatory signals are limiting, Jak3-dependent cytokine signals become necessary for the proliferation of naïve T cells. As CD28 signaling has been found to be dispensable for autoreactive T cell responses, these data suggest the potential for interfering with autoimmune T cell responses by inhibition of Jak3 signaling.
Materials and Methods
Mice and reagents
Jak3-/- mice (40) were backcrossed to C57BL/6 for ten generations. OTII-transgenic (41) and Bcl-2-transgenic (42) mice were purchased from Jackson laboratory (Bar Harbor, ME). Jak3-/- mice were crossed to OTII- and Bcl-2-transgenic mice to generate Jak3+/- and Jak3-/- OTII+ Bcl-2-transgenic mice. All mice were maintained in pathogen-free conditions and used between 6-10 weeks of age. PS078507 was developed at Pharmacopeia, Inc. (Princeton, NJ). A stock solution (10 mM) was prepared by dissolving PS078507 in dimethylsulfoxide (DMSO) (Sigma Chemical Co., St. Louis, MO). All working compound solutions were made by serial dilutions in buffers or culture medium. DMSO was used as a vehicle control.
Cell preparation and activation
Thymocytes were harvested from Jak3+/- and Jak3-/- OT-II Bcl-2 mice, red blood cells were lysed, and cells were incubated with anti-CD4-PE (BD Pharmingen, San Diego, CA) and anti-CD8-APC (BD Pharmingen) antibodies. CD4 SP thymocytes were sorted by flow cytometry on a Mo-Flo sorter (Cytomation, Fort Collins, CO) to a purity of >98%. Splenocytes from Jak3+/+ OTII-transgenic mice were incubated with anti-CD4 antibody-coated magnetic microbeads, and CD4+ T cells were purified by positive selection (Miltenyi, Auburn, CA) to a purity of >94%. T cells were stimulated with plate-bound anti-CD3 (1 μg/ml) plus anti-CD28 (4 μg/ml) antibodies (eBioscience, San Diego, CA), unless the concentrations were specified. T cells were cultured in 24-well plates at 1.2 × 106 cells/ml in RPMI 1640 supplemented with 10% heat inactivated fetal calf serum, 2 mM glutamine, 100 iu/ml penicillin, 100 ug/ml streptomycin, 50 μg/ml geneticin, 2 uM β-mercaptoethanol, and 25 mM HEPES.
Analysis of cell proliferation by thymidine incorporation
Isolated CD4+ CD44lo cells were plated out at a density of 1 × 105 cells /180 μl in triplicate in a 96-well flat-bottomed plate and stimulated with various concentrations of anti-CD3 and anti-CD28 or mitomycin C-treated C57BL/6 antigen presenting cells (APCs) plus the indicated concentrations of OVA323-339. Forty-eight hours later, cells were pulsed with one microcurie of [3H]-thymidine for 18h, and harvested on a Tomtec Harvester 96 (Orange, CT). Thymidine incorporation was quantified on a Trilux microbeta counter (PerkinElmer, Wellesley, MA).
Analysis of cell proliferation with carboxyfluorescein diacetate succinimidyl ester (CFSE)
Isolated cells were washed once in PBS and resuspended at a density of 2.5 × 107 cells/ml in PBS. CFSE was added to a final concentration of 2.5 μM. The cell suspension was mixed thoroughly and placed at 37°C for 12 min and the reaction was terminated by adding RPMI 1640 with 10% fetal calf serum. The cells were then plated at 1.2 × 106 cells/ml in 24-well plates, stimulated with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (4 μg/ml) antibodies or OVA323-339 peptide (1 μg) presented by mitomycin C-treated C57BL/6 APCs, in the absence or presence of the Jak3 inhibitor PS078507 (312nM) or IL-2 blocking antibodies (10 μg/ml of anti-IL-2, anti-CD25 and anti-CD122 antibodies; eBiosciences, Inc.). Three days later, the fluorescence of the cells was determined by flow cytometry.
Apoptotic analysis
Stimulated T cells were harvested, washed with cold PBS and resuspended in 1× BD Pharmingen™ Annexin V Binding Buffer to achieve a final concentration of 10.0 × 106 cells/ml. 5 μl of Annexin V-FITC and 7-AAD were added to 100 μl solution (∼1 × 106 cells) and incubated at room temperature for 15 min in the dark. Apoptosis was analyzed by flow cytometry within 1h.
Cell cycle analysis by flow cytometry
One million naïve or activated T cells were washed with cold PBS and fixed overnight at -20°C in 95% ethanol. Cells were then pelleted, washed and resuspended in 1 ml PBS with the propidium iodide (PI) at a final concentration of 20 μg/ml, ribonuclease at 20 μg/ml and EDTA at 2mM. The cells were incubated at 37°C for 30 min in the dark. PI content was assessed by flow cytometry.
Immunoblot
Naïve cells or activated cells were harvested at the indicated time points, washed and lysed in RIPA buffer for 20 min on ice. The protein fraction was separated by centrifugation at 13,000 rpm for 10 min at 4°C and protein level was quantified with the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Proteins were separated on SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were immunoblotted with antibodies to cyclin D2 (Santa Cruz Biotechnology, Santa Cruz, CA), cyclin D3 (Santa Cruz Biotechnology), cyclin E (Santa Cruz Biotechnology), cyclin A (Santa Cruz Biotechnology), p27kip1 (Santa Cruz Biotechnology), Stat5 (Santa Cruz Biotechnology), PI-3-kinase p85 (Cell Signaling Technology, Danvers, MA), phospho-Stat5 (Cell Signaling Technology) and β-actin (BD Pharmingen) antibodies.
Statistical Analysis
Statistical analysis was performed using the two-tailed paired student’s t test.
Results
Jak3-dependent cytokine signals are required for optimal T cell responses in vitro
To analyze the role of Jak3-dependent cytokine signals in T cell proliferation, we generated naïve T cells as described previously (43), by crossing Jak3-/- mice to the OT-II TCR transgenic mouse line, and then to a Bcl-2 transgenic line to improve the in vivo survival of the Jak3-/- T cells (hereafter referred to as Jak3-/- OT-II Bcl-2 mice). Previous studies have shown that IL-2-induced phosphorylation of Stat5 requires the activity of Jak3 (37). To confirm this result, we examined Stat5 phosphorylation in Jak3+/- and Jak3-/- OT-II Bcl-2 cells in response to IL-2 stimulation. As shown in Supplemental Figure 1, Stat5 phosphorylation in response to IL-2 stimulation is completely abolished in the absence of Jak3. Purified CD4+ CD44lo T cells from Jak3+/- or Jak3-/- OT-II Bcl-2 mice were then stimulated with varying concentrations of OVA peptide presented by mitomycin C-treated antigen presenting cells (APCs) from C57BL/6 mice (Fig. 1A) or anti-CD3 and anti-CD28 antibodies (Fig. 1B). As shown, Jak3-/- CD4+ CD44lo T cells were capable of proliferating in response to all stimuli, although the magnitude of the response was reduced in comparison to that of control cells. This reduced proliferation of Jak3-/- T cells could result from impaired T cell proliferation or impaired T cell survival, or both.
FIGURE 1. CD4+ T cell proliferation appears reduced in the absence of Jak3-dependent cytokine signals.
CD4+ CD44lo T cells were sorted from Jak3+/- and Jak3-/- OT-II Bcl-2 mice and stimulated with either mitomycin C-treated C57BL/6 APCs plus the indicated concentrations of OVA323-339 (A) or various concentrations of anti-CD3 and anti-CD28 antibodies (B) for 48h, then pulsed with [3H]-thymidine for the final 18h. Cell proliferation was measured by [3H]-thymidine incorporation. Data represent the mean ± SE of the triplicate reactions. Statistically significant differences were seen between Jak3+/- and Jak3-/- cells. The significant level is P < 0.05.
T cell survival, but not proliferation, is affected by Jak3-dependent cytokines
γc cytokines, especially IL-2, IL-4 and IL-7, promote T cell survival by up-regulating the anti-apoptotic factor Bcl-2 (44-47). In Jak3- or γc-deficient T cells, the expression of Bcl-2 is greatly decreased (17, 38). To determine whether constitutive expression of Bcl-2 is sufficient to reverse the survival defect of Jak3-/- T cells in vitro, we examined T cells for evidence of apoptosis. For these studies we used CD4+CD8- single-positive (CD4SP) thymocytes from Jak3+/- or Jak3-/- OT-II Bcl-2 mice as a source of homogeneous naïve T cells. Following 3 days of stimulation, cells from Jak3-/- mice showed a significantly higher degree of cell death compared to control cells (Fig. 2A). These findings indicate that Jak3-dependent cytokine signals normally induce a survival pathway that cannot be compensated for by constitutive expression of Bcl-2. Thus, in the absence of Jak3, the reduced level of 3H-thymidine incorporation following T cell activation may be due to enhanced apoptosis, rather than impaired proliferation.
FIGURE 2. Jak3-dependent cytokine signals affect T cell survival but not proliferation.
A, Purified CD4SP thymocytes from Jak3+/- and Jak3-/- OT-II Bcl-2 mice were stimulated with anti-CD3 (1 μg/ml) plus anti-CD28 (4 μg/ml) antibodies. Three days later, cells were stained with AnnexinV and 7-AAD, and analyzed by flow cytometry. Apoptotic cells were identified as AnnexinV+ 7-AAD+.
(B,C,D) CD4SP thymocytes were isolated from Jak3+/- and Jak3-/- OT-II Bcl-2 mice, labeled with CFSE, stimulated with anti-CD3 (1 μg/ml) plus anti-CD28 (4 μg/ml) antibodies, and analyzed by flow cytometry. B, representative histograms show the degree of CFSE dilution in Jak3+/- and Jak3-/- OT-II Bcl-2 T cells. C, Percentage of Jak3+/- and Jak3-/- OT-II Bcl-2 T cells that underwent cell division; data represent the mean ± SE from four independent experiments. D, Histograms were analyzed using the proliferation platform of the FlowJo software to estimate the percentage of cells in each round of cell division; representative data are shown above. The graph depicts the mean ± SE using data from four independent experiments. No significant differences were seen between Jak3+/- and Jak3-/- cells.
E, CD4+ T cells were isolated from Jak3+/+ OTII transgenic mice, labeled with CFSE, and stimulated with anti-CD3 (1 μg/ml) plus anti-CD28 (4 μg/ml) antibodies or 1 μg of OVA323-339 peptide presented by mitomycin C-treated C57BL/6 APCs (peptide stimulation). Cultures were supplemented with nothing (-), exogenous recombinant IL-2 (5 μg/ml; IL-2) or IL-2 blocking antibodies (10 μg/ml each of anti-IL-2, anti-CD25, and anti-CD122 antibodies; IL-2B). Three days later, cell proliferation was analyzed by flow cytometry.
F, CD4SP thymocytes were isolated from Jak3+/- and Jak3-/- OT-II Bcl-2 mice, labeled with CFSE, stimulated with anti-CD3 (1 μg/ml) plus anti-CD28 (4 μg/ml) antibodies, in the absence (-) or presence of IL-2 blocking antibodies (10 μg/ml each of anti-IL-2, anti-CD25 and anti-CD122 antibodies; IL-2B). Four days later, cell proliferation was analyzed by flow cytometry.
T cell proliferation can be assessed using the fluorescent dye, carboxyfluorescein succinimidyl ester (CFSE), providing a means of excluding dead cells in the population and of visualizing the proportion of cells representing each successive cell division cycle. As can be seen, after 3 days of stimulation with anti-CD3 and anti-CD28 antibodies, there was no significant difference in the percentage of divided cells between Jak3-deficient and Jak3-positive T cells, suggesting that Jak3-dependent cytokine signals do not regulate the proportion of cells entering the cell cycle (Fig. 2B, C). To examine this issue more carefully, we determined the percentage of cells in each round of cell division, and compiled the data from four independent experiments (Fig. 2D). This analysis confirmed that T cells lacking Jak3 showed comparable proliferative capacity to T cells expressing Jak3. Similarly, a comparison of the proliferation index as well as the division index between the two populations of T cells indicated no significant differences in the average number of divisions that cells of each genotype underwent. Taken together, these data indicate that Jak3-dependent cytokine signals are not required for naïve T cell proliferation in vitro, but are important in maintaining maximum T cell survival.
To confirm that IL-2 signals play no role in naïve T cell proliferation in vitro, we examined the consequences of adding exogenous IL-2 or blocking IL-2 in our experiments. As shown, addition of exogenous IL-2, or alternatively, addition of IL-2 blocking antibodies, did not alter the proliferation of wild-type naïve CD4+ T cells in response to either anti-CD3 and anti-CD28 antibody stimulation or OVA323-339 peptide stimulation (Fig. 2E). In addition, blocking of Jak3-/- CD4+ T cells with IL-2 neutralizing antibodies had no effect on T cell proliferation (Fig. 2F).
Pharmacological inhibition of Jak3 impairs T cell survival, but not proliferation
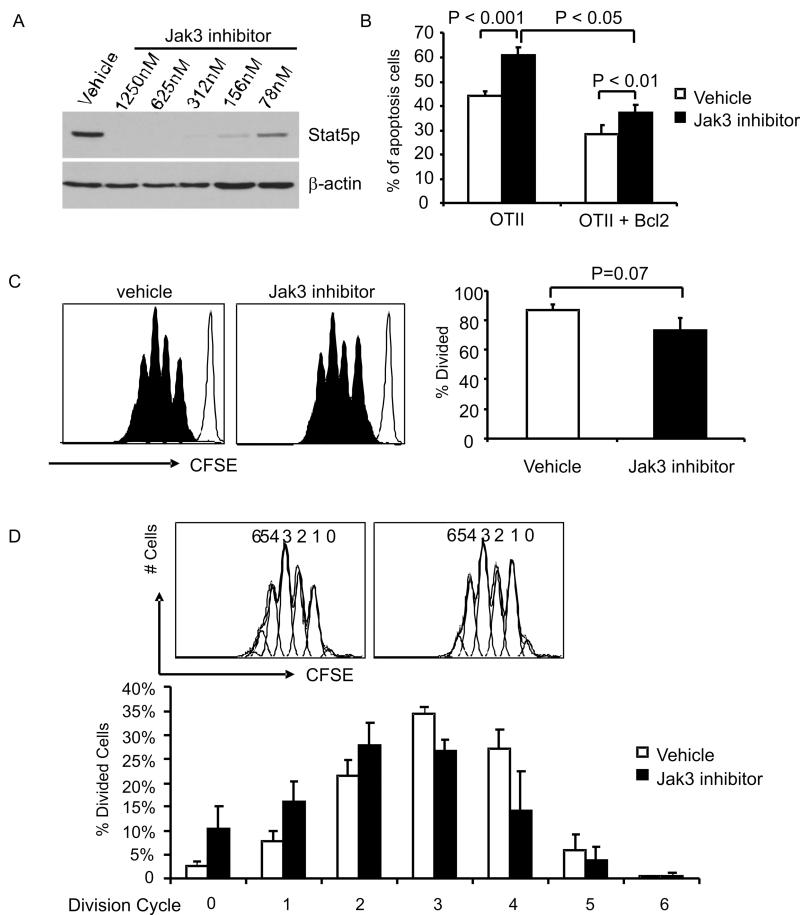
To rule out the possibility that Jak3-/- T cells are developmentally abnormal, leading to their independence from Jak3-dependent cytokine signals for T cell proliferation, we examined the responses of Jak3+/+ CD4+ T cells treated with a small molecule inhibitor of Jak3, PS078507(43). The optimal concentration of PS078507 for Jak3 inhibition in murine peripheral CD4+ T cells was determined by examining IL-2-induced Stat5 phosphorylation (Fig. 3A). The effect of PS078507 on cell survival was then assessed following 3 days of in vitro stimulation. As shown, naïve Jak3+/+ CD4+ T cells from OT-II transgenic mice displayed dramatically higher levels of apoptosis in the presence of the inhibitor, regardless of the expression of Bcl-2 (Fig. 3B). Consistent with the data shown above using Jak3-/- T cells, when cell proliferation was determined using CFSE dilution, Jak3+/+ naïve CD4+ T cells treated with PS078507 showed a comparable proportion of divided cells compared to cells treated with vehicle alone (Fig. 3C). Further, the percentage of divided cells in each successive cell division cycle also showed no significant difference compared to controls, when averaged over four independent experiments (Fig. 3D). Together, these data indicate that cell survival, but not proliferation, is regulated by Jak3-dependent cytokine signals.
FIGURE 3. CD4+ T cell survival but not proliferation is reduced by pharmacological inhibition of Jak3 activity.
A, CD4+ T cells were isolated from Jak3+/+ mice, stimulated with anti-CD3 and anti-CD28 antibodies for 2 days, rested for 4 hours, incubated with vehicle alone or serial-diluted PS078507 for 30 minutes, then stimulated with IL-2 (50 ng/ml) for 15 minutes. Cell lysates were prepared and immunoblotted for pStat5 and β-actin.
B, Purified CD4+ splenocytes from OT-II-transgenic or OT-II Bcl-2-transgenic mice were stimulated with anti-CD3 (1 μg/ml) and anti-CD28 (4 μg/ml) antibodies for 3 days in the presence of vehicle alone or PS078507 at 312 nM, stained with AnnexinV and 7-AAD, and analyzed by flow cytometry. Apoptotic cells were identified as AnnexinV+ 7-AAD+.
(C, D) Purified CD4+ splenocytes from OT-II-transgenic mice were labeled with CFSE and stimulated with anti-CD3 and anti-CD28 antibodies for 3 days in the presence of vehicle alone or PS078507 at 312 nM, and then analyzed by flow cytometry. C, Histograms show CFSE fluorescence. The bar graph indicates the percentage of T cells undergoing division in the absence or presence of PS078507; data show the mean ± SE compiled from four independent experiments. D, Histograms were analyzed using the proliferation platform of the FlowJo software to estimate the percentage of cells in each round of cell division; representative data are shown above. The graph depicts the mean ± SE using data from four independent experiments. No significant differences were seen between cells stimulated in the presence or absence of the Jak3 inhibitor.
Cell cycle regulation is independent of Jak3 signaling
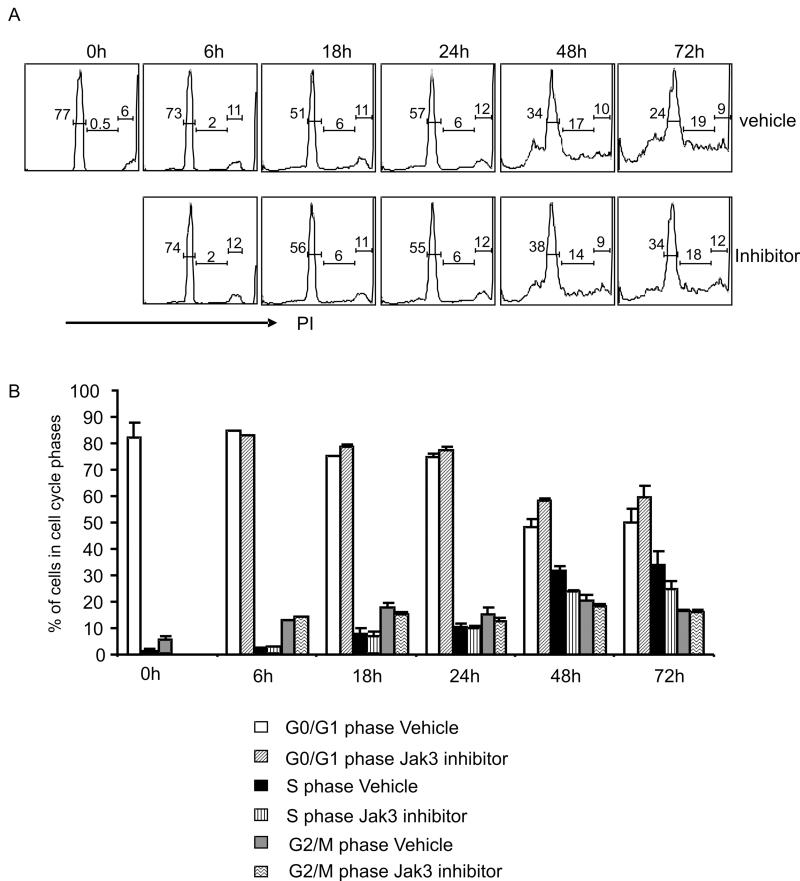
The precise function of TCR/CD28 signaling versus cytokine signaling in regulating naïve CD4+ T cell proliferation in vitro remains ambiguous. To address this issue, we examined cell cycle progression of naïve T cells stimulated in the presence or absence of the Jak3 inhibitor, PS078507. Jak3+/+ CD4+ T cells from OT-II transgenic mice were stimulated, harvested at a variety of timepoints, and analyzed for DNA content using propidium iodide (PI). The intensity of the PI signal is directly proportional to DNA content, with the rightmost peak on the histogram representing the cells in the G2/M phase, the leftmost peak showing the cells in the G0/G1 phase, and the area between the two peaks indicating the cells within the S phase (Fig. 4A). To compare cell cycle progression between samples, we calculated the percentage of live cells in each phase, and averaged the data from three independent experiments (Fig. 4B). This analysis indicated that CD4+ T cells lacking Jak3-dependent cytokine signals have indistinguishable cell cycle kinetics relative to cells receiving these cytokine signals (P > 0.05).
FIGURE 4. Cell cycle progression is not affect by Jak3 inhibition.
Purified CD4+ splenocytes from OT-II-transgenic mice were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of vehicle alone or PS078507 at 312 nM. At the indicated time points, cells were harvested, fixed, stained with propidium iodide (PI) and analyzed by flow cytometry. A, Histograms represent the DNA content of the cells after stimulation in the presence of vehicle or PS078507. B, Percentage of cells in G0/G1, S and G2/M phase is indicated for each timepoint; data represent the mean ± SE compiled from three independent experiments. No significant differences were seen between cells stimulated in the absence versus the presence of the Jak3 inhibitor.
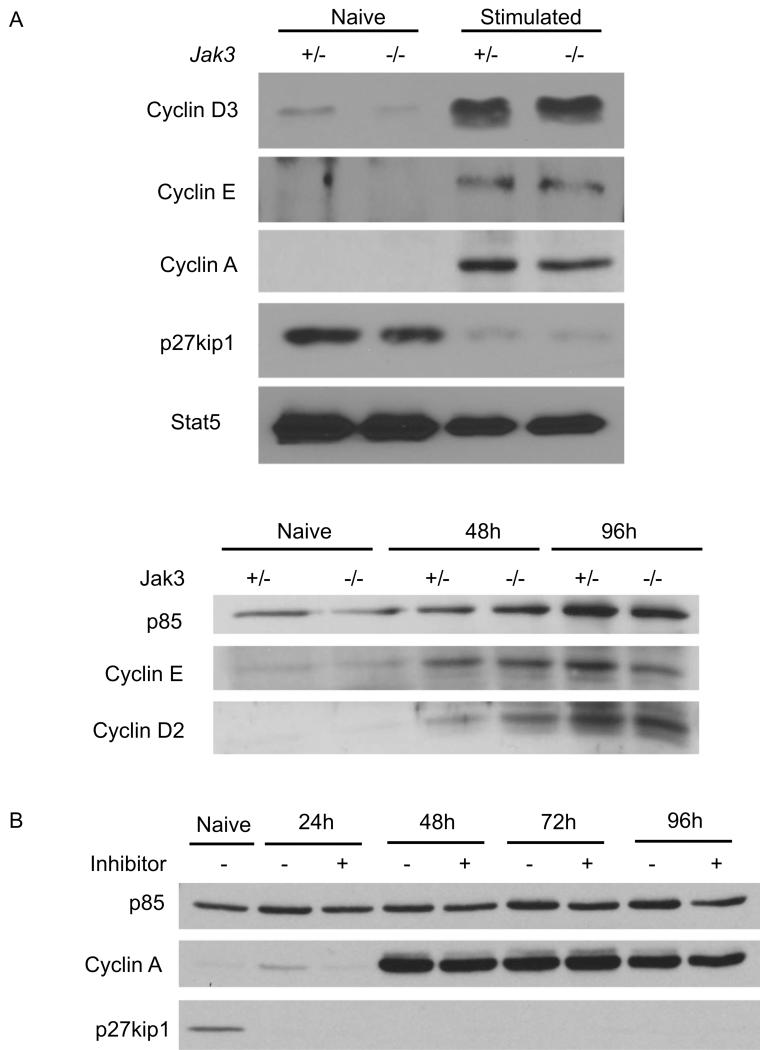
Cyclins and cyclin-dependent kinase inhibitors (CDKI) are the key regulators of cell cycle progression. The early stage of the G1 phase is regulated by D-type cyclins; in T cells, cyclin D2 is the first to be induced following activation, followed by cyclin D3 (48). To examine cell cycle progression at the molecular level, we determined whether TCR and CD28 stimulation could independently induce the expression of cyclin D3, in the absence of Jak3-dependent cytokine signals. As shown in Figure 5A, CD4 SP thymocytes from Jak3-/- OT-II Bcl-2 mice up-regulate cyclin D3 comparably to cells from control mice at day three of activation. Timepoint analysis further confirmed that the level of cyclin D2 increased at 48h and peaked at 96h after activation in both Jak3+/- and Jak3-/- OT-II Bcl-2 cells (Fig. 5A, lower panel). Whereas cyclin D2/D3 controls the entry of cells into the G1 phase, passage through the G1 restriction point into the late G1 stage requires the induction of cyclin E. When we examined cyclin E expression, we also saw no difference between Jak3+/- and Jak3-/- OT-II Bcl-2 cells at 48h or 96h post-activation (Fig. 5A).
FIGURE 5. Cell cycle proteins are regulated normally in the absence of Jak3-dependent cytokine signals.
A, Purified CD4SP thymocytes from Jak3+/- and Jak3-/- OT-II Bcl-2 mice were stimulated with anti-CD3 and anti-CD28 antibodies for 3 days (upper panel) or for indicated times (lower panel). Total cell lysates were prepared and immunoblotted for cyclin D3, cyclin D2, cyclin E, cyclin A, p27kip1, PI3-kinase p85, and total Stat5. Total Stat5 and PI3-kinase p85 are shown as loading controls.
B, Purified CD4+ splenocytes from OT-II-transgenic mice were stimulated with anti-CD3 and anti-CD28 antibodies in the presence or absence of PS078507 at 312 nM. At the indicated time points, cells were harvested and total cell lysates were prepared and innunolotted for p85 (loading control), cyclin A, and p27kip1.
To drive cell cycle progression, cyclins associate with cyclin-dependent kinases to form active holoenzymes. These holoenzymes are inhibited by CDKIs; specifically, p27kip1, which is constitutively expressed in resting naïve T cells, inhibits the activities of cyclinD2/cdk4/6 and cyclinE/cdk2 (27). Therefore, cell cycle progression depends on the down-regulation of p27kip1, in addition to the up-regulation of cyclins. When levels of p27kip1 were examined in activated Jak3+/- and Jak3-/- OT-II Bcl-2 cells, we found that p27kip1 was undetectable in both cell types by 3 days post-stimulation (Fig. 5A). Finally, we examined cyclin A, which is required for the cells to progress through S phase. Again, upregulation of cyclin A was comparable between Jak3+/- and Jak3-/- T cells (Fig. 5A). The upregulation of cyclin A and the loss of p27kip1 were confirmed with wild-type T cells stimulated in the presence of the Jak3 inhibitor, PS078507. Furthermore, an extensive timecourse indicated no differences in the kinetics of these changes with or without Jak3-dependent cytokine signals (Fig. 5B).
CD28 costimulation substitutes for cytokine signals to drive T cell proliferation
Our findings thus far indicated that Jak3-dependent cytokine signals are not required for T cell proliferation or cell cycle progression. However, it remained possible that cytokine-independent T cell proliferation requires strong TCR and/or CD28 stimulation. To examine whether Jak3-dependent cytokine signals are more essential under conditions of suboptimal T cell stimulation, we performed titration experiments in which the strength of TCR or CD28 stimulation was varied. As the anti-CD3 and anti-CD28 antibodies were titrated down, a substantial reduction in T cell proliferation was observed. The inhibition of Jak3 activity had no effect at any of the conditions tested, nor did addition of exogenous IL-2 (Fig. 6A). In a second set of experiments, the concentration of anti-CD28 antibody was fixed at 4 μg/ml and the anti-CD3 antibody was varied. As shown in figure 6A, T cell proliferation was largely unaffected by this wide range of TCR stimulation conditions, and was also independent of Jak3 signaling. Finally, we fixed the concentration of anti-CD3 antibody at 5 μg/ml and varied the concentration of anti-CD28 antibody. In the absence of the Jak3 inhibitor, CD4+ T cells proliferated robustly in response to each stimulation condition. However, when CD28 stimulation was limiting, inhibition of Jak3-dependent cytokine signaling led to a marked inhibition of T cell proliferation (Fig. 6A).
FIGURE 6. Jak3-dependent cytokine signals are required for T cell proliferation when CD28 costimulation is suboptimal.
A, Purified CD4+ splenocytes from OT-II-transgenic mice were labeled with CFSE and stimulated with indicated concentrations of anti-CD3 and anti-CD28 antibodies in the presence of vehicle alone or PS078507 at 312 nM, and then analyzed by flow cytometry. In the upper panel, recombinant IL-2 was added at 10 ng/ml. Bar graphs show the percentage of T cells that divided without or with the treatment of the inhibitor. Bar graphs represent one of the two independent experiments.
(B,C) Purified CD4+ splenocytes from Jak3+/+ OT-II-transgenic mice (B) or CD4SP thymocytes from Jak3+/- and Jak3-/- OT-II Bcl-2 mice (C), were stimulated with the indicated concentrations of anti-CD3 and anti-CD28 antibodies in the presence of vehicle alone or PS078507 at 312 nM. Cells were harvested after 3 days, total cell lysates were prepared and immunoblotted for cyclin D3, cyclin E, cyclin A and p27kip1 and total Stat5 (loading control). Freshly-isolated naïve cells are shown for comparison.
To investigate this issue at the biochemical level, we examined the expression of cell cycle regulatory proteins by immunoblotting. Consistent with the CFSE analysis of T cell proliferation, under conditions of strong CD28 stimulation (4 μg/ml), blocking of Jak3 signals by a Jak3 inhibitor did not affect the expression of cyclin D3, cyclin E, cyclin A or the degradation of p27 (Fig. 6B, lanes 2 and 3). When CD28 stimulation was limiting (0.1 μg/ml), Jak3-sufficient cells were still able to up-regulate the expression of cyclin D3, cyclin E and cyclin A, and down-regulate p27 (Fig 6B, lane 4); in contrast, inhibition of Jak3-dependent cytokine signals under these conditions resulted in a dramatic decrease in the expression cyclin D3, cyclin E and cylin A and an increase in p27 expression (Fig 6B, lane 5). Similar results can be seen when comparing Jak3+/- to Jak3-/- OT-II Bcl-2 cells (Fig 6C). These results indicate that optimal CD28 stimulation can replace Jak3-dependent cytokine signals to drive T cell cycle progression, and further, that when CD28 costimulatory signals, but not TCR signals, are limiting, T cell proliferation and cell cycle progression become cytokine-dependent.
Discussion
Generation of a pool of daughter cells from a small number of naïve T cells is an essential step for the adaptive immune response against pathogens. Following clearance of an infection, the expanded effector cells are eliminated to prevent the pathological accumulation of these potent cells. Thus, both cell proliferation and cell death must be tightly controlled to maintain the integrity of the immune system. In this report, we demonstrate that Jak3-dependent cytokine signals are not required for naïve CD4+ T cell in vitro proliferation; instead, they are critical for cell survival. Optimal TCR/CD28 signaling, in the absence of cytokine signals, induces cell cycle progression by modulating cell cycle regulators, such as cyclins and p27kip1. However, we find that under suboptimal stimulation conditions, CD28 signaling is critical in promoting IL-2-independent T cell proliferation.
T cell proliferation is generally accompanied by cell death, an essential aspect of preventing the over-expansion of immune cells. However, during a productive immune response, the proliferating T cells must be temporarily protected from cell death. The role of γc cytokines in promoting the survival of resting T cells, and in maintaining the population of naïve T cells in vivo, is well established (49). However, evidence demonstration that activated T cells can be killed by cytokine withdrawal also points out the key role of cytokine signals, in particular γc cytokine signals, in promoting the survival of activated T cells (50). This latter role of γc cytokine signals does not appear to be mediated solely through regulation of Bcl-2. Although constitutive expression of Bcl-2 leads to the accumulation of nearly normal numbers of naïve resting CD4+ T cells in Jak3-/- or γc-/- mice (43, 49), Bcl-2 expression only partially reverses the impaired survival of activated Jak3-/- T cells.
These data suggest that γc cytokine signals differentially regulate the survival of naïve versus activated CD4+ T cells. In this regard, a subgroup of Bcl-2 family proteins, the Bcl-2 homology (BH) 3-only proteins, play an essential role in activated T cell death (51, 52), leading to the conclusion that it is the ratio of BH3-only proteins (pro-apoptotic) to Bcl-2-like proteins (anti-apoptotic) that determines whether activated T cells live or die (50). In a second mechanism, the NFκB-regulator/coactivator Bcl-3 has also been implicated in activated T cell death (53, 54). Thus, Jak3-dependent cytokine signals acting in the first several days following T cell activation may prevent the upregulation of BH3-only proteins or induce the expression of Bcl-3.
While IL-2 has been shown to be important for many aspects of the immune response, including Th1, Th2, and Th17 differentiation (19, 43, 55), regulatory T cell development (56, 57), as well as CD4 and CD8 cell memory generation and maintenance (58-61), the issue of whether IL-2 is required for T cell proliferation has been a long-standing controversy. Initial experiments characterizing IL-2 demonstrated that this cytokine functions as a T cell growth factor, leading to the conclusion that IL-2 is crucial for T cell proliferation (3-5). More recent studies clearly indicate that T cells are capable of proliferating in the absence of IL-2R signals. However, the proliferative responses of IL-2- or IL-2R-deficient cells to antigen stimulation are highly variable among experiments. Some experiments show that these responses are only 5-10% of control T cells (20, 62, 63). Other experiments demonstrate that the proliferative responses of T cells lacking IL-2 signals are 50% of control T cells, and that the replication of these cells is limited to only 2-3 rounds of cell division (23).
In contrast to these earlier findings, our studies indicate that naïve T cell proliferation occurs independently of all γc cytokine signals, including IL-2. Further, we show that T cells lacking IL-2 signals are capable of undergoing at least 7 rounds of cell division in vitro. The discrepancy between our results and those reported previously is likely due to two factors. First, the traditional method used to measure T cell proliferation is by tritiated-thymidine incorporation. As this technique cannot distinguish between reduced proliferation and increased cell death, it is likely that the proliferative responses of T cells in the absence of IL-2 were underestimated in earlier studies. Second, some previous studies were performed with mixtures of naïve T cells and activated/memory like T cells. Since activated/memory like T cells may be more dependent on IL-2 for clonal expansion, this may also have led to conclusions not entirely applicable to naïve CD4+ T cells.
Since IL-2 is not the sole cytokine secreted by activated T cells, we expanded our study to simultaneously assess the role of all γc cytokines in T cell proliferation by targeting the downstream kinase, Jak3, required for all of these receptor signaling pathways. A previous study had indicated that γc-deficient CD4+ T cells could be activated and proliferate in vivo following antigenic stimulation (17). Our results, that naïve CD4+ T cell proliferation is independent of cytokines, are consistent these in vivo findings, as well as with a recent paper from Di Santo and colleagues, who reported robust in vitro proliferation of γc-/- T cells (49). These data indicated that signaling through the TCR plus CD28 is sufficient to induce T cell proliferation. However, this latter study limited their analysis to examining CFSE profiles of T cells at day 4-6 post-activation. No examination at early timepoints after activation, no cell cycle analysis, no biochemical analysis of cell cycle regulators, and no assessment of the differential roles of TCR versus CD28 signaling were included in this former study. Thus we have extended these previous findings with a more thorough examination of cell cycle progression in the absence of Jak3-dependent cytokine signals, and have also discovered an important role for cytokines when costimulatory signals are limiting.
Past studies using IL-2 and IL-2R blocking antibodies or IL-2-deficient cells, have shown that TCR/CD28 stimulation directly promotes the upregulation of cyclins and the downregulation p27kip1. However, in these studies it was difficult to determine whether the blocking antibodies were able to completely inhibit IL-2R signaling, and in addition, activated T cells secrete other γc cytokines that may contribute to the regulation of cell cycle proteins. Therefore, we examined this issue using two approaches, each of which leads to complete inhibition of all γc cytokine signaling pathways. Our results indicate that a genetic deficiency in Jak3, or pharmacological inhibition of Jak3, have no effect on cell cycle progression following optimal stimulation of the TCR plus CD28. Upregulation of cyclin D2, cyclin E and cyclin A, as well as downregulation of P27kip1, are indistinguishable in the presence versus the absence of cytokine signaling. This conclusion was also confirmed by a timecourse of cell cycle analysis quantitating the progression of cells through each phase of the cell cycle.
When antigen-presenting cells encounter pathogenic microorganisms, they up-regulate the expression of B7 to induce T cell responses (64, 65). When T cells are stimulated under these conditions, their proliferation is γc cytokine-independent. In our studies, this cytokine-independent proliferation was observed over an extensive dynamic range of TCR signaling. However, in the course of spontaneous autoimmune diseases, such as the type I diabetes observed in nonobese diabetic (NOD) mice, CD28 costimulatory signaling has been shown to be dispensable for autoreactive T cell responses; furthermore, in this system, the lack of CD28 signaling results in the exacerbation of disease (66, 67). While this latter phenomenon results from impaired regulatory T cell function, these findings also confirm that T cell activation, expansion, and effector cell differentiation can occur in a pathophysiological setting in the absence of CD28 costimulation. As our studies reveal that CD28-independent T cell proliferation is, instead, reliant on Jak3-dependent cytokine signals, these data suggest the potential for interfering with autoimmune T cell responses by inhibition of Jak3 signaling.
Supplementary Material
Acknowledgements
We thank Dr. Maria Webb for technical advice.
Footnotes
This work was supported by NIH grants AI46564 and AI37584. Core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
References
- 1.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 2.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. The common gamma-chain for multiple cytokine receptors. Adv Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- 3.Smith KA. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Gillis S, Smith KA. In vitro generation of tumor-specific cytotoxic lymphocytes. Secondary allogeneic mixed tumor lymphocyte culture of normal murine spleen cells. J Exp Med. 1977;146:468–482. doi: 10.1084/jem.146.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 6.Depper JM, Leonard WJ, Robb RJ, Waldmann TA, Greene WC. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol. 1983;131:690–696. [PubMed] [Google Scholar]
- 7.Malek TR, Ortega G, Jakway JP, Chan C, Shevach EM. The murine IL 2 receptor. II. Monoclonal anti-IL 2 receptor antibodies as specific inhibitors of T cell function in vitro. J Immunol. 1984;133:1976–1982. [PubMed] [Google Scholar]
- 8.Gillis S, Gillis AE, Henney CS. Monoclonal antibody directed against interleukin 2. I. Inhibition of T lymphocyte mitogenesis and the in vitro differentiation of alloreactive cytolytic T cells. J Exp Med. 1981;154:983–988. doi: 10.1084/jem.154.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modiano JF, Domenico J, Szepesi A, Lucas JJ, Gelfand EW. Differential requirements for interleukin-2 distinguish the expression and activity of the cyclin-dependent kinases Cdk4 and Cdk2 in human T cells. J Biol Chem. 1994;269:32972–32978. [PubMed] [Google Scholar]
- 10.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 11.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 12.Morice WG, Wiederrecht G, Brunn GJ, Siekierka JJ, Abraham RT. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993;268:22737–22745. [PubMed] [Google Scholar]
- 13.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 14.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung DT, Morefield S, Willerford DM. Regulation of lymphoid homeostasis by IL-2 receptor signals in vivo. J Immunol. 2000;164:3527–3534. doi: 10.4049/jimmunol.164.7.3527. [DOI] [PubMed] [Google Scholar]
- 16.Yu A, Zhou J, Marten N, Bergmann CC, Mammolenti M, Levy RB, Malek TR. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170:236–242. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]
- 17.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 18.Koretzky GA, Daniele RP, Greene WC, Nowell PC. Evidence for an interleukin-independent pathway for human lymphocyte activation. Proc Natl Acad Sci U S A. 1983;80:3444–3447. doi: 10.1073/pnas.80.11.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 21.Razi-Wolf Z, Hollander GA, Reiser H. Activation of CD4+ T lymphocytes form interleukin 2-deficient mice by costimulatory B7 molecules. Proc Natl Acad Sci U S A. 1996;93:2903–2908. doi: 10.1073/pnas.93.7.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Parijs L, Biuckians A, Ibragimov A, Alt FW, Willerford DM, Abbas AK. Functional responses and apoptosis of CD25 (IL-2R alpha)-deficient T cells expressing a transgenic antigen receptor. J Immunol. 1997;158:3738–3745. [PubMed] [Google Scholar]
- 23.Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001;166:1675–1683. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- 24.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 25.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 26.Boulougouris G, McLeod JD, Patel YI, Ellwood CN, Walker LS, Sansom DM. IL-2-independent activation and proliferation in human T cells induced by CD28. J Immunol. 1999;163:1809–1816. [PubMed] [Google Scholar]
- 27.Boonen GJ, van Dijk AM, Verdonck LF, van Lier RA, Rijksen G, Medema RH. CD28 induces cell cycle progression by IL-2-independent down-regulation of p27kip1 expression in human peripheral T lymphocytes. Eur J Immunol. 1999;29:789–798. doi: 10.1002/(SICI)1521-4141(199903)29:03<789::AID-IMMU789>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Parry RV, Reif K, Smith G, Sansom DM, Hemmings BA, Ward SG. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- 29.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J Immunol. 2002;169:6178–6186. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 30.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol. 2006;176:2730–2738. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 31.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson LM, Park DS, Mora AL, Goenka S, Boothby M. Sequence motifs in IL-4R alpha mediating cell-cycle progression of primary lymphocytes. J Immunol. 2005;175:5178–5185. doi: 10.4049/jimmunol.175.8.5178. [DOI] [PubMed] [Google Scholar]
- 33.Onoda T, Rahman M, Nara H, Araki A, Makabe K, Tsumoto K, Kumagai I, Kudo T, Ishii N, Tanaka N, Sugamura K, Hayasaka K, Asao H. Human CD4+ central and effector memory T cells produce IL-21: effect on cytokine-driven proliferation of CD4+ T cell subsets. Int Immunol. 2007;19:1191–1199. doi: 10.1093/intimm/dxm090. [DOI] [PubMed] [Google Scholar]
- 34.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annual review of immunology. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 35.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nature reviews. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 36.Nelson BH, Lord JD, Greenberg PD. A membrane-proximal region of the interleukin-2 receptor gamma c chain sufficient for Jak kinase activation and induction of proliferation in T cells. Molecular and cellular biology. 1996;16:309–317. doi: 10.1128/mcb.16.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakes SA, Candotti F, Johnston JA, Chen YQ, Ryan JJ, Taylor N, Liu X, Hennighausen L, Notarangelo LD, Paul WE, Blaese RM, O’Shea JJ. Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity. 1996;5:605–615. doi: 10.1016/s1074-7613(00)80274-5. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: comparative analysis of gamma(c), Jak3, and gamma(c) and Jak3 double-deficient mice. Int Immunol. 2000;12:123–132. doi: 10.1093/intimm/12.2.123. [DOI] [PubMed] [Google Scholar]
- 39.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome biology. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 41.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Harris AW, Corcoran LM, Cory S. Bcl-2 expression promotes B- but not T-lymphoid development in scid mice. Nature. 1994;368:457–460. doi: 10.1038/368457a0. [DOI] [PubMed] [Google Scholar]
- 43.Shi M, Lin TH, Appell KC, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–773. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi M, Sekiya M, Torigoe T, Takayama S, Reed JC, Miyazaki T, Minami Y, Taniguchi T, Imai K. Interleukin-2 (IL-2) upregulates BAG-1 gene expression through serine-rich region within IL-2 receptor beta c chain. Blood. 1996;88:4118–4123. [PubMed] [Google Scholar]
- 45.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 46.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ajchenbaum F, Ando K, DeCaprio JA, Griffin JD. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- 49.Masse GX, Corcuff E, Decaluwe H, Bommhardt U, Lantz O, Buer J, Di Santo JP. gamma(c) cytokines provide multiple homeostatic signals to naive CD4(+) T cells. Eur J Immunol. 2007;37:2606–2616. doi: 10.1002/eji.200737234. [DOI] [PubMed] [Google Scholar]
- 50.Hacker G, Bauer A, Villunger A. Apoptosis in activated T cells: what are the triggers, and what the signal transducers? Cell Cycle. 2006;5:2421–2424. doi: 10.4161/cc.5.21.3397. [DOI] [PubMed] [Google Scholar]
- 51.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 52.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, Zhu Y, Kappler J, Marrack P. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 54.Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, Schmid RM, Hacker G. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci U S A. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. J. [DOI] [PubMed] [Google Scholar]
- 56.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dooms H, Abbas AK. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol Rev. 2006;211:23–38. doi: 10.1111/j.0105-2896.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 59.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 63.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 64.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994;152:5208–5219. [PubMed] [Google Scholar]
- 65.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, Fuchs E, Singh B, Thompson CB, Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 67.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.