Abstract
Context
Postpartum depression (PPD) is common and has serious implications for the mother and her newborn. A possible link between placental corticotropin-releasing hormone (pCRH) and PPD incidence has been discussed, but there is a lack of empirical evidence.
Objective
To determine whether accelerated pCRH increases throughout pregnancy are associated with PPD symptoms.
Design
Pregnant women were recruited into this longitudinal cohort study. Blood samples were obtained at 15, 19, 25, 31 and 37 weeks gestational age (GA) for assessment of pCRH, cortisol and ACTH. Depressive symptoms were assessed with a standardized questionnaire at the last four pregnancy visits and postpartum.
Setting
Subjects were recruited from two Southern California Medical Centers, and visits were conducted in university research laboratories.
Participants
100 adult women with a singleton pregnancy.
Main Outcome Measure
PPD symptoms were assessed 8.7 weeks (SD = 2.94 wks) after delivery with the Edinburgh Postnatal Depression Scale.
Results
Sixteen women developed PPD symptoms. At 25 weeks GA, pCRH was a strong predictor of PPD symptoms (R2 = .21, β = .46, p < .001), an effect that remained significant after controlling for prenatal depressive symptoms. No significant associations were found for cortisol and ACTH. Receiver Operating Characteristic curve analyses revealed that pCRH at 25 weeks GA is a useful diagnostic test (area under the curve = .78, p = .001). Sensitivity (.75) and specificity (.74) at the ideal cut-off point (56.86 pg/ml pCRH) were high. Growth curve analyses indicated that pCRH trajectories in women with PPD symptoms are significantly accelerated between 23 and 26 weeks GA.
Conclusion
There is a critical period in mid-pregnancy during which pCRH is a sensitive and specific early diagnostic test for PPD symptoms. If replicated, these results have implications for identification and treatment of pregnant women at risk of PPD.
Introduction
Mood disorders in the postpartum period range from the mild and common “postpartum blues” to much rarer incidences of severe postpartum psychosis.1 The most commonly studied postpartum mood disorder is postpartum depression (PPD) which is similar to major depressive disorder but has its onset within the first four (ICD-10)2 to six weeks (DSM-IV)3 after delivery. PPD not only influences the well-being of the new mother, but also has adverse effects on the cognitive and behavioral development of her infant.4 Reports of PPD prevalence vary widely; a recent meta-analysis estimates it at 19.2% (7.1% for major PPD alone) within the first three months postpartum.5
The high incidence and severe consequences of PPD make the identification of women at risk an important research goal. The most consistently identified risk factors include previous PPD, prior history of depression, anxiety, stress and depression during pregnancy, stressful life events, lack of social support and low self-esteem,6, 7 but these risk factors only explain a portion of the variance in the incidence of PPD. Endocrine risk factors for PPD have been identified as well, including reproductive hormone changes during pregnancy, a history of premenstrual syndrome and a history of oral contraceptive-induced mood changes.8-10
Little research interest has been directed towards the role of corticotropin-releasing hormone (CRH), a 41-amino acid neuropeptide central in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis,11 as a potential predictor of PPD.12 This is surprising, because several lines of evidence suggest the possibility that increased CRH may be one of the risk factors for PPD. First, CRH plays an important role in the etiology of depression in the non-pregnant state.13 For example, depressed patients are more likely to have an increased number of hypothalamic CRH neurons, and these neurons tend to be hyperactive.14, 15 This evidence has led to the development of a CRH hypothesis of depression, suggesting that the hyperactivity of CRH neurons and the HPA axis may trigger depressive symptoms.16
Second, pregnancy is characterized by marked changes in maternal HPA axis regulation. In the central nervous system, CRH is produced in the paraventricular nucleus of the hypothalamus and released into the median eminence, a local portal system connecting the hypothalamus with the pituitary, where CRH stimulates the release of adrenocorticotropic hormone (ACTH). ACTH binding causes the adrenal cortex to release the glucocorticoid cortisol.17 During pregnancy, CRH is also produced by the placenta and, unlike CRH of hypothalamic origin, is detectable in maternal peripheral blood.18, 19 Placental (pCRH) and hypothalamic CRH (hCRH) are similar with regard to their structure, immunoreactivity and bioactivity.20, 21 However, in contrast to the role of cortisol in the negative feedback regulation of the HPA axis, cortisol stimulates CRH production in the placenta. As a result, levels of pCRH in maternal plasma increase exponentially throughout pregnancy18, 22 and reach levels similar to hCRH in the median eminence under conditions of acute stress.23 The sudden disappearance of the placenta after delivery results in a sharp drop of pCRH levels. The postpartum period is therefore characterized by pCRH withdrawal, resulting in transient suppression of hCRH release and HPA axis dysregulation. It has been suggested that this may explain the occurrence of postpartum depressive disorders.24-29
Finally, there are marked inter-individual differences in pCRH trajectories throughout pregnancy.22 It has been demonstrated that accelerated pCRH trajectories are associated with race/ethnicity30 and preterm birth.22, 31-34 Complications such as preeclampsia,35-37 fetal growth retardation,38 and diminished umbilical artery blood flow,39 are implicated in pregnancy and have consequences for the developing infant including lower newborn physical and neuromuscular maturity40 and increased irritability.41 Because of the established association between CRH and depression, accelerated pCRH increases throughout pregnancy may also serve as a potential early marker to identify women at high risk for PPD. It is the goal of the present study to address this possibility.
Methods
Participants
One hundred pregnant women with a singleton, intrauterine pregnancy were selected from a larger sample30, 40-42 that was recruited in a longitudinal study at Cedars-Sinai Medical Center in Los Angeles, California and the University of California, Irvine Medical Center. In this study, subjects with conditions known to affect HPA axis function, subjects with drug or alcohol abuse within six months before the index pregnancy, and non-English speaking subjects were excluded from participation.
The present sample comprised the 100 women with complete data for pCRH and depressive symptoms. Average age at delivery was 31.15 years (SD = 5.29). The ethnic composition was 54% non-Hispanic White, 22% Hispanic White, 12% Asian, 7% African-American, and 5% multi-ethnic or other. Most women were married (79%), had graduated from high-school (97%), and a majority were college graduates (52%). The annual household income varied between $5,000 and over $100,000, and median income was in the $80,000 - $90,000 range. All pregnancies resulted in live-births, and 53 girls and 47 boys were delivered. Deliveries were 72% vaginal and 28% c-section. Average infant birth weight was 3,514g (SD = 469.3, range: 2,340 − 4,450), and average gestational length at term was 39.4 weeks (SD = 1.25, range: 36.57 − 42.0). Since completion of the full study was an inclusion criterion, and the last study visit occurred around 37 weeks GA, most women had full term infants (97% had a gestational length > 37 wks), and no woman delivered before 36.6 weeks gestational age (GA). Most women had no previous live-born children (61%).
Overall Procedure
Blood samples were obtained at 15.3, 19.2, 25.0, 31.0, and 36.7 weeks GA (SD = 0.92, 0.72, 0.94, 0.76, and 0.70, respectively) for assessment of pCRH, cortisol and ACTH. Depressive symptoms were assessed at the last four time points during pregnancy and again at the postpartum visit (8.7 wks, SD = 2.94). Written informed consent was obtained prior to participation from all women. This protocol was approved by the Institutional Review Boards of the participating institutions.
Hormone Measures
A 25 ml blood sample was obtained by antecubital venipuncture. Samples were drawn into chilled EDTA vacutainers and spun for 15 minutes at 2,000 g. The plasma was then decanted into polypropylene tubes containing 500 KIU/ml aprotinin (Sigma Chemical, St. Louis) and stored at −70°C until assayed.
The concentration of total CRH was determined by radio-immunoassay (RIA) employing antiserum directed at human CRH (Bachem Peninsula Laboratories, San Carlos, CA). Plasma samples (1−2 ml) were extracted with three volumes of ice-cold methanol, mixed, allowed to stand for 10 min at 4°C, and then centrifuged (20 min, 1,700 g, 4°C).43 The pellets were washed with 0.5 ml methanol, and the combined supernatants dried in a SpeedVac concentrator (Savant Instruments, Holbrook, NY). Reconstituted samples were incubated (100 μl/assay tube) with antiserum (100 μl) for 48 h at 4°C followed by an overnight incubation with 125I-CRH at 4°C. Labeled and unlabeled CRH were collected by immunoprecipitation and the aspirated pellets were quantified using a gamma counter (ICN Biomedical, Isoflex Gamma Counter). Cross-reactivity was less than 0.01 for ovine CRH, 36% for bovine CRH and non-detectable for human ACTH. Intra- and inter-assay coefficients of variance (CV) were 5% and 15%, respectively. Using this technique, our laboratory has reliably detected pCRH as early as 15 weeks GA.30, 40-42
Plasma levels of ACTH were measured by a solid phase two-site immunoradiometric assay using human ACTH antibodies with non-significant cross-reactivity with beta-endorphin and ACTH fragments, and with reported detection limits of 1.0 pg/ml (Nichols Institute Diagnostics; San Juan Capistrano, CA). Briefly, samples (200 μl) combined with ACTH labeled antibody (100 μl) and a coated bead were incubated at room temperature for 20±1 hours. The bound radiolabeled antibody complex was quantified using an ICN Biomedical Isoflex Gamma Counter. Intra- and inter-assay CVs were 4.4% and 10.8%, respectively.
Plasma cortisol levels were determined by a competitive antibody-coated tube RIA with reported sensitivity of 0.22 μg/dl (American Laboratory Products Company, Windham, NH). Plasma samples (25 μl) were incubated with 125I-labeled cortisol (500 μl) in antibody-coated tubes for 45 min in a 37°C water bath. The aspirated antibody-bound labeled tubes were counted on a gamma counter. Cross-reactivities of the cortisol assay were <5% with 11-deoxycortisol, cortisone, prednisone, and <1% with other naturally occurring steroids. Intra- and inter-assay CVs were 7% and 11%, respectively.
Concentrations of CRH, ACTH, and cortisol were interpolated from standard curves computed by a four-parameter logistics program.44
Assessment of Depressive Symptoms
Depressive symptoms were assessed four times during pregnancy with a 9-item version of the Center for Epidemiological Studies – Depression (CES-D) scale.45 On a 4-point scale, participants indicated how often they experienced a symptom during the last week. Since validation analyses show higher associations with the Structured Interview for DSM-III-R when items are rescored into a bivariate score,45 each item was scored 0 if option 0 or 1 was endorsed, and was scored 1 if option 2 or 3 was endorsed. Bivariate scores ranged between 0 and 9, with a suggested cut-off score of 4 or more. This scale has good internal consistency (K-R 20 = .87), and scores correlate highly with the original scale (r=.97).
At the postpartum visit, participants completed the 10-item Edinburgh Postnatal Depression Scale (EPDS),46 a scale specifically developed to assess postpartum depressive symptoms. Participants indicated how often they experienced a symptom in the past week on a 4-point scale. Total scores varied between 0 and 30. A cut-off score of 10 or more has been suggested by the authors of the EPDS for studies including minor depression,46 and has been confirmed in other studies.47 The scale has good reliability (split-half: .88, standardized α: .87).46
Statistical Methods
All pCRH, cortisol and ACTH levels were log transformed to reduce skewness. Pearson Product moment correlations were performed to test for associations between relevant variables. Time of day was covaried when appropriate, and in no case changed the significance of the results.
Variables that were significantly correlated with PPD symptoms were included in a stepwise linear regression model. The model fit (adjusted R2), the change in R2, and the regression coefficient β are reported. Emerging significant predictors were included in a hierarchical linear regression model to assess the unique and separate contributions of each variable. A series of ancillary analyses (t-tests, χ2-tests) revealed no evidence that sociodemographic (i.e., ethnicity, marital status, education, household income) or pregnancy-related variables (i.e., birth weight, length of gestation, infant gender, mode of delivery, parity) were significantly associated with PPD symptoms (all χ2 < 9.33, p > 0.38; all t < 1.00, p > 0.32), with the exception of maternal age, t(98) = 2.58, p = 0.01. Controlling for maternal age, however, did not change the significance of the results. At the postpartum visit, no association was found between the number of weeks since delivery and PPD symptoms, t = 1.33, p = 0.19.
The sample was then divided into women with and without PPD symptoms (Table 1 for sample characteristics). Receiver Operating Characteristic (ROC) curves were computed to assess sensitivity and specificity of relevant variables as potential diagnostic markers for PPD symptoms. For this analysis, non-log-transformed pCRH values were used to provide practical guidelines for actual pCRH cut-off scores. Areas under the ROC curve were computed to compare the usefulness of each diagnostic test. ROC area under the curve values can vary between 0.5 and 1.0, with 1.0 indicating a perfect test. Youden's index, i.e., sensitivity + (specificity − 1), was computed to obtain an optimal cut-off score. Youden's index can range between −1 and +1, with +1 indicating a perfect test.48, 49 Positive and negative predictive values (PPV, NPV) were computed to express the probability that PPD is (a) present when the test is positive (PPV) and (b) absent when the test is negative (NPV), at the optimal cut-off.
Table 1.
Sample Characteristics for Women With and Without Postpartum Depressive Symptoms
| PPD (n = 16) | no PPD (n = 84) | |
|---|---|---|
| Maternal age at deliverya | 34.19 (SD = 4.16) | 30.57 (SD = 5.30) |
| Race/ethnicity | ||
| Non Hispanic White | 56.2% | 53.6% |
| Hispanic White | 18.8% | 22.6% |
| Asian | 25.0% | 9.5% |
| African American | - | 8.3% |
| Multiethnic/Other | - | 6.0% |
| Marital status (% married) | 81.0% | 73.3% |
| Education | ||
| High School Graduates | 93.7% | 98.8% |
| College Graduates | 62.6% | 50.0% |
| Annual Household Income | $5,000 - $>100,000 | $10,000 - >$100,000 |
| Mdn in $80,000 – $90,000 range | Mdn in $70,000 – $80,000 range | |
| Infant sex (% female) | 50.0% | 53.6% |
| Mode of delivery (% c-section) | 31.3% | 27.4% |
| Birth weight (g) | 3,532 (SD = 433) | 3,510 (SD = 478) |
| Length of gestation (wks) | 39.7 (SD = 1.45) | 39.4 (SD = 1.21) |
| Parity (% nulliparous) | 62.5% | 60.7% |
Women with PPD symptoms are significantly older than women without PPD symptoms, t(98) = 2.58, p = 0.01. All other comparisons are non significant.
To estimate when differences in pCRH emerge as a predictor of PPD symptoms, multilevel modeling techniques (HLM6)50 were used. First, an unconditional means model was computed to assess how much variance in pCRH can be attributed to between-subject (99.6%) and within-subject variation (0.4%). Two unconditional growth models were then computed to assess the linear (coefficient: 18.48, SE = 1.07, p < .001) and quadratic (coefficient: 0.85, SE = .13, p < .001) effects of time (level 1 predictor) on pCRH, which explained 68.90% and 80.37% of the variance in pCRH, respectively. A comparison of the deviance scores revealed that the quadratic model fit the data significantly better than the linear model, χ2(3) = 137.04, p < .001. PPD (coded: 0 = no, 1 = yes) was then included as a level 2 predictor into a series of quadratic models, testing differences in the intercept and the instantaneous rate of change at each GA within the range of actual pCRH assessments available (12 − 39 wks GA). The error term was allowed to vary randomly in each equation.
Results
Descriptives
Consistent with earlier reports, pCRH increased significantly throughout pregnancy, F(3.4, 332.5) = 586.83, < .001, η2 = .86. Likewise, significant increases in cortisol, F(3.8, 370.3) = 160.92, p < .001, η2 = .62, and ACTH, F(3.5, 351.4) = 186.63, p < .001, η2 = .65, were observed. Depressive symptoms did not change throughout pregnancy, F(2.8, 276.5) = 1.30, n.s.
Predictors of Postpartum Depressive Symptoms
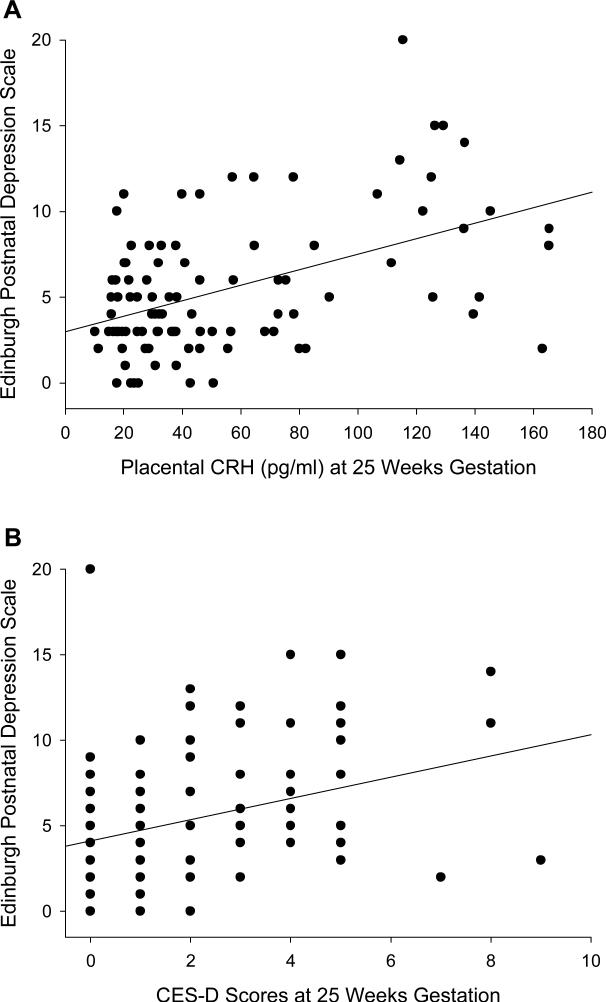
At no time point during pregnancy were any of the endocrine measures significantly associated with concurrent depressive symptoms (pCRH: r = .02 to r = .15, all p > .14; ACTH: r = −.01 to −.16, all p > .12; cortisol: r = −.002 to r = −.06, all p > .53). However, when pCRH, ACTH, cortisol and CES-D scores at each time point during pregnancy were correlated with PPD symptoms (Table 2), significant correlations emerged for pCRH at 25 and 31 weeks GA, for ACTH at 25 weeks GA, and for CES-D scores at 19, 25, 31 and 37 wks GA. The two strongest associations (pCRH and CES-D scores at 25 wks GA) are depicted in Figure 1. These correlational analyses suggest no significant association between HPA axis hormones and depressive symptoms when assessed concurrently, and provide evidence that HPA axis hormones (in mid-pregnancy) and depressive symptoms (throughout pregnancy) are significant predictors of PPD symptoms.
Table 2.
Correlation between pCRH, ACTH, Cortisol and CES-D Scores at Each Gestational Age with Postpartum Depressive Symptoms
| Correlation with Postpartum Depressive Symptoms | |||||
|---|---|---|---|---|---|
| 15 wks | 19 wks | 25 wks | 31 wks | 37 wks | |
| pCRH | .08 | .14 | .46*** | .22* | −.01 |
| ACTH | .06 | .11 | .21* | .14 | .17 |
| Cortisol | −.13 | .02 | .03 | −.06 | .04 |
| CES-Da | - | .31** | .33** | .26* | .25* |
p < .05
p < .01
p < .001
CES-D scores were not obtained at 15 weeks gestational age.
Figure 1.
Placental CRH (A) as well as CES-D scores (B) at 25 weeks gestational age and scores on the Edinburgh Postnatal Depression Scale.
To assess which variables are the strongest predictors of PPD symptoms, all variables that were significantly correlated with PPD symptoms (pCRH at 25 and 31 wks, ACTH at 25 wks, CES-D scores at 19, 25, 31 and 37 wks GA) were included in a stepwise linear regression. Elevated pCRH at 25 weeks GA emerged as the strongest predictor for PPD symptoms (Step 1; R2 = .21, β = .46, p < .001). The prediction of PPD symptoms was improved by including CES-D scores at 25 weeks GA into the model, accounting for seven percent additional variance (Step 2; βCRH = .42, βCES-D = .26, p < .001), while the influence of all other variables was not statistically significant. Because pCRH at 25 weeks GA emerged as the best predictor, a hierarchical linear regression was performed to test the unique predictive value of pCRH on PPD symptoms after controlling for CES-D scores at this time point. After CES-D scores were entered into the model in step 1, pCRH was still a significant and independent predictor of PPD symptoms (Step 2; R2 change = .17, p < .001). This further indicates that pCRH and CES-D scores explain different portions of the variance in the risk of developing PPD symptoms.
Placental CRH and CES-D Scores as Diagnostic Tests for Postpartum Depressive Symptoms
To test whether pCRH and CES-D scores at 25 weeks GA may be useful diagnostic tests for PPD symptoms, the sample was divided into women with (n = 16) and without PPD symptoms (n = 84). An ROC curve for pCRH was computed, and the area under the ROC curve was 0.78 (p = .001; 95% CI = .65 − .91), suggesting that pCRH at this time point is, in fact, a useful diagnostic test (Figure 2a). Areas under the ROC curves were lower at all other time points (15 wks: .53, n.s.; 19 wks: .62, n.s.; 31 wks: .66, p < .05; 37 wks: .61, n.s.). The optimal cut-off score for pCRH at 25 weeks GA (Youden's Index = .51) was 56.86 pg/ml. At this cut-off, 75% of cases would have been correctly identified (sensitivity; 95% CI = 47.6 − 92.6), whereas in 24% of euthymic women PPD symptoms would have been falsely predicted (1-specificity; 95% CI = 15.2 − 34.4). The PPV was 37.5% (95% CI = 21.1 − 56.3) and the NPV was 94.1% (95% CI = 85.6 − 98.3). Sensitivity and specificity for all possible cut-off scores are shown in Figure 2b.
Figure 2.
Receiver Operating Characteristic Curve (A) and Sensitivity and Specificity at Each Possible Cutpoint (B) for Placental CRH at 25 Weeks Gestation.
ROC curve analyses for CES-D scores at 25 weeks GA showed that this measure was a similarly strong predictor of PPD symptoms (ROC AUC = .77, p = .001; 95% CI = .65 − .89; Fig 3a), confirming previous research that identified depression during pregnancy as an important predictor. In contrast to the ROC analyses for pCRH, areas under the ROC curves for depressive symptoms were significant for all other time points (19 wks: 0.80, p < .001; 31 wks: .71, p < .01; 37 wks: .69, p < .05), suggesting that the predictive value of depressive symptoms is not specific to mid-pregnancy. At 25 weeks GA, the optimal CES-D cut-off score (Youden's Index = .45) was 1.5. With this cut-off (i.e., an actual score of 2 or more, since CES-D scores have no decimals), 88% (95% CI = 61.6 − 98.1) of women with PPD symptoms would have been correctly identified, however, 43% (95% CI = 32.1 − 54.1) of women without future PPD symptoms would have been misclassified. The PPV was 28.0% (95% CI = 16.2 − 42.5) and the NPV was 96.0 (95% CI = 86.3 − 99.4). Sensitivity and specificity for all possible cut-off scores are shown in Figure 3b. At the ideal cut-off points, the CES-D is the more sensitive diagnostic test (CES-D: 88% vs. pCRH: 75%) whereas pCRH is more specific (pCRH: 76% vs. CES-D: 57%) for the detection of PPD symptoms.
Figure 3.
Receiver Operating Characteristic Curve (A) and Sensitivity and Specificity at Each Possible Cutpoint (B) for CES-D Scores at 25 Weeks Gestation.
Time-Sensitive Periods for the Prediction of Postpartum Depressive Symptoms
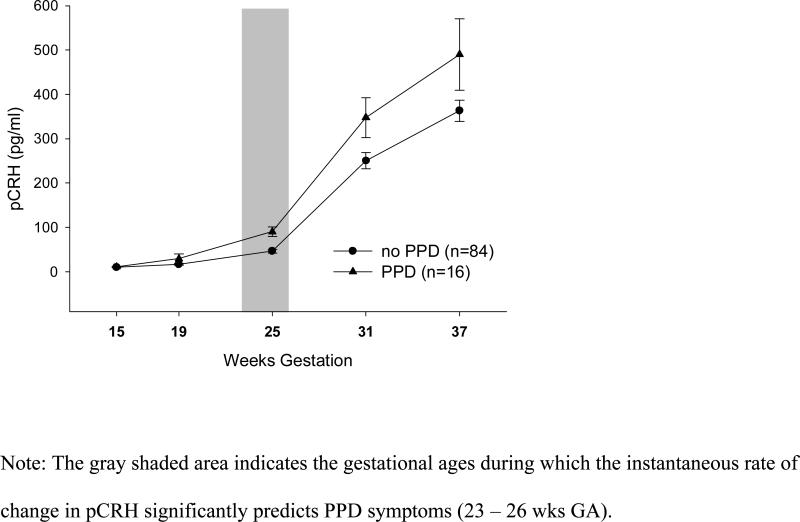
The above analyses suggest that the predictive value of pCRH for PPD symptoms may be limited to mid-pregnancy. With HLM analyses it is possible to model pCRH increases throughout pregnancy, and estimate (a) the time range during which the instantaneous rate of change in pCRH predicts PPD symptoms, and (b) the earliest time during gestation that differences in pCRH predict PPD symptoms. The growth curve analysis suggests that the instantaneous rate of change in pCRH in women with PPD symptoms is significantly accelerated between 23 and 26 weeks GA (Coefficients: 4.62 − 5.86, SE = 2.31 − 2.86, all p < .05), with a non-significant trend for weeks 22, 27 and 28 (all p < .10), compared to women without PPD symptoms (Fig 4). No differences in the instantaneous rate of change could be detected before 22 or after 28 weeks GA. Significant differences in the level of pCRH emerge at 18 weeks GA (Coefficient: 4.67, SE = 1.84, p<.01) and increase throughout the duration of pregnancy with greatest differences at 39 weeks GA (Coefficient: 38.79, SE = 4.43, p < .001). These data suggest that it is the rate of change in pCRH around 25 weeks GA, the time when differences in pCRH start to emerge, that makes some women more vulnerable to the development of PPD symptoms, and that pCRH in these women then remains at an accelerated trajectory until delivery.
Figure 4.
Placental CRH Across Gestation in Women With and Without Later Postpartum Depressive Symptoms.
Discussion
The present data are the first to suggest a sensitive period in mid-pregnancy during which pCRH, as measured in maternal plasma, is a strong and independent predictor of PPD symptoms. We propose that pCRH during this time period may serve as a sensitive and specific early diagnostic test to identify women at high risk for developing PPD symptoms. Our data also suggest that the predictive power of pCRH during this time period can be further increased by assessing mid-pregnancy depressive symptoms.
Our data indicate that pCRH is a useful marker to identify women at risk for the development of PPD symptoms. This is plausible from a neuroendocrine point of view. The postpartum period is characterized by a transient blunting of hypothalamic CRH secretion which has been implicated in the pathophysiology of PPD.25, 28 Consistent with this view, it has been shown that women who develop PPD show a more pronounced and longer lasting suppression of ACTH responses to stimulation with exogenous (ovine) CRH within the first 12 weeks postpartum, compared to women who remain euthymic.26 Our data now provide evidence that the HPA-placental system is already dysregulated during pregnancy among women at risk for PPD symptoms, such that they show accelerated pCRH increases. This is clinically relevant because the assessment of pCRH in maternal blood may provide a method to identify women at risk for PPD symptoms, months before symptoms occur.
Placental CRH in this study was a moderately sensitive and specific marker for PPD symptoms that allows for the correct identification of 75% of women with future PPD symptoms, and at the same time was characterized by a low misclassification rate (24%). The strength of pCRH as a diagnostic test for an early detection of PPD symptoms is indicated by an area under the ROC curve of 0.78 at 25 weeks GA. This association is high given that (a) a single endocrine marker was used to predict PPD symptoms, and (b) the area under the ROC curve for depressive symptoms, which are among the strongest and most consistently identified predictors of PPD in the previous literature,6, 7 is almost identical (0.77).
Our data also show that elevated pCRH, but neither cortisol nor ACTH, is a significant predictor of PPD symptoms (except for a correlation between ACTH at 25 wks GA and PPD symptoms that did not remain significant in the regression analyses). Few studies have investigated the link between cortisol or ACTH during pregnancy and PPD symptoms. Results are mixed, but the clearest evidence for an existing association comes from studies that have assessed the stimulated activity of these hormones.26, 51-53 In our study, however, baseline measures of cortisol and ACTH were used, which may explain the lack of association we found.
Remarkably, pCRH is an independent predictor of PPD symptoms. Placental CRH at 25 weeks GA has unique and significant predictive value for PPD symptoms, even after controlling for concurrent depressive symptoms. It indicates that pCRH assessments allow the identification of women at risk for developing PPD symptoms who would not be identified based on self-reports of their depressive symptoms during pregnancy. This is plausible since a hormone measure is independent of a woman's willingness to disclose feelings of depression. Thus, the combined assessment of both markers may be an ideal strategy to identify women at risk for the development of PPD symptoms.
Depressive symptoms at each time point during gestation are associated with PPD symptoms at least to a certain degree, however, the predictive value of pCRH for PPD symptoms is time-sensitive, and is maximized during mid-pregnancy (23 − 26 wks GA). The emergence of pCRH as a predictor of PPD symptoms around this time roughly coincides with a marked surge in pCRH.22, 42 Detrimental influences at the time of this initial surge may slightly accelerate the exponential pCRH trajectory, resulting in marked differences in pCRH towards the end of gestation. It is unknown what factors may precipitate the pCRH surge, but there is some evidence suggesting an association between elevated cortisol early in pregnancy and increased pCRH late in pregnancy.42
To date, only one other study has addressed the link between pCRH and PPD symptoms, and suggests a lack of association.12 In this study, pCRH was assessed only once within a wide range of gestational ages (24.6 − 37.4 wks). There are major changes in pCRH levels across pregnancy, and pCRH is characterized by significant individual differences.22 It is possible that we found an effect, because we were able to take advantage of a longitudinal study design. In addition, PPD symptoms in our study were assessed closer to parturition, around nine weeks after delivery, as compared to six months postpartum. These differences in timing may also explain, at least in part, our different results.
While pCRH in our sample emerged as a strong predictor of PPD symptoms, at no time point was it associated with concurrent depressive symptoms. There are two other studies that have investigated this association, one suggesting a positive12 and one suggesting a negative correlation54 between pCRH and concurrent depressive symptoms. These conflicting results could be explained by differences in maternal age (in one of the studies teenage pregnancies were studied54), GA at assessment, GA at delivery, and measures of depressive symptoms across studies. Future research should systematically address this question.
There is clear evidence that pCRH predicts length of gestation.22, 31-34 It is a strength of this study that our sample consists of women with full term deliveries (except for 3 women who delivered between 36.5 and 37 weeks GA), and that gestational ages at delivery were almost identical in women with and without PPD symptoms. Since pCRH predicts length of gestation, and we here show that pCRH predicts PPD symptoms in a sample of women who delivered full term, future research should investigate the link between pCRH and PPD symptoms in a sample including preterm deliveries.
There are two notable limitations to this study. First, our assessment of PPD symptoms relies on a self-report questionnaire and not on a clinical diagnosis. However, validation studies of the EPDS with the same cut-off score used in the present report document a high sensitivity (DSM-III criteria: 100%,55; Research Diagnostic Criteria 89%56) and specificity (DSM-III and Research Diagnostic Criteria: 82%55, 56) of this measure. Because of the quality of this measure, we are fairly confident that our results reflect clinically significant symptoms of depression. We acknowledge, however, the importance of replicating our findings using further diagnostic instruments. Second, although we did control for depressive symptoms in the index pregnancy, we did not have information about a life-time history of depression. Although it is reasonable to assume that the effects of current depressive symptoms would be much stronger than any additional variance explained by a history of depression, the general importance of this variable as a predictor of PPD is evident.6, 7 Future research, ideally prospective in nature, is needed to explain the importance of this variable.
The present study has important clinical and theoretical implications. If our results are replicable, it may be considered useful to implement a pCRH PPD screen into standard prenatal care. Since blood draws to screen for gestational diabetes are typically performed at 24 − 28 weeks GA,57 a PPD screen as suggested above would be relatively easy to implement. It should be noted, however, that the pCRH assay is relatively labor-intensive and is currently not readily available in many laboratories. In addition, a better understanding of the role of pCRH in the pathophysiology leading to PPD may contribute to the development of preventions targeted at this rather common disorder.
Acknowledgments
This research was supported by US PHS (NIH) research awards from the National Institute of Child Health and Human Development (HD28413 and HD51852 to Dr Sandman).
Footnotes
Portions of the data in this manuscript were presented on 7 November 2007, at the Fifth International Congress on Developmental Origins of Health and Disease, in Perth, Australia.
References
- 1.Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363(9405):303–310. doi: 10.1016/S0140-6736(03)15390-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . The international statistical classification of diseases and related health problems. 10 ed. World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4 ed. American Psychiatric Press; Washington (DC): 1994. [Google Scholar]
- 4.Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health. 2003;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- 5.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 6.Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50(5):275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44(3):234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 9.Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry. 2006;28(1):3–8. doi: 10.1016/j.genhosppsych.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.O'Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 12.Rich-Edwards JW, Mohllajee AP, Kleinman K, Hacker MR, Majzoub J, Wright RJ, Gillman MW. Elevated mid-pregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2007-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 14.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 15.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152(9):1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 16.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59(4):812–814. doi: 10.1210/jcem-59-4-812. [DOI] [PubMed] [Google Scholar]
- 19.Shibasaki T, Odagiri E, Shizume K, Ling N. Corticotropin-releasing factor-like activity in human placental extracts. J Clin Endocrinol Metab. 1982;55(2):384–386. doi: 10.1210/jcem-55-2-384. [DOI] [PubMed] [Google Scholar]
- 20.Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17(2):156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 21.Florio P, Zatelli MC, Reis FM, degli Uberti EC, Petraglia F. Corticotropin releasing hormone: a diagnostic marker for behavioral and reproductive disorders? Front Biosci. 2007;12:551–560. doi: 10.2741/2081. [DOI] [PubMed] [Google Scholar]
- 22.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 23.Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–115. discussion 115−128. [PubMed] [Google Scholar]
- 24.Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- 25.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81(5):1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- 27.Halbreich U. The association between pregnancy processes, preterm delivery, low birth weight, and postpartum depressions--the need for interdisciplinary integration. Am J Obstet Gynecol. 2005;193(4):1312–1322. doi: 10.1016/j.ajog.2005.02.103. [DOI] [PubMed] [Google Scholar]
- 28.Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G. “Reproductive” corticotropin-releasing hormone. Ann N Y Acad Sci. 2006;1092:310–318. doi: 10.1196/annals.1365.029. [DOI] [PubMed] [Google Scholar]
- 29.Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9(4):187–196. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- 30.Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28(6):1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 32.Leung TN, Chung TK, Madsen G, Lam PK, Sahota D, Smith R. Rate of rise in maternal plasma corticotrophin-releasing hormone and its relation to gestational length. Bjog. 2001;108(5):527–532. doi: 10.1111/j.1471-0528.2001.00112.x. [DOI] [PubMed] [Google Scholar]
- 33.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97(5 Pt 1):657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 34.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed I, Glynn BP, Perkins AV, Castro MG, Rowe J, Morrison E, Linton EA. Processing of procorticotropin-releasing hormone (pro-CRH): molecular forms of CRH in normal and preeclamptic pregnancy. J Clin Endocrinol Metab. 2000;85(2):755–764. doi: 10.1210/jcem.85.2.6351. [DOI] [PubMed] [Google Scholar]
- 36.Goland RS, Conwell IM, Jozak S. The effect of pre-eclampsia on human placental corticotrophin-releasing hormone content and processing. Placenta. 1995;16(4):375–382. doi: 10.1016/0143-4004(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 37.Florio P, Imperatore A, Sanseverino F, Torricelli M, Reis FM, Lowry PJ, Petraglia F. The measurement of maternal plasma corticotropin-releasing factor (CRF) and CRF-binding protein improves the early prediction of preeclampsia. J Clin Endocrinol Metab. 2004;89(9):4673–4677. doi: 10.1210/jc.2004-0186. [DOI] [PubMed] [Google Scholar]
- 38.Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab. 1993;77(5):1174–1179. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 39.Giles WB, McLean M, Davies JJ, Smith R. Abnormal umbilical artery Doppler waveforms and cord blood corticotropin-releasing hormone. Obstet Gynecol. 1996;87(1):107–111. doi: 10.1016/0029-7844(95)00338-x. [DOI] [PubMed] [Google Scholar]
- 40.Ellman LM, Dunkel Schetter C, Hobel CJ, Czics-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology. 2008;50(3):232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27(5):299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 42.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Linton EA, Perkins AV, Hagan P, Poole S, Bristow AF, Tilders F, Corder R, Wolfe CD. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: implications for direct CRH immunoassay. J Endocrinol. 1995;146(1):45–53. doi: 10.1677/joe.0.1460045. [DOI] [PubMed] [Google Scholar]
- 44.Rodbard D, Hutt D. Statistical analysis of radioimmunoassays and immunoradiometric (labeled antibody) assays. In: Rodbard D, Hutt D, editors. Proceedings, Symosium on Radioimmunoassays and Related Procedures in Medicine. Vol. 1. International Atomic Energy Agency; Vienna: 1974. pp. 165–192. [Google Scholar]
- 45.Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9(3):233–243. [Google Scholar]
- 46.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 47.Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale - implications for clinical and research practice. Arch Womens Ment Health. 2006;9:309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- 48.Hilden J, Glasziou P. Regret graphs, diagnostic uncertainty and Youden's Index. Stat Med. 1996;15(10):969–986. doi: 10.1002/(SICI)1097-0258(19960530)15:10<969::AID-SIM211>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Raudenbush SW, Bryk AS, Cheong YF, Congdon RT., Jr. HLM 5: Hierarchical linear and nonlinear modeling. Statistical software manual. Scientific Software International; Lincolnwood, IL: 2000. [Google Scholar]
- 51.Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006;68(6):931–937. doi: 10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- 52.Handley SL, Dunn TL, Waldron G, Baker JM. Tryptophan, cortisol and puerperal mood. Br J Psychiatry. 1980;136:498–508. doi: 10.1192/bjp.136.5.498. [DOI] [PubMed] [Google Scholar]
- 53.Okano T, Nomura J. Endocrine study of the maternity blues. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16(6):921–932. doi: 10.1016/0278-5846(92)90110-z. [DOI] [PubMed] [Google Scholar]
- 54.Schmeelk KH, Granger DA, Susman EJ, Chrousos GP. Maternal depression and risk for postpartum complications: Role of prenatal corticotropin-releasing hormone and interleukin-1 receptor antagonist. Behavioral Medicine. 1999;25(2):88–94. doi: 10.1080/08964289909595741. [DOI] [PubMed] [Google Scholar]
- 55.Harris B, Huckle P, Thomas R, Johns S, Fung H. The use of rating scales to identify post-natal depression. Br J Psychiatry. 1989;154:813–817. doi: 10.1192/bjp.154.6.813. [DOI] [PubMed] [Google Scholar]
- 56.Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry. 1990;157:288–290. doi: 10.1192/bjp.157.2.288. [DOI] [PubMed] [Google Scholar]
- 57.Hollander MH, Paarlberg KM, Huisjes AJ. Gestational diabetes: a review of the current literature and guidelines. Obstet Gynecol Surv. 2007;62(2):125–136. doi: 10.1097/01.ogx.0000253303.92229.59. [DOI] [PubMed] [Google Scholar]