Abstract
Leptin inhibition of bone mass accrual requires the integrity of specific hypothalamic neurons but not expression of its receptor on these neurons. The same is true for its regulation of appetite and energy expenditure. This suggests that leptin acts elsewhere in the brain to achieve these three functions. We show here that brainstem-derived serotonin (BDS) favors bone mass accrual following its binding to Htr2c receptors on ventromedial hypothalamic neurons and appetite via Htr1a and 2b receptors on arcuate neurons. Leptin inhibits these functions and increases energy expenditure because it reduces serotonin synthesis and firing of serotonergic neurons. Accordingly, while abrogating BDS synthesis corrects the bone, appetite and energy expenditure phenotypes caused by leptin deficiency, inactivation of the leptin receptor in serotonergic neurons recapitulates them fully. This study modifies the map of leptin signaling in the brain and identifies a molecular basis for the common regulation of bone and energy metabolisms.
Introduction
Leptin is an adipocyte-derived hormone that regulates a broad spectrum of homeostatic functions following its binding to the signaling form of its receptor, ObRb, present on neurons of the central nervous system (Friedman and Halaas, 1998; Spiegelman and Flier, 2001). It is widely assumed that the hypothalamus, where ObRb is expressed in several nuclei, is the main site where leptin acts in the brain (Elmquist, 2000).
One homeostatic function regulated by leptin in rodents, sheep and humans is bone remodeling, the mechanism whereby vertebrates renew their bones during adulthood (Karsenty, 2006; Pogoda et al., 2006). Leptin regulates, only through a central relay, both phases of this process, resorption and formation (Ducy et al., 2000; Shi et al., 2008). One mediator linking leptin signaling in the brain to bone remodeling is the sympathetic tone which inhibits bone formation and favors bone resorption through the β2 adrenergic receptor (Adrβ2) expressed in osteoblasts (Elefteriou et al., 2005; Takeda et al., 2002). Hence, sympathetic activity can be used as a readout of leptin regulation of bone mass.
The leptin-dependent central control of bone mass raises the question of the identity of the neurons mediating it. Chemical lesioning experiments performed in both WT and leptin-deficient (ob/ob) mice followed by leptin intracerebroventricular (ICV) infusion provided compelling evidence that, to regulate bone mass, leptin requires the integrity of neurons of the ventromedial hypothalamic (VMH) nuclei which in turn influence sympathetic activity (Takeda et al., 2002). Surprisingly however, VMH-specific deletion of ObRb does not affect bone mass (Balthasar et al., 2004). At least two interpretations of these experiments can be proposed. The first one is that they are contradicting each other and that, since chemical lesioning is less precise than cell-specific gene deletion, results obtained using the former technique are not reliable (Waddington et al., 2007). A second, more literal, interpretation views these two experiments as complementary and simply states that VMH neurons are necessary for leptin to regulate bone mass but signaling through ObRb on these neurons is not.
This latter interpretation gains further support if one looks at another function regulated centrally by leptin: appetite. Genetic inactivation of ObRb in all neurons and chemical destruction of the arcuate nuclei of hypothalamus increases appetite (Cohen et al., 2001), yet inactivation of ObRb selectively in arcuate, VMH or in both nuclei does not when mice are fed a normal diet (Balthasar et al., 2004). This inconsistency echoes the one noted above for the regulation of bone mass. Together they raise the prospect that leptin may first act elsewhere in the brain to affect synthesis of neuromediator(s) that in turn influences bone mass and energy metabolism by signaling to hypothalamic neurons.
Serotonin is an indoleamine produced in enterochromaffin cells of the duodenum and in serotonergic neurons of brainstem that does not cross the blood brain barrier (Mann et al., 1992). Thus, it is de facto a molecule with two distinct functional identities depending on its site of synthesis: a hormone when made in the gut and a neurotransmitter when made in the brain (Walther et al., 2003; Yadav et al., 2008). Although brain-derived serotonin (BDS) has many known roles (Heath and Hen, 1995) its potential function as a regulator of bone mass accrual or other homeostatic processes has not been thoroughly examined yet. This is an important question to address for several reasons. Firstly, the critical role exerted by gut-derived serotonin on bone formation (Yadav et al., 2008) raises questions regarding the role BDS may have in this process. Additionally, in invertebrates where it has been tested genetically, serotonin strongly enhances appetite (Horvitz et al., 1982; Srinivasan et al., 2008).
Here we show that, unlike leptin, BDS favors bone mass accrual and appetite, and decreases energy expenditure following its binding to distinct receptors located on two different hypothalamic nuclei. Cell-specific gene deletion of the leptin receptor show that leptin regulation of these functions occurs by inhibiting serotonin synthesis in neurons of the brainstem. These results reveal a different map of leptin action in the brain, expand the importance of BDS in physiology, they also identify a molecular basis for the common central control of bone mass and appetite.
Results
Low bone mass in mice deprived of serotonin in the brain
Serotonin synthesis is initiated by hydroxylation of tryptophan, a rate-limiting reaction performed by the enzyme tryptophan hydroxylase 2 (Tph2) in the brain (Walther et al., 2003). To determine whether BDS affects bone mass we disrupted Tph2 by inserting LacZ in its locus (Figure S1A) and first used this allele to study Tph2 pattern of expression.
The location of serotonergic neurons in the present study was defined according to Jensen et al (Jensen et al., 2008) as follows: Dorsal raphe (B4, B6 and B7), median raphe (B5, B8 and B9) and caudal raphe (B1, B2 and B3) nuclei. Together these neurons will be referred thereafter as serotonergic neurons of the brainstem in this manuscript. β-galactosidase staining of the whole brain showed that during embryonic development Tph2 expression was detected as early as E12.5 in neurons of the dorsal and median raphe nuclei in the brainstem (Figure 1A & data not shown). At E14.5, 15.5 and 18.5, β-galactosidase staining was also detected in neurons of the caudal raphe nuclei of the brainstem (Figure 1A & B) but not in other areas of the brain or in peripheral tissues (Figure S1B–D). To determine whether β-galactosidase staining is a faithful representation of Tph2 endogenous expression we performed in situ hybridization and co-immunolocalization of Tph2 and β-galactosidase. These experiments revealed a tight concordance between Tph2 expression and β-galactosidase staining (Figure 1C). After birth, Tph2 expression measured by real-time PCR was 4 orders of magnitude higher in the brainstem than in other parts of the brain or in peripheral tissues (Figure 1D). Based on these criteria, Tph2 expression is specific to serotonergic neurons of the brainstem.
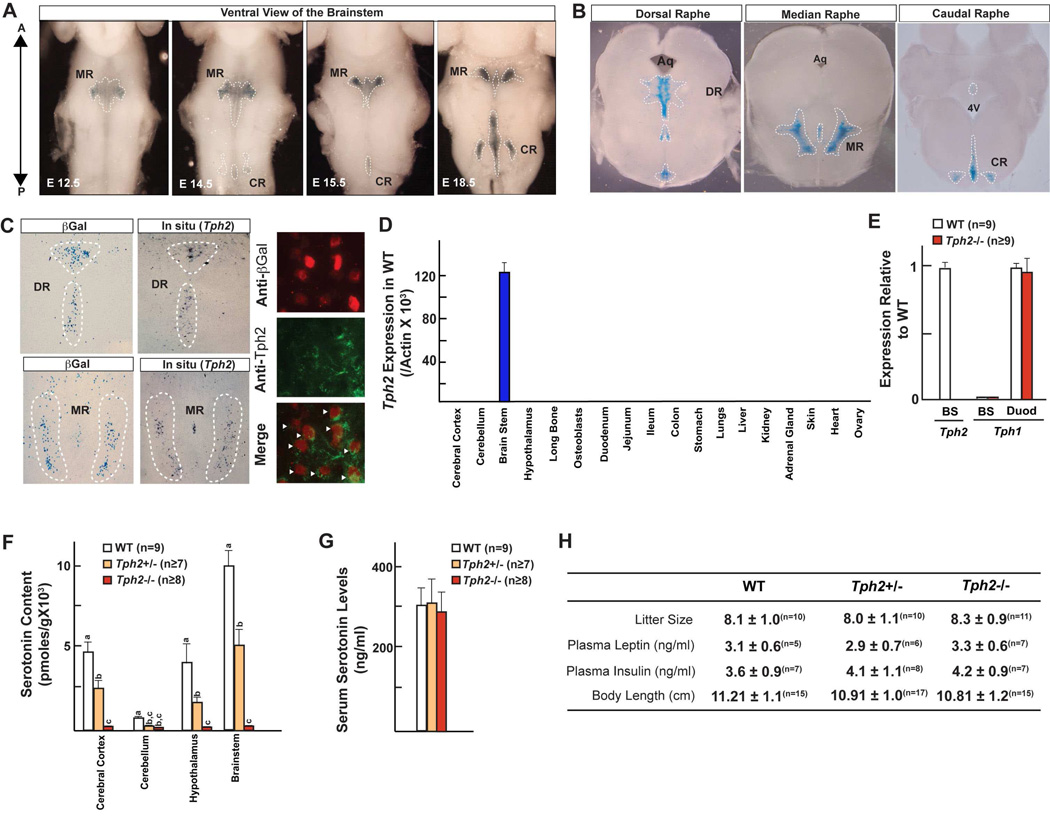
Figure 1. Generation of Tph2−/− mice.
(A) β-Galactosidase staining in the mouse brain during embryonic (E12.5–18.5) development. A: Anterior; P: Posterior.
(B) Localization of Tph2-expressing neurons in the Dorsal (DR; from Bregma −4.04 to −5.49), Median (MR; from Bregma −4.04 to −4.48) and Caudal raphe (CR; from Bregma −4.84 to −7.48) in coronal sections of a mouse brain.
(C) Tph2 expression by in situ hybridization, β-galactosidase staining and co-immunolocalization in Tph2LacZ/+ mice. Arrowheads indicate Tph2/ β-Gal double positive cells.
(D) Real-time PCR (qPCR) analysis of Tph2 expression in tissues of WT mice.
(E) qPCR analysis of Tph2 expression in brainstem (BS) and duodenum (Duod) of WT and Tph2−/− mice.
(F) HPLC analysis of serotonin levels in different regions of brain in WT, Tph2+/− and Tph2−/− mice.
(G) Serum serotonin levels in WT, Tph2+/− and Tph2−/− mice.
(H) Mean litter size, serum biochemistry and body length in WT, Tph2+/− and Tph2−/− mice (n is indicated in superscript above each value).
All panels (except F) * P < 0.05; ** P < 0.01 (Student’s t test). Error bars, SEM. Panel F (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
Tph2−/− mice were born at the expected Mendelian ratio, had a normal size and appearance and were normally fertile (Figure 1H & data not shown). The near complete absence of detectable serotonin in the brain of Tph2−/− mice verified that we had successfully inactivated this gene and was consistent with the fact that Tph1 expression in the brain was not enhanced, at least post-natally, by the Tph2 deletion (Figure 1E & F). Conversely, blood serotonin levels were normal in Tph2−/− mice (Figure 1G). Thus the Tph2−/− mouse is an animal model lacking serotonin selectively in the brain. Serum levels of leptin, insulin, corticosterone and T4 as well as body length were also normal in 3 month-old Tph2−/− animals (Figure 1H & Figure S1E).
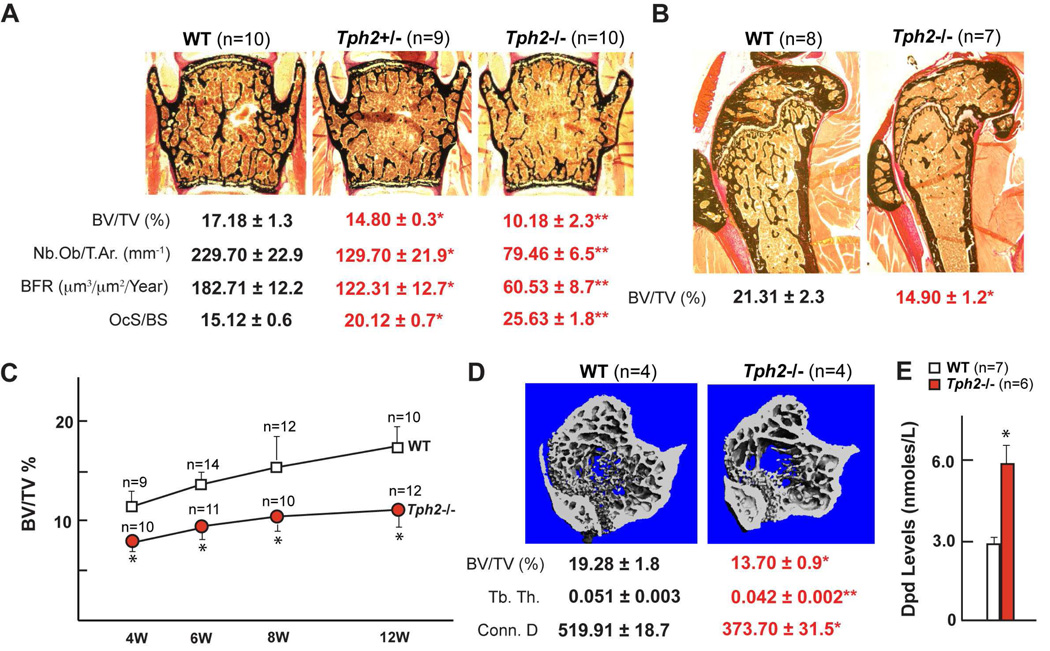
To assess the influence of BDS on bone remodeling histological, histomorphometric and microcomputed tomography (µCT) analyses of bones were performed in 4, 6 and 12 week-old wild type (WT) and Tph2−/− mice. The absence of serotonin in the brain resulted, at all time points, in a severe low bone mass phenotype affecting axial (vertebrae) and appendicular (long bones) skeleton while bone length and width were unaffected (Figures 2A–D & data not shown). Three month-old Tph2+/− mice also displayed a decrease in bone mass, albeit milder (Figure 2A). This phenotype was secondary to a decrease in bone formation parameters (osteoblast numbers and bone formation rate) and to an increase in bone resorption parameters [osteoclast surface and circulating levels of deoxypridinoline (Dpd), a degradation product of type I Collagen and a biomarker of bone resorption (Eyre et al., 1988)] (Figure 2A & E). Bone mineralization was normal in Tph2−/− mice (Figure S2). These results demonstrate that BDS is a positive and powerful regulator of bone mass accrual acting on both arms of bone remodeling. Since serotonin does not cross the blood brain barrier these observations provide a rare example of a regulation of bone mass by a neuromediator.
Figure 2. Low bone mass in Tph2−/− mice.
(A–B) Histological analysis of vertebrae (A) and long bones (B) of WT, Tph2+/− and Tph2−/− mice. Mineralized bone matrix is stained in black by von Kossa reagent. Histomorphometric parameters. BV/TV%, bone volume over trabecular volume; Nb.Ob/T.Ar., number of osteoblasts per trabecular area; BFR, bone formation rate; OcS/BS, osteoclast surface per bone surface.
(C) BV/TV% analysis in WT and Tph2−/− mice at 4, 6, 8 and 12 weeks after birth.
(D) Lower bone density in long bones of 12-week-old Tph2−/− mice by µCT analysis along with lower Tb.Th (trabecular thickness) and decreased connectivity density (Conn.D).
(E) Serum Dpd levels in WT and Tph2−/− mice.
All panels * P < 0.05; ** P < 0.01 Error bars, SEM)
The influence of brain-derived serotonin on bone mass prevails over the one of gut-derived serotonin
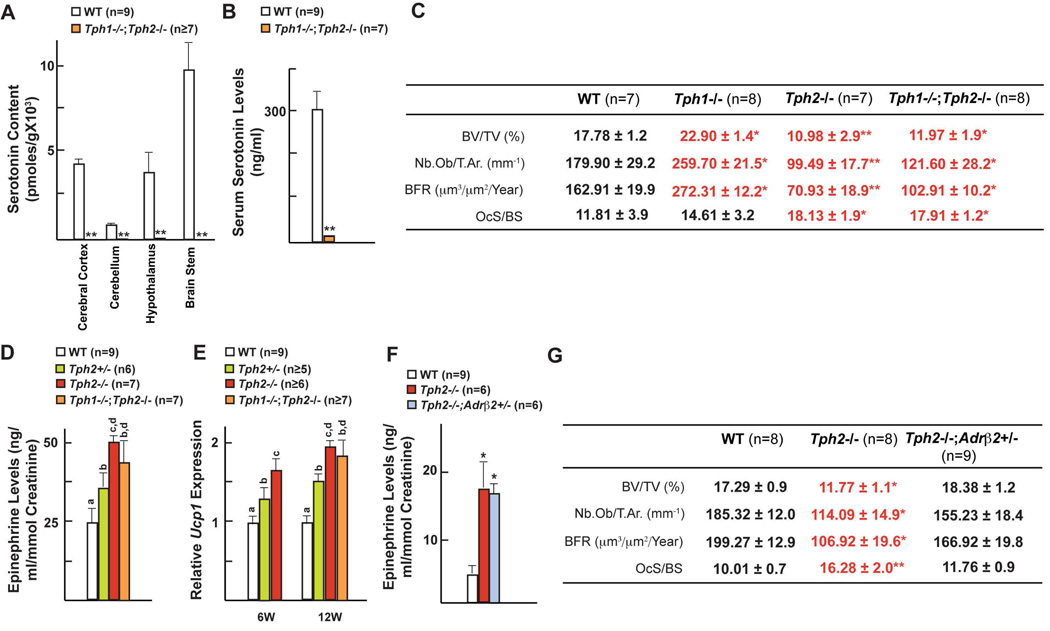
That serotonin exerts opposite influences on bone remodeling depending on its site of synthesis was unexpected. Since BDS accounts for only 5% of total serotonin we asked what was its actual contribution to the overall regulation of bone mass accrual by serotonin. To that end we generated mice unable to synthesize serotonin anywhere in their body by inactivating both Tph1 and Tph2 (Figure 3A & B). Tph1−/−;Tph2−/− mice were born at the expected Mendelian ratio and had normal size and life span (data not shown). To our surprise, like the Tph2−/− mice, Tph1−/−;Tph2−/− mice displayed a low bone mass secondary to a decrease in bone formation and to an increase in bone resorption parameters and affecting the axial and appendicular skeleton (Figure 3C & data not shown). By showing that the influence of BDS on bone remodeling prevails over the one exerted by gut-derived serotonin even though it accounts for only 5% of the total pool of serotonin this experiment underscored the importance of BDS in the regulation of bone mass and was an incentive to elucidate its mode of action.
Figure 3. Brain-derived serotonin inhibits sympathetic activity.
(A–B) HPLC analysis of serotonin levels in different regions of brain and serum serotonin levels in WT and Tph1−/−;Tph2−/− mice.
(C) Histomorphometric analysis of vertebrae of WT, Tph1−/−, Tph2−/− and Tph1−/−;Tph2−/− mice.
(D) Epinephrine levels in WT, Tph2+/−, Tph2−/− and Tph1−/−;Tph2−/− mice.
(E) qPCR analysis of Ucp1 expression in brown adipose tissue of WT, Tph2+/−, Tph2−/− and Tph1−/−;Tph2−/− mice.
(F) Epinephrine levels in the urine of WT, Tph2−/− and Tph2−/−;Adrβ2+/− mice.
(G) Histomorphometric analysis of vertebrae of WT, Tph2−/− and Tph2−/−;Adrβ2+/− mice.
All panels (except D and E) * P < 0.05; ** P < 0.01 (Student’s t test). Error bars, SEM. Panel D and E (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
Sympathetic mediation of brain-derived serotonin regulation of bone mass
The decrease in bone formation and the increase in bone resorption seen in Tph2−/− mice is the mirror image of what is observed in mice lacking the β2 adrenergic receptor (Adrβ2−/− mice) (Elefteriou et al., 2005). This feature suggested that the bone phenotype of the mice lacking serotonin in the brain could be secondary to an increase in sympathetic signaling in osteoblasts. That norepinephrine content in the brain, epinephrine elimination in the urine and Ucp1 expression in brown fat, 3 markers of the sympathetic tone, were all markedly increased in Tph2+/−, Tph2−/− and Tph1−/−;Tph2−/− mice at 6 and 12 weeks of age supported this hypothesis (Figure 3D–F & Figure S3). We also generated Tph2−/− mice in which one allele of Adrβ2 had been inactivated (Figures 3F & 3G). We removed one copy of this gene because Adrβ2 is the only adrenergic receptor expressed in osteoblasts (Takeda et al., 2002). Tph2−/−;Adrβ2+/− mice had normal bone formation and bone resorption parameters and a normal bone mass, the same was true for Tph2−/−;Adrβ2−/− mice (Figure 3G & data not shown). These results indicate that the regulation of bone mass accrual by BDS occurs by decreasing the sympathetic tone.
Brain-derived serotonin regulates bone mass through the hypothalamus
Since the sympathetic regulation of bone mass requires the integrity of the VMH neurons of the hypothalamus (Takeda et al., 2002) we next asked whether the BDS regulation of bone mass also occurs through a VMH relay.
To search for anatomical connections between Tph2-expressing and hypothalamic neurons we used the Rosa26R-Ecfp mice (Srinivas et al., 2001). In this latter mouse model the Ecfp (Enhanced Cyan fluorescent protein) reporter gene containing a floxed transcriptional blocker cassette inserted between the transcription start site and the ATG is placed downstream of the Rosa26 promoter. Thus, Ecfp can only be expressed after Cre-mediated deletion of the transcriptional blocker. We crossed Rosa26R-Ecfp mice with Sert-Cre transgenic mice that express Cre only in Tph2-expressing neurons (Zhuang et al., 2005). Ecfp immunostaining in Sert-Cre; Rosa26R-Ecfp mice showed that axons emanating from Tph2-expressing neurons of the brainstem projected to the hypothalamus (Figure 4A) and in situ hybridization performed on adjacent sections demonstrated that those axonal projections reached Sf1-expressing VMH neurons (Figure 4A). These findings were confirmed by fluorescent dextran tracing. Anterograde and retrograde labelling in Tph2+/− mice showed that VMH neurons were targeted by neuronal projections emanating from Tph2-expressing neurons in the brainstem (Figure 4B–C & Figure S4A–B). This morphological data suggesting that serotonin signals in neurons of the VMH nuclei was an incentive to search for serotonin receptor(s) on these neurons.
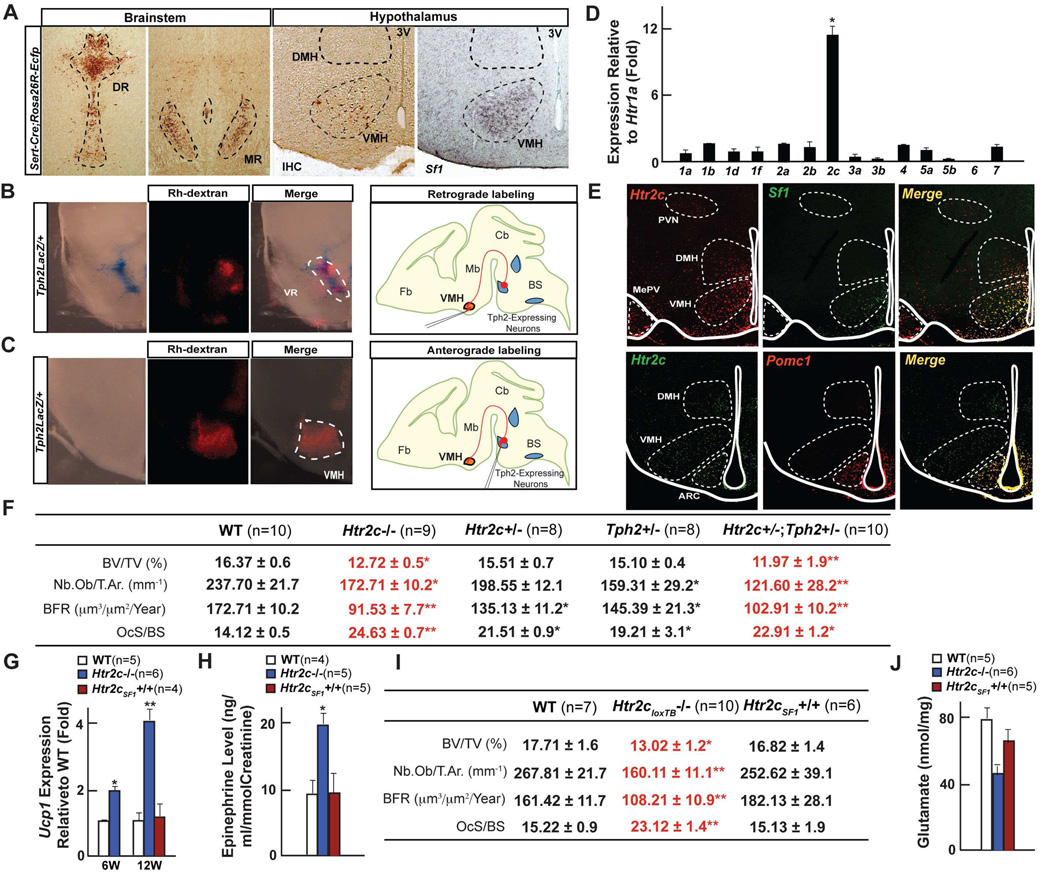
Figure 4. Serotonin promotes bone mass through Htr2c receptors in VMH.
(A–C) Analysis of axonal projections emanating from the serotonergic neurons of the brainstem. Coronal sections through the Dorsal (DR), Median (MR) raphe and ventromedial hypothalamus (VMH) nuclei from Sert-Cre;Rosa26REcfp mice identifying serotonergic neurons and their axonal projections to VMH neurons through Ecfp immunohistochemistry (A). Retrograde (B) and anterograde (C) Rhodamine dextran labeling (Rh-dextran) in Tph2LacZ/+ mice. Coronal sections through the brainstem and hypothalamus showing colocalization of β-galactosidase staining and Rh-dextran fluorescence.
(D) qPCR analysis of serotonin receptor expression in hypothalamus.
(E) Double fluorescence situ hybridization analysis of Htr2c expression with Pomc or Sf1 expression in anterior (Top panel) and posterior (Bottom panel) VMH and arcuate nuclei. The third ventricle is outlined by a white line.
(F) Histomorphometric analysis of vertebrae of WT, Htr2c−/−, Htr2c+/−, Tph2+/− and Htr2c+/− ;Tph2+/− mice.
(G–H) qPCR analysis of Ucp1 expression in brown adipose tissue (G) and epinephrine levels in urine (H) in WT, Htr2c−/− and Htr2cSF1+/+ mice.
(I) Histomorphometric analysis of vertebrae of WT, Htr2cloxTB−/− and Htr2cSF1+/+ mice.
(J) HPLC analysis of glutamate levels in hypothalamus of WT and Htr2c−/− mice
All panels (except J) * P < 0.05; ** P < 0.01 (Student’s t test). Error bars, SEM. Panel J (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
Real-time PCR analysis revealed that among the 14 serotonin receptors Htr2c was by far the most highly expressed in the hypothalamus albeit it was not the only one (Figure 4D) and double fluorescent in situ hybridization experiments showed that Htr2c was expressed in Sf1-expressing VMH and in Pomc-expressing arcuate neurons (Pasqualetti et al., 1998) (Figure 4E and Figure S4C). Moreover, Ecfp-positive neuronal arborizations originating from serotonergic neurons of the brainstem project preferentially to the anterior part of the VMH nucleus where Htr2c is expressed at its highest level (from Bregma −1.06 mm to −1.34 mm; Figure 4A, right panel). To determine the importance of serotonin signaling through Htr2c in the regulation of bone mass we first analyzed mice lacking Htr2c in all cells (Htr2c−/− mice). Since Htr2c−/− mice develop an increase in food intake and adiposity beyond 14 week of age (Tecott et al., 1995), we analyzed 6 and 12 week-old animals after verifying that at those ages appetite, energy expenditure, body weight, fat pad weights and hormonal profiles were identical in Htr2c−/− and WT mice (Figure S4D–H).
Histological analyses uncovered in both 6 and 12 week-old Htr2c−/− mice a severe low bone mass phenotype secondary to a decrease in the number of osteoblasts and bone formation rate, and to an increase in the number of osteoclasts and bone resorption parameters (Figure 4F & data not shown). Moreover, Ucp1 expression in brown fat and urinary elimination of epinephrine were both significantly higher in Htr2c−/− mice revealing the existence of a high sympathetic activity (Figures 4G–H). Thus, both in terms of bone remodeling parameters and sympathetic tone, Htr2c−/− mice are a phenocopy of Tph2−/− mice at time points when no metabolic abnormalities could be found. To establish that it is by signaling through Htr2c that BDS regulates bone mass we generated compound mutant mice lacking one allele of Tph2 and one allele of Htr2c (Tph2+/−;Htr2c+/− mice). These latter mutant mice presented at 6 and 12 weeks of age the same low bone mass/ high sympathetic activity phenotype than the Htr2c−/− and Tph2−/− mice (Figure 4F & data not shown). These results support the notion that BDS utilizes the Htr2c receptor to regulate sympathetic tone and bone mass independently of the influence it exerts through this receptor on energy metabolism.
To determine whether it is through its expression in VMH neurons that Htr2c regulates bone mass we used mutant mice harboring a loxP-flanked transcriptional blocking (loxTB) cassette inserted in the Htr2c gene (loxTB Htr2c mice) (Xu et al., 2008). In these mice disruption of Htr2c transcription can be alleviated, in a cell population of choice, by the Cre recombinase. Htr2c re-expression was targeted to VMH neurons by crossing loxTB Htr2c mice with Sf1-Cre mice (Figure S4J). Histological analyses showed that re-expression of Htr2c receptor in VMH neurons (Htr2cSF1+/+ mice) rescued entirely the bone mass phenotype observed in the absence of Htr2c (Figures 4G–I). Moreover, Ucp1 expression in brown fat and urinary elimination of epinephrine were also similar between WT and Htr2cSF1+/+ mice and levels of glutamate, an inhibitor of sympathetic tone that were suppressed in Htr2c−/− hypothalami were partially restored in Htr2cSF1+/+ hypothalami (Figures 4G, 4H & 4J). These findings echo previous observations indicating that serotonin attenuates activation of noradrenergic neurons in the locus coeruleus (Aston-Jones et al., 1991). Taken together, the results presented so far indicate that BDS acts on VMH neurons, through Htr2c, to decrease sympathetic activity and thereby favors bone mass accrual.
Leptin inhibits bone mass accrual by decreasing brain-derived serotonin synthesis
Although leptin and serotonin exert opposite influences on bone mass accrual, several features suggested that they might operate in the same pathway. For instance serotonin, like leptin, uses the sympathetic tone to regulate bone mass and, also like leptin, it requires VMH neurons integrity to achieve this function. As shown below, multiple lines of evidence indicate that it is by inhibiting BDS synthesis that leptin prevents bone mass accrual.
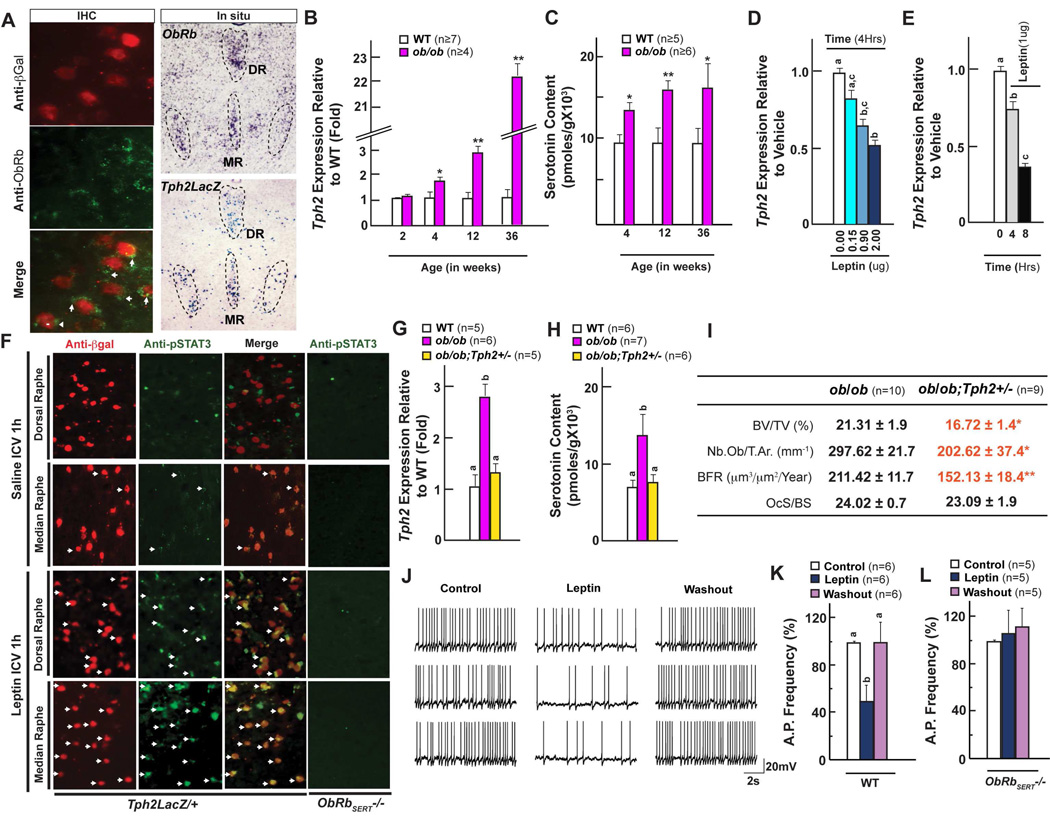
First, ObRb, the signaling form of the leptin receptor, is expressed in β-galactosidase-positive Tph2-expressing neurons (Figure 5A). Second, Tph2 expression increased steadily over time in ob/ob mice to eventually reach a level 10 fold higher than what is seen in WT mice at 6 months of age (Figure 5B) and conversely, serotonin content is significantly higher in the brainstem of ob/ob mice (Figure 5C). Third, leptin ICV infusion decreased Tph2 expression in a time- and dose-dependent manner in WT mice (Figures 5D–E). Fourth, co-immunolocalization studies revealed that the phosphorylation of Stat3, a transcription factor mediating leptin signaling, that was increased in β-galactosidase-positive serotonergic neurons of the brainstem following acute leptin ICV infusion in WT mice was dramatically reduced in ObRbSERT−/− mice (Figure 5F). In support of these correlative arguments ob/ob mice lacking one allele of Tph2 (ob/ob;Tph2+/− mice) displayed normal Tph2 expression, normal serotonin content in the brainstem, normal sympathetic tone and normal bone remodeling parameters and bone mass (Figure 5G–I & Figure S5). These data suggest a model whereby leptin regulates bone mass accrual through a double inhibitory loop. Leptin inhibits synthesis of BDS, which in turn reduces, by signaling in VMH neurons, the sympathetic tone; as a result leptin prevents bone mass accrual.
Figure 5. Leptin inhibits bone mass accrual by inhibiting brain-derived serotonin synthesis.
(A) In situ hybridization analysis and co-immunolocalization of ObRb expression in serotonergic neurons.
(B–C) qPCR analysis of Tph2 expression (B) and brainstem serotonin content (C) at different ages in WT and ob/ob female mice.
(D–E) qPCR analysis of Tph2 expression following intra-cerebroventricular (ICV) infusion of leptin at different doses (D) and at different time points (E) in WT mice.
(F) Immunohistochemical analysis of STAT3 phosphorylation in the dorsal and median raphe following leptin ICV. Arrows indicate pSTAT3/ β-Gal positive cells.
(G–H) qPCR analysis of Tph2 expression (G) and brainstem serotonin content (H) in WT, ob/ob and ob/ob;Tph2+/− mice.
(I) Histomorphometric analysis of vertebrae of ob/ob and ob/ob;Tph2+/− mice.
(J) Representative traces of action potentials recorded from WT mice before, during and after the application of leptin (100nM). R.M.P. −43.0 mV.
(K–L) Analysis of serotonergic neuron action potential (AP) frequency in brainstem slices from WT (K) and ObRbSERT−/− (L) mice.
All panels (except D, E, G, H and K) * P < 0.05; ** P < 0.01 (Student’s t test). Error bars, SEM. Panels D, E, G, H and K (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
Leptin inhibits the neuronal activity of serotonergic neurons
The mediation of peripheral hormone action on the output of the brain relies on altered circuit activity. Interaction between neuronal circuits hinges on electric properties of neurons, particularly on the generation of action potentials. Thus to test whether leptin directly alters serotonin output from brainstem neurons, we analyzed the responses of serotonin-producing cells to leptin with whole cell patch clamp recording in brain slices containing dorsal raphe (DR) (Supplemental methods). Slices were taken from WT animals and from mice lacking ObRb selectively in Tph2-expressing neurons (ObRbSERT−/− mice). Serotonergic neurons were identified according to their unique properties (long-duration action potential, activation by norepinephrine and inhibition by serotonin itself) (Liu et al., 2002). Since serotonergic neurons are usually quiescent in slices because of the loss of noradrenergic inputs, action potentials in these neurons were restored by application of alpha-1 adrenergic agonist phenylephrine (3 µM) in the bath (Liu et al., 2002). Whole cell patch recording showed that leptin significantly decreased action potential frequency in serotonergic neurons of WT mice, but not in serotonergic neurons of mice lacking ObRb in Tph2-expressing neurons (ObRbSERT−/− mice) (Figure 5J–L). These data show that leptin can alter directly activity of serotonergic neurons in the brainstem and that this effect of leptin is mediated by ObRb expressed on these neurons.
Brain-derived serotonin regulates appetite and energy expenditure
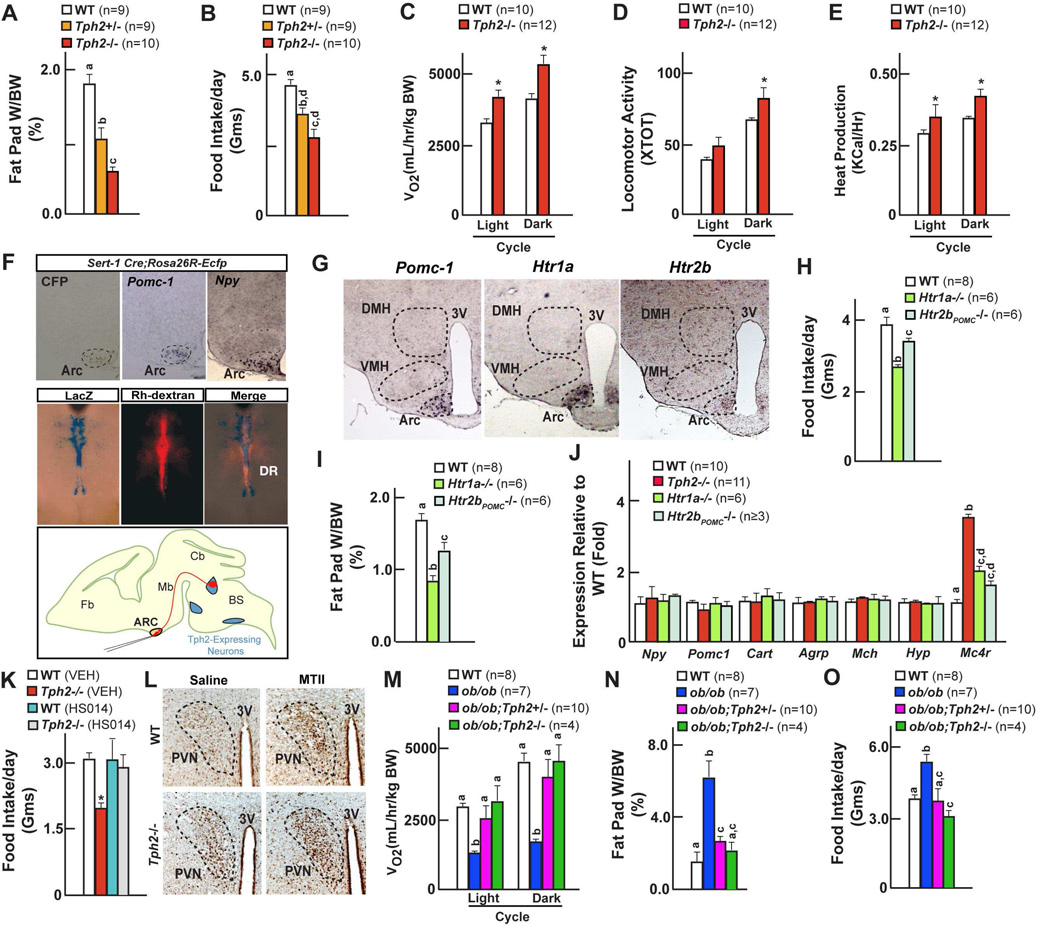
In addition to low bone mass, we consistently observed a significant decrease in fat pad weight in Tph2−/− mice (Figure 6A). This surprising observation led us to analyze in greater details energy metabolism in these mutant mice. At both 6 and 12 weeks of age there was a significant decrease in food intake in Tph2−/− (~31%) and Tph2+/− (~14%) mice compared to WT littermates along with an increase in energy expenditure (as measured by VO2, XTOT and Heat production) (Figures 6B–E). In contrast glucose metabolism, serum levels of leptin and other hormones were not affected in Tph2-deficient mice (Figure 1H, Figure S1E & Figure S6A–B).
Figure 6. Serotonin promotes food intake through Htr1a and Htr2b receptors on arcuate neurons.
(A–B) Fat pad weights (A) and food intake (B) in WT, Tph2+/− and Tph2−/− mice.
(C–E) Energy expenditure in WT and Tph2−/− mice; measured by volume of oxygen consumption (VO2) (C), locomotor activity (D) and Heat production (E).
(F) Analysis of axonal projections emanating from the serotonergic neurons. Cross of Sert-Cre and Rosa26REcfp mice identified projections reaching arcuate (Arc) nuclei in the hypothalamus through Ecfp immunohistochemistry colocalized to molecular markers of arcuate neurons (Pomc-1 and Npy) by in situ hybridization. Retrograde Rhodamine dextran labeling of the arcuate neurons identified serotonergic neurons in the brainstem in Tph2LacZ/+ mice through colocalization of β-galactosidase staining and Rh-dextran fluorescence in serotonergic neurons of the brainstem.
(G) In situ hybridization analysis of Htr1a, Htr2b in Pomc1-expressing arcuate neurons of the hypothalamus. 3V: third ventricle.
(H–I) Food intake (H) and fat pad weights (I) in WT, Htr1a−/− and Htr2bPOMC−/− mice.
(J) qPCR analysis of hypothalamic gene expression in WT, Htr1a−/− and Htr2bPOMC−/− mice.
(K) Food intake in WT, Tph2−/− mice before and after Mc4r antagonist (HS014) administration.
(L) cFos induction in paraventricular nucleus of hypothalamus in WT, Tph2−/− mice before and after acute administration Mc4r agonist (MTII). 3V: third ventricle.
(M–O) Volume of oxygen consumption (M), fat pad weight (N) and food intake (O) in WT, ob/ob, ob/ob;Tph2+/− and ob/ob;Tph2−/− mice.
All panels (except A–B, H–J and M–O) * P < 0.05; ** P < 0.01 (Student’s t test). Error bars, SEM. Panels A–B, H–J and M–O (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
This observation along with the fact that the control of appetite and energy expenditure requires the integrity of the arcuate nuclei of the hypothalamus raised the prospect that axonal projections emanating from Tph2-expressing neurons reach arcuate nuclei to regulate these functions. To address this question we crossed Rosa26-Ecfp mice with Sert-cre mice and analyzed on adjacent sections expression of Pomc-1 and Npy, two arcuate neuron-specific genes (Figure 6F & Figure S4B). This analysis verified that neurons of the arcuate nuclei were targeted by serotonergic innervations emanating from the brainstem, an observation confirmed in the Tph2+/− mice by retrograde labelling of the projections reaching the serotonergic neurons of the brainstem (Figure 6F). Among all serotonin receptors the most highly expressed in arcuate neurons were Htr1a, and, to a lower extent, Htr2b and Htr2c (Figures 6G & Figure S4C). While food intake was not affected in Htr2c−/− mice, it was significantly reduced in mice lacking Htr1a in all cells (~24% reduction) or lacking Htr2b in arcuate neurons only (~10% reduction); fat pad weight was also lower in Htr1a−/− and Htr2bPOMC−/− mice (Figure 6H, 6I & Figure S4D).
We next asked whether expression of genes expressed in hypothalamic neurons and that may mediate leptin regulation of appetite was perturbed in Tph2−/− mice. Among those tested the only gene whose expression was significantly increased in Tph2−/− mice was Mc4r (Figure 6J), a gene whose inactivation in mice and humans cause hyperphagia and obesity (Huszar et al., 1997; Yeo et al., 1998). Two experimental evidences support the notion that the appetite phenotype of the Tph2−/− mice was caused, at least in part, by an increase in melanocortin signaling. First, ICV infusion of a Mc4r antagonist (HS014) increased appetite ~50% in Tph2−/− mice (Figure 6K); second ICV infusion of a Mc4r agonist (MTII) increased c-Fos expression in neurons of the paraventricular and arcuate nuclei of both WT and Tph2−/− mice (Figure 6L & Figure S6C). Moreover, Mc4r expression was increased ~2 fold in Htr1a−/− and ~1.6 fold in Htr2bPOMC−/− mice, but was unaffected in Htr2c−/− mice (Figure 6J & data not shown). Energy expenditure was normal in Htr1a−/− and Htr2bPOMC−/− indicating that serotonin uses other receptors, yet to be identified, to regulate this function (Figure S6D–G). Taken together these results indicate that BDS regulates appetite and energy expenditure and that for the control of appetite this mediation occurs through the Htr1a and Htr2b receptors and involves melanocortin signaling.
Leptin signaling in serotonergic neurons regulates appetite, energy expenditure and bone mass
Three reasons led us to ask next whether the appetite and energy expenditure phenotypes of the ob/ob mice were serotonin-dependent. The first one is that the conjunction of a decrease in appetite and an increase in energy expenditure is the mirror image of what is seen in mice lacking leptin signaling; the second one is that leptin inhibition of serotonin synthesis in the brainstem is the mechanism used by this hormone to inhibit bone mass accrual; the third one is that no molecular mechanisms has been identified so far to explain the common control of bone mass and energy metabolism.
To test this hypothesis we first used ob/ob;Tph2+/− mice, that have a normal content of serotonin in the brain (Figure 5H). Remarkably, ob/ob;Tph2+/− mice also had appetite and energy expenditure parameters undistinguishable from WT littermates (Figures 6M–O & data not shown) suggesting that leptin must inhibit BDS synthesis in order to decrease appetite and to increase energy expenditure. Consistent with this hypothesis ob/ob mice unable to synthesize serotonin at all in the brain (ob/ob;Tph2−/−) had even a lower appetite than WT mice; as a result their fat pad weights were significantly smaller than the ones of ob/ob littermates (Figures 6M–O).
Next, to establish that serotonegic neurons of the brainstem and BDS are a critically important entry point and target of leptin in the brain, we analyzed bone mass, appetite and energy expenditure in mouse strains lacking ObRb in distinct neuronal populations in the brain (Figure S7A–B). This analysis was performed on mice fed a normal diet since leptin signaling-deficient mice develop a massive obesity on this diet. The specificity of Cre expression was verified for each mouse line by crossing them with RosaR26 mice and by in situ hybridization (Soriano, 1999) (Figure S7A–B).
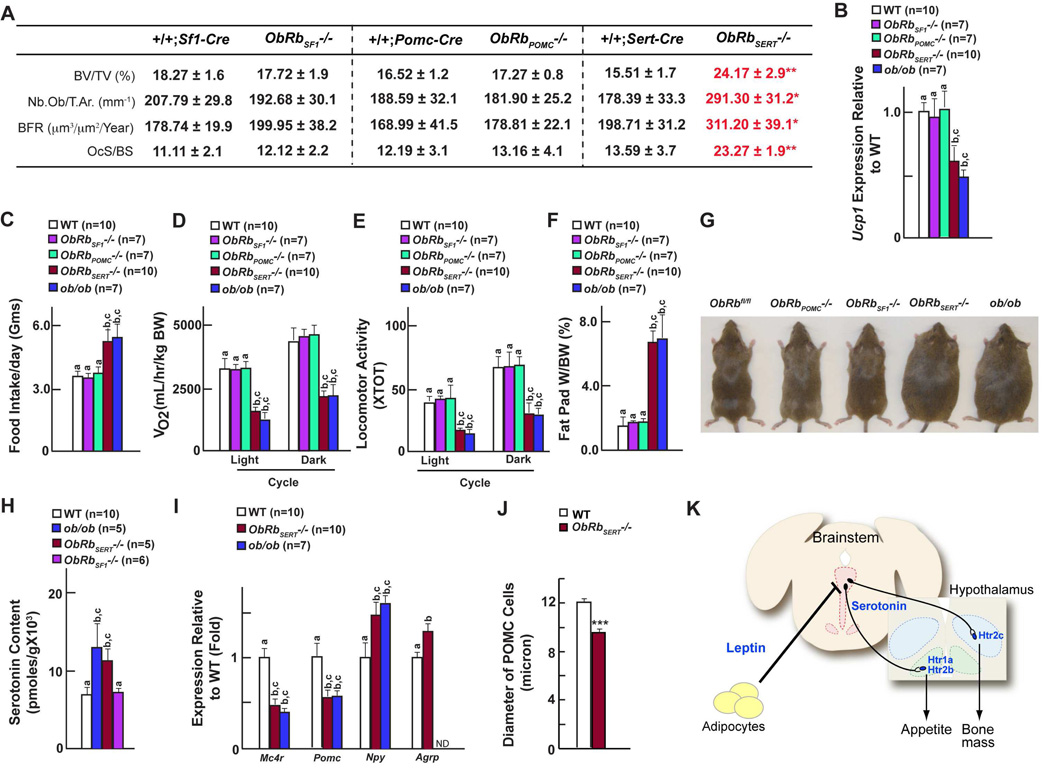
As reported previously mice lacking ObRb either in Sf1-expressing neurons of the VMH nuclei or in Pomc-expressing neurons of the arcuate nuclei had normal sympathetic activity, bone remodeling parameters and bone mass; they also had normal appetite, energy expenditure and body weight (Figures 7A–G & S7A–I) (Balthasar et al., 2004; Dhillon et al., 2006). In contrast ObRbSERT−/− mice lacking ObRb in serotonergic neurons of the brainstem developed rapidly, a low sympathetic activity, high bone mass phenotype and a similar increase in appetite as ob/ob mice; they also had low energy expenditure (Figures 7A–G). As a result ObRbSERT−/− mice, when fed a normal diet, developed an obesity phenotype of similar severity and at a similar pace than mice lacking leptin signaling (Figure 7G & Figure S7E). Serotonin in the brain of ObRbSERT−/− was elevated to the same extent as in ob/ob mice, while it was normal in the brain of ObRbSF1−/− mice (Figure 7H). Remarkably for our purpose hypothalamus gene expression analysis by real-time PCR revealed a decrease in Mc4r and Pomc expression, and an increase in Npy and Agrp expression in ObRbSERT−/− mice that is of similar severity to the one observed in ob/ob mice. (Figure 7I).
Figure 7. ObRb expression in serotonergic neurons is necessary and sufficient for leptin regulation of bone mass accrual, appetite and energy expenditure.
(A) Histomorphometric analysis (vertebrae) of +/+;Sf1-Cre, ObRbSF1−/−, +/+;Pomc1-Cre, ObRbPOMC−/−, +/+;Sert-Cre and ObRbSERT−/− mice.
(B) qPCR analysis of Ucp1 expression in brown adipose tissue in WT, ObRbSF1−/−, ObRbPOMC−/− and ObRbSERT−/− mice. WT refers to +/+;Sf1-Cre, +/+;Pomc1-Cre or +/+;Sert-Cre.
(C–F) Food intake (C) volume of oxygen consumption (D), locomoter activity (E) and fat pad weights (F) in WT, ObRbSF1−/−, ObRbPOMC−/− and ObRbSERT−/− mice.
(G) Representative photomicrographs of WT, ObRbSF1−/−, ObRbPOMC−/− and ObRbSERT−/− mice.
(H) Brainstem serotonin content in WT, ob/ob, ObRbSERT−/− and ObRbSF1−/− mice.
(I) qPCR analysis in the hypothalamus in WT, ObRbSERT−/− and ob/ob mice.
(J) Diameter of Pomc-expressing cells in WT and ObRbSERT−/− mice.
(K) Model of the leptin-dependent regulation of bone mass and appetite. Leptin inhibits release of brainstem-derived serotonin, which favors bone mass accrual and appetite. Adipocytes are in yellow; serotonergic neurons are in pink; VMH is in blue and arcuate is in green.
All panels (except B–F and H–I) * P < 0.05; ** P < 0.01, *** P < 0.001 (Student’s t test). Error bars, SEM. Panels B–F and H–I (One way ANOVA, Newman-Keuls test); Different letters on 2 or more bars indicate significant differences between the respective groups (P < 0.05).
ObRb deletion in Tph2-expressing neurons also had an organizational effect on Pomc-expressing neurons of the arcuate nuclei. Indeed, the average diameter of Pomc-expressing neurons in ObRbSERT−/− mice (n=42) was significantly lower than in WT mice (Figure 7J). The lower POMC perikaryal diameter of ob/ob mice is associated with a ~50% decrease in the number of perikaryal synapse density of POMC neurons (Pinto et al., 2004). Altered synaptic input organization of POMC neurons was also detected in ObRbSERT−/− mice (14.76±1.3 vs 27.31±2.03 synapses per 100 micron perikaryal membrane in ObRbSERT−/− and WT mice respectively). Thus, it is likely that the ob/ob phenotype of POMC neurons is determined, at least in part, by leptin signaling in serotonergic neurons of the brainstem.
Discussion
The results presented here demonstrate that in order to regulate bone mass accrual, appetite and energy expenditure leptin needs to inhibit the electrical activity and serotonin synthesis in Tph2-expressing neurons of the brainstem (Figure 7K). These results modify the map of leptin signaling in the brain and indicate that the serotonergic neuronal circuitry exerts a more fundamental influence on several homeostatic functions than previously thought. Moreover, they identify BDS as the long sought-after molecular basis for the common control of bone mass and energy metabolism.
Brain-derived serotonin regulation of bone mass
This effort to complete our understanding of the regulation of bone mass by serotonin led to two unexpected results. The first one is that, depending on its site of synthesis, serotonin regulates bone mass accrual in opposite directions: it inhibits it when synthesized in the duodenum and favors it when acting as a neurotransmitter. To our knowledge this is the first example of a molecule exerting different influences on bone remodeling depending on its site of synthesis. The central function of serotonin is mediated through the Htr2c receptor expressed in VMH neurons. Htr2c−/− mice are markedly osteopenic before any metabolic modification is detectable, indicating that serotonin regulation of bone mass occurs independently of its effects, through Htr2c, on energy metabolism. Given what is known about the molecular signaling of serotonin in osteoblasts (Yadav et al., 2008), future studies are needed to determine whether BDS recruits the same transcription factor(s) in VMH neurons.
The second surprising observation is that although it accounts only for a rather small portion of the total pool of serotonin in the body (~5%) BDS influence on bone remodeling is dominant over that exerted by gut-derived serotonin. Since BDS synthesis is regulated by leptin these results infer that leptin regulation of bone mass is more important than the one exerted by gut-derived serotonin (Yadav et al., 2008). This observation along with the fact that leptin appears during evolution with a bony skeleton underscores the importance of the regulation of bone remodeling in the panoply of leptin’s function; it also predicts that using leptin as a treatment for obesity would favor appearance of osteoporosis.
Patients taking chronically serotonin reuptake inhibitors (SSRIs) have an increased risk of osteoporotic fractures (Richards et al., 2007). Consistent with this observation an animal model reproducing this chronic use of SSRIs, namely mice lacking 5-hydroxytryptamine transporter (5HTT), a molecule responsible for serotonin reuptake by cells, also develop a low bone mass phenotype (Warden et al., 2005). Surprisingly however, this low bone mass phenotype cannot be ascribed to an increase in circulating serotonin levels i.e. to gut-derived serotonin, since serotonin is undetectable in the plasma of these mice (data not shown). The low bone mass of 5Htt−/− mice further supports the notion that BDS exerts a dominant role in the regulation of bone mass.
Serotonin regulation of appetite and energy expenditure
The study of BDS functions led to other unexpected findings beyond the control of bone mass. Indeed the deletion of Tph2 shows that BDS is a powerful orexigenic molecule in vertebrates. This function of BDS is similar to the function of serotonin in invertebrates (Nonogaki et al., 1998; Srinivasan et al., 2008). Genetic analysis showed that the orexigenic effect of serotonin occurs through Htr1a and Htr2b receptors, in a Mc4r-dependent manner (Heisler et al., 2002; Lam et al., 2008). This role of Htr1a in the regulation of appetite is consistent with the orexigenic action of Htr1a agonists (Gilbert et al., 1988; Neill and Cooper, 1988). The demonstration that it is through its expression in arcuate neurons that Htr1a regulates appetite will need generation of a cell-specific deletion of this gene.
The fact that removal of the ligand and addition of a pharmacological agonist of a receptor result in the same phenotype, i.e. anorexia, could have several explanations (Vickers et al., 1999). For instance, it is possible that signaling through some serotonin receptors may antagonize signaling through others. Likewise, it should be noted that in most settings the effect of pharmacologic agents are acute while by definition the effect of gene deletion is chronic. Lastly, it is also possible that different serotonin receptors regulate differently food intake possibly even on the same neuron (Xu et al., 2008).
Brain-derived serotonin as a target of leptin
Several lines of evidence suggested initially that leptin regulates bone mass, appetite and energy expenditure following binding to its receptors located on hypothalamic neurons. Chief among those are the facts that the hypothalamus is a known regulator of most homeostatic functions and that ObRb is highly expressed in various hypothalamic neuronal populations (Elmquist et al., 2005). Surprisingly however, deletion of ObRb in those hypothalamic neuronal populations did not affect any of these three functions (Balthasar et al., 2004; Dhillon et al., 2006). These latter findings suggested the following hypothesis: leptin would act in other parts of the brain where its receptor is expressed to affect synthesis of neurotransmitter(s), which would then act in hypothalamic neurons. That serotonin is the initial target in the brain of leptin regulation of bone mass accrual was an incentive to test whether it could also be implicated in the leptin regulation of appetite and energy expenditure.
We show here, through cell-specific gene deletion of Tph2 or the leptin receptor, that BDS enhances appetite and decreases energy expenditure and that leptin regulates these functions by inhibiting BDS synthesis. Thus leptin does use the same mechanism to regulate bone mass accrual, appetite and energy expenditure. We remain aware however, that these data do not exclude formally the possibility that leptin acts also on hypothalamic neurons at a level not detectable using cell-specific gene deletion experiments. For instance, our study does not rule out an involvement of Agrp-expressing neurons in the control of various aspects of energy metabolism (van de Wall et al., 2008). This is especially true since Agrp expression is similarly increased in ObRbSERT−/− and ob/ob mice. By identifying brainstem serotonergic neurons as an initial target of leptin this study provides a cellular and molecular explanation for the apparent contradiction between the fact that chemical lesioning of hypothalamic neurons hampers leptin signaling while inactivating ObRb in these neurons does not. A question raised by our work is to know what are the functions of the leptin receptor expressed in hypothalamic neurons? At the present time it seems that ObRb expression on these neurons is mainly needed for the regulation of insulin secretion and glucose metabolism (Coppari et al., 2005; Hinoi et al., 2008; van de Wall et al., 2008) a function not affected by serotonin or leptin signaling in serotonergic neurons (Figure S1). Indeed it is important to underscore that serotonin is not implicated in all functions of leptin, for instance ob/ob;Tph2+/−, as ob/ob mice, are sterile.
Co-regulation of bone remodeling and energy metabolism
Why would bone remodeling and energy metabolism need to be co-regulated in the first place? To answer this question, one needs to look at what was the original purpose of bone remodeling earlier during evolution. Through its ability to constantly renew bone the original function of bone remodeling was to repair micro and macro damages, i.e. fractures of bones. This function was, early on, absolutely necessary to maintain mobility and therefore to assure survival. In addition, bone remodeling is characterized by two opposing processes: destruction followed by de novo formation; these two cellular events are costly in terms of energy; even more so if one takes into account that they occur simultaneously in multiple locations. Thus, for bone remodeling to occur there must be a constant supply of energy channeled to osteoclasts and osteoblasts. This view of bone remodeling predicts that there should be one or several hormones, appearing during evolution with this function, and regulating it and energy metabolism. To date leptin is the only known hormone fulfilling all these criteria. This view of bone remodeling also implies that the metabolic importance of the skeleton that begins to be unraveled (Lee et al., 2007) has not been fully characterized yet.
Experimental Procedures
Mice Generation
Tph2-LacZ mice were generated by embryonic stem cell manipulations following standard protocols to obtain Tph2+/− mice. Tph2+/− mice were intercrossed to obtain the WT, Tph2+/− and Tph2−/− mice for analysis. Generation of Tph1−/−, Htr2c−/−, loxTB Htr2c, Htr1a−/−, ObRbfl/fl, Htr2bfl/fl, Sf1-Cre and Sert-Cre mice was previously reported (Balthasar et al., 2004; Dhillon et al., 2006; Klemenhagen et al., 2006; Tecott et al., 1995; van de Wall et al., 2008; Xu et al., 2008; Yadav et al., 2008; Zhuang et al., 2005). WT, Pomc1-Cre and ob/ob mice were obtained from The Jackson Laboratory.
Histological procedures, immunohistochemistry, in situ hybridization, axonal tracing and microcomputed tomography (µCT) analysis
Sections containing dorsal raphe were from bregma −4.04 to −5.40; median raphe from −4.04 to − 4.48; caudal raphe from −4.84 to −7.48; arcuate from −1.22 to −2.80; VMH from −1.06 to −2.06 and PVN from −0.58 to −1.22 according to Franklin and Paxinos mouse brain atlas. Immunohistochemistry was performed on paraffin-embedded specimens sectioned at 6 µm according to standard protocols. LacZ staining was performed on whole brain and coronal sections obtained from the Tph2+/− mice following standard procedures. In situ hybridization on brain sections was performed as described (Oury et al., 2006). Ex vivo axonal tracing was performed using Rhodamine-conjugated dextrans (Molecular Probes, Eugene, Oregonaxonal; See supplemental methods for details). Bone histomorphometric analyses were performed on undecalcified sections using the Osteomeasure analysis system (Osteometrics, Atlanta). Trabecular bone architecture of proximal tibia was assessed using a µCT system (VivaCT 40, SCANCO Medical AG, Switzerland) as described (Shi et al., 2008). Six to 12 animals were analyzed for each group.
Bioassays
Serotonin levels in the brain and serum were quantified as described (Yadav et al., 2008). Serum level of total deoxypyridinoline (DPD) cross-links was measured using the Metra tDPD kit (Quidel corp. San Diego, CA). Urinary elimination of catecholamines was measured in acidified spot urine samples by EIA (Bi-CAT, Alpco Diagnostics, Salem, NH) and creatinine (Metra creatinine kit, Quidel corp. San Diego, CA) used to standardize between urine samples.
Molecular studies
RNA isolation, cDNA preparation and qPCR analysis was carried out following standard protocols (See supplemental methods). Genotypes of all the mice were determined by PCR. All primer sequences for genotyping and DNA probes for southern hybridization are available upon request.
Electrophysiology, food intake and energy expenditure measurements
Brain slice preparation and electrophysiological recording were performed as described [(Liu et al., 2002; Rao et al., 2007) & supplemental methods]. Food intake was measured using metabolic cages (Nalgene, Rochester, NY) and energy expenditure by indirect calorimetry method as described [(Shi et al., 2008) & supplemental methods].
Statistical analyses
Statistical significance was assessed by Student’s t test or a one way ANOVA followed by Newman-Keuls test for comparison between more than 2 groups. P<0.05 was considered significant. Different letters indicate significant differences among groups.
Supplementary Material
Acknowledgement
We thank G. Ren for mouse genotyping, Yung-Yu Huang for HPLC analysis, X. Sherry Liu for MicroCT analysis, Marya Shanabrough for electrophysiological recordings and Dr. Patricia Ducy for critical reading of the manuscript. Special thanks to Drs. Bradford B. Lowell, Joel K. Elmquist, Streamson Chua Jr., Yong Xu, Harveen Dhillon and J.M. Zigman for generously providing loxTB Htr2c, ObRb floxed and Sf1-Cre mice. This work was supported by grants from the NIH (G.K.), a Gideon and Sevgi Rodan fellowship from IBMS (V.K.Y.), HFSP (F.O.), FRM (F.O.), foundation Bettencourt Schueller (F.O. and C.C.) and the Philippe foundation (F.O. and C.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Akaoka H, Charlety P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991;11:760–769. doi: 10.1523/JNEUROSCI.11-03-00760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr., Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr., et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Anatomic basis of leptin action in the hypothalamus. Front Horm Res. 2000;26:21–41. doi: 10.1159/000061020. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinum residues. Biochem. 1988;252:494–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gilbert F, Dourish CT, Brazell C, McClue S, Stahl SM. Relationship of increased food intake and plasma ACTH levels to 5-HT1A receptor activation in rats. Psychoneuroendocrinology. 1988;13:471–478. doi: 10.1016/0306-4530(88)90032-7. [DOI] [PubMed] [Google Scholar]
- Heath MJ, Hen R. Serotonin receptors. Genetic insights into serotonin function. Curr Biol. 1995;5:997–999. doi: 10.1016/s0960-9822(95)00199-0. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr., Chua SC, Jr., Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: Leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo M, Mieczkowski T, Myers JE, Stanley M. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry. 1992;49:442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- Neill JC, Cooper SJ. MDL 72832, a selective 5-HT1A receptor ligand, stereospecifically increases food intake. Eur J Pharmacol. 1988;151:329–332. doi: 10.1016/0014-2999(88)90818-7. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Marazziti D, Castagna M, Nardi I. Distribution of 5-HT2c and 5-ht5a receptor mRNA in human brain. Ann N Y Acad Sci. 1998;861:245. doi: 10.1111/j.1749-6632.1998.tb10202.x. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res. 2006;21:1591–1599. doi: 10.1359/jbmr.060709. [DOI] [PubMed] [Google Scholar]
- Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr., Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression ith the ROSA26 Cre reporter strain. Nat Genet. 1999;14:670–689. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Sadegh L, Elle IC, Christensen AG, Faergeman NJ, Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 2008;7:533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Buckley SM, David AL, Peebles DM, Rodeck CH, Coutelle C. Fetal gene transfer. Curr Opin Mol Ther. 2007;9:432–438. [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.