Abstract
Osteoarthritis (OA) is a multifactorial disease. Different risk factors have been identified such as aging and obesity and different models have been used to study the impact of obesity and overweight in this pathology.
The field the more studied is in vitro cartilage submitted to mechanical stresses. Four different stresses can be applied on this tissue: shear stress, loading, tensile stress (stretching) and hydrostatic pressure. The signal transduction to the chondrocyte and to the nucleus of the cell is a large field of investigation named mechano-transduction.
The response of cartilage depends on quality of subchondral bone as well. So, more and more teams are studying the impact of mechanical stresses on bone, mainly by stretching osteoblasts or by submitting them to a fluid shear stress. Recently, a new model of bone compression has been set up, with osteoblasts in their own extracellular matrix.
Finally the third field studied is the role of adipokines, mediators playing a key role in obesity, on the aetiology of OA. Adipokines like leptin, resistin, adiponectin and visfatin, seems to play a pro-inflammatory role in arthritis.
Studying the role of obesity in OA could be more complex than expected. The link between OA and obesity may not simply be due to high mechanical stresses applied on the tissues, but soluble mediators may play an important role in the onset of OA in obese patients.
Keywords: Osteoarthritis, Obesity, Cartilage, Bone, Mechanical stress, Adipokines
1. Introduction
Osteoarthritis (OA) is a multifactorial disease that leads to a complete alteration of articular cartilage and other articular tissues such as subchondral bone. Over the last decade, the impact of certain risk factors such as aging and overweight in OA has been actively studied. Since obesity has become a public health problem, various laboratories have set up in vitro models in order to study mechanical stresses involved in the weight-associated cartilage degradative process, as well as the role of mediators involved in obesity. In this review, we will discuss on the role of mechanical stresses on cartilage, on bone and the role of adipokines in OA.
2. Mechanical stress and cartilage
Joints, and more particularly cartilage and subchondral bone tissues, are always exposed to mechanical stresses.
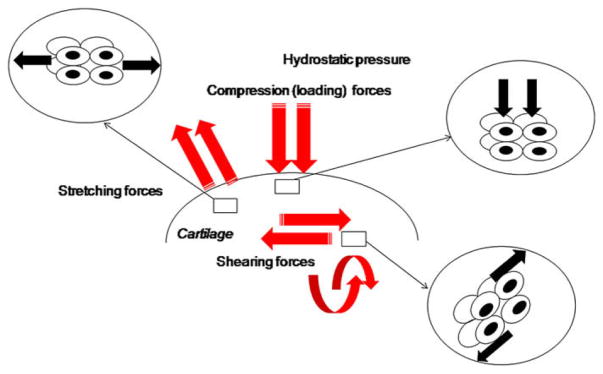
As outlined in Fig. 1, different stresses can be applied to a tissue, such as:
Fig. 1.
Different mechanical stresses applied on cartilage.
- Mechanical stress of compression (loading)
- Shear stress
- Tensile stress (stretching)
- Hydrostatic pressure
When standing, a load of around 0.7 MPa is applied to joints. When walking, a load ranging between 5 and 10 MPa is applied to cartilage [1] and when doing exercise, a load of over 18 MPa is applied on cartilage [2]. Numerous in vitro studies show that ExtraCellular Matrix (ECM) production by chondrocytes is highly sensitive to a variety of mechanical signals mediated by loading. Moderate exercise is beneficial for cartilage constitution [3], while excessive stresses or static stress, disrupt the homeostasis of anabolism and catabolism within cartilage [4]. The chondrocytes heterogeneity has to be taken into account as well [5].
The transmission of mechanical constraints to the cell could be described in four parts:
- Mechanical coupling which transforms the applied force into different signals to the cell.
- Mechano-signal transduction through sensitive mechano-receptors such as integrins (such as α5β1-integrin), the stretch-activated ion channels and the cytoskeleton. The chondrocyte cilia seems as well to be a mechanoreceptor as shown by Guilak and collaborators.
- Signal transduction which converts the mechanical signal into a biochemical signal in the cell, translocating to the nucleus.
- Cellular response: regulation of gene expression and release of paracrine–autocrine factors.
Different mechanical stresses have been studied in cartilage, in different animal or human models. Biomechanical signals are perceived by cartilage in magnitude-, frequency-, and time-dependent manners. Compression is generally associated with shear stress. Force intensity, frequency and nature of the sample are variable parameters in the different models studied. As a result, it is extremely difficult to compare the different results published in the literature.
Static loading, which has been mainly studied by Grodzinsky, Agarwal and Sharma, has been shown to inhibit matrix synthesis and induce pro-inflammatory genes [6–8]. Grodzinsky has recently shown that the inducing of degradative enzymes (MMP-13, ADAMTS-5) by a 50% static loading requires activation of p38 and ERK1/2 MAPKinases [9]. Sharma and collaborators have evidenced that static load has a degenerative effect in cartilage [8]. The fluid flow, which is associated with static loading, concentrates cations in the ECM and raises its osmolarity. Cyclic loading elicits a hyper-polarisation through the activation of Ca2+ channels [10] and can exert a pro-inflammatory role as shown by Guilak on bovine articular cartilage explants [11]. Chowdhury et al have used a 3D culture system of chondrocytes in agarose subsequently exposed to a cyclic compression, which thereby counteracts the effects of IL1β on iNOS and COX-2 activity [12]. Using newborn mouse rib cartilage explants Berenbaum et al have shown that mPGES-1, the last enzymatic step for PGE2 release, is a mechanosensitive gene [13]. Moreover, they have shown that NF-κB, ERK1/2 and p38 pathways are strongly activated by the mechanical stress of compression on cartilage. These signaling events, lead to the expression of the pro-inflammatory genes MMP-3, MMP-13 and PGE2 [14]. These results complete previous studies demonstrating that shear stress activates NF-κB, ERK1/2 and p38 pathways. Dynamic biomechanical signals of low-physiologic magnitudes studied by Agarwal, are potent anti-inflammatory signals that inhibit interleukin-1beta-induced pro-inflammatory gene expression and abrogate IL-1beta/tumor necrosis factor-alpha-induced inhibition of matrix synthesis [15,16]. Hydrostatic pressure also modulates matrix synthesis. Long term application of hydrostatic pressure greater than 20 MPa suppresses matrix synthesis.
Stretch and shear have been mainly studied by Agarwal, Guilak and Grodzinsky [17–19] using aspiration of the chondrocyte membrane with a micro-pipetus for example, and by Millward Sadler and Salter using a model of cyclic shear stress of human monolayer cell culture [20–22]. Cell monolayer cultures could only be studied with stretch or shear stress. They have shown that moderate levels of tensile stress act as a protective signal by decreasing the expression of catabolic mediators. A shear stress on bovine explants submitted to cyclic loading shows induction of the MAP ERK1/2 and p38 pathways [9].
Some teams have tried to modulate in vivo pressure on cartilage inside the joint by using a taping approach in order to treat lower limb OA focused on the knee. Recently, Richette and collaborators have reviewed data showing a lack of efficacy of this method in vivo [23].
The response of cartilage to load will also depend on the quality of subchondral bone.
3. Mechanical stress and bone
Osteoblast function is intimately linked to production of cytokines, growth factors and prostaglandins (PGs). The production of some of these factors is controlled by mechanical strain. Recently, a number of in vitro models attempted to identify genes and signaling pathways involved in this mechanism, mainly by stretching osteoblasts or by submitting them to a fluid shear stress. Osteoblasts possess mechanosensors which activate intracellular signals including ion channels, integrins, calveolar membrane structure and the cytoskeleton.
Fluid shear stress has been shown to elicit multiple intra-cellular signaling pathways involving elevation in intracellular calcium as well as ERK1/2 activation of c-Fos and nuclear factor (NF)-κB translocation [24,25]. Downstream of such signaling events, expression of various genes are induced, including type I collagen (COL1), osteopontin (OPN), insulin-like growth factor-I (IGF-1) and cyclooxygenase (COX)-2 [24].
Cyclic tensile stresses are also potent activators of the signaling cascade formed by ERK/c-fos/NF-κB [26]. Stretching increases the production of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β1 [27], alkaline phosphatase activity (ALP), osteocalcin (OC), osteoprotegerin (OPG), matrix metalloproteinases (MMP-1 and MMP-3) [28] COX-1 and -2, prostaglandin (PG)D2 synthase, peroxisome proliferator-activated receptor (PPAR) gamma-1 [29]. Stretching also decreases the release of the soluble receptor activator of nuclear factor ligand (sRANKL) by osteoblasts [30]. In contrast, no significant effect has been reported on MMP-2, tissue inhibitor of metalloproteinases (TIMP)-1 and -2, and PPARgamma-2 synthesis [29,31].
One major barrier to understanding bone physiology at a cellular level is the lack of models to study cells in their native environment. Usually, compression is generated by a bending system [26] or a glass cylinder and is applied on osteoblasts cultured in monolayer on flat surfaces [32]. In our study, loading was applied at a large amplitude (6–10% or 1–1.67 MPa) and at a frequency of 1 Hz. These loading conditions are included in the physiological range of amplitude and frequency of mechanical strains applied on bone during locomotion.
Recently, we have proposed an original model of 3D-osteoblast culture, allowing the study of compression on osteoblasts embedded in their own extracellular matrix. In our study, loading was applied at a large amplitude (6–10% or 1–1.67 MPa) and at a frequency of 1 Hz. These loading conditions are included in the physiological range of amplitude and frequency of mechanical strains applied on bone during locomotion. In this model, cell/matrix interactions are conserved and fluid flow through a three dimensional extra-cellular matrix is allowed. These loading conditions mimic the physiological range of amplitude and frequency of mechanical strains applied on bone during locomotion.
In these experimental conditions, we have observed a strong release of PGE2 in the culture medium of loaded 3D- osteoblasts. PGE2 is also a mediator involved in IL-6-induced osteoclast formation and bone resorption [26]. Further, we have shown for the first time that IL-6 is a highly mechano-sensitive gene. Moreover, in our experimental conditions, compression increased COX-2 expression but decreased 15-PGDH expression. Another important finding was that compression had no significant effects on COX-1 and mPGES gene expression. In addition, compressive stress stimulated MMP-2, MMP-3 and MMP-13 gene expression and MMP-3 synthesis, suggesting that osteoblasts may contribute to bone remodeling. Finally, using specific inhibitors we have identified some transcriptional factors involved in the compression-induced IL-6 and PGE2 production. It clearly appeared that alpha5beta1 integrin, intracellular Ca++, NF-κB and ERK1/2 were involved in these syntheses.
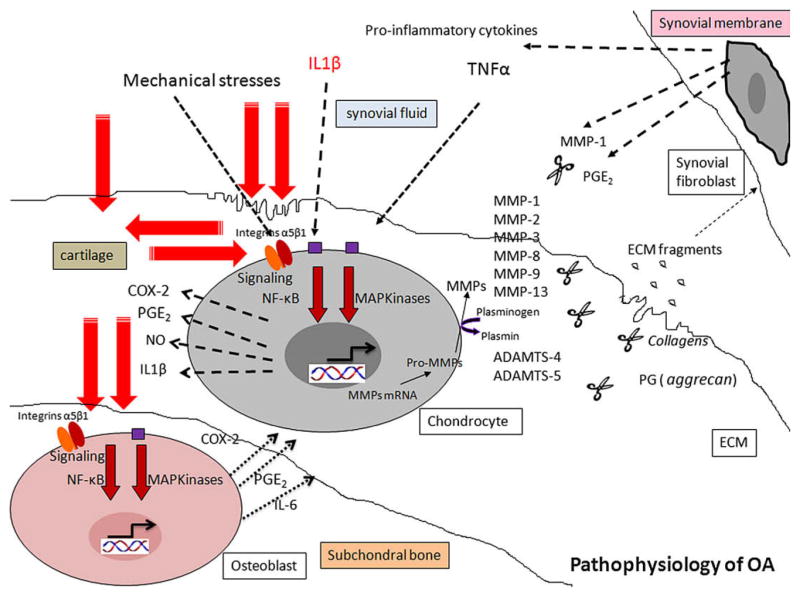
As summarized in Fig. 2, we provide in vitro evidence that compression stimulates IL-6, PGE2, and MMP production via Ca++/ERK1/2/NF-κB signaling pathways. Both PGE2 and IL-6 have demonstrated effects on bone remodeling, suggesting that these factors could play a key role in the mechanically-triggered bone remodeling [33].
Fig. 2.
Role of mechanical stress in pathophysiology of OA.
4. Role of adipokines in OA
Epidemiological studies have shown an intriguing correlation between hand OA and obesity [34]. Since mechanical stress cannot explain such a correlation, the hypothesis of one or several systemic factors has been proposed. It is thought that adipocytes share a common mesenchymal stem cell precursor with osteoblasts and chondrocytes, which suggests a link between lipid metabolism and connective tissues. As developing pre-adipocytes differentiate to become mature adipocytes, they acquire the ability to synthesize more than a hundred proteins (cytokines, growth factors, hormones). These proteins derived from adipose tissue were initially referred to as “adipocyto-kines” or “adipokines”.
The discovery of leptin in 1994 was a key development that led to our understanding of adipose tissue importance [35]. The role of leptin, the main adipokine, has since been widely studied. Among the adipokines, adiponectin and resistin are more specifically associated with leptin in playing key roles in the regulation of insulin action, inflammation, homeostasis and some pathologic events. Leptin, resistin and adiponectin are detected in the synovial fluid obtained from OA patients, but little is known about their contribution. Leptin is found in both osteophytes and cartilage obtained from OA patients and exhibits biological activity on chondrocytes. Available data related to the potential effects of these adipokines to joint disorders indicated that they may have an active role in the pathogenesis of chronic inflammatory joint diseases such as rheumatoid arthritis [36]. Leptin induces the expression of growth factors, stimulates proteoglycan and collagen synthesis, and increases the stimulatory effects of pro-inflammatory cytokines on nitric oxide production in chondrocytes [37]. Leptin also exerts regulatory effects on bone tissue [38].
Resistin might also be involved in the pathogenesis of RA since it has been found in the plasma and the synovial fluid of RA patients. Injection of resistin into mice joints induces an arthritis-like condition with leukocyte infiltration of synovial tissues, hypertrophy of the synovial layer and pannus formation [39].
Pre-B cell colony-enhancing factor (PBEF), also known as visfatin, has been shown to be an adipokine expressed by fat cells that exerts a number of insulin mimetic effects and plays a key role in the persistence of inflammation. Very recently, Gosset et al have shown that visfatin is also expressed in OA cartilage, stimulates the terminal enzyme of PGE2 synthesis, mPGES-1, and inhibits the degradative enzyme of PGE2, 15- PGDH. Visfatin seems to play a pro-inflammatory role in OA [40].
Given all this evidence, adipokines may be the metabolic link between obesity and OA. However, many questions remain concerning the role played by adipokines. Future research will be needed to clarify their roles in OA.
5. Conclusion
OA is a multifactorial disease and the process leading to the degradation of the joint seems to be more complex than expected. While there seems to be a link between obesity and OA this may not simply be due to the increased mechanical stresses on joint tissues resulting from increased weight gain in individuals. Additional soluble factors such as adipokines may also play an important role in the onset of OA in these obese patients.
Footnotes
Conflict of interest
None
References
- 1.Bergman BC, Martin DT, Wilkinson JG. Knee extensor torque and perceived discomfort during symmetrical biphasic electromyostimulation. J Strength Cond Res. 2001;15:1–5. [PubMed] [Google Scholar]
- 2.Kerin A, Patwari P, Kuettner K, et al. Molecular basis of osteoarthritis: biomechanical aspects. Cell Mol Life Sci. 2002;59:27–35. doi: 10.1007/s00018-002-8402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosami-noglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 4.Sharma L, Chang A. Overweight: advancing our understanding of its impact on the knee and the hip. Ann Rheum Dis. 2007;66:141–2. doi: 10.1136/ard.2006.059931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JB, Youn I, Cao L, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray ML, Pizzanelli AM, Lee RC, et al. Kinetics of the chondrocyte biosynthetic response to compressive load and release. Biochim Biophys Acta. 1989;991:415–25. doi: 10.1016/0304-4165(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 7.Knobloch TJ, Madhavan S, Nam J, et al. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139–50. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma G, Saxena RK, Mishra P. Differential effects of cyclic and static pressure on biochemical and morphological properties of chondrocytes from articular cartilage. Clin Biomech (Bristol, Avon) 2007;22:248–55. doi: 10.1016/j.clinbiomech.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald JB, Jin M, Chai DH, et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283:6735–43. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 10.Shimazaki A, Wright MO, Elliot K, et al. Calcium/calmodulin-dependent protein kinase II in human articular chondrocytes. Biorheology. 2006;43:223–33. [PubMed] [Google Scholar]
- 11.Guilak F, Meyer BC, Ratcliffe A, et al. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury T, Arghandawi S, Brand J, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosset M, Berenbaum F, Levy A, et al. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006;8:R135. doi: 10.1186/ar2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabay O, Gosset M, Levy A, et al. Stress-induced signaling pathways in hyalin chondrocytes: inhibition by Avocado–Soybean Unsaponifiables (ASU) Osteoarthritis Cartilage. 2008;16:373–84. doi: 10.1016/j.joca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Sowa G, Agarwal S. Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil. 2008;87:537–44. doi: 10.1097/PHM.0b013e31816197ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhavan S, Anghelina M, Sjostrom D, et al. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. J Immunol. 2007;179:6246–54. doi: 10.4049/jimmunol.179.9.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gassner RJ, Buckley MJ, Studer RK, et al. Interaction of strain and interleukin-1 in articular cartilage: effects on proteoglycan synthesis in chondrocytes. Int J Oral Maxillofac Surg. 2000;29:389–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–73. [PubMed] [Google Scholar]
- 19.LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47:46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Millward-Sadler SJ, Lin H, et al. Evidence for JNK-dependent up-regulation of proteoglycan synthesis and for activation of JNK1 following cyclical mechanical stimulation in a human chondrocyte culture model. Osteoarthritis Cartilage. 2007;15:884–93. doi: 10.1016/j.joca.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Orazizadeh M, Salter DM. The expression of signal regulatory protein-alpha in normal and osteoarthritic human articular cartilage and its involvement in chondrocyte mechano-transduction response. Iran Biomed J. 2007;11:119–24. [PubMed] [Google Scholar]
- 22.Chowdhury TT, Salter DM, Bader DL, et al. Integrin-mediated mechanotransduction processes in TGFbeta-stimulated monolayer-expanded chondrocytes. Biochem Biophys Res Commun. 2004;318:873–81. doi: 10.1016/j.bbrc.2004.04.107. [DOI] [PubMed] [Google Scholar]
- 23.Richette P, Sautreuil P, Coudeyre E, et al. Usefulness of taping in lower limb osteoarthritis. French clinical practice guidelines. Joint Bone Spine. 2008;75:475–8. doi: 10.1016/j.jbspin.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Chen NX, Geist DJ, Genetos DC, et al. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone Sep. 2003;33(3):399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 25.Inoue D, Kido S, Matsumoto T. Transcriptional induction of FosB/Del-taFosB gene by mechanical stress in osteoblasts. J Biol Chem. 2004;279:49795–803. doi: 10.1074/jbc.M404096200. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Liu T, Zheng Y, et al. Early responses of osteoblast-like cells to different mechanical signals through various signaling pathways. Biochem Biophys Res Commun. 2006;348:1167–73. doi: 10.1016/j.bbrc.2006.07.175. [DOI] [PubMed] [Google Scholar]
- 27.Singh SP, Chang EI, Gossain AK, et al. Cyclic mechanical strain increases production of regulators of bone healing in cultured murine osteoblasts. J Am Coll Surg. 2007;204:426–34. doi: 10.1016/j.jamcollsurg.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Kanno T, Takahashi T, Tsujisawa T, et al. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem. 2007;101:1266–77. doi: 10.1002/jcb.21249. [DOI] [PubMed] [Google Scholar]
- 29.Siddhivarn C, Banes A, Champagne C, et al. Prostaglandin D2 pathway and peroxisome proliferator-activated receptor gamma-1 expression are induced by mechanical loading in an osteoblastic cell line. J Periodontal Res. 2006;41:92–100. doi: 10.1111/j.1600-0765.2005.00834.x. [DOI] [PubMed] [Google Scholar]
- 30.Tang LL, Xian CY, Wang YL. The MGF expression of osteoblasts in response to mechanical overload. Arch Oral Biol. 2006;51:1080–5. doi: 10.1016/j.archoralbio.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki K, Takagi M, Konttinen YT, et al. Upregulation of matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human osteoblast by uniaxial cyclic stimulation. J Biomed Mater Res B Appl Biomater. 2007;80:491–8. doi: 10.1002/jbm.b.30622. [DOI] [PubMed] [Google Scholar]
- 32.Mitsui N, Suzuki N, Maeno M, et al. Optimal compressive force induces bone formation via increasing bone morphogenetic proteins production and decreasing their antagonists production by Saos-2 cells. Life Sci May 1. 2006;78(23):2697–706. doi: 10.1016/j.lfs.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez C, Gabay O, Salvat C, et al. Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2008.09.007. in press. [DOI] [PubMed] [Google Scholar]
- 34.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;23:1221–6. [PubMed] [Google Scholar]
- 35.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 36.Pottie P, Presle N, Terlain B, et al. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65:1403–5. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aspden RM, Scheven BA, Hutchison JD. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet. 2001;357:1118–20. doi: 10.1016/S0140-6736(00)04264-1. [DOI] [PubMed] [Google Scholar]
- 38.Takeda S, Elefteriou F, Karsenty G. Common endocrine control of body weight, reproduction, and bone mass. Annu Rev Nutr. 2003;23:403–11. doi: 10.1146/annurev.nutr.23.011702.073312. [DOI] [PubMed] [Google Scholar]
- 39.Lago F, Dieguez C, Gomez-Reino J, et al. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–24. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 40.Gosset M, Berenbaum F, Salvat C, et al. Crucial role of Visfatin/PBEF in matrix degradation and PGE2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 2008;58:1399–409. doi: 10.1002/art.23431. [DOI] [PubMed] [Google Scholar]