Abstract
Low-molecular-weight heparins (LMWH) appear to prolong survival of patients with cancer. Such a beneficial effect is thought to be associated with interruption of molecular mechanisms involving the heparan sulfate (HS) chains of cell surface and extracellular matrix proteoglycans (HSPGs), growth factors and their receptors, heparanase, and selectins. The beneficial effects of heparin species could also be associated with their ability to release tissue factor pathway inhibitor from endothelium. The utility of heparin and LMWH as anticancer drugs is limited due to their anticoagulant properties. Non-anticoagulant heparins can be obtained either by removing chains containing the antithrombin-binding sequence, or by inactivating critical functional groups or units of this sequence. The non-anticoagulant heparins most extensively studied are regioselectively desulfated heparins and ‘glycol-split’ heparins. Some modified heparins of both types are potent inhibitors of heparanase. A number of them also attenuate metastasis in experimental models. With cancer cells overexpressing selectins, heparin-mediated inhibition of tumor cells-platelets aggregation and tumor cell interaction with the vascular endothelium appears to be the prevalent mechanism of attenuation of early stages of metastasis. The structural requirements for inhibition of growth factors, heparanase, and selectins by heparin derivatives are somewhat different for the different activities. An N-acetylated, glycol-split heparin provides an example of application of a non-anticoagulant heparin that inhibits cancer in animal models without unwanted side effects. Delivery of this compound to mice bearing established myeloma tumors dramatically blocked tumor growth and progression.
Key Words: Non-anticoagulant heparins, Cancer, Angiogenesis, Metastasis, Growth factors, Heparanase, Selectins
Introduction
Low-molecular-weight species of heparin appear to prolong survival of patients with cancer. In recently published randomized controlled trials, different types of low-molecular-weight heparin (LMWH) increased the survival of patients with advanced cancer [1]. Animal studies using non-anticoagulant species of heparin indicate that it is possible to separate the antimetastatic and anticoagulant activities of heparin [2]. The use of heparin as an antitumor agent is limited due to its potent anticoagulant activity. Because LMWHs also retain some anticoagulant activity, non-anticoagulant heparins are preferable for potential clinical use because they could be administered at high doses, thereby fully exploiting the antimetastatic component of heparin, and because they could be applied to cancer patients with bleeding complications. The mechanism by which heparins and non-anticoagulant heparins inhibit metastasis is not fully understood. However, evidence suggests that heparin species inhibit mitogenic signaling mainly through inhibition of growth factors and their receptors [3], and/or by inhibition of the enzyme heparanase [4]. Another possibility is that heparin inhibits metastasis by blocking platelet-tumor cell interactions, thereby inhibiting aggregates of tumor cells lodging in the microvasculature. Heparin and non-anticoagulant heparins also inhibit selectin-mediated cell-cell interactions thus preventing extravasation of blood-borne cells [5]. The present overview covers some structural and functional aspects associated with the anticancer activities of heparin species, with special emphasis on non-anticoagulant heparins.
Structure and Functional Domains of Heparin
Heparin is a sulfated polysaccharide belonging to the family of glycosaminoglycans. The structure of heparin has been extensively investigated especially to unravel features associated with its potent anticoagulant activity. The emerging interest in non-anticoagulant properties of heparin and their prospective therapeutic applications has extended these studies with the aim of understanding the molecular basis and possible interplay of different activities. The anticoagulant properties of heparin have long been thought to be exclusively associated with the prevalent, ‘regular’ sequences of this polysaccharide. The unexpected discovery that these properties are largely dependent on small, antithrombin (AT)-binding domains that are present in no more than one third of the chains constituting heparins currently used in therapy has led to reappraisal of the role of minor sequences in determining specificities of biological interactions of heparin [6].
Heparin is constituted by alternating disaccharide sequences of a uronic acid and an amino sugar, the uronic acid residues being L-iduronic acid (IdoA) and D-glucuronic acid (GlcA), and the amino sugar exclusively D-glucosamine (GlcN). IdoA prevalently bears sulfate substituents at position 2; GlcN is prevalently N-sulfated (N-acetylated in minor sequences) and 6-O-sulfated.
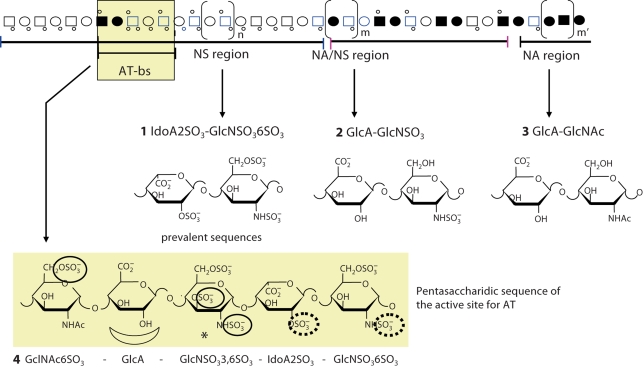
The main structural regions of heparin and the structure of its most represented disaccharide sequences are shown in figure 1, where 1 (2-O-sulfated iduronic acid – N,6-disulfated glucosamine) are major components of the N-sulfated (NS) region, which is prevalent (>70%) in heparin, and 3 (glucuronic acid – N-acetylated glucosamine) and 2 are components of the less abundant N-acetylated (NA) and mixed (NA/NS) regions, respectively. The minor but important sequence is the pentasaccharide 4, which is the AT-binding sequence (AT-bs). Though being contained in not more than one third of the chains, the AT-bs accounts for most of the anticoagulant activity of clinically used heparins. Sulfate groups essential for high affinity to AT are circled in formula 4; the central GlcA residue is also essential for high-affinity binding to AT [7, 8].
Fig. 1.
Idealized representation of a heparin chain constituted of N-acetylated (NA), N-sulfated (NS), and mixed NA/NS domains also containing an AT-binding domain (AT-bs). Formulas of major disaccharidic sequences within NS, NA/NS and NA domains (1–3) and of the AT-bd (4) are shown. Symbols are as explained in Casu and Lindahl [8].
Non-Anticoagulant Heparins
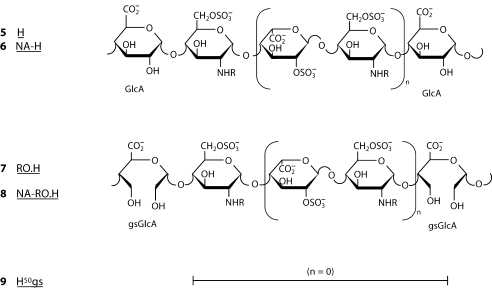
Non-anticoagulant heparins can be obtained either by removing from unfractionated heparin (UH) its chains containing sequence 4, which can be achieved by complexation with AT applying affinity chromatography or precipitation experiments. Alternatively, sequence 4 can be ‘inactivated’ by modification of one or more groups or residues that are essential for high affinity to AT. Thus, N-desulfation, i.e., removal of sulfate substituents from N-SO3 groups, involves removal also of the essential sulfamino group of the internal residue (*) of sequence 4 and implies a dramatic drop in anticoagulant activity. A similar drop can be achieved upon 2-O-desulfation, i.e., removal of the sulfate group of IdoA2SO3 residues. As illustrated in formula 4, this group is only marginally essential for high affinity for AT. However, under the basic conditions used for 2-O-desulfation, the internal residue (*), which is a marker of the active site for AT, loses its essential 3-O-sulfate group. The anticoagulant activity of heparin can be even more drastically decreased by modification of the GlcA residue of sequence 4, as achievable by reduction of its carboxyl group, or by cleavage of the bond between its two hydroxyl groups. This latter reaction, most often quantitatively performed by periodate oxidation of all non-sulfated uronic acid residues of heparin, leads to the so-called ‘glycol-split’ (gs) heparins. Glycol splitting does not involve the major sequences 1, to which most of the non-AT-mediated activities of UH and LMWH are ascribed. Periodate oxidization of heparin only at the level of the original non-sulfated uronic acid units (mostly GlcA, but also IdoA) are designated ‘reduced oxyheparins’ (RO.H), since they are stabilized by reduction (forming CH2OH groups) of the primary oxidation product (a polydialdehyde). A wide class of glycol-split heparin species can also be obtained by controlled 2-O-desulfation of heparins and periodate oxidation/reduction of both the preexisting and the newly generated non-sulfated IdoA residues [9]. The statistical structures of the non-anticoagulant heparins N-acetyl heparin (6, NA-H), reduced oxyheparin (7, RO.H), and N-acetylated reduced oxyheparin (8, NA-RO.H) are shown in figure 2 together with that of the parent heparin (5, H). Heparin fractions devoid of chains containing the AT-bs still retain the anticoagulant properties associated with non-AT-mediated mechanisms. Desulfation of heparin involving removal of sulfate groups in sequences located outside the AT-bs leads to impairment of thrombin inhibition mediated by heparin cofactor II (HC2) and release of vascular tissue factor pathway inhibitor (TFPI). It should be remembered that inhibition of factor Xa can be efficiently achieved with sequences as short as pentasaccharide provided it has the specific structure 4. For inhibition of thrombin, the AT-bs should be prolonged by about five additional disaccharide units (usually of the ‘regular’ type 1, with minor internal variants allowed). Like most of the AT-mediated heparin activities, the anticoagulant properties mediated by HC2 or induced by release of TFPI are molecular weight dependent and require a minimum chain length to express significant activities [7, 8].
Fig. 2.
Prevalent sequences of heparin (5), N-acetylated heparin (6), and the corresponding RO (reduced oxyheparin) derivatives RO.H (7) and NA-RO.H (8), where all non-sulfated uronic acid residues of H and NA-H are glycol split; for simplicity, only GlcA and glycol-split (gs) GlcA residues are shown. Formula 9 represents a heparin derivative where additional non-sulfated uronic acid residues (generated by selective 2-O-desulfation of IdoA2SO 3 residues) were glycol split, to reach about 1: 1 ratio of split to non-split residues. The value of n varies from 1 to 5 in 5–8 and is = 1 in 9 [9].
Interaction of Heparin and Non-Anticoagulant Heparins with Proteins Involved in Tumor Angiogenesis and Metastasis
Most of the anticancer mechanisms of heparin species are exerted through competition with functions of the heparan sulfate (HS) chains of HS proteoglycans (HSPGs) [10, 11]. HSPGs are ubiquitous macromolecules associated with the cell surface and the extracellular matrix (ECM) of a wide range of tissues. HSPGs within the ECM are either secreted by cells (e.g. perlecan) or initially expressed as cell surface HSPGs and then proteolytically shed from the cell surface (e.g. syndecans). HSPGs play a critical role in regulating the metastatic behavior of tumor cells. HS proteoglycans may at times act as inhibitors of cell invasion and at other times as promoters of cell invasion, with their function being determined by their location (cell surface or ECM), the heparin-binding molecules they associate with, the presence of modifying enzymes (i.e., heparanase) and the precise structural characteristics of the proteoglycan. Also, the tissue type and pathological state of the tumor influence proteoglycan function [12]. The basic HSPG structure consists of a polypeptide core to which several linear HS chains are covalently O-linked. HS binds to and assembles ECM proteins thus playing important roles in ECM integrity, barrier function, and cell-ECM interactions. The HS chains ensure that a wide variety of bioactive molecules bind to the cell surface and ECM and thereby function in the control of diverse normal and pathological processes. HSPGs not only provide a storage depot for heparin-binding molecules such as growth factors, but rather can decisively regulate their accessibility, function and mode of action [10, 11].
The structure of HS is similar to that of heparin, differences between the two glycosaminoglycans being mainly in the relative proportions of sequences shown in figure 1. HS is less sulfated than heparin and its NA and NA/NS domains are more extended than the NS domains. Nevertheless, HS sequences most commonly identified to interact with growth factors are the more ‘heparin-like’ sequences 1 located along the NS region [8]. Growth factors more extensively studied as implicated in tumor growth are fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). These factors are sequestered by HS chains of HSPGs, and are released upon cleavage by the enzyme heparanase [4]. Such a cleavage occurs only at a few sites (at the level of GlcA residues), resulting in fragments of still appreciable size (10–15 units) that activate the growth factors, facilitating their dimerization, binding to receptors, and signaling. Enzymatic degradation of HS leads to disassembly of the ECM and is therefore involved in tissue remodeling and cell migration, including cancer angiogenesis and metastasis [4].
The involvement of heparanase in cancer is well documented. Heparanase is preferentially expressed at early stages of carcinoma progression and is upregulated in essentially all human tumors examined. There is also a correlation between heparanase expression levels and tumor vascularity in cancer patients, indicating a significant role in tumor angiogenesis [4].
Apart of direct involvement in basement membrane invasion by endothelial cells and the indirect angiogenic response elicited by releasing HS-bound angiogenic growth factors from ECM, heparanase has been recently demonstrated to exert tumorigenic effects independent of its enzymatic activity, such as Akt-dependent endothelial cell invasion and migration, and upregulation of VEGF gene expression through activation of Src [4].
Cleavage of HS by heparanase may result in disassembly of the ECM and is a prerequisite for tumor invasion and extravasation of blood-borne cells. Heparanase immunostaining pattern and/or expression levels correlate with the metastatic potential of human tumors. Moreover, elevated levels of heparanase were detected in the urine and plasma of patients with aggressive metastatic disease [4]. Heparanase also stimulates tumor growth and metastasis by enhancing shedding of syndecan-1 [13] (see later).
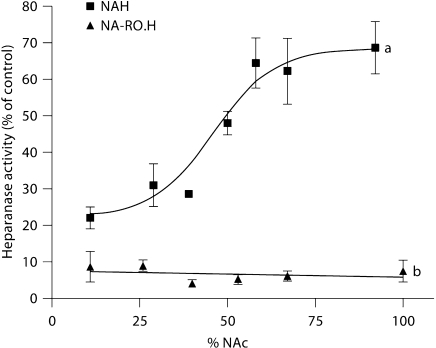
As a close mimic of HS, heparin has been investigated as potential inhibitor of heparanase. In fact, heparin efficiently inhibits tumor cell heparanase and experimental metastasis [2, 4]. However, its GlcA-containing sequences are susceptible to cleavage by heparanase so that heparin acts also as a substrate for the enzyme [14]. In a search for tumor-inhibiting non-anticoagulant alternatives to heparin, regioselectively desulfated heparins (i.e., heparins where either N-sulfate, 2-O-sulfate, or 6-O-sulfate groups were removed) have been screened [2, 15]. In early studies, NA-H (formula 6) was identified as a heparin derivative endowed with anti-heparanase and antimetastatic activities [16], though less potent than heparin [17]. The heparanase-inhibiting activity of heparin derivatives increases with increasing degrees of sulfation. However, 2-O-desulfated derivatives were found to retain the heparanase inhibition activity of heparin. In fact, neither 2-O-sulfate nor 6-O-sulfate groups are essential for efficient inhibition of heparanase, provided that at least one of these positions is extensively sulfated. Complete removal of N-sulfate groups followed by N-acetylation resulted in a substantial decrease in the inhibitory activity. However, as illustrated in figure 3a, this effect was only noted for N-acetylation degrees higher than approximately 50%. In the framework of a systematic study aimed at obtaining heparanase-inhibiting species of heparin devoid of anticoagulant and pro-angiogenic activities, a pronounced gain of heparanase-inhibiting activity has been obtained following glycol splitting of both N-sulfated and N-acetylated heparins, as in derivatives RO.H (7) and NA-RO.H (8), respectively. Such a pronounced effect is illustrated by a comparison of graphs a and b in figure 3. NA-RO.H emerged as the most potent heparanase inhibitor tested by us thus far [17, 18]. Glycol-split heparins of the RO.H type (general formula 7) were found to inhibit metastasis in animal models [19, 20]. Certain non-acetylated, glycol-split heparins, such as H50gs (formula 9), obtained by glycol splitting also of some IdoA residues generated by controlled 2-O-desulfation of sequences 1, were found to exert a more direct antiangiogenic effect by inhibiting FGF-2 [21] and VEGF [22]. At least for FGF-2, such an effect is attributable to inhibition of dimerization of the growth factor [21]. Following another approach (i.e., by inhibiting interaction of the growth factor with its receptor), FGF-2-dependent angiogenesis was inhibited using 6-O-desulfated heparin [23]. Molecular modeling studies confirmed the assumption that glycol-split residues along the heparin chains act as flexible joints, thus favoring docking to the protein of the heparin sequences involved in binding to either the growth factor or heparanase [9]. H50gs showed a potent antiangiogenic activity also in wound-healing animal models [24]. The dual action of some glycol-split heparin derivatives is attributed to different ways of binding to different heparin sequences. Thus, whereas H50gs (formula 9) interacts with FGF-2 through one of its disaccharide sequences 1 at a typical FGF heparin/HS-binding region [21], heparanase-inhibiting glycol-split heparins are thought to span through two heparin/HS-binding regions of heparanase, with one of their glycol-split residues positioned between the two basic regions, close to the active site of the enzyme [18, 25].
Fig. 3.
Heparanase inhibition activity of N-acetyl heparins (a) and of glycol-split N-acetyl heparins of the RO type (b), as a function of the degree of N-acetylation. The anti-heparanase activity is expressed in terms of residual activity [17].
The antimetastatic action of heparin and non-anticoagulant heparins can be exerted also through inhibition of selectins [26,27,28]. P- and L-selectin mediate aggregation of cancer cells with platelets and leukocytes, respectively. Selectin inhibition is a clear component of heparin inhibition of metastasis while anticoagulation is not a necessary component of such inhibition. In fact, studies in animal models have demonstrated that some non-anticoagulant heparins are even more potent inhibitors of metastasis than unmodified heparin [29,30,31]. Inhibition of selectins plays a major role in attenuating early stages of metastasis. However, some additional antimetastatic effects are observed using doses of heparin higher than those used for prevention and therapy of thrombosis [30]. The two modes of action on metastasis could be dissected, as observed for selected glycol-split heparanase inhibitors devoid of anti-selectin activity [[28, 29]; unpubl. results].
The molecular weight dependence of most of the cancer-related activities of heparin and non-anticoagulant heparins has not been yet systematically investigated. However, on the basis of biochemical and structural studies using heparin-derived oligosaccharides [32], it can be predicted that such a size dependence is different for different protein targets. Whereas short heparin chains may directly bind FGF2 and VEGF, thus interfering with HS-mediated dimerization of these and other growth factors and eventual mitogenic signaling through activation of their receptors, relatively longer chains are expected to induce the adverse effect of potentiating the mitogenic signaling [3].
Octasaccharides are somewhat at the borderline between angiogenesis-inhibiting and -activating heparin fragments [3]. Indeed, they were shown to inhibit angiogenesis in vivo with decreasing efficacies correlated with decreasing involvement of FGF-2 in different models [33]. On the other hand, heparin oligosaccharides shorter than about eight monosaccharide residues are poor inhibitors of heparanase [18] as well as of selectins [34]. LMWH and – even more so – very-low MW (VLMW) heparins are consistently more effective than full-length UH in preventing angiogenesis in some animal models [35]. Since LMWHs have almost invariably shown more pronounced beneficial effects on cancer patients than UH [1], it is conceivable that their antitumor activities are prevalently exerted by their shortest chain components, suggesting that the resulting action is associated more with growth factors-mediated inhibition of signaling than with other mechanisms – although they are poorer inhibitors of selectins than UH [34]. LMWHs contain species that could preferentially inhibit either growth factors, heparanase, or selectins, or act on all these target proteins. Thus, the relative contribution of LMWH to different mechanisms of antitumor actions appears to be dependent, at least in part, on the relative content of species with different MWs. Information on in vivo antitumor activities of small non-anticoagulant heparin species is scanty. However, comparison of UH and its fragments for their ability to inhibit effects on growth and metastasis of breast cancer cells indicated that UH modulates these effects with mechanisms of actions more complex than a heparin dodecasaccharide [36]. Although small heparin fragments are non-anticoagulant, especially if they do not contain the AT-binding domain, the subject of heparin fragments and synthetic oligosaccharides is beyond the scope of this overview. With non-anticoagulant heparins where anticoagulation was hampered without major changes in the internal structure, as, for example, by removal of chains containing the AT-bs or inactivation of this domain, the molecular weight dependence of antitumor activities is expected to parallel that of the corresponding coagulant species.
There is a general consensus that the beneficial effects of heparin species on cancer patients are multifactorial and may also involve mechanisms other than those mediated by growth factors and their receptors, heparanase, and selectins. In fact, release of TFPI from endothelium induced by heparin and non-anticoagulant heparins may significantly contribute to the antiangiogenic and antimetastatic activity of this TF inhibitor [37, 38]. Like most of the non-AT-mediated effects, also TFPI release from the vascular endothelium is thought to be mostly associated with NS regions of heparin and non-anticoagulant heparins. A minimum chain length of these heparin species is also expected for expression of a significant inhibitory activity. The interplay of actions of coagulation and tumor-promoting factors, though complex, is well documented [38,39,40], as indicated also by the influence of heparanase on anticoagulation properties of heparin and LMWH [39]. Although the mechanisms of the anticancer properties of heparins and their possible interplay are complex and still largely unclarified [40], some heparin-derived non-anticoagulant inhibitors of major activities involved in cancer growth and progression are emerging as drug candidates. The following section describes an example of such a potential application.
An N-Acetylated, Glycol-Split Heparin Effectively Blocks Myeloma Growth in vivo
Myeloma tumors are replete with HS due to high levels of syndecan-1 (CD138) on the surface of tumor cells [41, 42]. In addition, syndecan-1 is shed from the surface of myeloma cells and is an indicator of poor prognosis when present at high levels in myeloma patient serum [43]. A recent study of 324 myeloma patients demonstrated that serum syndecan-1 is an independent prognostic factor both at diagnosis and at the plateau phase of disease [44]. Studies in animal models have confirmed that shed syndecan-1 plays an active role in promoting myeloma progression by enhancing tumor growth, angiogenesis and metastasis within the bone [45, 46]. This likely occurs via HS chains on syndecan-1 binding to, and regulating the function of, multiple effector molecules (e.g. IL-6, IL-7, IL-8, HGF, FGF-2, VEGF, EGF-family ligands). Through these interactions, syndecan-1 acts to manage the cross-talk between the tumor and the bone marrow microenvironment.
The HS-degrading enzyme heparanase is also present and active in the bone marrow of myeloma patients where it correlates with high microvessel density and poor prognosis [47, 48]. Enhanced expression of heparanase stimulates expression and shedding of syndecan-1 [13, 48], implying that in myeloma, syndecan-1 and heparanase act synergistically to drive tumor growth and progression [49]. In animal models, enhanced expression of heparanase increased growth and angiogenesis of myeloma tumors in addition to promoting spontaneous metastasis from subcutaneous sites to bone [50]. The importance of HS regulation of cross-talk within the myeloma marrow and the fact that heparanase can regulate HS function led to the hypothesis that targeting the HS/heparanase axis would be an effective therapy for myeloma [51].
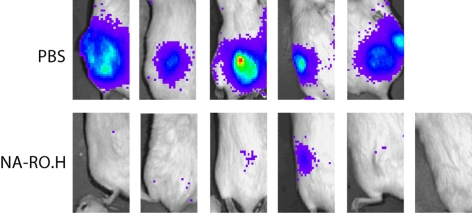
In animal models of myeloma, we found that the heparanase inhibitor NA-RO.H (formula 8: 100% N-acetylated and 25% glycol-split heparin, corresponding to 100NA-RO.H in Naggi et al. [17]) has potent anti-myeloma activity [50]. In animals bearing an established subcutaneous myeloma tumor treated for 28 days with 18 mg/kg/day of glycol-split heparin, 6/7 animals treated had detectable tumor with a mean wet weight of 30 mg. The mean tumor weight in controls was 450 mg. In animals treated with twice the concentration of glycol-split heparin, 36 mg/ kg/day, only 1/6 animals had a detectable tumor (weight 5 mg) at the end of the treatment period (fig. 4). Analysis of levels of human κ light chain in the serum of the treated mice confirmed that tumor burden was dramatically reduced in the animals treated with glycol-split heparin (mean κ levels of 7,341, 176 and 34 ng/ml, for control, 18 and 36 mg/kg/day treatment), respectively [50].
Fig. 4.
A modified non-anticoagulant heparin inhibits myeloma growth in vivo. Animals were treated with either PBS or 100% Nacetylated and 25% glycol-split heparin (NA-RO.H) for 28 days beginning 10 days after subcutaneous injection of heparanaseexpressing myeloma tumor cells. Bioluminescence imaging of luciferase-expressing tumor cells just prior to euthanasia of animals reveals all 5 animals treated with PBS have large tumors. In contrast, only 1/6 animals treated with NA-RO.H has clearly detectable tumor. This was the only tumor that was found in any of the 6 animals at necropsy [50].
These results demonstrate that this modified heparin can potently inhibit myeloma tumor growth in vivo without obvious side effects and reflect the potential for this as an anti-myeloma drug. Although the mechanism of action of NA-RO.H is not yet clearly defined, its anti-heparanase effects likely contribute to tumor growth inhibition. In addition, because it is a modified heparin, the compound may also interfere with normal HS function within the tumor microenvironment by acting as a competitive inhibitor. Such inhibition could interfere with the localization and bioavailability of heparin-binding factors. If indeed these heparins are targeting and disrupting the myeloma microenvironment, they may prove effective anti-tumor agents either singly or in combination with conventional chemotherapeutic drugs designed to target the tumor cell compartment.
Acknowledgements
This work was supported by the G. Ronzoni Foundation and EU Grant QLK3-CT-2002-02049 to B.C., by NIH grants CA103054, CA55819 and the Veterans Administration to R.D.S., and by grants from the DKFZ-MOST cooperation program in cancer research, NIH (CA 106456), and the Israel Cancer Research Fund (ICRF) to I.V. We wish to thank the Molecular Imaging Core at the UAB Comprehensive Cancer Center for assistance with bioluminescence imaging.
References
- 1.Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 2.Vlodavsky I, Ishai-Michaeli R, Mohsen M, Bar-Shavit R, Catane R, Ekre HPT, Svahn CM. Modulation of neovascularization and metastasis by species of heparin. In: Lane DA, Björk I, Lindahl U, editors. Heparin and Related Polysaccharides. New York: Plenum Press; 2006. pp. 317–327. [Google Scholar]
- 3.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Borsig L. Selectins facilitate carcinoma metastasis and heparin can prevent them. News Physiol Sci. 2004;19:16–21. doi: 10.1152/nips.01450.2003. [DOI] [PubMed] [Google Scholar]
- 6.Garg HG, Linhardt RJ. In: Chemistry and Biology of Heparin and Heparan Sulfate. Hales CA, editor. Amsterdam: Elsevier; 2006. [Google Scholar]
- 7.Casu B. Structure and active domains of heparin. In: Garg HG, Linhardt RJ, Hales CA, editors. Chemistry and Biology of Heparin and Heparan Sulfate. Amsterdam: Elsevier; 2006. pp. 1–28. [Google Scholar]
- 8.Casu B, Lindahl U. Structure and biological interactions of heparin and heparan sulfate. Adv Carbohydr Chem Biochem. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]
- 9.Naggi A. Glycol splitting as a device for modulating inhibition of growth factors and heparanase by heparin and heparin derivatives. J Thromb Haemost. 2007;5:461–481. [Google Scholar]
- 10.Iozzo RV. Matrix proteoglycans; from molecular design to cellular functions. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 11.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson RD. Heparan sulfate proteoglycans in invasion and metastasis. Cell Dev Biol. 2000;12:89–98. doi: 10.1006/scdb.2000.0241. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, MacLeod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 14.Sandbäck-Pikas D, Li JP, Vlodavsky I, Lindahl U. Substrate specificity of heparanase from human hepatoma and platelets. J Biol Chem. 1988;273:18770–18777. doi: 10.1074/jbc.273.30.18770. [DOI] [PubMed] [Google Scholar]
- 15.Presta M, Leali D, Stabile H, Ronca R, Camozzi M, Coco L, Moroni E, Liekens S, Rusnati M. Heparin derivatives as angiogenesis inhibitors. Curr Pharm Des. 2003;9:553–556. doi: 10.2174/1381612033391379. [DOI] [PubMed] [Google Scholar]
- 16.Nakajiama M, Irimura T, Di Ferrante N, Nicolson GL. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984;259:2283–2290. [PubMed] [Google Scholar]
- 17.Naggi A, Casu B, Perez M, Torri G, Cassinelli G, Penco S, Pisano C, Giannini G, Ishai-Michaeli R, Vlodavsky I. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005;280:12103–12113. doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]
- 18.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Design. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 19.Lapierre F, Holme K, Lam L, Tresser RJ, Storm N, Wee J, Stack RJ, Castellot J, Tyrrell DJ. Chemical modifications of heparin that diminish its anticoagulant but preserve its heparanase-inhibitory, angiostatic, anti-tumor and anti-metastatic properties. Glycobiology. 1966;6:355–366. doi: 10.1093/glycob/6.3.355. [DOI] [PubMed] [Google Scholar]
- 20.Krag M, Binderup L, Vig Hjarnaa PJ, Bramm E, Johansen KB, Petersen CF. Non-anticoagulant heparin inhibits metastasis but not primary tumor growth. Oncol Rep. 2005;14:99–104. [PubMed] [Google Scholar]
- 21.Casu B, Guerrini M, Guglieri S, Naggi A, Perez M, Torri G, Cassinelli G, Ribatti D, Carminati P, Giannini G, Penco S, Pisano C, Belleri M, Rusnati M, Presta M. Undersulfated and glycol-split heparin derivatives endowed with antiangiogenic activity. J Med Chem. 2004;47:838–848. doi: 10.1021/jm030893g. [DOI] [PubMed] [Google Scholar]
- 22.Pisano C, Aulicino C, Vesci L, Casu B, Naggi A, Torri G, Ribatti D, Belleri M, Rusnati M, Presta M. Undersulfated, low-molecular-weight glycol-split heparin as an antiangiogenic VEGF antagonist. Glycobiology. 2005;15:1C–6C. doi: 10.1093/glycob/cwi007. [DOI] [PubMed] [Google Scholar]
- 23.Lundin L, Larsson H, Kreuger J, Kanda S, Lindahl U, Salmivirta M, Claesson-Welsh L. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem. 2003;75:155–164. doi: 10.1074/jbc.M908930199. [DOI] [PubMed] [Google Scholar]
- 24.Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, Vlodavsky I, Abramovitch R. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J. 2005;19:211–221. doi: 10.1096/fj.04-1970com. [DOI] [PubMed] [Google Scholar]
- 25.Vlodavsky I, Abboud-Jarrous G, Elkin M, Naggi A, Casu B, Sasisekharan R, Ilan N. The impact of heparanase and heparin on cancer metastasis and angiogenesis. J Pathophys Haemost Thromb. 2006;35:116–127. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- 26.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvig RJ, Boehme G, Podda M, Henschler R, Jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R, Gille J. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–2750. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- 28.Borsig L. Antimetastatic activities of modified heparins: selectin inhibition by heparin attenuates metastasis. Semin Thromb Hemost. 2007;33:540–546. doi: 10.1055/s-2007-982086. [DOI] [PubMed] [Google Scholar]
- 29.Hostettler N, Naggi A, Torri G, Isahai-Michaeli R, Casu B, Vlodavsky I, Borsig L. P-selectin and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. FASEB J. 2007;21:3562–3572. doi: 10.1096/fj.07-8450com. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson JL, Varki, A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb Res. 2007;120(suppl 2):S107–S111. doi: 10.1016/S0049-3848(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparin – correlation with selectin inhibition, not antithrombotic activity. Clin Cancer Res. 2005;11:7003–7011. doi: 10.1158/1078-0432.CCR-05-1131. [DOI] [PubMed] [Google Scholar]
- 32.Ashikari-Ada S, Habuchi H, Karya Y, Itoh N, Reddi AH, Kimata K. Characterization of growth factor binding structures in heparin/heparan sulfate using an octasaccharide library. J Biol Chem. 2004;279:12326–12354. doi: 10.1074/jbc.M313523200. [DOI] [PubMed] [Google Scholar]
- 33.Hasan J, Shnyder SD, Clamp AR, McGown AT, Bicknell R, Presta M, Bibby M, Double J, Craig S, Leeming D, Stevenson K, Gallagher JT, Jayson GC. Heparin octasaccharides inhibit angiogenesis in vivo. Clin Cancer Res. 2005;11:8172–8179. doi: 10.1158/1078-0432.CCR-05-0452. [DOI] [PubMed] [Google Scholar]
- 34.Koenig A, Norgard-Sumnicht, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low-molecular-weight heparin as therapeutic agents. J Clin Inv. 1988;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norrby K. Low-molecular-weight heparins and angiogenesis. Acta Pathol Microb Immunol. 2006;114:79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x. [DOI] [PubMed] [Google Scholar]
- 36.Mellor P, Harvey JR, Murphy KJ, Pye D, O’Boyle G, Lennard TWJ, Kirby JA, Ali S. Modulatory effects of heparin and short-length oligosaccharides of heparin on the metastasis and growth of LMD-MB 231 breast cancer cells in vivo. Br J Cancer. 2007;97:761–768. doi: 10.1038/sj.bjc.6603928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlodavsky I, Ilan N, Nadir Y, Brenner B, Katz BZ, Naggi A, Torri G, Casu B, Sasisekharan R. Heparanase, heparin and the coagulation system in cancer progression. Thromb Res. 2007;120(suppl 2):112–120. doi: 10.1016/S0049-3848(07)70139-1. [DOI] [PubMed] [Google Scholar]
- 38.Amirkhosravi A, Meyer T, Amaya M, Davila M, Mousa SA, Robson Y, Francis JL. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin Thromb Hemost. 2007;33:634–652. doi: 10.1055/s-2007-991531. [DOI] [PubMed] [Google Scholar]
- 39.Nasser NJ, Sarig G, Brenner B, Nevo E, Ilan N, Goldschmidt O, Zhacaria E, Li JP, Vlodavsky I. Heparanase neutralizes the anti-coagulation properties of heparin and low-molecular-weight heparin. J Thromb Haemost. 2006;4:560–565. doi: 10.1111/j.1538-7836.2006.01792.x. [DOI] [PubMed] [Google Scholar]
- 40.Rickles FR. If heparanase is the answer, what is the question? J Thromb Haemost. 2006;4:557–559. doi: 10.1111/j.1538-7836.2006.01828.x. [DOI] [PubMed] [Google Scholar]
- 41.Sanderson RD, Yang Y. 2007 Syndecan-1: a dynamic regulator of the myeloma microenvironment. Clin Exp Metastasis. 2008;25:149–159. doi: 10.1007/s10585-007-9125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijdenes J, Vooijs WC, Clement C, Post J, Morard F, Vita N, Laurent P, Sun RX, Klein B, Dore JM. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94:318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 43.Seidel C, Sundan A, Hjorth M, Turesson I, Dahl IM, Abildgaard N, Waage A, Borset M. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000;95:388–392. [PubMed] [Google Scholar]
- 44.Lovell R, Dunn JA, Begum G, Barth NJ, Plant T, Moss PA, Drayson MT, Pratt G. Soluble syndecan-1 level at diagnosis is an independent prognostic factor in multiple myeloma and the extent of fall from diagnosis to plateau predicts for overall survival. Br J Haematol. 2005;130:542–548. doi: 10.1111/j.1365-2141.2005.05647.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, Epstein J, Sanderson RD. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- 46.Andersen NF, Standal T, Nielsen JL, Heickendorff L, Borset M, Sorensen FB, Abildgaard N. Syndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survival. Br J Haematol. 2005;128:210–217. doi: 10.1111/j.1365-2141.2004.05299.x. [DOI] [PubMed] [Google Scholar]
- 47.Kelly T, Miao HQ, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, Shaughnessy J, Sanderson RD. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–8756. [PubMed] [Google Scholar]
- 48.Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, De Vos J, Larroque M, Moehler T, Rossi JF, Reme T, Goldschmidt H, Klein B. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, Sasisekharan R, Naggi A, Torri G, Casu B, Vlodavsky I, Suva LJ, Epstein J, Yaccoby S, Shaughnessy JD, Jr, Barlogie B, Sanderson R. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, MacLeod V, Bendre M, Huang Y, Theus AM, Miao HQ, Kussie P, Yaccoby S, Epstein J, Suva LJ, Kelly T, Sanderson RD. Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood. 2005;105:1303–1309. doi: 10.1182/blood-2004-06-2141. [DOI] [PubMed] [Google Scholar]