Abstract
Objective
To assess cerclage to prevent recurrent preterm birth in women with short cervix.
Study Design
Women with prior spontaneous preterm birth <34 weeks were screened for short cervix, and randomly assigned to cerclage if cervical length was <25 mm.
Results
Of 1014 women screened, 302 were randomized; 42% of women not assigned and 32% of those assigned to cerclage delivered <35 weeks (p=0.09). In planned analyses, birth <24 weeks (p=0.03) and perinatal mortality (p=0.046) were less frequent in the cerclage group. There was a significant interaction between cervical length and cerclage. Birth <35 weeks (p = 0.006) was reduced in the <15 mm stratum with a null effect in the 15–24 mm stratum.
Conclusion
In women with a prior spontaneous preterm birth <34 weeks and cervical length <25 mm, cerclage reduced previable birth and perinatal mortality but did not prevent birth <35 weeks, unless cervical length was <15 mm.
Keywords: cervical length, cerclage, prior preterm birth, vaginal sonography
The role of cervical cerclage to prevent preterm birth is controversial.1,2 Originally proposed for use in women with recurrent mid-trimester pregnancy loss that was unaccompanied by bleeding, contractions, infection, or ruptured membranes,3 cerclage has subsequently been more broadly recommended for women with a history of preterm birth, especially if the gestational age at birth was less than 26 weeks.4 Ultrasound studies5,6 showing that the cervix appeared to shorten without contractions in women destined for preterm birth led many to consider cerclage as prophylaxis,7–9 but several randomized trials have not supported this practice.10–12
Althuisius et al. observed a significant benefit in a small clinical trial of women whose history or symptoms suggested cervical insufficiency; preterm birth before 35 weeks of gestation was observed in 44% of the no-cerclage group versus none of the women who were assigned to receive cerclage (p = 0.002).13 Larger trials10, 11 included women with various historic risk factors for spontaneous preterm birth: Rust et al.10 observed rates of preterm birth before 34 weeks of gestation in 35% of cerclage-group women versus 36% of controls. Berghella et al.11 also observed similar rates of preterm birth before 35 weeks of gestation regardless of group assignment: 45% in the cerclage group versus 47% in controls. Finally, a large multinational trial12 enrolled unselected, but mostly low-risk women with shortened cervical length of 15 mm or less and also found no significant reduction in preterm birth before 33 weeks of gestation in women randomly assigned to treatment with cerclage (22%) versus controls (26%). More recently a patient-level meta-analysis14 of these 4 randomized cerclage trials uncovered a relationship between pregnancy history and cerclage: intervention was effective only in singleton pregnancies (there was significant harm observed in women with a multiple gestation), and it was especially beneficial in women who had a prior preterm birth (adjusted odds ratio, 0.6). Thus, significant controversy remains regarding appropriate candidate selection for cerclage.15, 16 We hypothesized that cerclage would reduce the rate of preterm birth before 35 weeks gestation in women with a prior early spontaneous preterm birth before 34 weeks’ gestation and whose mid-trimester cervical length was less than 25 mm.
METHODS
This randomized controlled trial was performed by a consortium of 15 U.S. Clinical Centers between January, 2003 and November, 2007. Healthy multiparous women carrying a singleton gestation who enrolled for prenatal care were screened to identify those with at least one prior spontaneous preterm birth between 170/7 and 336/7 weeks’ gestation, confirmed by a review of the patient’s medical records. When efforts to retrieve the records of the prior birth were unsuccessful, we women as eligible if the events surrounding the prior birth included spontaneous causes such as preterm labor or preterm membrane rupture, and the reported birth weight was less than 2 kg.
Exclusion criteria were fetal anomaly, planned history-indicated cerclage for a clinical diagnosis of cervical insufficiency, and clinically significant maternal-fetal complications (e.g. fetal red cell isoimmunization, treated chronic hypertension, insulin-dependent diabetes) which would increase the risk of an indicated preterm birth and potentially confound the primary study outcome. Women with cerclage in a prior pregnancy were not excluded if review indicated that the cerclage had been placed for an indication other than classically defined cervical insufficiency. Qualifying women were invited to enroll in the ultrasound screening phase of the study.
Gestational age was established by a certain last menstrual period (if available), confirmed by standard sonographic biometric measurements at less than 20 weeks’ gestation. If a certain last menstrual period was not reported, gestational age was defined using the earliest available sonographic biometric information, and a second-trimester fetal anatomic assessment was performed to rule out structural anomalies. The conception date was used for women whose pregnancies were conceived by assisted reproductive techniques. As part of routine obstetric care, women were screened for N. gonorrhoeae and C. trachomatis, and treatment was prescribed for those who were culture-positive.
Fifty-six sonologists underwent a uniform certification process by a single investigator (J.O.) to ensure uniformity in sonographic equipment, measurement technique, completion of study forms, and adherence to protocol. Specifics of this sonographic evaluation based on the technique of Iams have been previously described. 17 Briefly, the cervical length at each visit was measured along a closed endocervical canal, where minimal degrees of apparent dilation (i.e. echolucency along the entire canal) less than 5 mm were considered closed. Fundal pressure was also applied for 30 seconds as a provocative maneuver, and each scan included an evaluation period of at least 5 minutes to detect spontaneously occurring cervical shortening. The shortest cervical length for each examination that clearly displayed the internal and external cervical os with equivalent thickness of the anterior and posterior cervix was recorded as the cervical length, regardless of whether the measurement was obtained with pressure or was the result of spontaneous dynamic shortening.
Eligible women consented to serial transvaginal ultrasound examinations to measure cervical length, the first of which was scheduled in the gestational age window 160/7 to 216/7 weeks’ gestation. Follow-up scans were scheduled every 2 weeks unless the cervical length was observed to be 25–29 mm, after which scan frequency was increased to weekly. Women with a cervical length that remained at least 25 mm by the final sonographic evaluation, scheduled to be no later than 226/7 weeks, were ineligible for randomization and resumed their obstetric care.
If on any evaluation the cervical length was <25 mm, the woman became eligible for randomization. Informed consent was then obtained for centralized random assignment to cerclage or no cerclage. Since the cerclage intervention was not masked, managing physicians might infer that the cervical length was less than 25 mm, but they were otherwise masked to the results of the sonographic evaluations except in cases of complete placenta previa, oligohydramnios or fetal death.17 At the qualifying cervical length evaluation, a sterile speculum examination was also performed to rule out acute cervical insufficiency which we defined as a cervical dilation of at least 2 cm with membranes visible. In these cases, managing physicians were notified, and women became ineligible for randomization.
Consenting women assigned to the cerclage intervention group were to be scheduled for their surgery within 96 hours of the qualifying scan, and a McDonald procedure3 with non-absorbable suture was the cerclage technique of choice. The use of perioperative prophylactic antibiotics and tocolytic medications was not specified in the protocol and left to the discretion of the managing physicians. Post-randomization patient management was similar in both cerclage and no-cerclage groups and included the recommendation for pelvic rest, described as abstinence from any sexual activity involving penetration of the vagina, no use of tampons, and no douching. Recommended physical activity restrictions consisted of no prolonged standing >4 hours, no heavy physical work involving lifting >20 pounds or straining, exercise only in moderation with no impact aerobics or other activity that involves straining or valsalva, such as weight training, and avoidance of any activity that brings on symptoms of pelvic pressure or discomfort. Women were also educated regarding the signs and symptoms of preterm labor and preterm membrane rupture and instructed to report any changes in vaginal discharge, vaginal fluid, bleeding or abdominal pain to their care providers. Research nurses at each center maintained weekly contact with participants. Otherwise, management was directed by clinical practice at each center. Women in the no-cerclage group could receive a physical-examination indicated cerclage for acute cervical insufficiency diagnosed on clinical examination, while women who had undergone cerclage as their trial intervention could undergo cerclage revision if clinically indicated; post randomization transvaginal ultrasound information was not utilized for clinical decision making. In the absence of pregnancy complications requiring earlier removal (e.g. chorioamnion rupture, labor, hemorrhage), the cerclage suture was removed at 37 weeks’ gestation.
The protocol and consent forms received local institutional review board approval at all centers.
Assessment of outcome and statistical analysis
The primary study outcome was birth at <35 weeks’ project gestational age. From a previous report17 we estimated that 57% of women in the no-cerclage group would experience a preterm birth before 35 weeks’ gestation. The study was designed to have 80% power to detect a 30% reduction in the rate of preterm birth, or to an absolute rate of 40%. Allowing also for a maximum 10% lost-to-follow-up rate, we planned to enroll 300 women in the randomized intervention trial.
Because of previous observations demonstrating a preponderance of mid-trimester births in these high-risk women with shortened cervical length,18 planned secondary outcomes of interest included the rates of birth <7 days from randomization, previable birth (<24 weeks) and perinatal death, defined as either a stillbirth or a postnatal death prior to hospital discharge. We also planned to evaluate preterm birth <37 weeks. Since cervical length as a surrogate for cervical competence is believed to operate on a continuum19 with a well-documented inverse relationship between shortest mid-trimester cervical length and the risk of preterm birth,17 we had also hypothesized an interaction between cerclage efficacy and cervical length at randomization. Thus, we planned an analysis to assess the interaction between cervical length and treatment, and if found significant at the p = 0.10 level, associated analyses similar to the primary aims and within cervical length strata (less than 15 mm versus 15–24 mm) would be performed.
Randomization in predetermined blocks was stratified by each center and qualifying cervical length <20 mm versus 20–24 mm. Early in the trial (May, 2003), the results of a randomized trial of 17-alpha hydroxyprogesterone caproate became available.20 In response to this report the steering committee and an independent data and safety monitoring board recommended that the use of progesterone for preterm birth prevention be an option for study participants. This was included in the informed consent process, and an additional randomization stratum, reflecting the woman’s stated intent to use progesterone, was added.
Intergroup comparisons were performed using the principle of intent to treat. The primary study outcome and other categorical variables were analyzed with chi-square, while continuous variables were analyzed using a t-test or Wilcoxon rank-sum test. Treatment differences in time to birth were assessed by Kaplan-Meier curves and the log-rank test. Multivariable logistic regression and Cox proportional hazard models considered possible confounders for the outcomes of preterm birth <35 weeks and time to birth respectively.
A single interim analysis was performed after half the planned sample had been randomized (yielding approximately 1/3 of the planned 300 with pregnancy outcomes) using O’Brien-Fleming boundaries with critical values of p = 0.0064 at the interim assessment and p = 0.0498 for the final assessment.
RESULTS
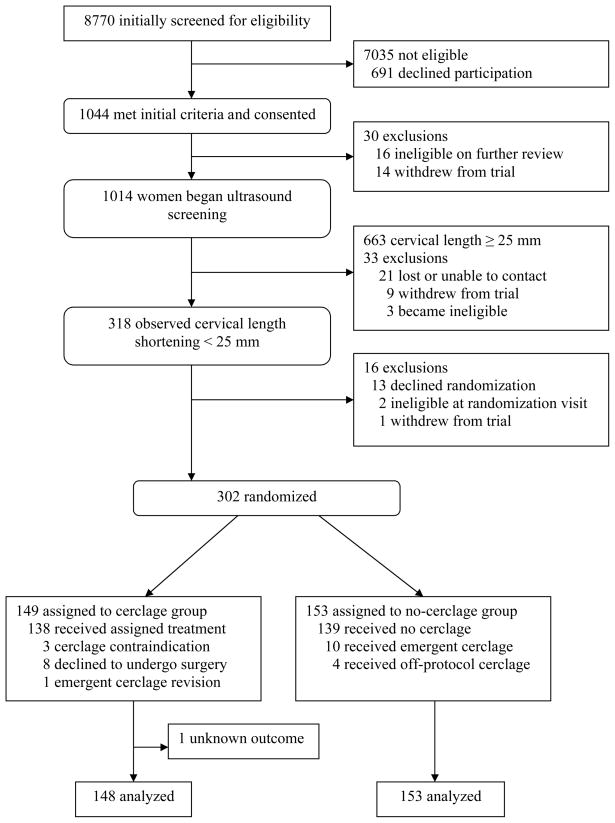
Of the 1044 women who were determined to have a qualifying prior preterm birth, 1014 (99%) were consented and underwent their initial sonographic assessment of cervical length. Review of prior pregnancy information indicated that of these 1014, 831 (82%) entered screening after medical record review confirmed a qualifying prior preterm birth. From this cohort, we observed 318 (31%) who experienced cervical length shortening less than 25 mm. Sixteen patients did not consent to randomization, and 302 (95%) were randomly assigned to no-cerclage or cerclage groups. Primary outcome information was available for all 153 in the no-cerclage group and for 148 of 149 in the cerclage group, leaving a total of 301 women in the analysis (Fig. 1). Only one patient was excluded from randomization because of the diagnosis of acute cervical insufficiency at the randomization visit. Selected baseline characteristics of the study population are shown in Table 1, showing the 2 groups to be well-balanced.
Fig. 1.
Table 1.
Baseline characteristics and treatment group differences for 301 women randomly assigned to cerclage or to no-cerclage groups.
| No-cerclage (n = 153) | Cerclage (n = 148) | |
|---|---|---|
| Race/ethnicity* - no. (%) | ||
| Black (non-Hispanic) | 93 (61) | 80 (54) |
| White (non-Hispanic) | 28 (18) | 25 (16.9) |
| Hispanic | 17 (11) | 27 (18.2) |
| Asian | 0 (0) | 1 (0.7) |
| Other | 15 (9.8) | 15 (0.1) |
| Marital Status - no. (%) | ||
| Single/never married | 99 (65) | 85 (57) |
| Married | 42 (27) | 49 (33) |
| Divorced | 10 (6.5) | 13 (8.8) |
| Widowed | 1 (0.7) | 0 (0.0) |
| Other | 1 (0.7) | 1 (0.7) |
| Cigarette use - no. (%) | 30 (20) | 24 (16) |
| Any drug abuse - no. (%) | 10 (6.5) | 5 (3.4) |
| Cervicovaginal microbiology - no. (%) | ||
| Chlamydia | 8 (5.2) | 6 (4.0) |
| Gonorrhea | 2 (1.3) | 1 (0.7) |
| One or more prior Induced abortion - no. (%) | 25 (16) | 25 (17) |
| Prior cerclage - no. (%) | 12 (7.8) | 8 (5.4) |
| Maternal age (y) | 26.6 ± 5.1 | 26.4 ± 5.5 |
| Body mass index (kg/m2) | 29.9 ± 7.5 | 29.2 ± 7.8 |
| Number of prior births (n) | 2 (1, 4)† | 2 (1,4)† |
| Years of education (n) | 11.9 ± 2.4 | 12.0 ± 2.8 |
| Gestational age of qualifying birth (wks) | 24.9 ± 4.7 | 24.4 ± 4.9 |
| Weeks of gestation at first vaginal sonogram (wks) | 17.4 ± 1.4 | 17.4 ± 1.2 |
| Cervical length at first vaginal sonogram (mm) | 29.5 ± 12.9 | 28.5 ± 12.7 |
| Weeks of gestation at randomization (wks) | 19.5 ± 2.0 | 19.4 ± 1.9 |
| Cervical length at randomization (mm) | 19.5 ± 5.3 | 18.6 ± 6.3 |
| Total number of vaginal sonograms (N) | 2 (1, 4)† | 2 (1, 4)† |
Plus-minus values are means and one standard deviation.
Race and ethnic group are self-reported
Median and interdecile range
Compliance with the intervention was good; a total of 14 (9.1%) women assigned to the no-cerclage group underwent the procedure, 4 solely at the discretion of their managing physicians (off-protocol treatment crossover), while 10 were placed for a diagnosis of acute cervical insufficiency (protocol-sanctioned treatment crossover), confirmed by review of the maternal records. Eleven (7.4%) women in the cerclage group did not receive the planned intervention: 8 declined to undergo surgery, while 3 procedures were contraindicated due to obstetric complications (intraamniotic infection, fetal death and cervicitis) and were cancelled by the managing physicians.
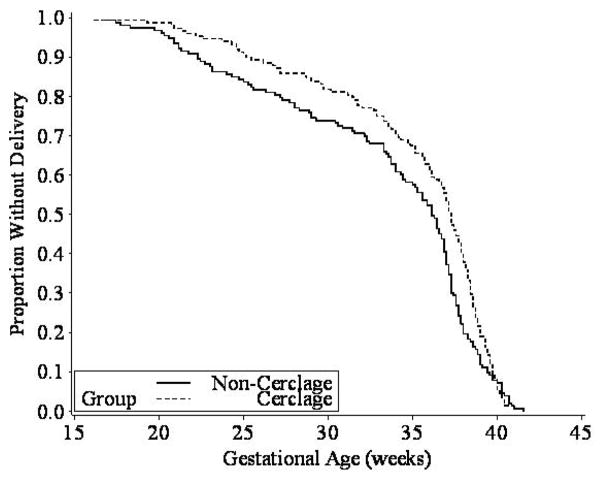
The primary outcome of preterm birth <35 weeks’ gestation was observed in 32% of women in the cerclage group versus 42% in the no-cerclage group (odds ratio, 0.67, 95% CI, 0.42 to 1.07; p = 0.09). As depicted in Figure 2, the Kaplan-Meier survival analysis, considering the time to birth (i.e. duration of gestation), suggested an overall benefit from cerclage (log-rank test p = 0.053).
Fig. 2.
The addition of cervical length at randomization as a continuous variable to a logistic regression model strengthened the association between cerclage and preterm birth less than 35 weeks (odds ratio, 0.60, 95% CI, 0.37 to 0.98; p = 0.04). We further evaluated the effect of cervical length at randomization in the 2 strata. The interaction between randomization cervical length strata less than 15 mm (n=64) versus 16–24 mm (n=237) and treatment was significant (p = 0.03). Stratified analyses indicated that in the less-than-15 mm stratum, there was a significant benefit from cerclage assignment (odds ratio=0.23; 95% CI 0.08 to 0.66; p = 0.006) versus a null finding in the 15–24 mm stratum (odds ratio, 0.84, 95% CI, 0.49 to 1.4; p = 0.52).
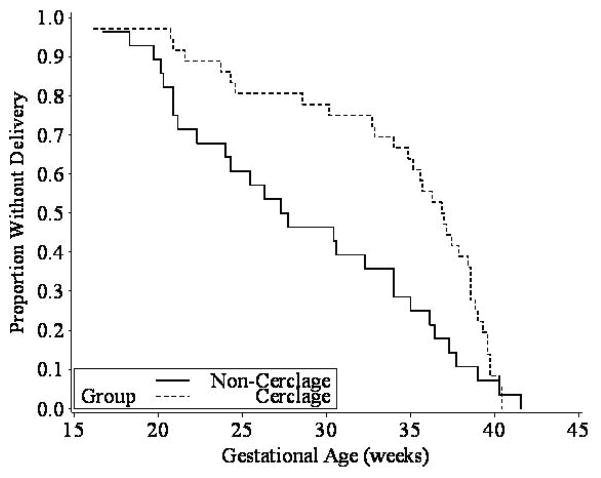
As depicted in Figure 3, the Kaplan-Meier graph and associated log-rank test (p = 0.024) demonstrated a significant beneficial effect of cerclage in the less-than-15 mm stratum. Similarly, a Cox proportional hazards model demonstrated that the women in the less-than-15 mm cervical length stratum who were assigned to cerclage had a significantly lower hazard for an earlier birth as compared to the no-cerclage group (hazard ratio, 0.57, 95% CI, 0.34 to 0.95; p = 0.03). As observed in the logistic regression analysis above, the relationship between cerclage assignment and pregnancy duration in the 15–24 mm stratum in the survival analysis was also null (hazard ratio, 0.84, 95% CI, 0.65 to 1.09; p = 0.20).
Fig. 3.
When the progesterone-use stratum was introduced, only 10 of the eventual 302 (3.3%) women had been randomized. Of the subsequent 292, 117 were randomized within the progesterone stratum: 56 were assigned to the cerclage group and 61 to no cerclage. Of the 175 who did not plan to use progesterone, 89 were assigned to cerclage and 86 to no cerclage (p = 0.62). The single woman who was lost-to-follow-up was randomized both to the cerclage group and with the intent to use progesterone. In a logistic regression model, the effect of the patient’s plan to use progesterone on preterm birth less than 35 weeks was null (odds ratio, 0.97, 95% CI, 0.6 to 1.6). We also included the progesterone strata in a multivariable model with the intervention group and an interaction term. The interaction term was not significant (p = 0.94). The inclusion of the patient’s plan to use progesterone in the model had no appreciable effect on the relationship between cerclage intervention and birth less than 35 weeks (adjusted odds ratio, 0.67, 95% CI, 0.42 to 1.1; p = 0.09).
Secondary perinatal outcomes are depicted in Table 2. Delivery less than 7 days from randomization was very uncommon, affecting only 7 (2.3%) women, and the intergroup distribution was not significantly different (p = 0.72). However, previable birth less than 24 weeks occurred in 14% of the no-cerclage group versus 6.1% of the cerclage group (p = 0.03), and preterm birth less than 37 weeks was also less common in the cerclage group (p = 0.01). Intergroup rates of perinatal death were also significantly different: 8.8% in the cerclage group versus 16% in the no-cerclage group (p = 0.046).
Table 2.
Secondary perinatal outcomes for 301 women randomly assigned to cerclage or to no-cerclage groups.
| No-cerclage (n = 153) | Cerclage (n = 148) | P value | |
|---|---|---|---|
| Birth < 7 days from randomization - no. (%) | 3 (2.0) | 4 (2.7) | 0.72 |
| Previable birth < 24 weeks - no. (%) | 21 (14) | 9 (6.1) | 0.03 |
| Preterm birth < 37 weeks - no. (%) | 91 (60) | 66 (45) | 0.01 |
| Perinatal death - no. (%)* | 25 (16) | 13 (8.8) | 0.046 |
One neonate in the cerclage group was lost-to-follow-up
We also examined the homogeneity of the effect of cerclage on preterm birth outcomes across the participating centers with the Breslow-Day test. There was no significant heterogeneity across sites for birth less than 35 weeks (p = 0.06), birth less than 37 weeks (p=0.33), less than 24 weeks (p = 0.067), or perinatal death (p = 0.24).
Surgical adverse events associated with cerclage placement were uncommon. Of the women who underwent either protocol-directed cerclage (cerclage group, N = 138), emergent cerclage (no-cerclage group, N = 14) or a cerclage revision (cerclage group, N = 1), only 2 experienced a reported complication: one experienced chorioamnion rupture during the procedure, and one experienced a postoperative hemorrhage. There were 2 reported surgical anesthetic complications: one failed spinal and one post-spinal headache.
COMMENT
We did not observe a statistically significant benefit from cerclage in preventing birth before a gestational age of 35 weeks, the primary outcome for the trial. While somewhat arbitrary, this gestational age endpoint was chosen to avoid cases of near-term birth, which are associated with much lower rates of neonatal morbidity and only rare mortality. Nevertheless, the weight of our findings suggests that cerclage, utilized for shortened cervical length in selected women with a prior early spontaneous preterm birth, can improve pregnancy outcomes with essentially no demonstrable harm.
We believe that the most clinically important finding from this randomized trial is the interaction between cervical length at randomization and cerclage effectiveness. The risk of prematurity is inversely proportional to cervical length measured with endovaginal sonography at various times in gestation, and the mid-trimester has been the focus of most of the research in this area.1,5,17 We have demonstrated a biologically predictable, differential benefit of cerclage when the cervical length is very short, less than 15 mm. We chose a priori to examine 15 mm as an alternate cutoff to define shortened cervical length, because this has been utilized by other investigators to assess both the predictive value of sonographic cervical length 6 and cerclage effectiveness for shortened cervical length.7,13 Still unclear are the factors which incite pathologic cervical shortening in these women.15 Similarly, the precise mechanism by which cerclage confers a benefit is unknown, but it may support the immunological barrier between the chorioamnion-extraovular space and the vaginal microbiologic flora.21
Because of the well-known relationship between preterm birth history and subsequent pregnancy outcome,2 we had also considered the possible effect of the gestational age of the prior preterm birth on cerclage efficacy with regard to the trial’s primary outcome; the effect here was null (data not shown). However, based on prerandomization data from this trial, we recently reported the relationship between birth history and cervical length.22 Women with a prior birth <24 weeks were significantly more likely to experience cervical shortening (<25mm) and did so at an earlier gestational age than women whose earliest prior birth occurred at 24–336/7 weeks. Thus, we conclude that, while birth history affects cervical length in a subsequent pregnancy, once shortening <25 mm is observed, this history does not significantly affect the cerclage intervention.
Possible limitations to our trial include the open treatment, as blinding may only have been possible with sham surgery. Even then, evidence of the cerclage suture would be readily visible during a pelvic examination. However, since the primary and secondary outcomes were objective, the potential impact from lack of blinding may be minimal. The possibility of missing women who underwent rapid shortening and delivery during the sonographic screening was a concern, but only one woman was excluded from the randomized trial because of acute cervical insufficiency.
Another possible limitation was our decision to cap the upper gestational age cutoff for screening and randomization at 226/7 weeks of gestation, potentially limiting the generalizability of results beyond this gestational age. While somewhat arbitrary, we were concerned about the possibility of cerclage-associated complications at the threshold of viability and the possibility of an interaction with other common post-viability treatments for women with threatened preterm birth. We recognize that other investigators have extended this temporal window to include more of the mid-trimester.10 To the extent that some of our high-risk patients may have continued to experience pathologic cervical shortening after completion of ultrasound screening (as evidenced by the 10 women who later presented with acute cervical insufficiency and underwent physical exam-indicated cerclage), and who may also have benefitted from earlier cerclage placement, our findings may have underestimated the utility of the intervention in this population.
The finding of no interaction between cerclage and progesterone and the complete lack of effect of progesterone on preterm birth in this trial was surprising. We purposefully added the progesterone stratum after a large randomized trial reported a reduced rate of recurrent preterm birth in women treated with 17 alpha-hydroxy progesterone caproate.20 Nevertheless, in spite of that demonstrated benefit, only 39% of our participants stated their intent to use progesterone for preterm birth prevention. However, because 17 alpha-hydroxyprogesterone caproate has to be extemporaneously compounded, and is variably covered by third party payers, we could not control the precise form of progestin locally available to participants or the gestational age at the initiation of treatment. Moreover, the stratum was based only on a subject’s intended use of progesterone at the time of randomization, not an intent to treat by the managing physicians.
We emphasize that this screening and treatment regimen was limited to a highly selected population of women with a prior spontaneous preterm birth of a non-anomalous singleton at less than 34 weeks of gestation, primarily confirmed by history and review of maternal records. We have demonstrated that women with a prior early spontaneous preterm birth represent a population that can benefit from endovaginal sonographic cervical assessment. We recommend that women with this history be considered for serial cervical length measurement at 2 week intervals, beginning as early as 16 weeks of gestation. Our screening schedule included weekly assessment if the cervical length was within 5 mm of our action point for randomization (25–29 mm). Nevertheless, our findings may not be prescriptive regarding the optimal cervical length cutoff for cerclage for the indication of shortened cervical length in these women at risk for recurrent preterm birth. In planned secondary analyses we demonstrated improved obstetric outcomes in the form of lower rates of previable birth and perinatal mortality using the trial’s entry cervical length cutoff of 25 mm. However, we also recognize that the beneficial effect of cerclage for pregnancy prolongation varies, depending on the degree of cervical length shortening prior to 23 weeks of gestation, and is significantly more pronounced in women with very shortened cervical length less than 15 mm.
Acknowledgments
Source of funding: The Eunice Kennedy Shriver National Institute of Child Health and Development provided funding via grant U01 HD039939-05; from the same agency, Dr. Owen also received support via grant 5K24 HD43314-5.
Data safety and monitoring committee: Dr. Andrew Satin (Chair), Dr. Cora McPherson, Dr. Alessandro Ghidini, Dr. Roger Soll, and Heidi Maloni.
We also acknowledge George Howard, DrPH, Rachel Copper, RN, MSN and Robin Steele for their many contributions to the trial.
Footnotes
Presented in abstract form on 1/29/09 at the 2009 annual meeting of the Society for Maternal-Fetal Medicine in San Diego, CA.
Publisher's Disclaimer: This is a PDF file of an unedited that has been for publication. As a service to our customers we are providing this early version of the. The will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owen J, Iams JD, Hauth JC. Vaginal sonography and cervical incompetence. Am J Obstet Gynecol. 2003;188:586–96. doi: 10.1067/mob.2003.137. [DOI] [PubMed] [Google Scholar]
- 2.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 3.McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Empire. 1957;64:346–53. doi: 10.1111/j.1471-0528.1957.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 4.Cromblehome WR, Minkooff HL, Delke I, Schwarz RH. Cervical cerclage: An aggressive approach to threatened or recurrent pregnancy wastage. Am J Obstet Gynecol. 1983;146:168–74. doi: 10.1016/0002-9378(83)91048-7. [DOI] [PubMed] [Google Scholar]
- 5.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM for the National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 6.Heath VCF, Southall TR, Souka AP, Elliseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: Prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–7. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 7.Heath VCF, Souka AP, Erasmus I, Gibb DMF, Nicolaides KH. Cervical length at 23 weeks of gestation: The value of Shirodkar suture for the short cervix. Ultrasound Obstet Gynecol. 1998;12:318–22. doi: 10.1046/j.1469-0705.1998.12050318.x. [DOI] [PubMed] [Google Scholar]
- 8.Berghella V, Daly SF, Tolosa JE, DiVito MM, Chalmers R, Gorg N, Bhullar A, Wapner RJ. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: Does cerclage prevent prematurity? Am J Obstet Gynecol. 1999;181:809–15. doi: 10.1016/s0002-9378(99)70306-6. [DOI] [PubMed] [Google Scholar]
- 9.Hassan SS, Romero R, Maymon E, Berry SM, Blackwell SC, Treadwell MC, Tomlinson M. Does cervical cerclage prevent preterm delivery in patients with a short cervix? Am J Obstet Gynecol. 2001;184:1325–31. doi: 10.1067/mob.2001.115119. [DOI] [PubMed] [Google Scholar]
- 10.Rust OA, Atlas RO, Reed J, van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: Why cerclage may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 11.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound: A randomized trial. Am J Obstet Gynecol. 2004;191:1311–7. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 12.To MS, Alfirevic Z, Heath VCF, Cicero S, Cacho AM, Williamson PR, Nicolaides KH on behalf of the Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–53. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 13.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the cervical incompetence prevention randomized cerclage trial (CIPRACT): Therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001a;185:1106–12. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 14.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for Short Cervix on Ultrasonography, Meta-Analysis of Trials Using Individual Patient-Level Data. Obstet Gynecol. 2005;106(1):181–9. doi: 10.1097/01.AOG.0000168435.17200.53. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Espinoza J, Erez O, Hassan S. The role of cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfirevic Z. Cerclage: we all know how to do it but can’t agree when to do it. Obstet Gynecol. 2006;107:219–20. doi: 10.1097/01.AOG.0000194479.93493.2c. [DOI] [PubMed] [Google Scholar]
- 17.Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, Miodovnik M, Langer D, Sibai BM, McNellis D for the National Institute for Child Health and Human Development Maternal Fetal Medicine Unit Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–8. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 18.Owen J, Yost N, Berghella V, et al. Can shortened mid-trimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: A study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–106. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 20.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm birth by 17 alpha-hydroxyprogesterone caproate. NEJM. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 21.Jones G. The weak cervix: Failing to keep the baby in or infection out? Br J Obstet and Gynaecol. 1998;105:1214–5. doi: 10.1111/j.1471-0528.1998.tb09979.x. [DOI] [PubMed] [Google Scholar]
- 22.Szychowski JM, Owen J, Hankins G, et al. Timing of mid-trimester cervical length shortening in high-risk women. Ultrasound Obstet Gynecol. 2009;33:70–5. doi: 10.1002/uog.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]