Abstract
The peptide 4F is known to have potent anti-atherogenic activity. 4F is an 18 residue peptide that has a sequence capable of forming a class A amphipathic helix. Several other class A amphipathic helical, 18 residue peptides with the same polar face but with increasing Phe residues on the nonpolar face have been synthesized with varying degrees of biological activity. In this work we compared the properties of the original 2F peptide, modeled on the consensus sequence of the amphipathic helical segments of the apolipoprotein A–I with the peptide 4F that has two Leu residues replaced with Phe. We demonstrate that the more biologically active 4F peptide has the greatest affinity for binding to several molecular species of oxidized lipids. Lipoprotein particles can be formed by solubilizing 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC). These solubilized lipoprotein particles extract oxidized lipid from liposomes of POPC containing 5 mol% of oxidized lipid. The peptides with the strongest anti-atherogenic activity interact most strongly with the oxidized lipid. The results show that there is a correlation between the biological potency of these peptides and their ability to interact with certain specific cytotoxic lipids, suggesting that this interaction may contribute favourably to their biological properties.
Keywords: atherosclerosis, amphipathic helices, lipid oxidation products, peptide-lipid interactions, fluorescence
Introduction
There is increasing evidence that inflammation is an important causative factor in atherosclerosis [1–9]. It has recently been suggested that a promising approach to develop drugs against atherosclerosis is through blocking inflammatory processes [10]. One of the consequences of inflammation is the formation of lipid and protein oxidation products. Polyunsaturated acyl chains are particularly susceptible to oxidation, generally resulting in the sn-2 chain becoming shorter, with less unsaturation as well as the introduction of polar groups [11,12]. Lipid oxidation products accumulate in LDL and are found in atherosclerotic lesions [13]. One of the most abundant lipid oxidation products of LDL is hexadecyl azelaoyl phosphatidylcholine. This oxidized lipid induces apoptosis at low (micromolar) concentrations [14]. The oxidized LDL is recognized by scavenger receptors on macrophages that promote their unregulated uptake, eventually leading to the formation of foam cells. The macrophage scavenger receptor, CD36, has particularly high affinity for phospholipids with an acyl chain at the sn-2 position that incorporates a γ-hydroxy (or oxo)-α,β unsaturated carbonyl [15,16]. The oxidized lipids, 1-palmitoyl-2-(4-keto-dodec-3-ene-dioyl)phosphatidylcholine (KDdiA-PC) and 1-palmitoyl-2-(5-keto-6-octene-dioyl)phosphatidylcholine (KOdiA-PC), used in the present work, are examples of such lipids that bind to the CD36 receptor with particularly high affinity. Oxidized phospholipids also represent ligands for another member of the scavenger receptor class B, type I (SR-BI) [17]. The presence of these oxidized lipids prevents binding of HDL to SR-BI and thereby interferes with SR-BI-mediated selective uptake of cholesteryl esters in hepatocytes. Oxidative stress plays an important role in atherosclerosis even in the absence of elevated levels of cholesterol [18] and leads to the accumulation of specific oxidized phospholipids in plasma that have both an inhibitory effect on reverse cholesterol transport as well as promoting an accumulation of cholesterol esters in macrophages leading to foam cell formation and eventual deposition of cholesterol in the form of atherosclerotic plaque. Removal of oxidized lipids from LDL will decrease the infiltration of monocytes into the artery wall thus avoiding their eventual deposition. Reducing the levels of oxidized lipids in LDL has been proposed as a potential target to prevent cardiovascular disease [19,20].
Recent evidence has suggested that shortened acyl chain in the sn-2 position of oxidized phospholipids reorients itself so that it protrudes from the membrane bilayer. This has been referred to as the “lipid whisker model” [21]. Molecular dynamic simulations provide support for this conformation of oxidized lipids [22]. This leaves only one acyl chain of the oxidized lipids buried in the membrane. The presence of these oxidized acyl chains in the sn-2 position plays an important role in their biological activity [23]. This is likely a consequence of their configuration that would be expected to allow facile extraction of oxidized lipids from the membrane. In support of this is the finding that fluorescently labelled forms of these oxidized lipids are readily transferred from aqueous phospholipid dispersions or preloaded LDL to vascular smooth muscle [24].
The protective effect of HDL against atherosclerosis is not only dependent on a higher concentration of HDL in the blood but is also dependent on the molecular composition of the HDL [25]. Small variations in apo A–I can have major consequences in its ability to protect against atherosclerosis. For example, chlorination of apo A–I by myeloperoxidase reduces its effectiveness in stimulating cholesterol efflux [26]. A natural mutant form of human apo A–I, apo A–IMilano, has been recently considered for therapy in atherosclerosis [27–36]. One way in which the properties of HDL can be modified is by the binding of peptides. It is known that bound peptides represent about 1% of the protein mass of HDL [37]. In addition, we find that an anti-atherogenic amphipathic helical peptide 3F-2, analogous to 4F, binds to the HDL fraction in vivo to a much greater extent than does a homologous amphipathic helical peptide 3F14, that is devoid of biological function [38]. It is clear now that simple modifications in the sequence of 4F, such as changing Phe-3 or Phe-14 to Leu changes the in vivo properties and HDL binding capacity of the original peptide dramatically [38]. Recently biotinyl-4F was studied and it was concluded that 4F does not associate with HDL. It is possible that the authors followed the biotinyl label and not intact 4F, which may have degraded. Thus the biotin- containing fragments may have appeared away from HDL. Since the self-association and lipid or lipoprotein associating properties depend on the end protecting groups, this may account for the differing results obtained by Getz et al. [39] with the biotinyl-4F peptide.
Some peptide mimetics of apo A–I are effective in inhibiting atherosclerosis in atherosclerosis-sensitive animal models [40–47] as well as other inflammatory disorders [48–51]. They have been shown in vitro to inhibit oxidized phospholipids-induced monocyte chemotaxis and to scavenger oxidized lipids from LDL. We have earlier shown that the lipid binding ability does not determine the biological activity of these peptide analogs. For example, using the same amino acids in different arrangements of the nonpolar face of the class A amphipathic helix, despite their ability to associate with normal lipids with high affinity, possess different biological activities. This is correlated to their ability to inhibit LDL-induced monocyte chemotaxis. Among these peptides, peptide 4F was an ideal candidate to study in detail. The peptide 4F is active in inhibiting lipopolysaccharide-induced inflammatory responses [52]. The enantiomer of 4F, the most effective peptide, D-4F, is orally active in vivo and was shown to reduce inflammatory responses to influenza virus in mice [40] and to synergize with statin to cause regression of lesions [53]. This peptide also improves vascular reactivity in a rat model of diabetes [54]. D-4F has also been shown to protect against the production of oxidized cholesterol [55].
Recently several 18-residue peptide analogs that are active in synergizing with statin to even regress already existing atherosclerotic lesions in apo E-null mice [53] were shown (by surface plasmon resonance) to bind to oxidized lipids more avidly by several fold than either apo A–I or a less active analogous peptide, 3F14 [56]. We have extended this observation by measuring the binding of several different forms of oxidized phosphatidylcholine in solution without the use of solid supports. We demonstrate the ability of these peptides to interact with oxidized lipids present in a bilayer, in addition to oxidized lipid that is free in solution. We have also compared the affinity of the peptide 4F with that of 2F that was not used in the previous study. Thus this study demonstrates dramatic differences in the properties of two peptides with respect to their anti-inflammatory properties as a consequence of just by replacing two central Leu residues with Phe. The difference between Leu and Phe, especially in the context of membrane binding proteins and their properties is not well appreciated despite one being an aromatic side chain and the other an aliphatic sidechain, capable of stronger interdigitation with the lipid acyl chains, as pointed out by us in previous papers [38,57]. The properties of lipid complexes with 2F and 4F have recently been compared using NMR methods [58]. One of the mechanisms by which apo A–I and the antiatherogenic peptides can protect against atherosclerosis is by removal of oxidized lipid components from LDL [59,60]. While co-incubation of apoA–I with LDL did not inhibit LDL-induced monocyte chemotaxis, co-incubation with peptide 4F was highly effective and 2F had moderate activity. These results correlate well with their ability to inhibit atherosclerosis in mouse models of atherosclerosis. Therefore, we have tested peptides 2F and 4F for their ability to interact with specific oxidized phospholipids in bilayers using various methods and the relationship between the strength of this interaction and the biological properties of these molecules.
Experimental methods
Materials
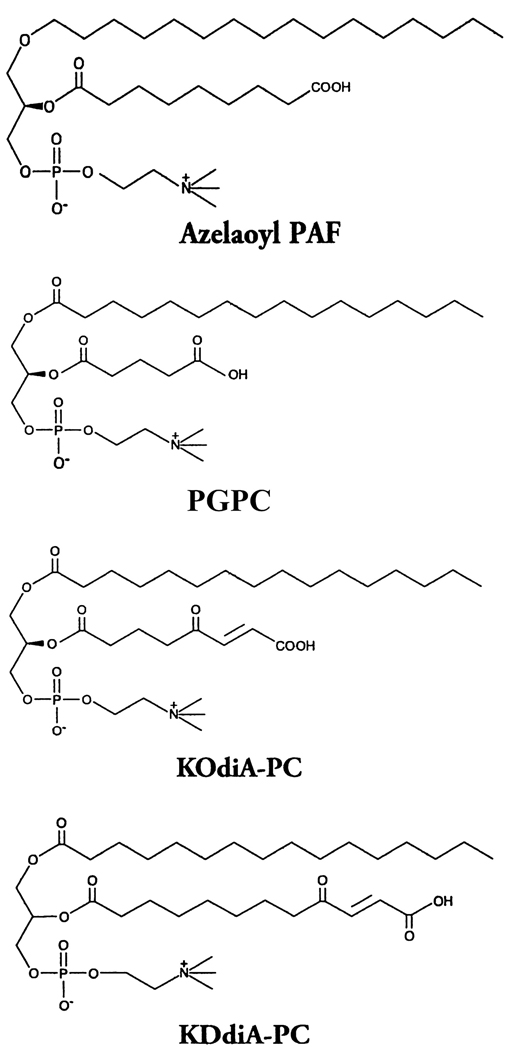
Peptides were synthesized by the solid phase method with a Protein Technologies PS-3 automatic peptide synthesizer using the procedures described previously [45,61]. Peptides were purified using a preparative HPLC system (Beckman Gold) and the purity of the peptides was ascertained by mass spectral analysis and analytical HPLC. The sequence of 2F is Ac-DWLKAFYDKVAEKLKEAF-NH2 and of 4F is Ac-DWFKAFYDKVAEKFKEAF-NH2. The phospholipids 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC), 1-stearoyl-2-oleoyl phosphatidylcholine (SOPC) and didielaidoyl phophatidylethanolamine (DEPE) as well as the oxidized lipids 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC) and 1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine (Azelaoyl PAF) were obtained from Avanti Polar Lipids (Alabaster, AL). The oxidized lipids, 1-palmitoyl-2-(4-keto-dodec-3-ene-dioyl) phosphatidylcholine (KDdiA-PC) and 1-palmitoyl-2-(5-keto-6-octene-dioyl) phosphatidylcholine (KOdiA-PC) were purchased from Cayman Chemical (Ann Arbor, MI). The structures of the oxidized lipids are shown in Figure 1.
Figure 1.
Structure of the oxidized forms of phosphatidylcholine used in this work.
Differential scanning calorimetry
Measurements were made using a Nano II Differential Scanning Calorimeter (Calorimetry Sciences Corporation, Lindon, UT). The scan rate was 2°C/min with a delay of 5 minutes between sequential scans in a series to allow for thermal equilibration. The features of the design of this instrument have been described [62]. DSC curves were analyzed by using the fitting program, DA-2, provided by Microcal Inc. (Northampton, MA) and plotted with Origin, version 5.0. Dry lipid films of DEPE, SOPC or SOPC + 10 mol% KOdiAPC were suspended in 20 mM PIPES, 1 mM EDTA, 150 mM NaCl with 0.002% NaN3, pH 7.40 with or without addition of the peptide 4F by vortexing above the phase transition temperature of the lipid to form multilamellar vesicles (MLVs). The concentration of MLV in the samples studied was maintained at 5 mg/mL for the DEPE samples and 2.5 mg/mL for the SOPC samples. The cell volume is 340 µL.
Binding isotherm for the interaction of 4F or 2F with oxidized lipid
The change in Trp fluorescence from the peptide was used to establish a binding curve for the interaction of each of the oxidized lipids with peptide. A solution of 7.5 µM peptide was placed in the fluorescence cuvette with stirring and small aliquots of a solution of oxidized lipids in ethanol solution were diluted into buffer to the desired concentration. The reaction was carried out in 10 mM HEPES buffer, 0.14 M NaCl, 1 mM EDTA, pH 7.4 at 25°C. Tryptophan fluorescence was monitored by recording the spectrum between 310 and 370 nm after each addition of oxidized lipid. The excitation wavelength was 295 nm with excitation and emission slits set at 8 and 4 nm bandpass, respectively. Scattered light intensity was reduced by the use of polarizing filters, setting the excitation filter at 90° and the emission filter at 0°. Appropriate correction factors were applied and the intensity at the emission maximum of the peptide alone was subtracted from the values of samples with lipid in order to construct a binding isotherm.
The binding affinity was calculated as a partition binding constant, using the equation:
| (1) |
Where Pb and Pf are the concentrations of peptide bound and peptide free, respectively. L and W are the concentrations of lipid and water, respectively.
Preparation of large unilamellar vesicles (LUV)
Lipids were codissolved in chloroform/methanol (2/1, v/v). The solvent was then evaporated under a stream of nitrogen with constant rotation of a test tube so as to deposit a uniform film of lipid over the bottom third of the tube. Last traces of solvent were removed by placing the tube under high vacuum for three hours. The lipid film was then hydrated with buffer, as indicated for specific experiments. LUVs were made from this suspension by 5 freeze-thaw cycles and extruded 10 times through two polycarbonate filters with 100 nm pore size, in a Lipex barrel extruder under nitrogen pressure (Lipex Biomembranes Inc.). Vesicles were kept on ice under Argon gas and used within a few hours of preparation. Lipid concentration was determined with the phosphate assay of Ames [63].
Interaction between discoidal lipopeptides and liposomes with oxidized lipids
The peptides 4F and 2F were used to solubilize POPC in the form of lipoprotein particles. 5 µM peptide was incubated for one hour at room temperature with MLVs of POPC at a lipid to peptide molar ratio of 3. The time course of the solubilization was followed by right angle light scattering in an SLM Aminco, Model II spectrofluorometer, setting both excitation and emission monochrometers at 400 nm. The clarification of the turbidity was complete within the one-two hour of incubation. These lipoprotein particles were then titrated with LUVs of POPC and the Trp fluorescence of the peptide monitored over time. No change of fluorescence was observed, suggesting that the lipoprotein particles were saturated with phospholipid.
These lipoprotein particles were then titrated at 25°C with successive additions of small aliquots of solutions of LUVs composed of either POPC:KDdiA-PC (95:5) or POPC:KOdiA-PC (95:5). The decrease in the fluorescence from Trp of the peptide was monitored as a function of time.
Isothermal Titration Calorimetry (ITC)
A 32 µM solution of 4F or 2F was made in 20 mM PIPES buffer, pH 7.4, containing 0.14 M NaCl and 1 mM EDTA and used to fill the calorimeter cell (1.4276 mL) which was equilibrated to 30°C in a MicroCal VP-ITC instrument (MicroCal Inc, Southampton, MA). Solutions of 1-palmitoyl-lysophosphatidylcholine (LPC) were made at a concentration of 860 µM in matching buffer and placed in the syringe. A volume of 20 µL of LPC was titrated into the solution of peptide with a constant stirring at 300 rpm. Data was analyzed and plotted using the program Origin 5.0 and fitted with programs for two sites binding models provided by the manufacturer.
Results
Oxidized phospholipids promote positive membrane curvature
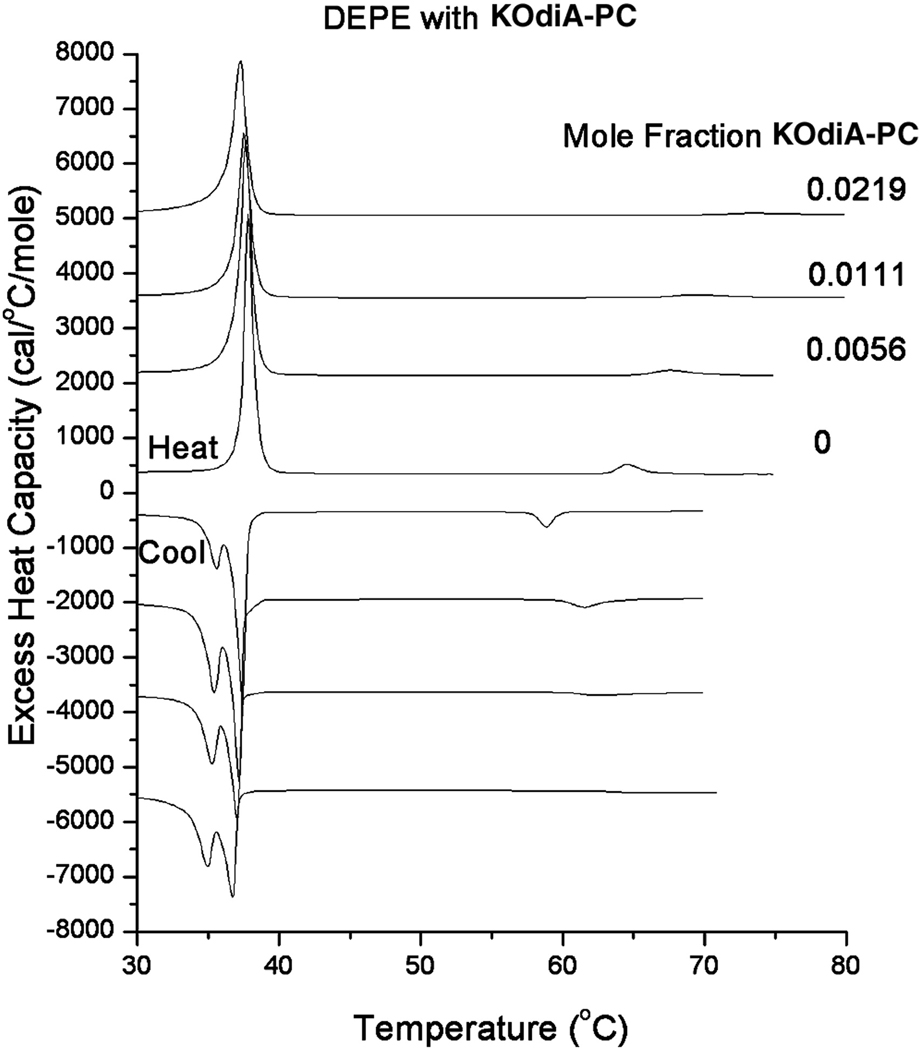
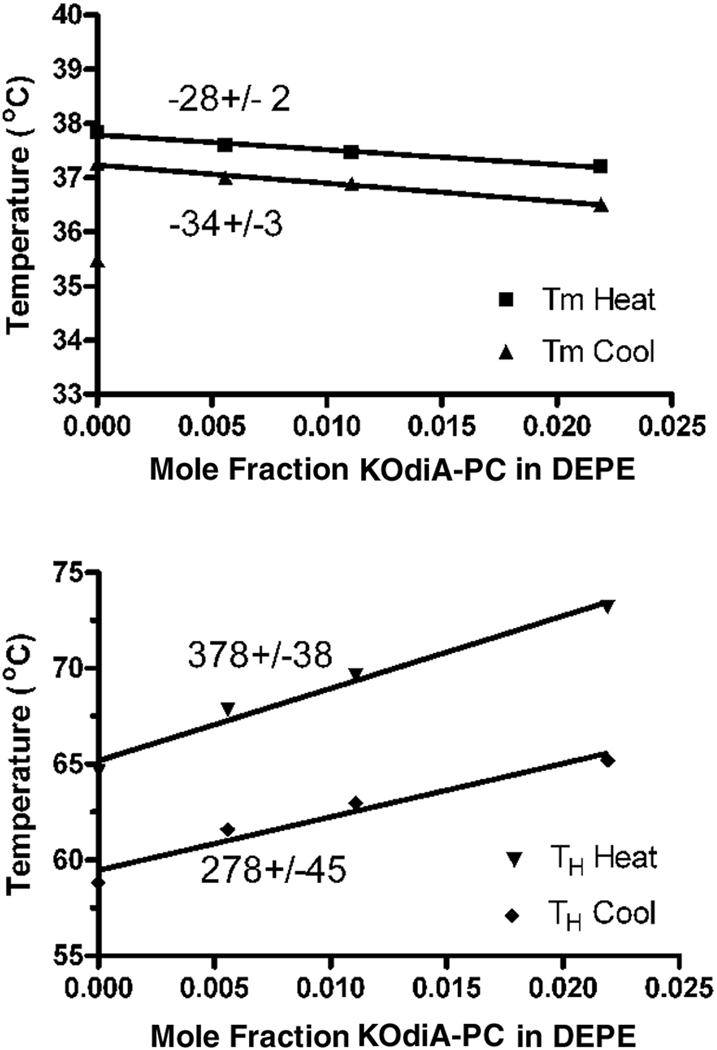
Using DSC we show that the oxidized lipids raise the bilayer to hexagonal phase transition temperature of DEPE, indicating that they promote positive curvature and stabilize bilayer properties. As an example we show a family of heating and cooling DSC curves of DEPE with the addition of several different mole fractions of KOdiA-PC (Fig. 2). The shift of the bilayer to hexagonal transition temperature is proportional to the amount of KOdiA-PC added (Fig. 3), with the value for the heating curves being 378 ± 38, which is comparatively high. Similar values were obtained introducing other oxidized lipids into DEPE.
Figure 2.
DSC curves of DEPE with increasing mol fractions of KOdiA-PC. Top four scans are heating scans, bottom four are cooling scans. DSC scans of DEPE alone (0) and in the presence of increasing mole fractions of KOdiA-PC. Lipid concentration 5 mg/mL in 20 mM PIPES, 1 mM EDTA, 150 mM NaCl with 0.002% NaN3, pH 7.40. Scan rate 2°/min. Curves have been displaced along the y-axis for presentation. Excess heat capacity is expressed per mole of DEPE.
Figure 3.
Shift of the gel to liquid crystalline phase transition temperature (Top curves) and the bilayer to hexagonal phase transition temperature (Bottom curves) with mol fraction of KOdiA-PC. Numbers near each line correspond to the slope of the regression line. The positive linear regression for the dependence of TH provides a measure of the promotion of positive curvature by the peptide.
Binding isotherm for the interaction of 4F or 2F with oxidized lipid
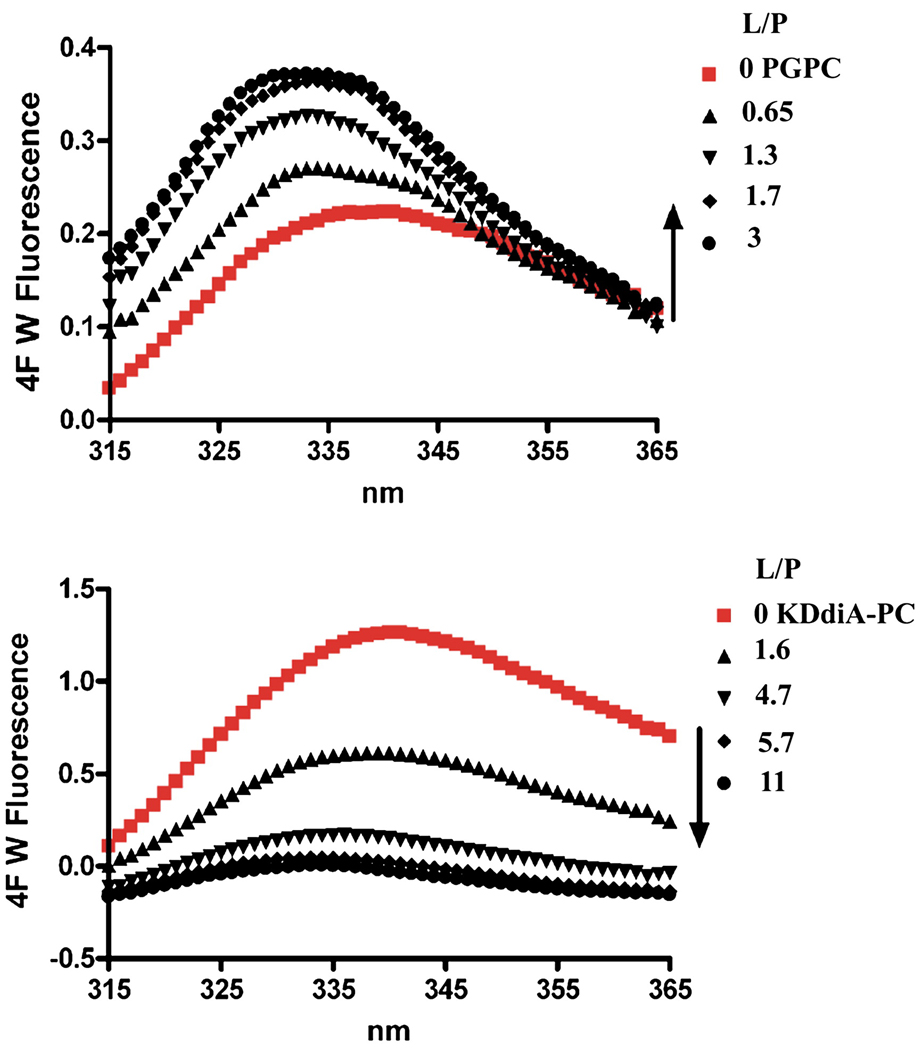
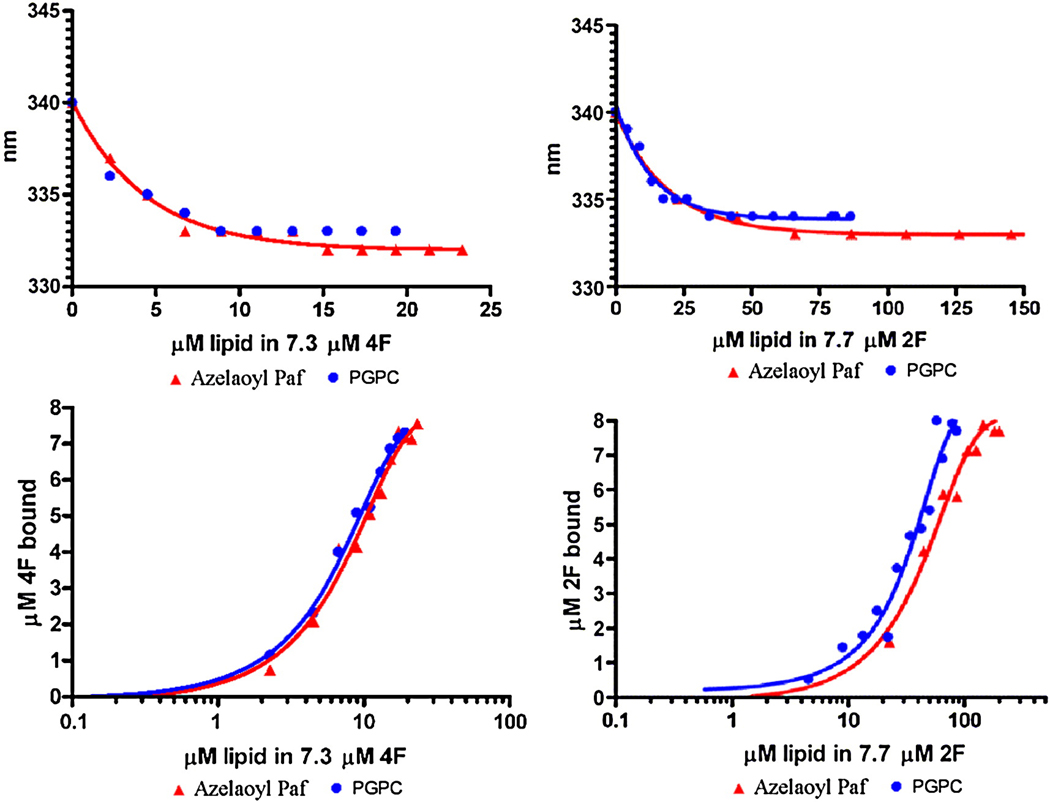
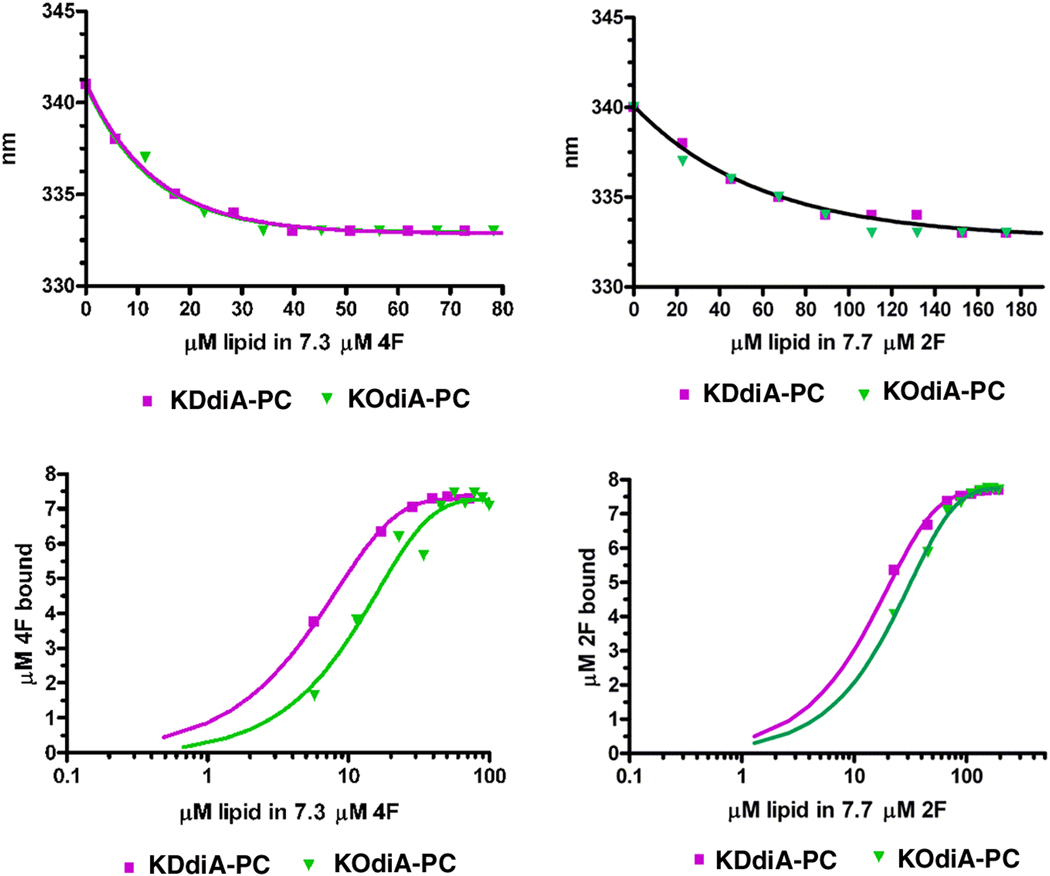
We initially studied the binding of these peptides with oxidized lipids by adding them directly to a solution of the peptide, in the absence of any phospholipid or liposomes. Titrating the peptides with the oxidized lipids Azelaoyl PAF or with PGPC there was a progressive increase in the intensity of Trp emission from the peptide shown for PGPC (Fig 4, Top graph). In contrast, the two oxidized lipids with α,β unsaturated ketones, KOdiA-PC and KDdiA-PC, caused a quenching of the peptides Trp fluorescence (Fig. 4, bottom). This quenching was not a result of an inner filter effect because of the low absorbance of the mixtures at the excitation wavelength of 295 nm nor was it the result of light scattering, as verified by the wavelength dependence of the absorbance. In general when lipids bind to peptides with a Trp residue one would expect that the fluorescence emission intensity would increase. However, highly polarizable groups are known to be good quenching agents. This is likely the reason why the oxidized lipids with α,β unsaturated ketones induce quenching of the Trp of these peptides, while the lipids with simple unconjugated carboxylic acids do not have this effect. With Azelaoyl PAF and PGPC, the emission wavelength of the Trp decreased from about 340 nm to about 333 nm, confirming the binding of the oxidized lipids to both 2F and 4F (see Fig. 5, top curves). This is also the case for KOdiA-PC and KDdiA-PC (Fig. 6, top curves). The binding isotherms are shown in the bottom curves of Figure 5 and Figure 6 and the values for the equilibrium binding constant and free energy of binding are given in Table 1.
Figure 4.
Changes in the fluorescence emission spectrum of 4F (7.3 µM in the cuvette) with successive additions of oxidized lipid in solution at 25°C.
Figure 5.
Shift in the Trp emission wavelength upon addition of Azelaoyl PC or PGPC (Top curves) and the binding isotherms for the interaction of Azelaoyl PC or PGPC with 4F (left) or with 2F (right).
Figure 6.
Shift in the Trp emission wavelength upon addition of KDdiA-PC or KOdiA-PC (Top curves) and the binding isotherms for the interaction of KDdiA-PC or KOdiA-PC with 4F (left) or with 2F (right).
Table 1.
Equilibrium binding constants for the binding of oxidized lipids with the peptides 2F and 4F
| Lipid | 4F | 2F | ||
|---|---|---|---|---|
| K (M−1) | ΔG (kcal/mol) | K (M−1) | ΔG (kcal/mol) | |
| PGPC | 1.6 × 107 | −9.9 | 3.1 ×106 | −8.9 |
| Azelaoyl PAF | 1.4 × 107 | −9.8 | 2.7 × 106 | −8.6 |
| KOdiA-PC | 1.4 × 107 | −9.8 | 9.9 × 106 | −9.6 |
| KDdiA-PC | 1 × 108 | −11 | 1.4 × 107 | −9.8 |
Interaction between discoidal lipopeptides and liposomes with oxidized lipids
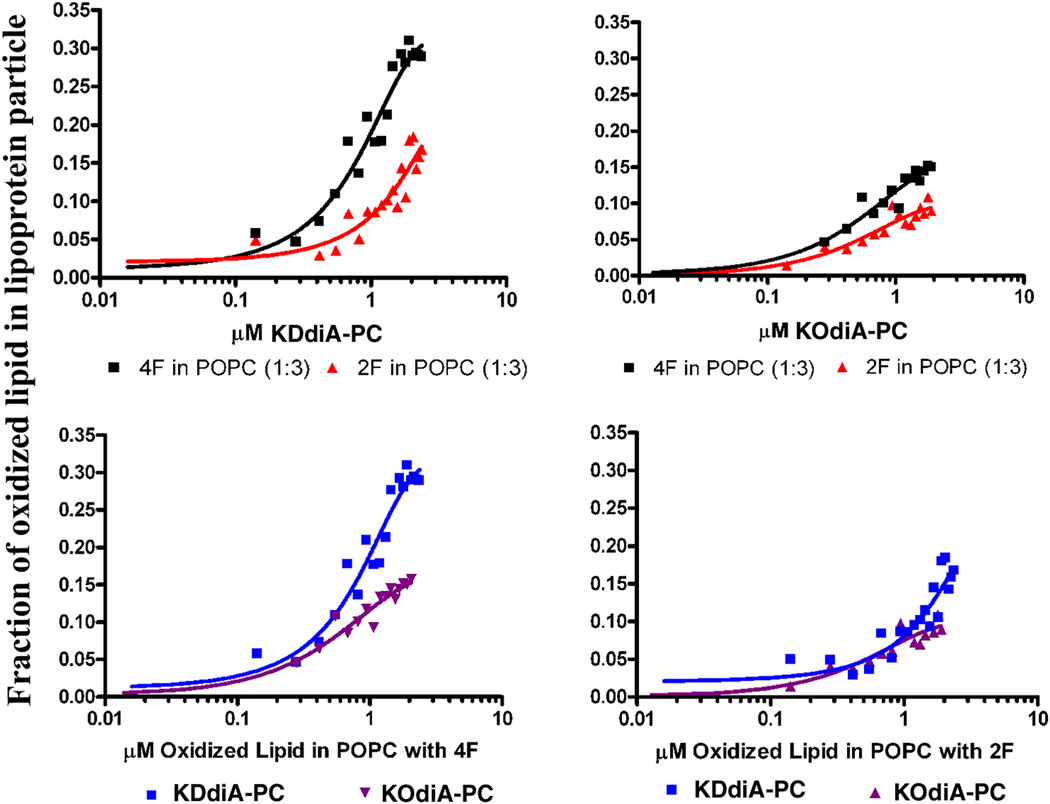
Lipoprotein particles of POPC and 2F or 4F were titrated with successive additions of small aliquots of LUV made from POPC and containing 5 mol% of either KOdiA-PC or KDdiA-PC. This resulted in a quenching of the Trp fluorescence of the peptide in the lipoprotein particle. There was no quenching of the Trp fluorescence of these peptides when the lipoprotein particles were titrated with POPC not containing oxidized lipid. Hence the inclusion of only 5 mole% oxidized lipid into the membrane resulted in the peptides binding to the liposomes with high affinity. This demonstrates the ability of these peptides to interact with oxidized lipids already contained in a membrane bilayer. The peptide 4F is more potent than 2F (Fig. 7, top panels). Of the two oxidized lipids, the KDdiA-PC, having the longer sn-2 chain, is more potent to interact with the peptides (Fig. 7, bottom panels), particularly for 4F (Fig. 7, bottom, left panel).
Figure 7.
Interaction between POPC liposomes containing 5% oxidized lipids and lipopeptide particles composed of POPC and either 2F or 4F at a lipid to peptide ratio of 1 to 3. Top, left panel shows titration with LUVs containing KDdiA-PC or top, right panel LUVs containing KOdiA-PC, into a solution of lipopeptide of 2F (▲) or 4F (▀). Lower, left panel titration of 4F lipopeptide titrated with LUVs containing KDdiA-PC (▀) or KOdiA-PC (▼) lower right hand panel of 2F lipopeptide, titrated with LUVs containing KDdiA-PC (▀) or KOdiA-PC (▲).
Centrifugation of the mixture after titration resulted in some of the LUVs sedimenting, however, the total amount of each peptide remained in the supernatant. This demonstrates that the loss of Trp fluorescence was not caused by the peptide binding to the oxidized lipid in the LUVs, but rather that the oxidized lipids were being extracted into the lipopeptide particle.
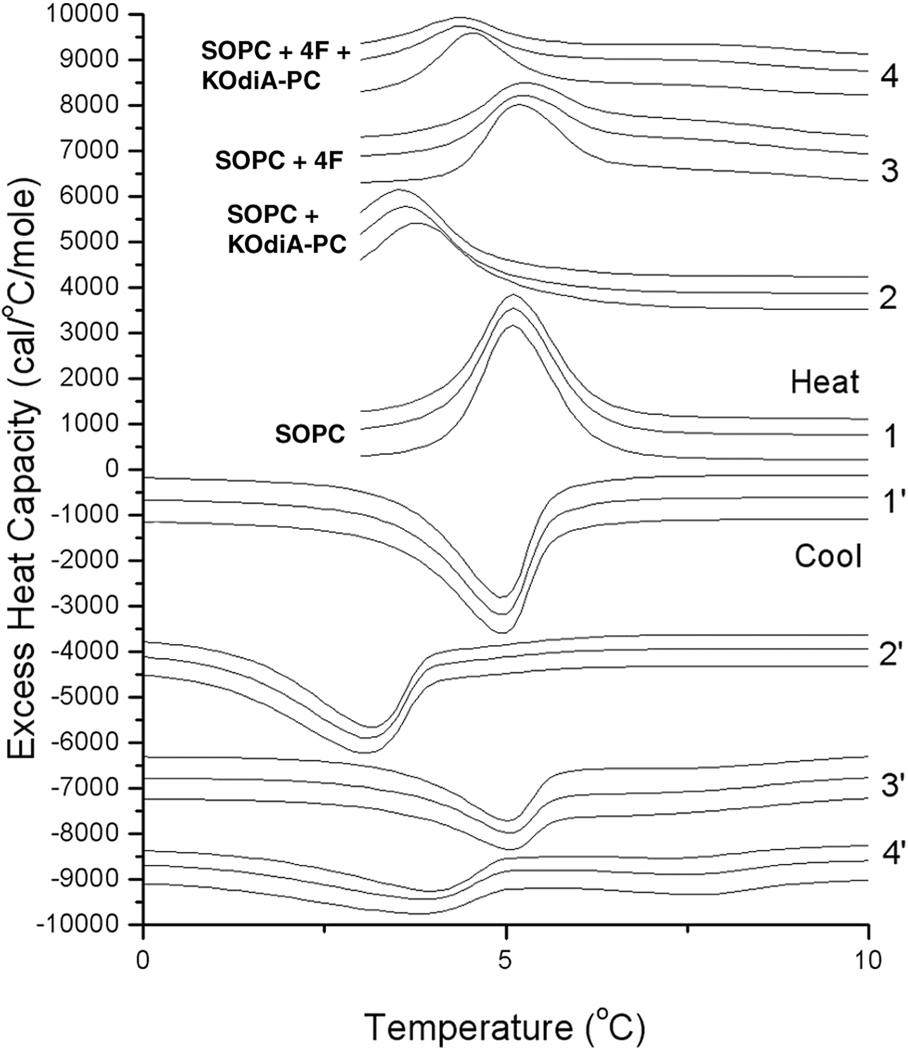
Evidence that the interaction of the oxidized lipid with the peptide results in the extraction of the oxidized lipid from the membrane comes from DSC studies (Fig. 8). SOPC was chosen for this because this lipid has the structure of phospholipids common to biological membranes with a saturated acyl chain at sn-1 and an unsaturated chain at sn-2. SOPC is also a lipid with a low gel to liquid crystalline phase transition at 4.9°C. Addition of KOdiA-PC lowers the transition temperature to 3.1°C, while addition of 4F has a negligible effect on the phase transition temperature. However, addition of the same amount of 4F to the mixture of SOPC + KOdiA-PC significantly reverses the lowering of the phase transition temperature caused by the oxidized lipid (Fig. 8). This is consistent with the 4F extracting KOdiA-PC from the membrane and reversing its effect in lowering the phase transition temperature of SOPC.
Figure 8.
DSC of SOPC (1 and 1’) or SOPC + 10 mol% KOdiA-PC (2 and 2’) alone or with the addition of 4F to SOPC (3 and 3’) or to SOPC + 10 mol% KOdiA-PC (4 and 4’). The lipid to peptide molar ratio is 500. Upper half of the Figure: heating curves, while the lower half (curves with ‘) are cooling curves. Each specimen was heated and cooled three times. Lipid concentration is 2.5 mg/mL in 20 mM PIPES, 1 mM EDTA, 150 mM NaCl with 0.002% NaN3, pH 7.40. Scan rate 1°/min. Curves have been displaced along the y-axis for presentation. Excess heat capacity is expressed per mole of SOPC.
Isothermal Titration Calorimetry (ITC)
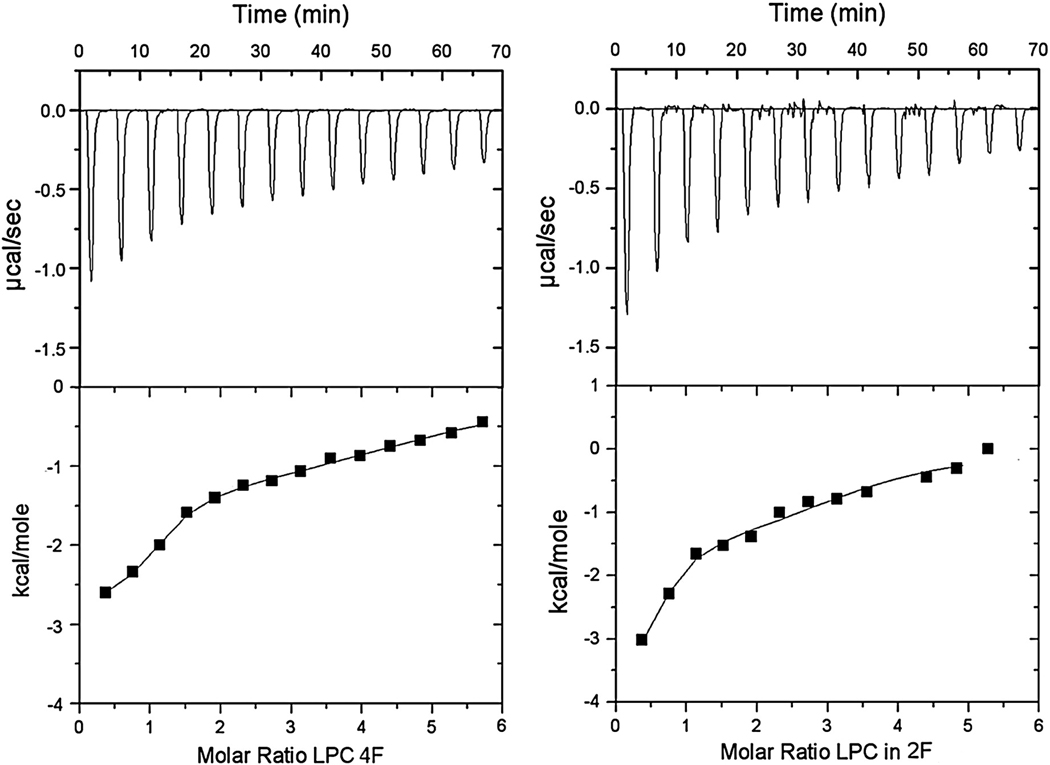
Because these oxidized lipids have shorter sn-2 acyl chains and they also promote positive membrane curvature (Fig. 2), these lipids have some relationship in terms of chemical structure and effects on membrane properties to lysophosphatidylcholine (LPC). Although there are differences between the structures of LPC and of oxidized lipids, the comparison allows one to determine if the observed interactions have some degree of specificity. Because LPC is more readily available than the specific forms of oxidized lipids, we used LPC to titrate 4F and 2F using both ITC and fluorescence titration. These measurements serve to compare the binding of an amphiphile, LPC, that does not have an sn-2 acyl chain that can protrude from the membrane surface with the binding of oxidized lipids to these peptides. In addition, it provides an independent determination of binding affinity that can be compared with that obtained from fluorescent titration, as a test of the validity of the latter method. The ITC data are presented (Fig. 9). The curves were fitted to two binding constants of different affinities and the values for the enthalpy and entropy of the reaction are summarized (Table 2). The results show that there is no significant difference in the binding affinity of 4F vs. 2F to LPC, unlike the case of the oxidized lipids in which 4F binds about 5-fold more strongly (Table 1).
Figure 9.
ITC data obtained by injection of successive aliquots of LPC into 32 µM 4F (left) or 32 µM 2F (right). Upper curves are the experimentally observed heat changes and the lower curves are the calculated enthalpy.
Table 2.
Thermodynamics of binding of LPC to 4F or 2F Data from ITC fitted with an equation for two independent binding sites.
| Peptide | K1 (M−1) | ΔH1 (kcal/mol) | ΔS1 | K2 (M−1) | ΔH2 (kcal/mol) | ΔS2 |
|---|---|---|---|---|---|---|
| 4F | 1.8 × 106 | −2.8 | 19.3 | 5 × 104 | −1.5 | 16.6 |
| 2F | 1.6 × 106 | −4.0 | 15.3 | 8.6 ×104 | −1.6 | 17.3 |
We also measured the binding of LPC to these peptides by monitoring the Trp fluorescence of the peptides. This titration could be fitted with a single binding isotherm with values of 6.8 × 105 M−1 and 9.4 × 105 M−1 for the partition binding constants of 4F and 2F, respectively. These values are close to, but somewhat lower than the high affinity binding constant obtained by ITC. In addition, both methods indicate that 2F and 4F have similar binding affinities for LPC. The more complex binding profile found with ITC, requiring two binding constants to fit the data, may be the result of interactions between LPC and peptide that have enthalpic contributions but do not cause change in the Trp fluorescence of the peptide.
Discussion
Although peptides such as 4F have been designed from a consensus sequence of the amphipathic helical segments of the apolipoprotein A–I, this peptide is not just a simple apo A–I mimetic, since it exhibits protective effects against cardiovascular disease at much lower concentrations than that apo A–I. This is also shown by the fact that orders of magnitude more apo A–IMilano are required to attain a cardio-protective effect, compared with the peptide 4F. In the present study we compare 4F with 2F, a similar 18 residue amphipathic helical peptide that has low cardiovascular protective potency even though it differs from 4F only in the substitution of two Leu residues of 2F with Phe in 4F. Peptide 2F has also been shown to be less effective than 4F in vitro in inhibiting LDL-induced monocyte chemotaxis in a co-culture system [64]. Thus we investigated the abilities of these two peptides to bind to the different oxidized lipids shown in Fig. 1.
We find that oxidized lipids raise the bilayer to hexagonal phase transition temperature. This is the same effect on membrane curvature as we had found with the amphipathic helical peptides [65]. The amphipathic helical peptides used in this work are known to bind more strongly to smaller vesicles having higher positive curvature on the exterior. This could be a factor promoting the attraction of the peptides to regions of the membrane containing oxidized lipids and hence higher local positive curvature. Earlier we had suggested that 4F because of clustering of pi-electrons at the center of the nonpolar face, would possess high affinity to oxidized lipids [66]. Indeed, this was supported by a recent study using surface plasmon resonance, which demonstrated higher affinity of 4F for several forms of oxidized lipids, compared with apo A–I. In the present study we have analyzed the binding of 4F and 2F in solution with several forms of oxidized lipids as well as demonstrating the interaction of these peptides in the form of lipoprotein particles with bilayer liposomes containing oxidized lipids. This latter arrangement mimics the situation in vivo since 4F has been shown to associate with small preβ-HDL-like particles that would resemble those we have made for this in vitro study [64]. These lipoprotein particles could then extract oxidized lipids from other lipid structures, such as oxidized LDL. Evidence from the reversal of the effect of KOdiA-PC by 4F (FIG. 8), supports this mechanism.
The binding affinity of PGPC and of KOdiA-PC for 4F (Table 1) is 9 and 4.5 fold lower than that previously found using surface plasmon resonance [56]. The difference may reflect a stabilization of the lipid-peptide complex by the solid support required for surface plasmon resonance. Our results demonstrate that 4F binds to oxidized lipids with almost 10-fold higher affinity than 2F, except for KOdiA-PC, where the difference in binding affinity to the two peptides is smaller (Table 1). We cannot explain why KOdiA-PC is the only form to show a smaller difference in binding affinity to the two peptides. The oxidized lipid with the highest affinity for binding these peptides is KDdiA-PC. Interestingly, it binds more avidly than the structurally similar KOdiA-PC, particularly to 4F. The difference between these two forms of oxidized phosphatidylcholine is the length of the acyl chain at the sn-2 position, with KDdiA-PC having a 12 carbon chain at this position, at least 4 carbons longer than any of the other forms of oxidized lipid. This observation would fit well the whisker hypothesis that demonstrates a reorientation of the sn-2 acyl chain to extend out of the membrane [21]. We envisage that if the sn-2 oxidized acyl chain were able to extend out of the membrane, then the longer it is, the greater the length of the segment of the lipid that would be accessible for interactions with the amphipathic helical peptides. This hypothesis is supported by the findings that LPC that does not have an sn-2 chain, binds to the peptides with one or two orders of magnitude lower affinity. In addition, the binding affinity of 2F and 4F to LPC is similar, unlike the situation with the oxidized lipids in which 4F binds with a 10-fold higher affinity. The correlation between the protective effect of the peptide and the affinity of binding to oxidized lipids also agrees with the findings using surface plasmon resonance [56].
Just as the apolipoprotein A–I affects several functions in contributing a protective cardiovascular effect, so too would these anti-atherosclerotic peptides be expected to have more than one site of action. However, many of the important effects of these peptides can be correlated with removal of oxidized lipids resulting in lowering the inflammatory response and also lowering the arterial deposition of cholesterol. These peptides are a component of the pre-β HDL fraction of circulating lipoprotein particles. The demonstration in this work that small lipoprotein particles containing 4F are able to interact with oxidized lipid components in a different membrane provides a molecular basis by which these peptides can neutralize the effects of oxidized lipids.
Acknowledgements
This work was funded by grant NA 6178 from the Heart and Stroke Foundation of Ontario (to RME) and by a PPG grant NIHHL 34343 from NIH (to GMA).
Abbreviations used
- POPC
1-palmitoyl-2-oleoyl phosphatidylcholine
- SOPC
1-stearoyl-2-oleoyl phosphatidylcholine
- DEPE
dielaidoyl phophatidylethanolamine
- PGPC
1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- Azelaoyl PAF
1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine
- KDdiA-PC
1-palmitoyl-2-(4-keto-dodec-3-ene-dioyl)phosphatidylcholine
- KOdiA-PC
1-palmitoyl-2-(5-keto-6-octene-dioyl) phosphatidylcholine
- LPC
lysophosphatidylcholine
- LUV
large unilamellar vesicles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am. Coll. Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 3.Haskard DO. Accelerated atherosclerosis in inflammatory rheumatic diseases. Scand. J Rheumatol. 2004;33:281–292. doi: 10.1080/03009740410010281. [DOI] [PubMed] [Google Scholar]
- 4.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 5.Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109:III20–III26. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- 6.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 7.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 8.Linton MF, Fazio S. Cyclooxygenase-2 and inflammation in atherosclerosis. Curr. Opin. Pharmacol. 2004;4:116–123. doi: 10.1016/j.coph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Spieker LE, Ruschitzka F, Luscher TF, Noll G. HDL and inflammation in atherosclerosis. Curr. Drug Targets. Immune. Endocr. Metabol. Disord. 2004;4:51–57. doi: 10.2174/1568008043340044. [DOI] [PubMed] [Google Scholar]
- 10.Meng CQ. Inflammation in atherosclerosis: new opportunities for drug discovery. Mini. Rev. Med. Chem. 2005;5:33–40. doi: 10.2174/1389557053402792. [DOI] [PubMed] [Google Scholar]
- 11.Domingues MR, Reis A, Domingues P. Mass spectrometry analysis of oxidized phospholipids. Chem. Phys. Lipids. 2008;156:1–12. doi: 10.1016/j.chemphyslip.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem. 2008;107:1614–1633. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berliner JA, Gharavi NM. Endothelial cell regulation by phospholipid oxidation products. Free Radic. Biol Med. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J Biol Chem. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a Novel Family of Oxidized Phospholipids That Serve as Ligands for the Macrophage Scavenger Receptor CD36. J. Biol. Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 16.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashraf MZ, Kar NS, Chen X, Choi J, Salomon RG, Febbraio M, Podrez EA. Specific Oxidized Phospholipids Inhibit Scavenger Receptor BI-mediated Selective Uptake of Cholesteryl Esters. J. Biol. Chem. 2008;283:10408–10414. doi: 10.1074/jbc.M710474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435:502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 19.Le NA. Reducing oxidized lipids to prevent cardiovascular disease. Curr. Treat. Options. Cardiovasc. Med. 2008;10:263–272. doi: 10.1007/s11936-008-0047-4. [DOI] [PubMed] [Google Scholar]
- 20.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 22.Khandelia H, Mouritsen OG. Lipid gymnastics: evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations. Biophys. J. 2009;96:2734–2743. doi: 10.1016/j.bpj.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 24.Moumtzi A, Trenker M, Flicker K, Zenzmaier E, Saf R, Hermetter A. Import and fate of fluorescent analogs of oxidized phospholipids in vascular smooth muscle cells. J. Lipid Res. 2007;48:565–582. doi: 10.1194/jlr.M600394-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell B, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis A, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 2005;15:212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW. Recombinant apolipoprotein A–I Milano effects on coronary atherosclerosis. Curr. Cardiol. Rep. 2004;6:438. [PubMed] [Google Scholar]
- 28.Brewer HB., Jr Focus on high-density lipoproteins in reducing cardiovascular risk. Am. Heart J. 2004;148:S14–S18. doi: 10.1016/j.ahj.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Sirtori CR. ApoA-1 Milano and regression of atherosclerosis. JAMA. 2004;291:1319. doi: 10.1001/jama.291.11.1319-b. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Jacobson EL, Jacobson MK, Lacko AG. ApoA-1 Milano and regression of atherosclerosis. JAMA. 2004;291:1319. doi: 10.1001/jama.291.11.1319-a. [DOI] [PubMed] [Google Scholar]
- 31.Davidson MH. Biologic therapies for dyslipidemia. Curr. Atheroscler. Rep. 2004;6:69–72. doi: 10.1007/s11883-004-0118-2. [DOI] [PubMed] [Google Scholar]
- 32.Duffy D, Rader DJ. Drugs in development: targeting high-density lipoprotein metabolism and reverse cholesterol transport. Curr. Opin. Cardiol. 2005;20:301–306. doi: 10.1097/01.hco.0000168532.69342.26. [DOI] [PubMed] [Google Scholar]
- 33.Futterman LG, Lemberg L. Apo a–I Milano. Am. J. Crit. Care. 2005;14:244–247. [PubMed] [Google Scholar]
- 34.Kaul S, Coin B, Hedayiti A, Yano J, Cercek B, Chyu KY, Shah PK. Rapid reversal of endothelial dysfunction in hypercholesterolemic apolipoprotein E-null mice by recombinant apolipoprotein A–I(Milano)-phospholipid complex. J. Am. Coll. Cardiol. 2004;44:1311–1319. doi: 10.1016/j.jacc.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Wu G, Zeng W, Xue H, Chen B. Cysteine mutants of human apolipoprotein A–I: a study of secondary structural and functional properties. J. Lipid. Res. 2005;46:1303–1311. doi: 10.1194/jlr.M400401-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Hortin GL, Shen RF, Martin BM, Remaley AT. Diverse range of small peptides associated with high-density lipoprotein. Biochem. Biophys. Res. Commun. 2006;340:909–915. doi: 10.1016/j.bbrc.2005.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handattu SP, Garber DW, Horn DC, Hughes DW, Berno B, Bain AD, Mishra VK, Palgunachari MN, Datta G, Anantharamaiah GM, Epand RM. ApoA-I mimetic peptides with differing ability to inhibit atherosclerosis also exhibit differences in their interactions with membrane bilayers. J Biol Chem. 2007;282:1980–1988. doi: 10.1074/jbc.M606231200. [DOI] [PubMed] [Google Scholar]
- 39.Wool GD, Vaisar T, Reardon CA, Getz GS. An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J. Lipid Res. 2009:M900151–MJLR200. doi: 10.1194/jlr.M900151-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A–I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 41.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Datta G, Garber D, Fogelman AM. Human apolipoprotein A–I and A–I mimetic peptides: potential for atherosclerosis reversal. Curr. Opin. Lipidol. 2004;15:645–649. doi: 10.1097/00041433-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 43.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Hough G, Wagner A, Nakamura K, Garber DW, Datta G, Segrest JP, Hama S, Fogelman AM. Human apolipoprotein AI mimetic peptides for the treatment of atherosclerosis. Curr. Opin. Investig. Drugs. 2003;4:1100–1104. [PubMed] [Google Scholar]
- 44.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral administration of an Apo A–I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 45.Garber DW, Datta G, Chaddha M, Palgunachari MN, Hama SY, Navab M, Fogelman AM, Segrest JP, Anantharamaiah GM. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 46.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. Apolipoprotein A–I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 2005;25:1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 47.Getz GS, Wool GD, Reardon CA. Apoprotein A–I mimetic peptides and their potential anti-atherogenic mechanisms of action. Curr. Opin. Lipidol. 2009;20:171–175. doi: 10.1097/MOL.0b013e32832ac051. [DOI] [PubMed] [Google Scholar]
- 48.Navab M, Anantharamaiah GM, Fogelman AM. The effect of apolipoprotein mimetic peptides in inflammatory disorders other than atherosclerosis. Trends Cardiovasc. Med. 2008;18:61–66. doi: 10.1016/j.tcm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Lenten BJ, Navab M, Anantharamaiah GM, Buga GM, Reddy ST, Fogelman AM. Multiple indications for anti-inflammatory apolipoprotein mimetic peptides. Curr. Opin. Investig. Drugs. 2008;9:1157–1162. [PMC free article] [PubMed] [Google Scholar]
- 50.Deigner HP, Hermetter A. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr. Opin. Lipidol. 2008;19:289–294. doi: 10.1097/MOL.0b013e3282fe1d0e. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL proinflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009 doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ. Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 53.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, Garber DW, Handattu S, Fogelman AM. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:1426–1432. doi: 10.1161/01.ATV.0000167412.98221.1a. [DOI] [PubMed] [Google Scholar]
- 54.Kruger AL, Peterson S, Turkseven s, Kaminski PM, Zhang FE, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 55.Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, Stacchiotti A, Rezzani R, McClung JA, Aronow WS, Ikehara S, Abraham NG. Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Pharmacol Exp Ther. 2007;322:514–520. doi: 10.1124/jpet.107.119479. [DOI] [PubMed] [Google Scholar]
- 56.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA–I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Epand RM, Epand RF, Sayer BG, Datta G, Chaddha M, Anantharamaiah GM. Two homologous apolipoprotein AI mimetic peptides. Relationship between membrane interactions and biological activity. J Biol. Chem. 2004;279:51404–51414. doi: 10.1074/jbc.M408581200. [DOI] [PubMed] [Google Scholar]
- 58.Mishra VK, Palgunachari MN, Krishna R, Glushka J, Segrest JP, Anantharamaiah GM. Effect of leucine to phenylalanine substitution on the nonpolar face of a class A amphipathic helical peptide on its interaction with lipid: high resolution solution NMR studies of 4F-dimyristoylphosphatidylcholine discoidal complex. J Biol Chem. 2008;283:34393–34402. doi: 10.1074/jbc.M806384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 60.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 61.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 62.Privalov G, Kavina V, Freire E, Privalov PL. Precise scanning calorimeter for studying thermal properties of biological macromolecules in dilute solution. Anal. Biochem. 1995;232:79–85. doi: 10.1006/abio.1995.9957. [DOI] [PubMed] [Google Scholar]
- 63.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology. 1966;8:115–118. [Google Scholar]
- 64.Datta G, Epand RF, Epand RM, Chaddha M, Kirksey MA, Garber DW, Lund-Katz S, Phillips MC, Hama S, Navab M, Fogelman AM, Palgunachari MN, Segrest JP, Anantharamaiah GM. Aromatic residue position on the nonpolar face of class A amphipathic helical peptides determines biological activity. J. Biol. Chem. 2004;279:26509–26517. doi: 10.1074/jbc.M314276200. [DOI] [PubMed] [Google Scholar]
- 65.Venkatachalapathi YV, Phillips MC, Epand RM, Epand RF, Tytler EM, Segrest JP, Anantharamaiah GM. Effect of end group blockage on the properties of a class A amphipathic helical peptide. Proteins. 1993;15:349–359. doi: 10.1002/prot.340150403. [DOI] [PubMed] [Google Scholar]
- 66.Anantharamaiah GM, Mishra VK, Garber DW, Datta G, Handattu SP, Palgunachari MN, Chaddha M, Navab M, Reddy ST, Segrest JP, Fogelman AM. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A–I mimetic peptides. J. Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]