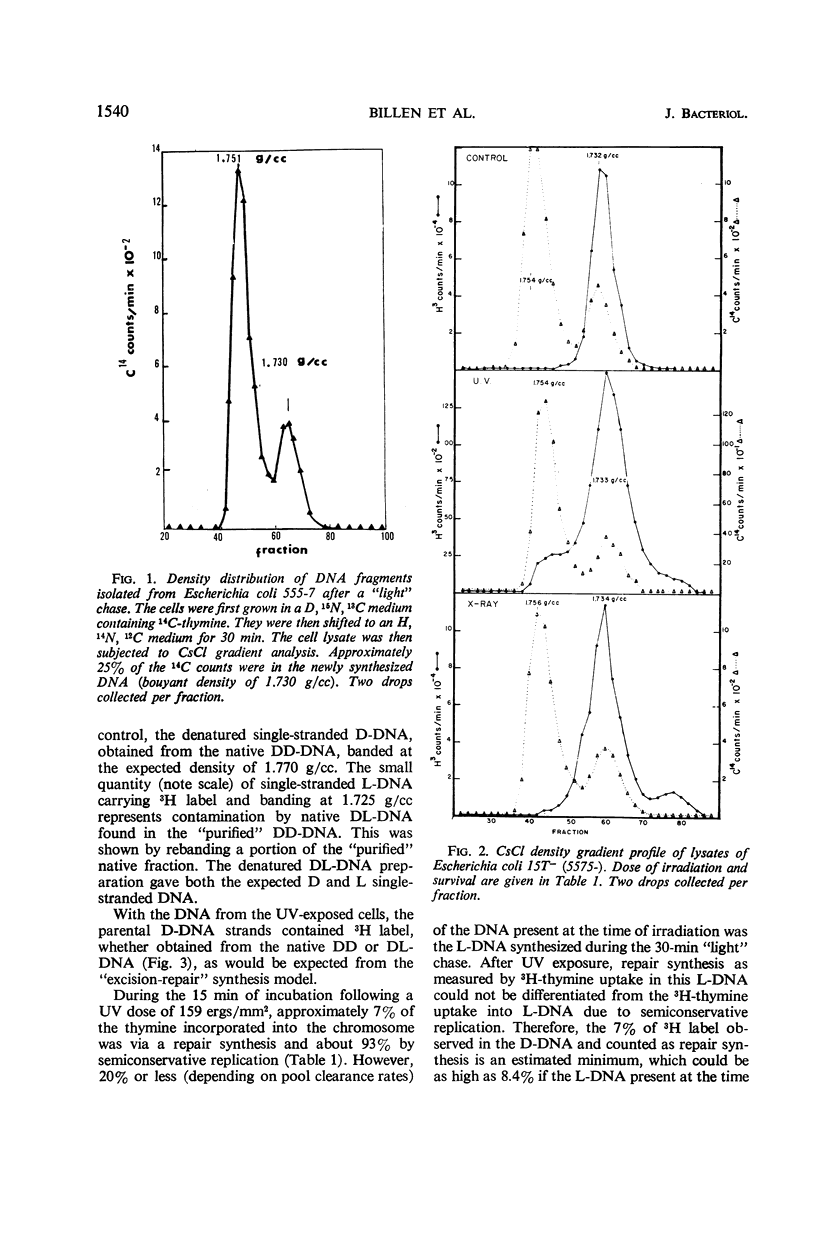
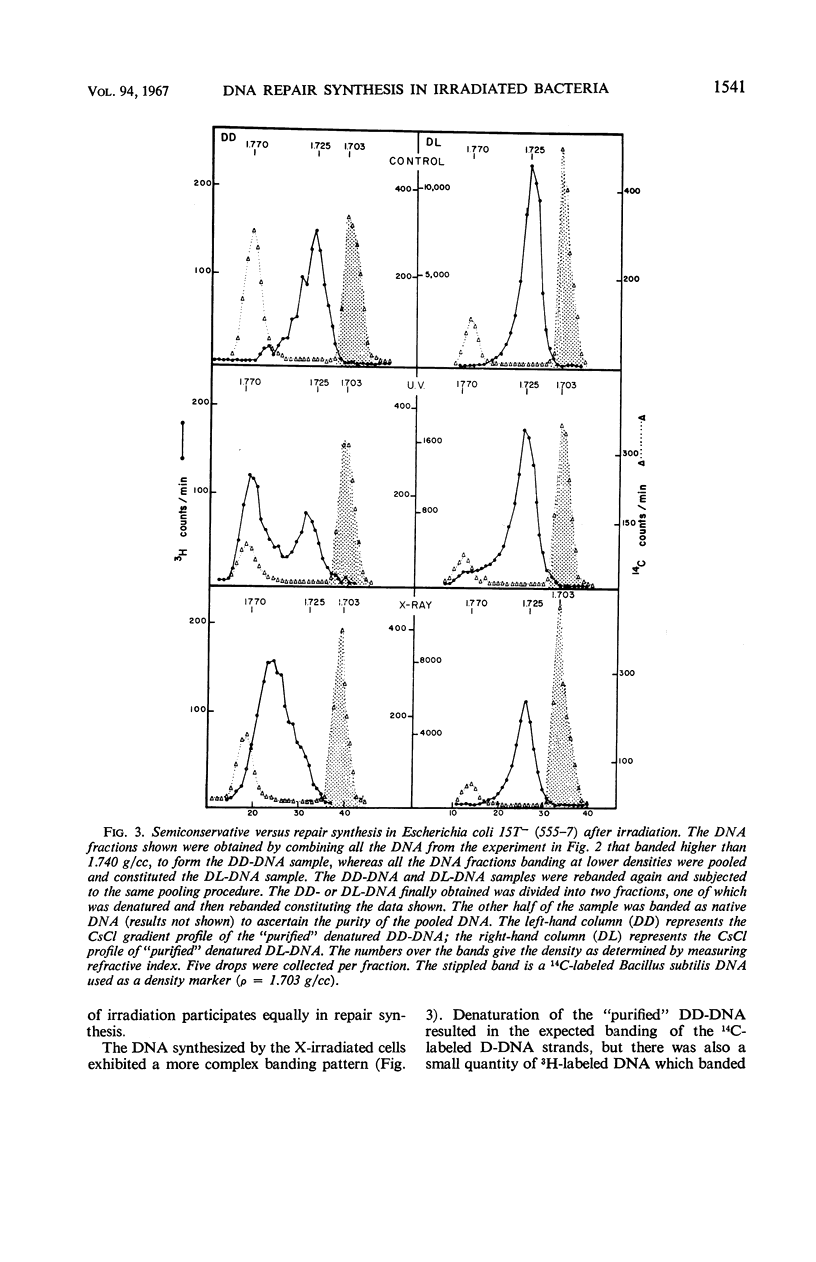
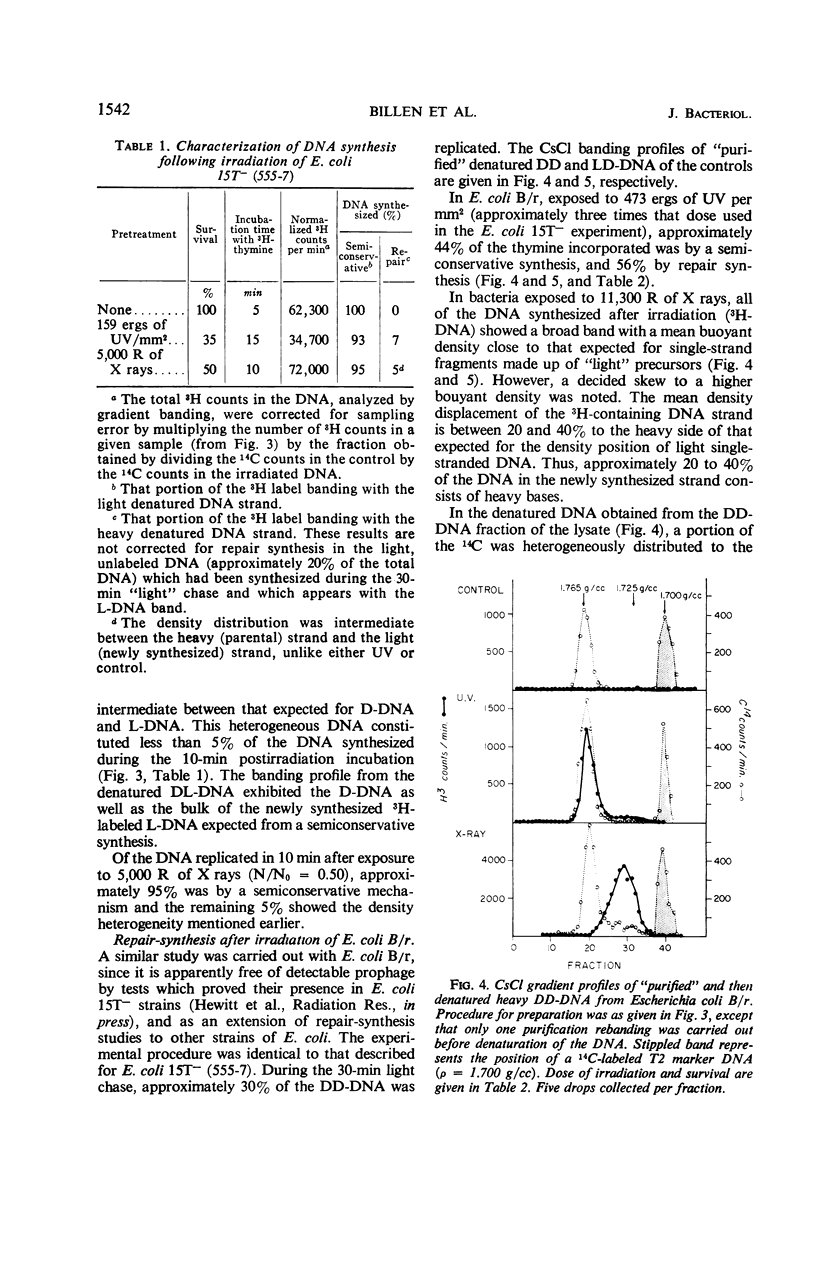
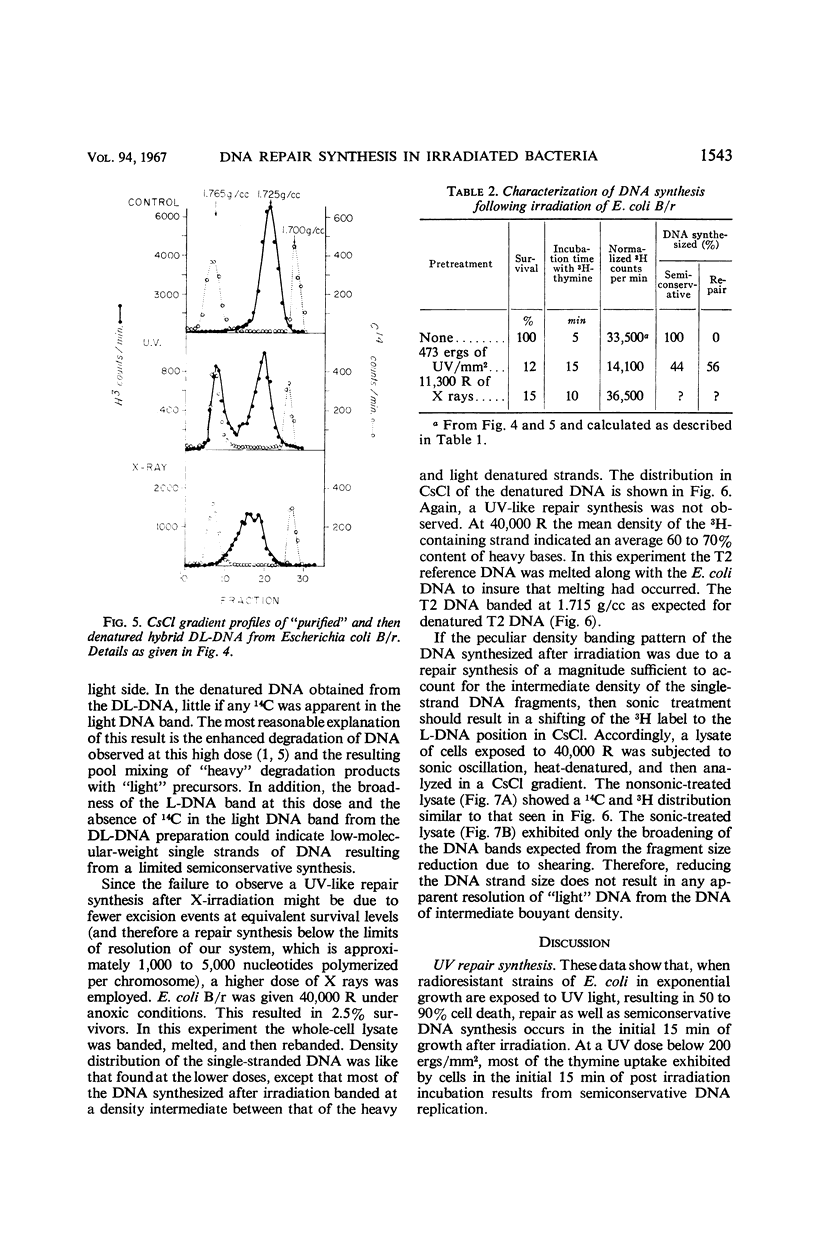
Abstract
A comparison of repair synthesis after ultraviolet light (UV) or X-ray exposure was made in Escherichia coli strains 15T− (555–7) and B/r by use of a D, 15N, 13C density labeling system. During the initial 15 min of incubation after UV irradiation, both a “repair” synthesis and a reduced semiconservative deoxyribonucleic acid (DNA) synthesis occurred. In the so-called “physiological” dose range used, the latter was greater than the former. X-irradiation of cells, at doses producing similar levels of cell death as in the UV-exposed cultures, did not lead to a similar repair replication process. However, a density heterogeneity of the DNA synthesized in the initial 10 min after exposure was observed. This is interpreted in terms of X ray-induced DNA degradation. Normal cells showed only a semiconservative type of replication and, therefore, within the limits of resolution of the system used (the incorporation of 1,000 to 5,000 nucleotides per replicating chromosome could be measured), DNA in normal cells did not appear to undergo a repair synthesis involving thymine exchange. These results indicate that not all repair mechanisms mimic that found after UV exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P. M., Pollard E. C. Studies on the radiation-induced breakdown of deoxyribonucleic acid in Escherichia coli 15 T L. Radiat Res. 1967 May;31(1):47–62. [PubMed] [Google Scholar]
- BILLEN D. Alterations in the radiosensitivity of Escherichia coli through modification of cellular macromolecular components. Biochim Biophys Acta. 1959 Jul;34:110–116. doi: 10.1016/0006-3002(59)90238-0. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Hewitt R. Influence of starvation for methionine and other amino acids on subsequent bacterial deoxyribonucleic acid replication. J Bacteriol. 1966 Sep;92(3):609–617. doi: 10.1128/jb.92.3.609-617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton E. W., Billen D. X-ray-induced degradation of deoxyribonucleic acid in a polyauxotrophic bacterium. Radiat Res. 1966 May;28(1):109–120. [PubMed] [Google Scholar]
- Freifelder D. Lethal changes in bacteriophage DNA produced by x-rays. Radiat Res. 1966;(Suppl):80–96. [PubMed] [Google Scholar]
- HANAWALT P. C., HAYNES R. H. REPAIR REPLICATION OF DNA IN BACTERIA: IRRELEVANCE OF CHEMICAL NATURE OF BASE DEFECT. Biochem Biophys Res Commun. 1965 May 3;19:462–467. doi: 10.1016/0006-291x(65)90147-6. [DOI] [PubMed] [Google Scholar]
- Hewitt R., Suit J. C., Billen D. Utilization of 5-bromouracil by thymineless bacteria. J Bacteriol. 1967 Jan;93(1):86–89. doi: 10.1128/jb.93.1.86-89.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- Kaplan H. S. DNA-strand scission and loss of viability after x irradiation of normal and sensitized bacterial cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1442–1446. doi: 10.1073/pnas.55.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON G. E., BILLEN D., HOLLAENDER A. Recovery of x-irradiated bacteria at suboptimal incubation temperatures. J Cell Physiol. 1953 Apr;41(2):345–357. doi: 10.1002/jcp.1030410211. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Setlow R. B. Effects of ultraviolet radiation on macromolecular synthesis in Escherichia coli. J Mol Biol. 1966 Jan;15(1):201–219. doi: 10.1016/s0022-2836(66)80221-8. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., DE GIOVANNI R., RICH K. Escherichia coli containing unnatural pyrimidines in its deoxyribonucleic acid. J Bacteriol. 1956 Jan;71(1):60–69. doi: 10.1128/jb.71.1.60-69.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


