Abstract
Longitudinal axons transmit all signals between the brain and spinal cord. Their axon tracts through the brain stem are established by a simple set of pioneer axons with precise trajectories parallel to the floor plate. To identify longitudinal guidance mechanisms in vivo, the overall role of floor plate tissue and the specific roles of Slit/Robo signals were tested. Ectopic induction or genetic deletion of the floor plate diverted longitudinal axons into abnormal trajectories. The expression patterns of the diffusible cues of the Slit family were altered in the floor plate experiments, suggesting their involvement in longitudinal guidance. Genetic tests of Slit1 and Slit2, and the Slit receptors Robo1 and Robo2 were carried out in mutant mice. Slit1;Slit2 double mutants had severe longitudinal errors, particularly for ventral axons, including midline crossing and wandering longitudinal trajectories. Robo1 and Robo2 were largely genetically redundant, and neither appeared to specify specific tract positions. However, combined Robo1 and Robo2 mutations strongly disrupted each pioneer tract. Thus, pioneer axons depend on long-range floor plate cues, with Slit/Robo signaling required for precise longitudinal trajectories.
Keywords: Axon guidance, Floor plate, Longitudinal axon, Robo, Slit, Hindbrain
INTRODUCTION
Longitudinal axons transmit all signals between the brain and the spinal cord. Longitudinal tracts are the first axon pathways to be established in the embryonic vertebrate brain, pioneered by a small number of axons organized into a parallel array of longitudinal tracts (Chedotal et al., 1995; Chitnis and Kuwada, 1991; Easter et al., 1994; Easter et al., 1993; Glover and Petursdottir, 1991; Mastick and Easter, 1996; Taylor, 1991; Wilson et al., 1990). Despite the importance of these tracts, longitudinal guidance mechanisms remain largely undefined. To project longitudinally during development, axons must orient anteriorly or posteriorly, choose specific dorsoventral (DV) positions for their trajectories, and precisely maintain those positions while growing great distances. Unlike extensively studied commissural axon systems, longitudinal axons do not grow towards or away from any common CNS structures, and thus lack a shared intermediate target. One clue is that they grow parallel to the longitudinal axis, suggesting that their molecular guidance cues are produced and distributed in a continuum along the length of the neural tube.
Along the ventral midline lies the floor plate, a well-characterized source of secreted and local signals for growing axons (Charron and Tessier-Lavigne, 2005; Colamarino and Tessier-Lavigne, 1995; Tessier-Lavigne et al., 1988). The floor plate has key roles in guiding spinal cord commissural axons, mediated by several molecular activities that initially attract axons, then promote midline crossing, and finally repulse axons to ensure that they cross the midline only once (Charron et al., 2003; Kennedy et al., 1994; Long et al., 2004; Serafini et al., 1996; Serafini et al., 1994). Additional floor plate signals dictate the direction of turns after crossing (Bourikas et al., 2005; Lyuksyutova et al., 2003). Longitudinal axons project through the same environment, and thus are potentially influenced by the same floor-plate cues, albeit resulting in distinct trajectories. The floor plate repels cultures of longitudinal axons in vitro (Tamada et al., 1995), while certain longitudinal axon bundles wander into the midline in zebrafish lacking floor plate (Hatta, 1992). These experiments suggest that the ability of longitudinal axons to remain ipsilateral and sustain longitudinal growth at specific DV positions is due to floor plate repulsive signals.
The Slit proteins are secreted by the ventral midline (Brose et al., 1999; Holmes et al., 1998; Zou et al., 2000) and have conserved roles as repellents in C. elegans (Hao et al., 2001), fruit flies (Kidd et al., 1999), rodents (Long et al., 2004) and probably humans (Jen et al., 2004). The Slits are expressed along the anteroposterior (AP) axis (Rothberg et al., 1990), making them candidates for longitudinal axon guidance. In Drosophila, Slit repellent activity prevents commissural axons from re-crossing the midline (Kidd et al., 1998b), and forces longitudinal axons to remain ispilateral (Kidd et al., 1998a; Seeger et al., 1993). Slit is also responsible for setting tracts at specific positions parallel to the midline (Rajagopalan et al., 2000b; Simpson et al., 2000b). The positioning of fly longitudinal tracts presumably results from Slit repulsion balanced by positive cues, in part including attractive cues from the midline (Garbe and Bashaw, 2007; Simpson et al., 2000a). In the mouse spinal cord, the loss of all three Slit proteins causes commissural axons to stall and re-cross the midline (Long et al., 2004), while the loss of Slit1 and Slit2 disrupts several late-forming forebrain tracts, with shifts towards and into otherwise Slit-positive regions (Bagri et al., 2002). The early expression of Slits in the developing brain and their roles in other systems make the Slits likely candidates to guide longitudinal axons in the early brain.
The Robo transmembrane protein family members are the canonical Slit receptors that mediate the repulsive effects of the Slits (Kidd et al., 1999), although recent evidence suggests that there are additional Slit receptors (Fujisawa et al., 2007). Robo proteins are expressed on axons that avoid the midline, such as ipsilateral longitudinal axons in flies, and post-crossing axons in flies and rodents (Kidd et al., 1998a; Long et al., 2004; Mambetisaeva et al., 2005; Sundaresan et al., 2004). Loss of multiple Robo proteins in Drosophila causes the collapse of longitudinal axons onto the midline, resembling Slit mutants (Rajagopalan et al., 2000b; Simpson et al., 2000b). Additionally, the expression of specific Robo isoforms sets the lateral position of longitudinal tracts in the fly nerve cord, as altering Robo expression can re-position tracts (Rajagopalan et al., 2000a; Simpson et al., 2000a). In mice, Robos may play similar roles in post-crossing commissural axons (Long et al., 2004). Robo function is widespread in CNS tract formation, as Robos also function in the forebrain to organize several late-forming tracts (Andrews et al., 2006; Fouquet et al., 2007; Lopez-Bendito et al., 2007). Together, these studies suggest that longitudinal guidance depends on Robos to mediate Slit repulsive signals from the midline, keeping longitudinal axons ispilateral, away from the midline, and setting specific tract positions.
To investigate pioneer longitudinal guidance mechanisms, we tested the potential roles of floor plate in guiding the first brain longitudinal axons. We used genetic gain- and loss-of-function strategies in chick and mouse embryos to demonstrate a crucial role of floor plate-derived signals. To begin to define the molecular signals involved, we examined tract formation in Slit and Robo mutant mouse embryos. These results indicate that Slit/Robo signaling is responsible for many aspects of longitudinal guidance.
MATERIALS AND METHODS
Chicken embryo electroporation
Eggs were incubated at 39°C for 48 hours to 10-12 somites, windowed, and a DNA/PBS solution injected into the hindbrain ventricle. Plasmids included 2 μg/μl pCS-Shh-IG (Oberg et al., 2002) and 1 μg/μl pCAX-GFP. After culture and GFP imaging, embryos were fixed overnight at 4°C in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4).
Mouse embryos
Mouse experiments were approved by UNR IACUC, following NIH guidelines. Expression analyses were performed with CD1 embryos. Gli2 mutant mice have been previously reported (Mo et al., 1997). Slit1–/–;Slit2+/–, Robo1–/–, Robo2–/– and Robo1+/–;Robo2+/– double mutant mice were a gift of Marc Tessier-Lavigne, Genentech (Grieshammer et al., 2004; Long et al., 2004; Lopez-Bendito et al., 2007). Embryos were collected at embryonic day 10 (E10) or E10.5, with noon of the day of vaginal plug detection designated as E0.5. The lipophilic fluorescent axon tracer DiI was used (Mastick and Easter, 1996; Nural and Mastick, 2004).
In situ hybridization
Whole-mount in situ hybridization was carried out as described (Mastick et al., 1997). Slit and Robo probes were provided by Marc Tessier-Lavigne, Genentech. Chick Slit1 and chick Slit2 cDNA was provided by Ed Laufer, Columbia. Chick Hnf3β cDNA was provided by Ariel Ruiz Altaba, NYU.
Immunohistochemistry
Whole embryos were prepared by dissecting out the neural tube, and washing for several hours in PBS containing 10% FBS and 1% Triton X-100 (PBTS). Primary antibody in PBTS was applied for ~3 days: rabbit anti-βIII tubulin (Covance) 1:1000, rabbit anti-GFP (Molecular Probes) 1:500 or Robo1 antibody (R&D Systems) 1:500 (pre-treatment at 95°C in 10 mM sodium citrate for 20 minutes). After washing in PBTS overnight, secondary antibodies (Jackson Immuno Laboratories) were applied in PBTS at 1:200 for 1-2 days, followed by overnight washes.
X-gal staining
To visualize Robo2+ axons, β-galactosidase staining was performed on Robo2–/– embryos. Embryos were fixed in 0.2% gluteraldehyde, 5 mM EDTA and 2 mM MgCl2 for 30 minutes. After fixation, embryos were washed with PBS for 3×15 minutes. Embryos were stained in 1 mg/ml X-gal, 2.12 mg/ml ferrocyanide and 1.64 mg/ml ferricyanide for 24-48 hours. After staining, embryos were washed in PBS containing 2 mM MgCl2 and 0.02% NP-40.
Statistical analysis of ILF axon trajectories
Axon angles were measured in images of ILF diI labels (see Fig. S1 in the supplementary material). The direction of >100 axon segments per embryo (n=3/genotype) was traced using ImageJ (NIH). Segments were initiated at each change in angle or intersection. In control embryos, straight axons were probably under-counted due to tract bundles. The variability of segment angles was compared by Levene's test for homogeneity of variance.
RESULTS
To study longitudinal guidance mechanisms, we focused on the relatively simple set of pioneer axons that descend through the midbrain and the hindbrain (Easter et al., 1993; Mastick and Easter, 1996). Their precise trajectories form an array of tracts in mouse and chick embryos (Fig. 1). This array consists of longitudinal fascicles (LF) at dorsal, intermediate and ventral positions (or equivalently, lateral, intermediate and medial positions), referred to here as LLF, ILF and MLF populations.
Fig. 1. Schematic of pioneer longitudinal tracts.
The first neurons form an array of longitudinal axons with precise trajectories at specific DV positions (Easter et al., 1993; Mastick and Easter, 1996). The tracts include: ventral axons of the medial longitudinal fasciculus (MLF); dorsal axons of the lateral longitudinal fasciculus (LLF, originating from dorsal midbrain neurons, also known as the tract of the mesencephalic nucleus of the trigeminal nerve, tmesV); and a broad set of intermediate axons (termed here the ILF).
Longitudinal trajectories can be diverted by Shh-transfected tissue
To test the role of the floor plate in vivo, we first challenged pioneer longitudinal axons with transfection of sonic hedgehog (Shh), an inducer of ventral fates including floor plate markers (Roelink et al., 1994; Ruiz i Altaba et al., 1995). A Shh expression plasmid was electroporated unilaterally into the anterior hindbrain of chick embryos prior to axon outgrowth. After culture, longitudinal tracts were disrupted in every electroporated embryo, as visualized by the labeling of whole mounts with axonal antibodies (n=12) and tracing with diI (n=4; Fig. 2).
Fig. 2. Sonic hedgehog transfection can divert longitudinal axon trajectories.
A Sonic hedgehog (Shh) expression plasmid was co-electroporated with a GFP reporter plasmid into the hindbrain of 10- to 12-somite chick embryos. All panels show side views of whole hindbrain labels, anterior to left, dorsal to top. Axons were labeled with (A-C) DiI (red), with crystal locations shown in the insert of A, or (D) anti-β-tubulin (red). (A) Hindbrain of a Shh transfected embryo, as an open book with MLF and ILF axons labeled on both electroporated (top) and control sides. (B) Diffuse Shh electroporation showing both ventral and dorsal turning LLF responses. (C) Example of LLF axons turning towards the anterior (asterisks) near a Shh+ region. (D) Axonal antibody (red), showing reorientation of both longitudinal and commissural axon trajectories. (E) MLF axons (arrowheads) fan out within Shh-transfected patches. (F-I) Shh electroporation induces the ectopic floor plate markers Hnf3β and Slit1 (arrowheads); control (F,H) and electroporated hindbrains (G,I). fp, floor plate. Scale bars: 200 μm.
To visualize how specific populations of longitudinal axons responded to Shh-transfected tissue, axon labels were compared with a GFP transfection marker (Fig. 2). Many axons turned gradually, at a distance, towards transfected patches, but then reoriented sharply to avoid directly transiting the patches. Axons generally diverted dorsally, and interestingly returned to their normal DV positions after passing the patches. Different conformations of transfected cells resulted in varying axon phenotypes. For example, small clusters of Shh+ cells could divert the LLF both dorsally and ventrally (Fig. 2B), while large dorsal Shh+ patches forced sharp reversing turns (Fig. 2C). Large ventral patches caused MLF fanning within the GFP+ regions, rather than diversion, so transfected tissue was not simply inhibitory (Fig. 2E). Thus, when confronted with Shh-transfected tissue, longitudinal axons displayed both positive and negative responses, depending on the distance from the transfected tissue and the configuration of the transfected cells.
To characterize the effects of Shh transfection on the expression of floor plate and midbrain markers, and on axon guidance cues, we carried out in situ hybridization analyses. The floorplate marker Hnf3β showed ectopic expression (Fig. 2F,G). Lateral and dorsal markers in the midbrain were suppressed (Agarwala and Ragsdale, 2002), which we verified in the hindbrain for Pax6 and Pax3 (V. Lisowski and G.S.M., unpublished). The axon guidance cue Slit1 showed local induction of ectopic expression within and near electroporated patches (Fig. 2H,I), as did netrin 1 (not shown). Thus, Shh transfection causes the induction of potential long-range cues, which is associated with the strong responses of longitudinal axons.
Loss of floor plate disrupts longitudinal trajectories
In a converse approach to testing floor plate function, we examined how longitudinal pioneers navigate in the absence of floor plate-derived guidance cues by analyzing Gli2 mutant mouse embryos (see Table S1 in the supplementary material). In Gli2 mutants, floor plate is not induced by notochordal Shh, yet DV patterning of neural tube genes and neurons remains mostly normal (Lebel et al., 2007; Matise et al., 1998; Matise et al., 1999). In spinal cord, the expression of Slits 1-3 and netrin 1 is reduced, but some expression is retained in ventral and lateral tissue (Charron et al., 2003; Kadison et al., 2006).
Antibody labeling for hindbrain longitudinal tracts when they were first established (E10 and E10.5) showed many altered trajectories in Gli2–/– embryos (Fig. 3A,B,B′). The anterior hindbrain was narrower, and the midline was invaded by axon bundles. The MLF bundle failed to form. Many axons at ventral and intermediate positions had non-parallel trajectories (Fig. 3B′). LLF axons had parallel trajectories, but were reduced in number. The posterior hindbrain was wider, and longitudinal axons angled dorsally to form a more organized array.
Fig. 3. Loss of floor plate disrupts ipsilateral trajectories, dorsoventral position, and anteroposterior direction.
(A,B,B′) Hindbrain of E10.5 control and Gli2–/– mouse embryos; open book whole mounts labeled with βIII tubulin antibody. Anterior to the left. In mutants, many axons project at angles and encroach into the midline. No MLF is visible. Dashed box enlarged in B′ highlights the mutant midline. (C,D) Axon tracing using DiI crystals placed at an intermediate position in r1 (letters in A and B). The midline is indicated by dashed lines. Control axons project posteriorly at intermediate positions, with a small number of commissural axons (CA). In mutants, many axons cross the midline and descend in bilaterally symmetrical tracts, with a few axons turning anteriorly. (Note that the DiI label site is similar in size and position in C and D, but appears larger in D because the mutant image in D was taken at a longer exposure, required to show the more widespread but less intensely labeled fibers.) (E,F) βIII tubulin antibody labeling of forebrain (FB) and midbrain (MB), side views of bisected whole mounts, truncated at the MB/HB boundary. In mutants, no MLF is visible, and several abnormal axon bundles are seen (arrowheads). (G-I) DiI tracing of projections from the ventral forebrain; side views of whole mounts. (H) MLF label of mutant shows no axons descending, but many axons ascending and crossing the midline. (I) Contralateral side of the same embryo, showing the anterior turning of abnormal post-crossing axons. (J-L) DiI tracing of dorsal midbrain axons; side views of whole midbrains. (K,L) Two mutant embryos, with numerous axons projecting at abnormal angles and/or anteriorly (arrowheads). The bar (J,K) indicates the distance between the descending LLF axons and the ventral midline. (C′,D′,G′-L′) Schematics of DiI labels. cf, cephalic flexure; FB, forebrain; HB, hindbrain; MB, midbrain; r2/r4, rhombomeres.
To visualize axon trajectories, diI was used to trace specific tracts. Intermediate axons made disorganized projections in the anterior hindbrain. These split into two main trajectories. Some axons descended ispilaterally but angled away from the midline, while, surprisingly, half projected first across the midline before descending, forming a contralateral tract. A few axons turned anteriorly after crossing. Forebrain and midbrain axons also made errors (Fig. 3E-L). Tracing of the MLF labeled a single large descending axon bundle in wild type, but no descending axons in the mutant (Fig. 3G-I). Instead, many axons projected anteriorly, some after first crossing the midline, forming bilaterally symmetrical tracts. LLF axons in the midbrain projected in abnormal directions, including anteriorly and angling toward hindbrain midline (Fig. 3J-L).
In summary, the loss of the floor plate disrupts navigation by several distant longitudinal populations, resulting in midline crossing, AP mis-direction, and DV positioning errors. These diverse effects suggest that floor plate signals coordinate several aspects of longitudinal navigation.
Gli2 mutations disrupt Slit expression
To evaluate the molecular basis for axon errors, Slit1-3 mRNA expression was examined (Fig. 4; see also Fig. S1 in the supplementary material). In wild type, the mRNAs for all three Slits were expressed primarily in the floor plate, with additional zones outside of the floor plate. Slit2 and Slit3 were expressed in narrow bands adjacent to the floor plate, and Slit1 at low levels in a stripe just dorsal to the LLF. In Gli2 mutants, Slit midline expression was abolished in r1 and r5, was weakened in some segments, but was retained in r4 and in posterior hindbrain. Another study found partial retention of other midline genes in the Gli2–/– hindbrain (Lebel et al., 2007). We further observed that dorsal Slit1 expression was still present. Thus, the longitudinal errors in Gli2 mutants are associated with altered Slit mRNA, and presumably altered patterns of diffusing Slit proteins.
Fig. 4. Changes in midline Slit expression correlate with axon guidance defects in Gli2 mutants.
(A,B) βIII tubulin antibody labels in Gli2+/– and Gli2–/– embryos, showing the severe disruption of tracts. (C-H) In situ hybridization of three Slits, showing reduced Slit expression, with retention in r4 and the posterior hindbrain. In C, note the dorsal expression of low levels of Slit1 (see Fig. S1 in the supplementary material for midbrain expression of Slit1 and Slit2, but not Slit3). Scale bar: 200 μm.
Slit1 and Slit2 are essential for DV positioning of longitudinal axons
To test Slit function in pioneer longitudinal guidance, we examined mouse embryos carrying Slit1 and Slit2 mutations (see Table S1 in the supplementary material). Overall tract patterns were largely normal in Slit1–/–; Slit2+/– embryos, consistent with the functional overlap of Slit1 and Slit2 (Fig. 5A). By contrast, Slit1–/–;Slit2–/– double mutants had disrupted tracts (Fig. 5B,C), although these were not as disorganized as in Gli2 mutants. ILF bundles were sparse, and axons in the MLF position were diffuse and lacked a tight bundle (Fig. 5B,C). Interestingly, many axon bundles entered the midline in the midbrain and the anterior hindbrain, but the rest of the hindbrain floor plate remained clear of longitudinal bundles, consistent with Slit3 remaining in hindbrain. We noted several neuronal landmarks in their normal positions (ventral motoneurons and projections to exit points, dorsal neurons and their commissural projections), suggesting that the longitudinal errors resulted from direct guidance errors rather than general patterning defects.
Fig. 5. Slit1 and Slit2 are required for many aspects of longitudinal axon guidance.
Open-book hindbrain preparations of Slit1–/–;Slit2+/– and Slit1–/–;Slit2–/– E10.5 embryos, showing (A-C) βIII tubulin antibody and (D-O) DiI labeling of the LLF, ILF and MLF. Anterior, left; floor plate, down (or shown by dashed lines). (A,B) In Slit1–/–;Slit2–/– embryos, intermediate axons appear more diffuse, and the MLF is not visible. (C) Midline closeup showing longitudinal axons projecting in midbrain and anterior r1 floor plate. (D-F) DiI labels of LLF, from dorsal label sites in anterior r1. Most LLF axons in Slit1–/–;Slit2–/– mutants project normally, but a subset wander ventrally (arrowheads; E,F). (G-I) DiI labels of ILF, from intermediate anterior r1. In Slit1–/–;Slit2–/– mutants, the ILF appears shorter (H), and more axons wandering at divergent angles (I, arrowheads). (J-L) MLF labels from DiI placed adjacent to the floor plate in ventral midbrain. (K) Slit1–/–;Slit2+/– mutants show some axons that loop dorsally (arrowhead) in the anterior hindbrain. (L) The MLF in double mutants shows severe errors, including midline crossing and dorsal looping (arrowheads). (M-O) Slit1–/–;Slit2–/– double mutants. Midline sites back-label many MLF neurons, as well as bundles entering midbrain floor plate (N). (O) MLF axons project along the sides of hindbrain floor plate, crossing at multiple points. Some axons wander dorsally (arrowheads). These trajectory errors were verified by Robo1 antibody labeling (see Fig. S4 in the supplementary material). Scale bars: 200 μm in A,B,G,H,M; 50 μm in C,F,I,N; 100 μm in D,E,J-L,O.
The tracing of specific tracts with diI showed altered trajectories in all three longitudinal populations. Most of the axons in the LLF had normal trajectories, but a few ventral deviations were seen. Despite the loss of dorsal Slit1, no axons were seen deviating dorsally (Fig. 5E). The ILF in Slit double mutants was shorter than in controls, and had unusual non-parallel trajectories. Some axon bundles arced dorsally, while others diverged ventrally (Fig. 5H,I). Individual axon trajectories often showed wandering, with switching from dorsal to ventral angles, or vice versa. To examine wandering quantitatively, ILF axon trajectories were measured in hindbrain, and their variability was significantly increased in Slit double mutants (see Fig. S2 in the supplementary material). Therefore, ILF trajectories require Slit1/Slit2 signaling for precise and directed growth.
The MLF was most strongly affected in Slit double mutants. MLF tracing involved two types of diI labels. First, diI crystals were placed in the usual MLF position, adjacent to the midbrain floor plate, which in controls labeled straight bundles (Fig. 5J). With the removal of Slit2 alleles, axons in Slit1–/–; Slit2+/– embryos diverged dorsally in the midbrain, but then returned ventrally in the hindbrain (Fig. 5K). In Slit1–/–;Slit2–/– double mutants fewer axons were labeled, but bundles grew at dorsal angles and axons crossed the floor plate in midbrain and hindbrain (Fig. 5L). Retrograde labeling showed a mixture of mostly ILF cell bodies in lateral mid- and forebrain, with few MLF cell bodies being back-labeled (not shown). Thus, ILF axons are likely to account for many dorsal angling axons.
Using a second labeling strategy to account for the missing MLF axons, we shifted the diI label site to the ventral midline of the midbrain (Fig. 5M). In control embryos, this labeling strategy labeled very few MLF axons (see Fig. S3 in the supplementary material). In Slit1–/–;Slit2–/– double mutants, many MLF cell bodies were retrogradely labeled (Fig. 5N). Bundles of MLF axons projected into the midbrain floor plate, but then bifurcated, with wandering axons forming wide disorganized fascicles on either side of the hindbrain floor plate (Fig. 5O). Axons crossed but did not project longitudinally within the hindbrain floor plate. These trajectory errors were also verified by Robo1 antibody labeling, a marker for MLF axons (see next section; see also Fig. S4 in the supplementary material).
Overall, a graded response to Slit1/Slit2 mutations was evident, with errors being less severe in the LLF and most severe in the MLF. Entry of MLF axons into the midbrain floor plate in Slit double mutants is consistent with an inhibitory or repulsive role for floor plate Slits. In addition, many axons had wandering trajectories, indicating a requirement for Slits in precise longitudinal growth.
Pioneer longitudinal axons express Robo1 and Robo2
The Slit phenotypes suggested that longitudinal pioneers would express Robo receptors. Therefore, we tested pioneer neurons for Robo1 and Robo2 mRNA, protein and reporter gene expression (Fig. 6). Robo1 mRNA was present at the source of the MLF, and in intermediate positions, consistent with ILF neurons. Robo2 expression was more restricted, with dorsal midbrain labeling being consistent with tmesV/LLF neurons, and additional labeling observed in intermediate positions. Rig1/Robo3 was not predicted to be expressed in longitudinal axons (Sabatier et al., 2004) and in fact was restricted to an intermediate region that was clearly dorsal to the MLF and likely ventral to the LLF neurons, so was not further tested (H.F.N. and G.S.M., unpublished).
Fig. 6. Robo1 and Robo2 expression in longitudinal pioneer neurons.
E10.5 whole mounts. (A,B) Differential expression of Robo1 and Robo2 mRNA by ISH. Insets show close-ups of midbrain with strong Robo1 signal in MLF cell bodies (arrowhead). Low signal is seen in the ILF for both Robo1 and Robo2 (dashed box). In the dorsal midbrain, tmesV cell bodies of LLF express Robo2 (dashed box). (C) Anti-Robo1 antibody labels axons of the MLF, and of the ILF, although at lower levels; anterior up. (D) X-gal labeling of homozygous Robo2 tau-lacZ knock-in embryos labels LLF axons (arrowheads) and possibly a subset of ILF, but not MLF.
Robo1 protein expression was examined directly by antibody labeling. MLF axons were Robo1+ as they descended into hindbrain, and a subset of ILF axons showed low expression (Fig. 6C). Two different Robo2 antibodies failed to give sufficient signal, so instead we performed X-gal labeling on embryos carrying a Robo2 tau-lacZ knock-in allele (Long et al., 2004). LLF axons in midbrain were Robo2+, as were an adjacent subset of ILF axons (Fig. 6D). Thus, the major sites of Robo expression were Robo1 in ventral axons, MLF and some ILF, and Robo2 in dorsal axons, some ILF and LLF (although the genetic analysis below suggest a more widespread function of both Robos).
Robo1 and Robo2 receptors are required for precise longitudinal trajectories
To test Robo functions, we examined mutant mice (see Table S1 in the supplementary material). The MLF was specifically affected in Robo1–/– mutants, forming a slightly wider tract as a result of dorsal wandering (Fig. 7). However, the MLF axons did not enter the floor plate (compare with Fig. 5L,O). Further examination of Robo1–/– single mutants showed wild-type patterns of dorsal and intermediate tracts (data not shown). Robo2–/– mutants appeared to be completely normal in all three axon populations.
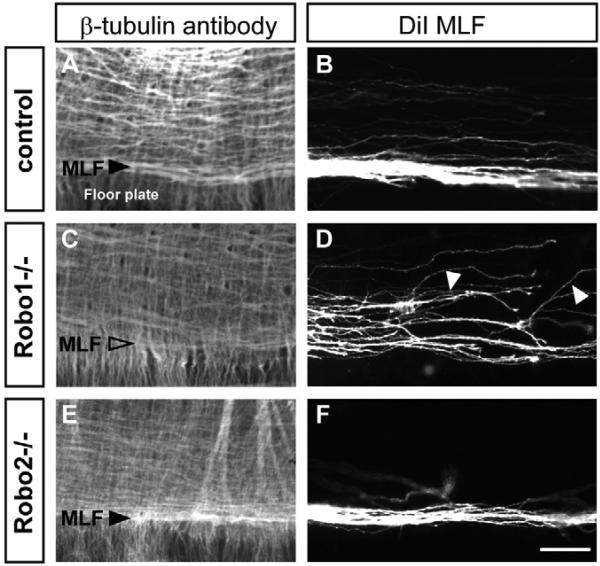
Fig. 7. Robo1 mutations alter MLF pathfinding.
(A,B) Close-up views of the MLF in control embryos, showing a wild-type MLF bundle (A) and trajectories (B). (C,D) Robo1 mutants lack a distinct MLF bundle. (D) DiI labels show a widened MLF with some axons traversing a more dorsal position or taking a dorsal trajectory (arrowheads). (E,F) Robo2 mutants have a normal MLF (and other tracts). Scale bar: 50 μm.
Robo1–/–;Robo2–/– double mutants had severely disorganized tracts, with many axons failing to project parallel to the floor plate (Fig. 8B). No MLF bundle was visible, but the floor plate did contain longitudinal bundles. Along the dorsal margin of the hindbrain, axons deviated dorsally at several positions (Fig. 8C), which contrasted with Slit1–/–;Slit2–/– double mutants (see Fig. 5D-F).
Fig. 8. Robo1 and Robo2 are required for longitudinal axon guidance.
(A-C) Axonal antibody labels. Robo1–/–;Robo2–/– errors include axons diverging dorsally, a large number of axons in the floor plate, and disorganized intermediate axons. (C) Close up of dorsal looping axons in the posterior hindbrain. (D-F) Broad DiI labels of many longitudinal axons. Mutant trajectories are abnormal, tending to divert ventrally (arrowheads). (G-I) LLF labels, DiI in dorsal r1. (G) Control LLF projections parallel the bend in r2 tissue. (H,I) In Robo1–/–;Robo2–/– double mutant embryos, some LLF axons have a normal trajectory, but others make dorsal and ventral deviations (arrowheads). (J-L) ILF labels from r1. Few mutant ILF axons project straight longitudinally, and instead diverge dorsally or ventrally (arrowheads, K,L). (L) An ILF axon wandering dorsally then ventrally. Asterisks indicate the DiI label site. Scale bars: 200 μm in A,B; 50 μm in C,I,L; 100 μm in H,K (for G,J, respectively).
Tract tracing showed more severe errors in Robo1–/–;Robo2–/– double mutants than in Slit1–/–;Slit2–/– double mutants. A wide DiI site was used to label LLF and ILF axons. The axon trajectories often deviated at sharp angles to the floor plate (Fig. 8E). Some mutant axons projected to the contralateral side, sometimes anteriorly. By using specific LLF and ILF labels, it could be seen that some axons diverged dorsally or ventrally (Fig. 8H,I,K,L). Some ILF axons radically changed direction, resulting in a significantly increased variance in angles (supplementary material Fig. S5).
As in Slit mutants, MLF axons were labeled using two sites (Fig. 9). From tract label sites adjacent to the floor plate, Robo double mutant axons fanned out both ventrally into the midline and dorsally (Fig. 9B), and, as in Slit mutants, appeared in retrograde labels to be a combination of mis-directed ILF and MLF axons (W.T.F. and J.P.D., unpublished). Midline label sites showed that most MLF axons converged into the midbrain floor plate (Fig. 9D,E), similar to the Slit double mutants. However, unlike Slit double mutants, MLF axons continued into the hindbrain floor plate, and axon bundles occasionally left the floor plate at sharp angles (Fig. 9F).
Fig. 9. Robo1 and Robo2 are required for MLF guidance.
(A-C) MLF labeling adjacent to the floor plate. A single DiI crystal was placed adjacent to the floor plate. (B) In Robo1–/–;Robo2–/– double mutants, DiI labels MLF axons that fan out and cross midline. (C) Close up of wandering axons. (D-F) DiI labeling in the midline of anterior r1 of Robo1–/–;Robo2–/– double mutants back-labels many MLF cell bodies and midline bundles. (The MLF cell bodies are faintly labeled in the example shown.) Note that numerous commissural axons (CA) were labeled in mutant embryos (D, arrowhead), but not in controls. (E) Closeup of back-labeled MLF cell bodies. Their axons project ventrally and bundle in the midline. (F) Closeup of axon bundles in the hindbrain floor plate, and diverging dorsal bundles. Scale bar: 200 μm in A,B,D; 50 μm in C,E,F.
Overall, Robo1–/–;Robo2–/– double mutant axons were disrupted in their ability to navigate longitudinally, with errors in all three axon populations. The errors made by each population include midline entry, and dorsal and ventral wandering trajectories.
DISCUSSION
Longitudinal axons grow through a landscape of neural tissue, and must use signals laid down within this landscape to navigate with precise trajectories. Longitudinal pioneers in the brain precede commissural axons, and may share the same cues, but they respond with distinct longitudinal projections. As summarized in Fig. 10, our main findings are that the floor plate influences several aspects of longitudinal guidance, and that Slit/Robo signaling plays a predominant role. Specifically, Slit signals are crucial for preventing entry of ventral axons into the midline, and they thereby force ipsilateral projections. Furthermore, Slit/Robo signaling has an unanticipated function in maintaining precise longitudinal trajectories.
Fig. 10. Longitudinal axon trajectories in Gli2, Slit and Robo mutants.
Schematic diagram of the axon trajectories, with thick and thin arrows indicating common and rare axon paths. Forks show where axons diverge from major bundles, not actual axon bifurcation. The floor plate is designated by the yellow box (residual in Gli2 mutants). In the Slit mutant diagram, the yellow box represents Slit3 expression.
The role of the floor plate in longitudinal guidance
Shh transfection profoundly affects longitudinal axon guidance. Conversely, disruption of the floor plate severely impairs the ability of longitudinal axons to navigate. Taken together, these results implicate floor plate-derived signals in guiding longitudinal axons in vivo. Shh electroporation shifts longitudinal tracts, forcing them to turn, at a distance, towards a transfected patch, but then to divert around it. The complex axon responses suggest multiple guidance signals, probably a combination of attractive and repulsive activities. At the same time, ectopic Shh induces re-patterning of the substrate tissue (Agarwala et al., 2001), so these potent effects could include both long range and local cues. Direct guidance by Shh itself is possible, via Boc or Cdon (Charron et al., 2003; Okada et al., 2006).
Similarly, all pioneer longitudinal populations have diverse reactions to Gli2 mutations. Previous studies of an analogous genetic ablation of the floor plate using the zebrafish mutant cyclops (nodal) observed MLF entry and crossing of the ventral midline, but no LLF errors (Hatta, 1992). This confirms the importance of floor plate cues for the MLF, but differs from the Gli2 mutant errors reported here, including MLF direction, and the widespread dorsal axon errors. These differences may be due to an incomplete loss of floor plate cues, or to species differences. Our results lead to the conclusion that Gli2-dependent cues are important for keeping axons ipsilateral, as many axons crossed the midline in Gli2 mutants. Additionally, their post-crossing trajectories were longitudinal, implying retention of the signals for longitudinal growth. A previous spinal cord study provided evidence that Slits are more crucial than other Gli2-dependent signals (Kadison et al., 2006). We observed partial retention of Slits in the hindbrain floor plate (Fig. 4). The reduced and fragmented Slit sources were insufficient to prevent midline crossing or to organize parallel trajectories. Surprisingly, the remnant Slit expression in r4 did not restore discernable order in nearby longitudinal tracts. We speculate that more extensive AP or DV Slit expression is needed for effective guidance. Another major floor plate activity determines AP direction, particularly for MLF axons, which switch AP directions in Gli2 mutants. Possible candidates for directional cues are Wnt and Shh signals (Bourikas et al., 2005; Lyuksyutova et al., 2003), localized in the floor plate, or potentially emanating from other signaling centers, such as the ZLI in the forebrain.
Slit/Robo signals are crucial for several aspects of longitudinal pioneer navigation
The Slits are expressed in and adjacent to the floor plate throughout the length of the neural tube, and the initial expression of Robo1 and Robo2 is predominantly in the longitudinal pioneers. Our analysis of Slit and Robo mutants shows that longitudinal axons make extensive errors of several types. Thus, the floor plate-derived Slit1 and Slit2 signals, as mediated by Robo1 and Robo2, are required for pioneer axons to establish the major descending longitudinal tracts.
A primary role for Slit/Robo signaling is to keep MLF trajectories ipsilateral, although ILF axons also occasionally crossed the midline. Only Slit1 and Slit2 are expressed in the midbrain, and here their loss results in MLF axons traveling into and within the floor plate in disorganized bundles. In the hindbrain, where Slit3 is retained in Slit1/Slit2 mutants, MLF axons projected mainly in bundles on both sides of the floor plate, but with many axons crossing at several locations. Thus, Slit3 alone does not have enough repulsive activity to prevent midline entry, but can prevent lingering. Slit prevention of floor plate entry confirms a previous suggestion that ventral Slits pair with an unknown dorsal repellent, creating a permissive window for longitudinal pioneer axons to pass from midbrain to hindbrain (Molle et al., 2004). Our genetic tests of Slits and Robos extend this model, showing that ventral Slits function continuously along the axis, not just at the midbrain-hindbrain border, and further support the existence of a Slit-independent dorsal repellent. However, the dorsal deflections and the wandering of longitudinal axons in Slit and Robo mutants are not consistent with a purely repellent role for Slits.
The Robo1/Robo2 double mutants likely provide a loss of Slit/Robo responses [with the caveat that the Robo alleles are severe hypomorphs, based on the detection of remnant mis-trafficked Robo protein (Long et al., 2004)]. In the midbrain, the MLF in Robo1/Robo2 double mutants resembles that in Slit double mutants, as expected. In the hindbrain, the Robo double mutant MLF extends within the floor plate, suggesting a complete loss of midline repulsion, reminiscent of the collapse of longitudinal tracts in Drosophila Slit mutants (Kidd et al., 1999; Rothberg et al., 1990).
The collapse of MLF axons into the midline shows that Slits prevent MLF axons from entering the floor plate, and that the loss of Slit activity unmasks an attractive midline cue. These opposing activities, Slits and the midline attractant, could work independently, as in the Drosophila midline (Garbe and Bashaw, 2007), or possibly they could work by Slits silencing the attractant (Stein and Tessier-Lavigne, 2001). The simplest model would be that MLF axons integrate a repulsive Slit signal balanced by attractive cues emanating from the floor plate, possibly netrin or Shh. This would present a combined ‘push-pull’ to position the MLF fascicle at the edge of the floor plate. This relationship of the MLF with the floor plate has been suggested recently (Ahsan et al., 2007).
Interestingly, the ILF and the LLF depend on Slit/Robo signaling to project straight longitudinally, but fail to make a concerted shift toward the floor plate. This indicates that these types of longitudinal axons differ in their use of Slit/Robo signaling. The distinct molecular or signaling basis for distinct types of longitudinal axons will require further investigation.
Outside of the floor plate, Slit1 is also expressed at low levels in dorsal hindbrain (Fig. 4), similar to in spinal cord (Yuan et al., 1999). The dorsal-deviating bundles observed in Robo1/Robo2 double mutants are not seen in Slit1 and Slit1/Slit2 mutants, suggesting that Slits are not the sole ligands for Robos. A distinct dorsal repellent signal may act through Robos. Other mis-matches between Slit and Robo mutant phenotypes include the exuberant forebrain axons observed in Robo mutants (Andrews et al., 2006).
The roles of Robo1 and Robo2 in longitudinal guidance
Robo1 and Robo2 are expressed in longitudinal axons; our analysis detected Robo1 in the MLF and an adjacent subset of intermediate axons, and Robo2 in the LLF pioneers. The ventral Robo1, dorsal Robo2 pattern was somewhat similar to post-crossing commissural axons in the spinal cord (Long et al., 2004). A type of Robo code may specify spinal cord axon positions, as Robo single mutants showed changes in fascicle cross-sectional areas. In the brain, however, pioneer axons had weak reactions to Robo single mutations: Robo1 mutant axons formed a wider MLF tract but did not make a concerted dorsal shift, and Robo2 mutant axons projected normally. Moreover, combined Robo1 and Robo2 double mutations disrupted longitudinal trajectories at all positions. This genetic evidence indicates widespread and largely redundant Robo1 and Robo2 functions. The implication is that both Robos are in fact expressed by axons at all positions, perhaps at levels below the available detection methods.
Importantly, Robo1/Robo2 double mutant phenotypes were similar to Slit1/Slit2 double mutant phenotypes. This is consistent with Robo reception mediating long-range Slit signals. As an alternative mechanism, Robos could directly mediate axon-axon interactions, such as through Robo homophilic binding (Hivert et al., 2002). Pioneers cannot rely on axon-axon interactions, as they navigate in the absence of pre-existing tracts, but later axons may track along pioneers. Pioneers may be particularly important for the tightly fasciculated MLF, but less so for the broader ILF and LLF. Reduced adhesion to pioneers would result in axons wandering to widen tracts. Testing pioneer-follower interactions will require molecular markers for pioneers, and methods to test their influences on followers. We note that Robo1/Robo2 mutant axons can fasciculate, particularly MLF bundles in the floor plate.
In regards to Robo regulation of tract widths, a recent study tested the Slit/Robo guidance of a set of forebrain longitudinal pioneers through Slit1+ substrate tissue in the zebrafish (Devine and Key, 2008). Knockdown of zebrafish Robo1 caused a DV spreading of forebrain pioneers, similar to the MLF phenotype in Robo1–/– mice, but zebrafish Robo2 and Slit1 perturbations condensed the tract, and midline entry was not reported. It is not clear whether these differing observations originate from different tracts, experimental methods, or species.
The precision of longitudinal trajectories: integrating Slit/Robo signals with other cues
The remarkable precision of longitudinal trajectories is disrupted by the loss of Slit/Robo signaling. All three pioneer populations wandered, most prominently in Robo1/Robo2 double mutants. The wandering trajectories were not anticipated from previous fly or mouse studies. One possible explanation would be distinct subpopulations of axons with varying Slit responses, yet individual axons wandered both towards and away from the floor plate (see Figs 1, 2 in the supplementary material). Formally, dorsal deflections would provide evidence for an attractive Slit activity, and although there is precedence for positive Slit roles (Englund et al., 2002; Kramer et al., 2001), dorsal deflections may simply represent wandering. Wandering errors have also been observed in astray (robo2) mutant zebrafish, a result of increased errors combined with a failure to correct common wild-type errors (Hutson and Chien, 2002). Although we did not observe errors in Robo2 single mutant mice, similar increased errors may underlie the wandering in Robo1/Robo2 double mutants.
The identity and relative importance of other longitudinal cues awaits investigation. Axon growth was extensive in Slit and Robo mutants, suggesting widespread permissive or growth-promoting cues. Also, the general posterior direction was retained for most axons, even when DV wandering provided ample opportunity to reverse direction. The floor plate could be the source of these directional cues, as many axons switched to ascending trajectories in Gli2 mutants. For DV positioning, local cues are likely to include cadherins (Mastick and Andrews, 2001). Ephrin signals may also set the dorsal limit of post-crossing commissural axons (Imondi and Kaprielian, 2001). We suggest that Slit/Robo signaling could be required for the coordination of multiple cues, such as the coupling of Robos to a larger complex of receptors (Stein and Tessier-Lavigne, 2001), to prevent wandering and, ultimately, to promote precise longitudinal trajectories. MLF navigation is likely to depend on multiple types of cue, including Sema3D, Tag1, and laminin, alpha 1 (Wolman et al., 2004; Wolman et al., 2008), emphasizing the need for integrating multiple signals.
In conclusion, longitudinal pioneers have diverse reactions to Slit/Robo mutations that were not anticipated by commissural phenotypes. This implies that longitudinal growth cones use Slits in novel ways or contexts, presumably through distinct intracellular signaling or novel interactions with other cues that combine to steer axons along precise longitudinal trajectories.
Supplementary Material
Acknowledgments
The Slit and Robo mutant founder mice, and mouse Slit and Robo probes were a gift of Marc Tessier-Lavigne (Stanford, Genentech). The Shh expression plasmid was a gift of Kerby Oberg (Loma Linda). Gli2 mutant mice were a gift from C. C. Hui. We are grateful to Le Ma (Stanford, UCLA), who contributed initial Slit mutant embryos. Several people provided technical assistance with genotyping and other experiments, including Laura Popko, Victoria Lisowski, Michael Berberoglu, Mebrat Mebrahtu, Hector Miguel, Samuel McMahon, Andrea Stratton, Matt Beuhler and Minkyung Kim. We thank Gracie Andrews for help with microscopy. G.S.M. is grateful for helpful discussions at the University of Queensland from Linda Richards, Geoff Goodhill, Divya Unni, Zac Pujic, Mike Piper, Tom Fothergill, Charlotta Lindwall, Duncan Mortimer, Guy Barry, Will Rosoff, Helen Cooper and Brian Key. We are grateful to Erick Leurken, UNR Center for Research Design and Analysis, for statistics advice. Use of the Nevada Genomics Center was supported by P20 RR-016464 from INBRE (NCRR). We are grateful to the March of Dimes for grant #1-FY06-387, and for NIH grants HD38069 and NS054740 to G.S.M.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/22/3643/DC1
References
- Agarwala S, Ragsdale CW. A role for midbrain arcs in nucleogenesis. Development. 2002;129:5779–5788. doi: 10.1242/dev.00179. [DOI] [PubMed] [Google Scholar]
- Agarwala S, Sanders TA, Ragsdale CW. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–2150. doi: 10.1126/science.1058624. [DOI] [PubMed] [Google Scholar]
- Ahsan M, Riley KL, Schubert FR. Molecular mechanisms in the formation of the medial longitudinal fascicle. J. Anat. 2007;211:177–187. doi: 10.1111/j.1469-7580.2007.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat. Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chedotal A, Pourquie O, Sotelo C. Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur. J. Neurosci. 1995;7:198–212. doi: 10.1111/j.1460-9568.1995.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron. 1991;7:277–285. doi: 10.1016/0896-6273(91)90266-3. [DOI] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu. Rev. Neurosci. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev. Biol. 2008;313:371–383. doi: 10.1016/j.ydbio.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J. Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter SS, Jr, Burrill J, Marcus RC, Ross LS, Taylor JS, Wilson SW. Initial tract formation in the vertebrate brain. Prog. Brain Res. 1994;102:79–93. doi: 10.1016/S0079-6123(08)60533-6. [DOI] [PubMed] [Google Scholar]
- Englund C, Steneberg P, Falileeva L, Xylourgidis N, Samakovlis C. Attractive and repulsive functions of Slit are mediated by different receptors in the Drosophila trachea. Development. 2002;129:4941–4951. doi: 10.1242/dev.129.21.4941. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Di Meglio T, Ma L, Kawasaki T, Long H, Hirata T, Tessier-Lavigne M, Chedotal A, Nguyen-Ba-Charvet KT. Robo1 and robo2 control the development of the lateral olfactory tract. J. Neurosci. 2007;27:3037–3045. doi: 10.1523/JNEUROSCI.0172-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa K, Wrana JL, Culotti JG. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science. 2007;317:1934–1938. doi: 10.1126/science.1144874. [DOI] [PubMed] [Google Scholar]
- Garbe DS, Bashaw GJ. Independent functions of Slit-Robo repulsion and Netrin-Frazzled attraction regulate axon crossing at the midline in Drosophila. J. Neurosci. 2007;27:3584–3592. doi: 10.1523/JNEUROSCI.0301-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JC, Petursdottir G. Regional specificity of developing reticulospinal, vestibulospinal, and vestibulo-ocular projections in the chicken embryo. J. Neurobiol. 1991;22:353–376. doi: 10.1002/neu.480220405. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Hatta K. Role of the floor plate in axonal patterning in the zebrafish CNS. Neuron. 1992;9:629–642. doi: 10.1016/0896-6273(92)90027-b. [DOI] [PubMed] [Google Scholar]
- Hivert B, Liu Z, Chuang CY, Doherty P, Sundaresan V. Robo1 and Robo2 are homophilic binding molecules that promote axonal growth. Mol. Cell Neurosci. 2002;21:534–545. doi: 10.1006/mcne.2002.1193. [DOI] [PubMed] [Google Scholar]
- Holmes GP, Negus K, Burridge L, Raman S, Algar E, Yamada T, Little MH. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech. Dev. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- Hutson LD, Chien CB. Pathfinding and error correction by retinal axons: the role of astray/robo2. Neuron. 2002;33:205–217. doi: 10.1016/s0896-6273(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Imondi R, Kaprielian Z. Commissural axon pathfinding on the contralateral side of the floor plate: a role for B-class ephrins in specifying the dorsoventral position of longitudinally projecting commissural axons. Development. 2001;128:4859–4871. doi: 10.1242/dev.128.23.4859. [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadison SR, Murakami F, Matise MP, Kaprielian Z. The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord. Dev. Biol. 2006;296:499–513. doi: 10.1016/j.ydbio.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998a;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998b;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- Lebel M, Mo R, Shimamura K, Hui CC. Gli2 and Gli3 play distinct roles in the dorsoventral patterning of the mouse hindbrain. Dev. Biol. 2007;302:345–355. doi: 10.1016/j.ydbio.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marin O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J. Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Mambetisaeva ET, Andrews W, Camurri L, Annan A, Sundaresan V. Robo family of proteins exhibit differential expression in mouse spinal cord and Robo-Slit interaction is required for midline crossing in vertebrate spinal cord. Dev. Dyn. 2005;233:41–51. doi: 10.1002/dvdy.20324. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Easter SS., Jr Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev. Biol. 1996;173:79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Andrews GL. Pax6 regulates the identity of embryonic diencephalic neurons. Mol. Cell Neurosci. 2001;17:190–207. doi: 10.1006/mcne.2000.0924. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Davis NM, Andrew GL, Easter SS., Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997;124:1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- Matise MP, Lustig M, Sakurai T, Grumet M, Joyner AL. Ventral midline cells are required for the local control of commissural axon guidance in the mouse spinal cord. Development. 1999;126:3649–3659. doi: 10.1242/dev.126.16.3649. [DOI] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Molle KD, Chedotal A, Rao Y, Lumsden A, Wizenmann A. Local inhibition guides the trajectory of early longitudinal tracts in the developing chick brain. Mech. Dev. 2004;121:143–156. doi: 10.1016/j.mod.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Nural HF, Mastick GS. Pax6 guides a relay of pioneer longitudinal axons in the embryonic mouse forebrain. J. Comp. Neurol. 2004;479:399–409. doi: 10.1002/cne.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg KC, Pira CU, Revelli JP, Ratz B, Aguilar-Cordova E, Eichele G. Efficient ectopic gene expression targeting chick mesoderm. Dev. Dyn. 2002;224:291–302. doi: 10.1002/dvdy.10104. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000a;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000b;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell TM, Roelink H. Restrictions to floor plate induction by hedgehog and winged-helix genes in the neural tube of frog embryos. Mol. Cell Neurosci. 1995;6:106–121. doi: 10.1006/mcne.1995.1011. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000a;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000b;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Mambetisaeva E, Andrews W, Annan A, Knoll B, Tear G, Bannister L. Dynamic expression patterns of Robo (Robo1 and Robo2) in the developing murine central nervous system. J. Comp. Neurol. 2004;468:467–481. doi: 10.1002/cne.10984. [DOI] [PubMed] [Google Scholar]
- Tamada A, Shirasaki R, Murakami F. Floor plate chemoattracts crossed axons and chemorepels uncrossed axons in the vertebrate brain. Neuron. 1995;14:1083–1093. doi: 10.1016/0896-6273(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Taylor JS. The early development of the frog retinotectal projection. Development. 1991;(Suppl 2):95–104. [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Ross LS, Parrett T, Easter SS., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Wolman MA, Liu Y, Tawarayama H, Shoji W, Halloran MC. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J. Neurosci. 2004;24:8428–8435. doi: 10.1523/JNEUROSCI.2349-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Sittaramane VK, Essner JJ, Yost HJ, Chandrasekhar A, Halloran MC. Transient axonal glycoprotein-1 (TAG-1) and laminin-alpha1 regulate dynamic growth cone behaviors and initial axon direction in vivo. Neural Develop. 2008;3:6. doi: 10.1186/1749-8104-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev. Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.