Abstract
It has been known for decades that the neuroendocrine system can both directly and indirectly influence the developmental and functional activity of the immune system. In contrast, far less is known about the extent to which the immune system collaborates in the regulation of endocrine activity. This is particularly true for immune-endocrine interactions of the hypothalamus-pituitary-thyroid axis. Although thyroid stimulating hormone (TSH) can be produced by many types of extra-pituitary cells — including T cells, B cells, splenic dendritic cells, bone marrow hematopoietic cells, intestinal epithelial cells and lymphocytes — the functional significance of those TSH pathways remain elusive and historically have been largely ignored from a research perspective. There is now, however, evidence linking cells of the immune system to the regulation of thyroid hormone activity in normal physiological conditions as well as during times of immunological stress. Although the mechanisms behind this are poorly understood, they appear to reflect a process of local intrathyroidal synthesis of TSH mediated by a population of bone marrow cells that traffic to the thyroid. This hitherto undescribed cell population has the potential to microregulate thyroid hormone secretion leading to critical alterations in metabolic activity independent of pituitary TSH output, and it has expansive implications for understanding mechanisms by which the immune system may act to modulate neuroendocrine function during times of host stress. In this article, the basic underpinnings of the hematopoietic-thyroid connection are described, and a model is presented in which the immune system participates in the regulation of thyroid hormone activity during acute infection.
Keywords: immune-endocrine, homeostasis, metabolic, infection, hormone
Synthesis and Use of Thyroid Stimulating Hormone by Hematopoietic Cells
TSH Producing Cells of the Immune System
The primary function of the hypothalamus-pituitary-thyroid (HPT) axis is to regulate thyroid hormone synthesis and production. Thyrotropin releasing hormone (TRH) — a tripeptide hormone produced by the hypothalamus — serves as an inductive signal for the release of thyrotropin (thyroid stimulating hormone [TSH]) from the anterior pituitary. Similar to other glycoprotein hormones, TSH is a heterodimer consisting of α-chain and β-chain molecules. Because the α-chain is shared with follicle stimulating hormone, luteinizing hormone, and chorionic gonadotropin, the TSHβ component is responsible for conferring hormone activity and specificity on TSH. Once released from the anterior pituitary and disseminated via the blood to the thyroid, its target tissue, TSH induces the release of thyroxine (T4) and tri-iodothyronine (T3) (1). T4 may be converted into the more biologically-active T3 form in target tissues. Levels of circulating thyroid hormones provide feedback mechanisms, in both positive and negative ways, that contribute to the regulation of TRH and TSH synthesis (2).
Evidence for the production of TSH by cells of the immune system was first demonstrated over twenty years ago (3, 4). Initial studies used human leukocytes, which produced TSH following stimulation with staphylococcus enterotoxin A or upon exposure to TRH (3-5). Thyroid hormones also may serve as negative regulators of hematopoietic TSH in a fashion similar to that of the HPT axis (6). In secondary lymphoid tissues, splenic dendritic cells (DCs) have been shown to be a particularly strong source of TSH. Following in vitro culture in medium without stimulation, or when cultured with staphylococcus enterotoxin B, DCs produced 3-6 times more TSH than was produced by purified B cells or T cells (7). The capacity of DCs to produce TSH was confirmed in vivo by immunofluorescence staining of splenic tissues from normal mice, which revealed that the majority of TSH-producing cells were localized in the marginal zones surrounding T cell areas and in germinal centers where DCs are enriched (8). Additionally, studies in our laboratory demonstrate that the number of TSH producing cells in the marginal zone of the spleen and lymph nodes is markedly increased following in vivo challenge with bacterial lipopolysaccharide (LPS) (unpublished observation), suggesting that TSH producing cells of the immune system respond to strong antigenic stimuli with an aggressive burst of TSH production.
TSH has been shown to be produced by a sub-population of bone marrow hematopoietic cells. This has been demonstrated by intracellular staining in combination with CD45 (leukocyte-common antigen [LCA]) or CD11b staining. TSH+ bone marrow cells were exclusively associated with the LCA+ cells (9), thus they were not bone marrow stromal cells. Moreover, most TSH+ cell belonged to a CD11b+ monocyte/macrophage precursor or granulocyte precursor population; considerably fewer TSH+ cells were lymphocyte precursors (9). That bone marrow cells actively secreted TSH was confirmed using cell-sorted CD11b+ bone marrow cells in an enzyme-linked assay with anti-mouse TSH antibody (9, 10). Although both CD11b+ and CD11b− cells produced TSH, the CD11b+ population was the predominant TSH-producing cell population (9) — a finding that will have significance later in this article.
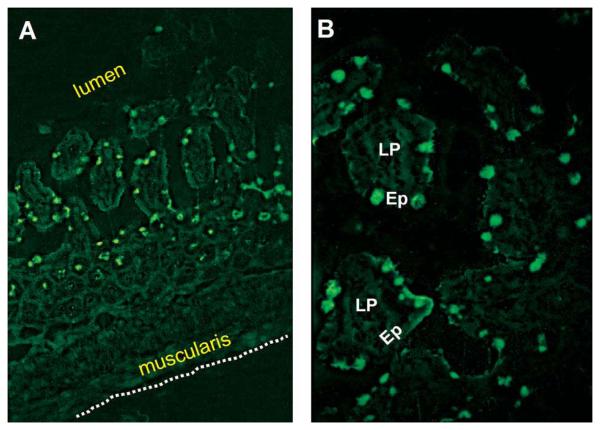
The small intestine in mice also has been shown to be a site of active TSH synthesis as documented at both the transcriptional and cell surface levels for intestinal epithelial cells and intestinal T cells, and the presence of the TSH receptor (TSHR) on intestinal cells (11). TSH synthesis is localized in sub-villus crypt regions (12) — a site where local T cell development occurs (13) — and also in focal areas of the epithelium (12). Moreover, during acute rotavirus infection, TSH staining in the epithelium increases considerably, particularly in areas of virus infection (12). Similar findings now have been observed in our laboratory during acute intestinal reovirus infection (Fig. 1). Given the potential involvement of TSH in immune regulation of the intestine (14, 15), those studies provide evidence for local paracrine action of TSH, and thus they lend credence to the likelihood that immune system-derived TSH may operate in a paracrine manner elsewhere in the organism as will be discussed for the thyroid. These findings are summarized in Table 1.
Fig. 1.
Small intestinal jejunum section from a day 4 reovirus serotype 3-infected mouse stained with biotinylated anti-TSHβ antibody 1B11 (10) and streptavidin fluorescein isothiocyanate. Note the predominance of TSH+ cells in the intestinal epithelium. Staining was rare in epithelial regions of non-infected mice or using isotype control antibody (data not shown).
Table 1.
Sources of Extra-pituitary TSH
| Cells | Stimulus | Reference |
|---|---|---|
| Leukocytes | SEAa, TRHband immune activation | 3, 4, 5 |
| Dendritic cells | SEBc | 7 |
| Bone marrow cells | spontaneous | 9 |
| Intestinal epithelial cells and T cells | spontaneous and virus-induced | 11 |
| CD11b+ intra-thyroidal cells | spontaneous; possibly after activation |
8 |
Staphylococcus enterotoxin A
Thyrotropin releasing hormone
Staphylococcus enterotoxin B
Two Venues for TSH Modulation of Immune Function
Despite evidence for a hematopoietic source of TSH, questions remain as to how immune system TSH participates in the immunophysiological process during health and disease. There are at least two ways through which extra-pituitary TSH could exert an effect on cells of the body: one being a direct effect of TSH on cells of the immune system, the other being an indirect effect mediated by TSH-induced thyroid hormone.
In as much as TSH can be produced by leukocytes, it is reasonable to assume that TSH may act as a cytokine-like regulatory molecule within the immune system. Support for this comes from studies demonstrating the expression of TSHR on lymphoid and myeloid cells (16, 17), and as inferred from studies demonstrating the ability of TSH to influence lymphocyte functional behavior (18, 19). TSH has been shown to elicit elevated antibody responses in in vitro plaque assays (4, 5, 20), and to have potentiating effects on concanavalin-A- and phytohemagglutinin-induced proliferation of lymphocytes (21). TSH also has costimulatory activity for natural killer cells in combination with interleukin (IL)-2 (21). TSH stimulation of splenic DCs results in a stronger phagocytic response in vitro, and increases the cytokine activity of IL-1β and IL-12 in the presence of phagocytic stimuli (17). In the bone marrow, the TSHR is expressed on some but not all lymphocyte, monocyte, and granulocyte precursors, and stimulation of bone marrow cells with TSH results in increased secretion of TNFα by the CD11b− population (9, 22). TSH stimulation of bone marrow cells results in a classical cAMP response and rapid phosphorylation of the Jak2 kinase (22). Because those studies utilized in vitro systems, and thus were less affected by secondary or ancillary events mediated by TSH in vivo, the likelihood is high that they reflected direct activity of TSH on TSHR+ immune cells.
Alternatively, TSH also could act indirectly on the immune system by altering T3 and T4 release from the thyroid, which then would affect the functional or developmental activity of cells in the bone marrow and/or in secondary lymphoid tissues. Evidence for this comes from studies showing impaired immune function in situations of low circulating thyroid hormones. In the TSHR defective C.RF-hyt/hyt mouse, which are unable to utilize TSH and consequently are severely hypothyroidic, B cell development in the bone marrow is curtailed (23, 24). Administration of T4 to C.RF-hyt/hyt mice increased the percent and absolute numbers of pro-B cells in cell cycle (23). Similarly, mice with gene deletion in the T3 receptors α1 and α2 had significantly reduced numbers of cells in the bone marrow, thymus, and the spleen, with all leukocyte populations in the spleen (B cells, T cells, granulocytes, and macrophages) being affected (25). Taken together, these studies point to a range of activities exerted by TSH and thyroid hormone on central and peripheral immune function.
A Novel Role for Hematopoietic Cell-Derived TSH in Metabolic Regulation
The targeted effects of TSH on immunological function notwithstanding, an additional role of immune system TSH may be to microregulate thyroid hormone synthesis. This would occur under highly specialized conditions in which there is a fundamental necessity for communication between the immune system and the thyroid. Several suppositions would need to be fulfilled for this to occur.
There must exist a TSH-producing cell with the potential to traffic to the thyroid. This could consist of a cell of known characteristics and properties, possibly one of the TSH-producing cells described in the previous section, or it could involve a novel hematopoietic cells with a dedicated function aimed at thyroid regulation.
The operative cell must migrate to the thyroid with the primary purpose of producing intrathyroidal TSH.
There must be a rationale for a system such as this to be maintained.
In the following sections, the empirical and conceptual underpinnings of an immune-thyroid communication network are laid out.
Immune-Thyroid Networks
To begin to understand how immune system TSH might be involved in thyroid hormone regulation, it will be necessary to predict the biological context in which this would occur. There are at present several examples by which immune-thyroid interactions might come into play under natural or experimental conditions. Two of these are described here.
Non-Thyroidal Illness — A Complex and Enigmatic Process that Involves Multiple Interactive Physiological Events with Salient Features of an Immune Regulated Process
Non-thyroidal illness, also known as euthyroid sick syndrome (ESS), is a hypothyroidic condition of humans that occurs in the absence of overt thyroid disease. In mild forms, ESS is characterized by a reduction circulating T3, usually resulting from a failure to convert T4 to T3 in the liver or kidneys (2, 26, 27). In severe forms, however, levels of both T3 and T4 can be reduced, the latter caused by impaired T4 output from the thyroid (2). ESS can result from a wide variety of causes including fasting, infection, sepsis, trauma, myocardial infarction, coronary artery bypass surgery, and bone marrow transplantation (27-31). Although the full significance of ESS is not known, it may represent a basic host mechanism used to conserve energy during periods of physiological stress (27, 32, 33), and it likely represents an essential, albeit poorly understood, phase of the host immuno-physiological defense against infection. The mechanisms responsible for the hypothyroidic state in ESS differ depending upon the inducing stimuli. During fasting, suppressed serum leptin levels lead to lower TRH and TSH levels with a concomitant lowering of T3 and T4 output and suppressed host metabolic activity (34-36). After the cessation of fasting, leptin levels increase and the physiological process is reversed, resulting in increased metabolic activity.
The mechanistic pathway involved in ESS during infection is different from that of fasting in that, among other things, it is not significantly regulated by leptin (38-41). In experimental systems involving infection or exposure to lipopolysaccharide (LPS), two mechanisms (not necessarily mutually exclusive) have been described that could account for suppressed TSH levels. First, infusion of rats with the proinflammatory cytokines, IL-1, IL-6, or TNFα, has been shown to lower serum TSH levels (42-45). Thus, the mere presence of those potent inflammatory mediators could indirectly influence thyroid hormone synthesis. Second, studies have demonstrated that, following LPS exposure, T4 is converted to T3 in tanycytes of the third ventricle near the hypothalamic median eminence, thereby causing a surge in T3 locally (33, 36). This, in essence, would constitute a localized T3 feedback mechanism that would suppress TRH and TSH output and curtail the release of thyroid hormones. As yet, however, there is no known compensatory mechanism to account for the increase in T3/T4 synthesis during the recovery phase of infection. Thus, a critical question remains as to how the neuroendocrine system ‘perceives’ that the time is right to re-adjust thyroid hormone production. The hypothesis proposed here, drawn from extant empirical evidence, is that it is the immune system — not the endocrine system — that re-starts the process of thyroid hormone output. The immune system, which is indeed capable of TSH production, would be well suited to determine the optimal time to initiate the thyroid hormone recovery phase. A critical factor in this would be the inherent ability of the immune system to continually assess the status of the infectious condition, and thus to determine whether or not it is safe for the host to return to a state of normal metabolic activity.
Thyroid Activity following Bone Marrow Transplantation
A substantial body of literature has accumulated over the years demonstrating a relationship between thyroid hormone dysfunction and bone marrow transplantation. This can occur following total body irradiation (46-53) or after chemotherapy in the absence of irradiation (53, 54). In both situations, an ESS-like condition is commonly observed. Characteristically, T3, and occasionally T4 levels, are low even though TSH output may be only marginally altered. Because the thyroid is primarily resistant to the effects of clinical radiation (55), the basis for changes in thyroid hormone levels are not well understood; however, they are not likely due to damage to the thyroid gland by the immunosuppressive treatment itself. Furthermore, it is unclear why thyroid hormone levels in bone marrow transplant patients continue to be suppressed in the face of otherwise normal circulating TSH levels. Indeed, as is frequently observed in ESS, the effects of available circulating TSH appear to have minimal influence on thyroid hormone levels in bone marrow transplant patients. Thus, the question emerges as to whether — as a consequence of bone marrow transplantation — there is a disturbance of a bone marrow cell population vested with TSH production.
Experimental Evidence for Immune-Thyroid Interactions
Following Immunological Stress, Mice Display a Precipitous though Transient Drop in Circulating Thyroid Hormone Levels
In experimental systems using rodents, several studies have reported a drop in circulating thyroid hormones following systemic exposure to natural or synthetic antigens (e.g., sheep red blood cells or trinitrophenyl hemocyanin) (56), as well as to tumor inducing agents such as methylcholanthrene or dimethylbenzanthracene (57). Studies in our laboratory have explored this phenomenon in two experimental murine systems (7). In the first, the effects of acute antigen exposure on circulating thyroid hormone levels were evaluated in mice primed intraperitoneally with allogeneic lymphoid cells — a situation that most closely approximates the condition that occurs in humans following transplantation with non-autologous bone marrow. In the second experimental system, mice expressing a transgenic T cell receptor for hen-egg lysozyme (HEL) were primed with HEL. That system was selected since it represented a strong T cell-mediated response to a nominal antigen. Serum T3 and T4 levels were monitored daily in mice after antigen priming (7). Notably, in both experimental models, there was a sharp though transient drop in circulating T3 and T4 24-48 hrs after antigen exposure; recovery in circulating thyroid hormone levels began 3-4 days post-antigen challenge (7). As with ESS, there was minimal change in levels of circulating TSH during or after the hypothyroidic phase (7), and thus these findings replicated experimentally using defined antigen systems the general observations seen in ESS. The reason for the immune-modulating effects of those stimuli on circulating thyroid hormone levels remains unclear, although a key feature of all is that they involve some type of acute immunological stress.
A Compensatory Response in Thyroid Hormone Production Arising via an Extra-Pituitary Pathway
Because circulating TSH levels did not fluctuate significantly during the hypothyroidic period in the experimental systems described above, the question emerges as to what mechanisms might be responsible for the recovery of thyroid hormone activity. One possibility is that this occurs from a natural source of extra-pituitary TSH. To test that hypothesis, experiments were done in which hypophysectomized (HPX) mice were used for in vivo alloantigen challenge (7). Owing to the fact that HPX mice are incapable of producing pituitary-derived TSH, an increase in thyroid hormone levels following antigen priming would have to come from an extra-pituitary pool of TSH. Sera were collected from tail veins of HPX mice immediately prior to alloantigen injection and also at the time of sacrifice on days 1, 2, 3, or 4 after antigen priming and comparisons were made of T4 levels in matched pre- and post-challenge sera for each animal (7). Findings from those experiments were remarkable in that there was a significant increase in serum T4 in all HPX mice after antigen priming compared to pre-antigen-challenge levels (7). Most important, because HPX mice were unable to synthesize pituitary TSH, the increase in serum T4 following antigen exposure must have been due to a compensatory event similar to that observed in non-HPX mice during days 3-4 post-antigen challenge (7).
A Bone Marrow-Thyroid TSH Network
Bone Marrow-Derived TSH Producing Cells Actively Traffic to the Thyroid
As described above, there is now evidence that bone marrow cells are an active source of extra-pituitary TSH, that the predominant TSH-producing cell population is a CD11b+ subset (9), and that those cells routinely migrate to the thyroid (8). When thyroid tissue sections from normal mice were stained with a panel of antibodies to lymphoid and myeloid cell surface markers, there were a surprisingly high number of cells clustering near thyroid follicles that expressed CD11b but lacked expression of CD3, CD8α, CD11c, CD19, CD40, Ly-6G, and F4/80 (8). That particularly unusual phenotype suggested that the intrathyroidal CD11b+ cells were not conventional macrophages, plasmacytoid or lymphoid dendritic cells, activated dendritic cells, T cells, or B cells, but rather that they may be a novel, previously uncharacterized type of hematopoietic cell.
To trace the origins of those cells, bone marrow chimeras were constructed in which normal mice were exposed to 900 rad total body irradiation to destroy host hematopoietic cell precursors, and the immune system was reconstituted using bone marrow from syngeneic mice that expressed an enhanced-green fluorescent protein (EGFP) transgene (58). By using EGFP donor bone marrow, it was possible to accurately follow the trafficking of cells to the thyroid, and to confirm that they were of donor origin. Thyroid tissue sections from chimeric mice were studied from 1-20 weeks after bone marrow transfer using immunofluorescence microscopy to monitor the appearance of EGFP+ cells. The striking finding from these experiments was the rapid accumulation of EGFP+ cells in the thyroid after bone marrow transfer, and the presence of EGFP+ cells throughout the 20 week period of study (8). Moreover, the location and basic morphological features of the EGFP+ cells suggested that they were identical to the CD11b+ intrathyroidal cells found in normal non-chimeric mice (8).
Evidence for Intrathyroidal TSH Synthesis by Bone Marrow-Derived Cells
Clearly, the presence of migrating bone marrow cells in the thyroid would not, of itself, be proof of intrathyroidal TSH synthesis. Compelling evidence of this came in the form of two empirical observations. First, RT-PCR analysis of thyroid tissues revealed gene expression of the TSHβ gene, thus providing evidence that in situ TSH production occurs (8). Second, active production of intrathyroidal TSH was confirmed by in situ staining using an anti-mouse TSHβ antibody, which revealed TSHβ+ cells with morphological features similar to the CD11b+ cells and the migratory EGFP+ cells found in the thyroid (8). Since there is no a priori evidence for TSH synthesis by thyrocytes themselves, expression of the TSHβ gene and TSHβ staining in thyroid tissues strongly favor a process of local intrathyroidal production by hematopoietic cells. Collectively, these findings make a strong case for the likelihood that there is a unique population of bone marrow-derived hematopoietic cells that traffic to the thyroid for the purpose of intrathyroidal TSH synthesis. Clearly, this system would operate on a paracrine basis involving the local synthesis and utilization of thyroid hormone. The best evidence to date that TSH has the potential to act through a paracrine rather than an endocrine delivery system come from the experiments of rotavirus infection in mice, as described earlier, in which local TSH synthesis was tracked to areas of virus infection (12), with the awareness that TSH has been shown to modulate immune activity in the intestine (11, 14, 15). Additional studies will be needed to confirm that locally produced TSH directly participates in thyroid hormone regulation.
A Model of Immune Regulation of Thyroid Hormone Synthesis
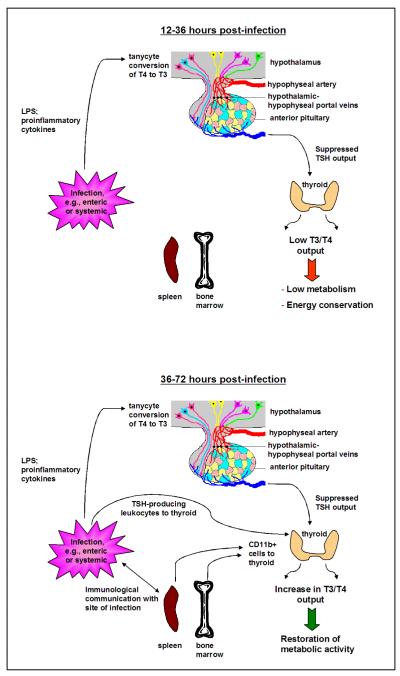
How, then, do all of these observations bear on a potential role for hematopoietic TSH regulation of thyroid hormone activity? Perhaps the best example comes from the infectious model as detailed in Fig. 2. During acute infection, inflammatory mediators released into the circulation would stimulate the conversion of T4 to T3 in tanycytes of the third ventricle. This in turn would hold TSH in check, lowering thyroid hormone output. Physiologically, this would initiate and sustain an overall condition of curtailed metabolic activity. Low metabolic activity would encourage energy conservation by the host and discourage attempts to over-exert during the period of infection — a time when rest would be important. Once the infection is under control through the generation of innate and adaptive immune responses, the immune system would provide the initial signal (through intrathyroidal TSH synthesis by CD11b+ cells from the bone marrow and possibly also from the peripheral immune system) that would lead to an adjustment in thyroid hormone activity with concomitant recovery in metabolic activity. This system offers the added advantage, particularly during times of acute infection, of drawing upon the inherent power of the immune system into the process of metabolic regulation in ways that could not be accomplished solely through classical HPT circuitry. Furthermore, many aspects of this model are amenable to additional experimental study, such as a detailed analysis of the intrathyroidal TSH-producing hematopoietic cell(s), which may be the key element in understanding the immune-thyroid pathway.
Fig. 2.
Model of immune regulation of thyroid hormone activity during acute infection. (A, top) During the early phase of infection (12-36 hrs) such as with enteric virus infection, e.g., rotavirus or reovirus (12) or systemic viral or bacterial infection, proinflammatory cytokines and/or bacterial-derived products stimulate the conversion of T4 to T3 in tanycytes of the third ventricle, causing suppression of TRH and TSH release and a decrease in T3 and T4 output. A general sense of malaise ensues due to curtailed metabolic activity, leading to energy conservation at a critical time of host physiological stress. (B, bottom) The immune system responds to the infectious process through the generation of innate and adaptive immune responses. As the infection is controlled, CD11b+ cells selectively mobilized in the bone marrow, peripheral lymphoid tissues, and possibly in the site of infection itself, traffic to the thyroid where they secrete TSH, prompting thyroid hormone release, which in turn leads to an elevation of metabolic activity. A critical feature of this model pertains to the readjustment in thyroid hormone activity by the immune system, since it is the immune system that would be most capable of determining the optimal time for this to occur in the context of the host’s response to infectious challenge.
Acknowledgements
The assistance of Dina Montufar-Solis in the staining of intestinal tissue sections is gratefully appreciated. This work was supported by NIH grant DK035566.
References
- 1.Szkudlinski M, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 2.Kelley G. Peripheral metabolism of thyroid hormones: A review. Alternative Med Rev. 2000;5:306–333. [PubMed] [Google Scholar]
- 3.Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci USA. 1982;80:6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruger TE, Blalock JE. Cellular requirements for thyrotropin enhancement of in vitro antibody production. J Immunol. 1986;137:197–200. [PubMed] [Google Scholar]
- 5.Kruger TE, Smith EM, Harbour DV, Blalock JE. Thyrotropin: an endogenous regulator of the in vitro immune response. J Immunol. 1989;142:744–747. [PubMed] [Google Scholar]
- 6.Harbour DV, Kruger TE, Coppenhaver D, Smith EM, Meyer WJ., III Differential expression and regulation of thyrotropin (TSH) in T cell lines. Mol Cell Endocrinol. 1989;64:229–241. doi: 10.1016/0303-7207(89)90150-0. [DOI] [PubMed] [Google Scholar]
- 7.Bagriacik EU, Zhou Q, Wang H-C, Klein J. Rapid and transient reduction in circulating thyroid hormones following systemic antigen priming: Implications for functional collaboration between dendritic cells and thyroid. Cell Immunol. 2001;212:92–100. doi: 10.1006/cimm.2001.1846. [DOI] [PubMed] [Google Scholar]
- 8.Klein JR, Wang H-C. Characterization of a novel set of resident intrathyroidal bone marrow-derived hematopoietic cells: potential for immune-endocrine interactions in thyroid homeostasis. J Exp Biol. 2004;207:55–65. doi: 10.1242/jeb.00710. [DOI] [PubMed] [Google Scholar]
- 9.Wang H-C, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNFα production by bone marrow cells. Blood. 2003;101:119–123. doi: 10.1182/blood-2002-02-0544. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Wang H-C, Klein JR. Characterization of a novel anti-mouse thyrotropin monoclonal antibody. Hybridoma Hybridomics. 2002;21:75–79. doi: 10.1089/15368590252917674. [DOI] [PubMed] [Google Scholar]
- 11.Wang H-C, Whetsell M, Klein JR. Local hormone networks and intestinal T cell homeostasis. Science. 1997;275:1937–1939. doi: 10.1126/science.275.5308.1937. [DOI] [PubMed] [Google Scholar]
- 12.Scofield VL, Montufar-Solis D, Cheng E, Estes MK, Klein JR. Intestinal TSH production is localized in villus ‘hotblocks’ and is coupled to IL-7 production: evidence for involvement of TSH during acute virus infection. Immunol Let. 2005;99:36–44. doi: 10.1016/j.imlet.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanga H, Iwanga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Klein JR. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994;265:1860–1862. doi: 10.1126/science.8091211. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Klein JR. Hormonal regulation of extrathymic gut T cell development: Involvement of thyroid stimulating hormone. Cell Immunol. 1995;161:299–302. doi: 10.1006/cimm.1995.1040. [DOI] [PubMed] [Google Scholar]
- 16.Coutelier JP, Kehrl JH, Bellur SS, Kohn LD, Notkins AL, Prabhakar BS. Binding and functional effects of thyroid stimulating hormone to human immune cells. J Clin Immunol. 1990;10:204–210. doi: 10.1007/BF00918653. [DOI] [PubMed] [Google Scholar]
- 17.Bagriacik EU, Klein JR. The thyrotropin (thyroid stimulating hormone) receptor is expressed on murine dendritic cells and on a subset of CD43RBhigh lymph node T cells: Functional role of thyroid stimulating hormone during immune activation. J Immunol. 2000;164:6158–6165. doi: 10.4049/jimmunol.164.12.6158. [DOI] [PubMed] [Google Scholar]
- 18.Kruger TE. Immunomodulation of peripheral lymphocytes by hormones of the hypothalamus-pituitary-thyroid axis. Adv Neuroimmunol. 1996;6:387–395. doi: 10.1016/s0960-5428(97)00033-2. [DOI] [PubMed] [Google Scholar]
- 19.Fabris N, Mochegiani E, Provinciali M. Pituitary-thyroid axis and immune system: A reciprocal neuroendocrine-immune interaction. Horm Res. 1995;43:29–38. doi: 10.1159/000184234. [DOI] [PubMed] [Google Scholar]
- 20.Blalock JE, Johnson HM, Smith EM, Torres BA. Enhancement of the in vitro antibody response by thyrotropin. Biochem Biophys Res Comm. 1984;25:30–34. doi: 10.1016/s0006-291x(84)80329-0. [DOI] [PubMed] [Google Scholar]
- 21.Provinciali M, Di Stefano G, Fabris N. Improvement in the proliferative capacity and natural killer cell activity of murine spleen lymphocytes by thyrotropin. Int J Immunopharmacol. 1992;14:865–870. doi: 10.1016/0192-0561(92)90085-y. [DOI] [PubMed] [Google Scholar]
- 22.Whetsell M, Bagriacik EU, Seetharamaiah GS, Prabhakar BS, Klein JR. Neuroendocrine-induced synthesis of bone marrow-derived cytokines with inflammatory immunomodulating properties. Cell Immunol. 1999;192:159–166. doi: 10.1006/cimm.1998.1444. [DOI] [PubMed] [Google Scholar]
- 23.Foster MP, Montecino-Rodriguez E, Dorshkind K. Proliferation of bone marrow pro-B cells is dependent on stimulation by the pituitary/thyroid axis. J Immunol. 1999;163:5883–5889. [PubMed] [Google Scholar]
- 24.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: Insights from genetic models of hormone and hormone receptor deficiency. Endocrine Rev. 2005;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- 25.Aprin C, Philgren M, Fraichard A, Aubert D, Samarut J, Chassande O, Marvel J. Effects of T3Rα1 and T3Rα2 gene deletion on T and B lymphocyte development. J Immunol. 2000;164:152–160. doi: 10.4049/jimmunol.164.1.152. [DOI] [PubMed] [Google Scholar]
- 26.Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Sick euthyroid syndrome is associated with decreased TR expression and DNA binding in mouse liver. Amer J Physiol Endocrinol Metab. 2003;284:E228–E236. doi: 10.1152/ajpendo.00155.2002. [DOI] [PubMed] [Google Scholar]
- 27.De Groot LJ. Dangerous dogmas in medicine: The nonthyrodial illness syndrome. J Clin Endocrinol Metab. 1999;84:151–164. doi: 10.1210/jcem.84.1.5364. [DOI] [PubMed] [Google Scholar]
- 28.Papanicolaou DA. Euthyroid sick syndrome and the role of cytokines. Rev Endocrine Metabolic Disorders. 2000;1:43–48. doi: 10.1023/a:1010060303031. [DOI] [PubMed] [Google Scholar]
- 29.Inan M, Koyuncu A, Aydin C, Turan M, Gokgoz S, Sen M. Thyroid hormone supplementation in sepsis: An experimental study. Surg Today. 2003;33:24–29. doi: 10.1007/s005950300004. [DOI] [PubMed] [Google Scholar]
- 30.Klemperer JD. Thyroid hormone and cardiac surgery. Thyroid. 2002;12:517–521. doi: 10.1089/105072502760143917. [DOI] [PubMed] [Google Scholar]
- 31.Brierre S, Kumari R, Deboisblanc BP. The endocrine system during sepsis. Amer J Med Sci. 2004;328:238–247. doi: 10.1097/00000441-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Larsen PR, Davies TF, Schumberger MJ, Hay ID. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Plonsky KS, editor. Williams Textbook of Endocrinology. 10th edit Saunders; Philadelphia: 2002. p. 350. [Google Scholar]
- 33.Fakete C, Gereben B, Doleschall M, Harney JW, Dora JM, Bianco AC, Sarkar S, Liposits Z, Rand W, Emerson C, Kacskovics I, Larsen PR, Lechan RM. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: Implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–1655. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 34.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 35.Legardi G, Emerson CH, Ahima RS, Fliers JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:105–119. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 36.Fakete C, Singru PS, Sarkar S, Rand WM, Lechan RM. Ascending brainstem pathways are not involved in lipopolysaccharide-induced suppression of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology. 2005;146:1357–1363. doi: 10.1210/en.2004-1429. [DOI] [PubMed] [Google Scholar]
- 37.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo K, Harbuz MS, Levy A, Lightman SL. Inhibition of the hypothalamic-pituitary-thyroid axis in response to lipopolysaccharide is independent of changes in circulating corticosteroids. Neuoimmunomodulation. 1997;4:188–194. doi: 10.1159/000097337. [DOI] [PubMed] [Google Scholar]
- 39.Marks DL, Cone RD. Central malanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res. 2001;56:359–375. doi: 10.1210/rp.56.1.359. [DOI] [PubMed] [Google Scholar]
- 40.Segeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NYP, galanin, CART and MCH in mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 41.Lechan RM, Fekete C. Feedback regulation of thyrotropin-releasing hormones (TRH): mechanisms for the non-thyroidal syndrome. J Endocrinol Invest. 2004;27:105–119. [PubMed] [Google Scholar]
- 42.Pang XP, Hershman JM, Chung M, Pekary AE. Characterization of tumor necrosis factor-alpha receptors by thyrotropin. Endocrinology. 1989;125:1783–1788. doi: 10.1210/endo-125-4-1783. [DOI] [PubMed] [Google Scholar]
- 43.Pang XP, Hershman JM, Smith V, Pekary AE, Sugawara M. The mechanisms of action of tumor necrosis factor-alpha and interleukin 1 on FRTL-5 thyroid cells. Acta Endocrinol. 1990;123:203–210. doi: 10.1530/acta.0.1230203. [DOI] [PubMed] [Google Scholar]
- 44.Lyson K, McCann SM. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology. 1991;54:262–266. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- 45.Dubuis JM, Dayer JM, Siegrist-Kaiser CA, Burger AG. Human recombinant-1 beta decreases plasma thyroid hormone and thyroid stimulating hormone levels in rats. Endocrinology. 1988;123:2175–2181. doi: 10.1210/endo-123-5-2175. [DOI] [PubMed] [Google Scholar]
- 46.Wehmann RE, Gregerman RI, Burns WH, Saral R, Santos GW. Suppression of thyrotropin in the low-thyroxine state of sever nonthyroidal illness. N Eng J Med. 1985;312:546–552. doi: 10.1056/NEJM198502283120904. [DOI] [PubMed] [Google Scholar]
- 47.Lio S, Arcese W, Papa G, D’Armiento M. Thyroid and pituitary function following bone marrow transplantation. Arch Intern Med. 1988;148:1066–1077. [PubMed] [Google Scholar]
- 48.Hershman JM, Eriksen E, Kaufman N, Chaplin RE. Thyroid function tests in patients undergoing bone marrow transplantation. Bone Marrow Trans. 1990;6:49–51. [PubMed] [Google Scholar]
- 49.Vexiau P, Perez-Castiglioni P, Socie G, Devergie A, Toubert ME, Aractingi S, Gluckman E. The ‘euthyroid sick syndrome’: incidence, risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Haematol. 1993;85:778–782. doi: 10.1111/j.1365-2141.1993.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 50.Kauppilla M, Koskinen P, Irjala K, Remes K, Viikari J. Long-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Trans. 1998;22:331–337. doi: 10.1038/sj.bmt.1701337. [DOI] [PubMed] [Google Scholar]
- 51.Ishiguro H, Yasuda Y, Omita Y, Shinagawa T, Shimizu T, Morimoto T, Hattori K, Matsumoto M, Inoue H, Yabe H, Yabe M, Shinohara O, Kato S. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescences. J Clin Endocrin Metab. 2004;89:5981–5986. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto M, Ishiguro H, Tomita Y, Inoue H, Yasuda Y, Shimizu T, Shinagawa T, Hattori K, Yabe H, Kubota C, Yabe M, Kato S, Shinohara O. Changes in thyroid function after bone marrow transplant in young patients. Ped Int. 2004;46:291–295. doi: 10.1111/j.1442-200x.2004.01894.x. [DOI] [PubMed] [Google Scholar]
- 53.Toubert ME, Socie G, Gluckman E, Aractingi S, Esperou H, Devergie A, Ribaud P, Parquet N, Schlageter MH, Beressi JP, Rain JD, Vexiau P. Short-and long-term follow-up of thyroid dysfunction after allogeneic bone marrow transplantation without the use of preparative total body irradiation. Br J Haematol. 1997;98:453–457. doi: 10.1046/j.1365-2141.1997.2433060.x. [DOI] [PubMed] [Google Scholar]
- 54.Slatter MA, Gennery AR, Cheetham TD, Bhattacherya A, Crooks BN, Flood TJ, Cant AJ, Abinun M. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Trans. 2004;33:949–953. doi: 10.1038/sj.bmt.1704456. [DOI] [PubMed] [Google Scholar]
- 55.Carlson K, Lonnerholm G, Smedmyr B, Oberg G, Simonsson B. Thyroid function after autologous bone marrow transplantation. Bone Marrow Trans. 1992;10:123–127. [PubMed] [Google Scholar]
- 56.Besedovsky HO, Sorkin E, Keiler M, Muller J. Changes in plasma hormone levels during the immune response. Proc Soc Exp Biol Med. 1975;150:466–502. doi: 10.3181/00379727-150-39057. [DOI] [PubMed] [Google Scholar]
- 57.Besedovsky HO, del Rey A, Sorkin E. Chanes in plasma hormone profile after tumor transplantation into syngeneic and allogeneic rats. Int J Cancer. 1985;36:209–216. doi: 10.1002/ijc.2910360213. [DOI] [PubMed] [Google Scholar]
- 58.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS lett. 1997;407:319–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]