Abstract
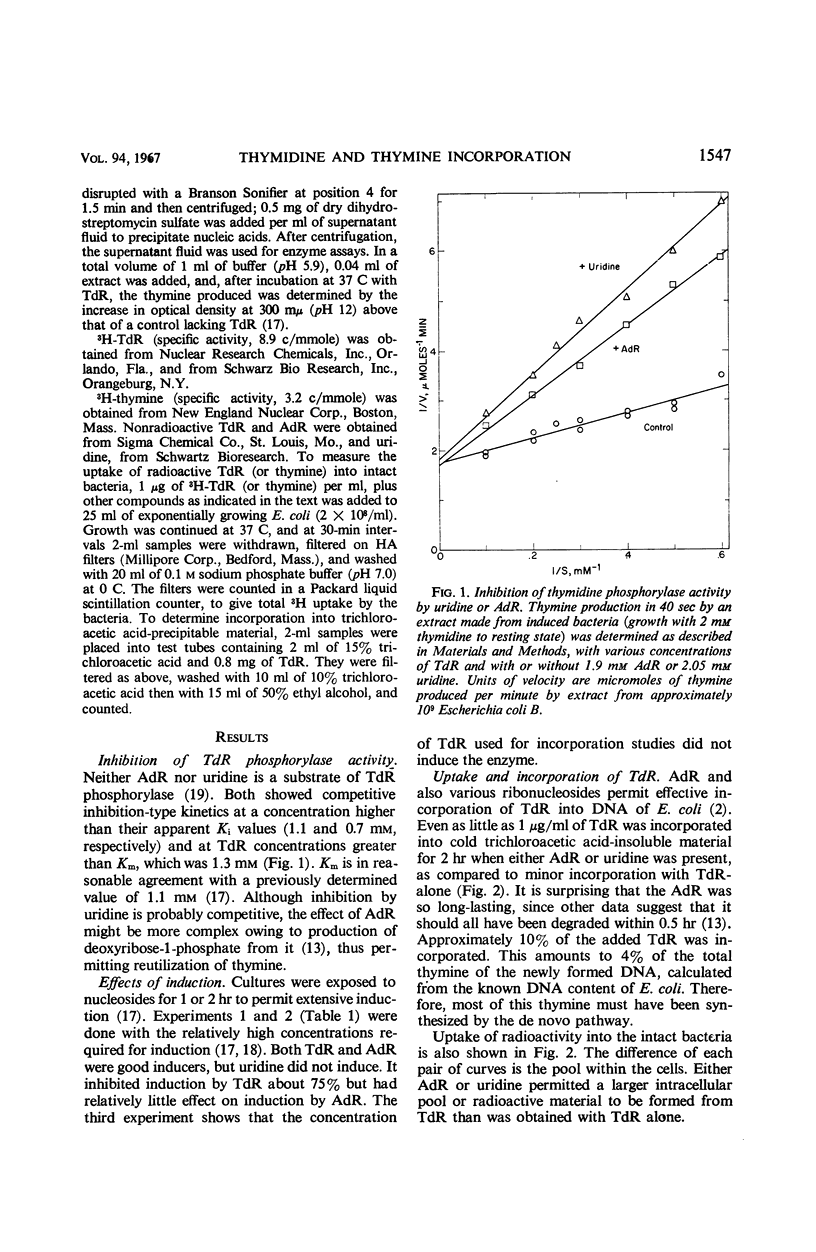
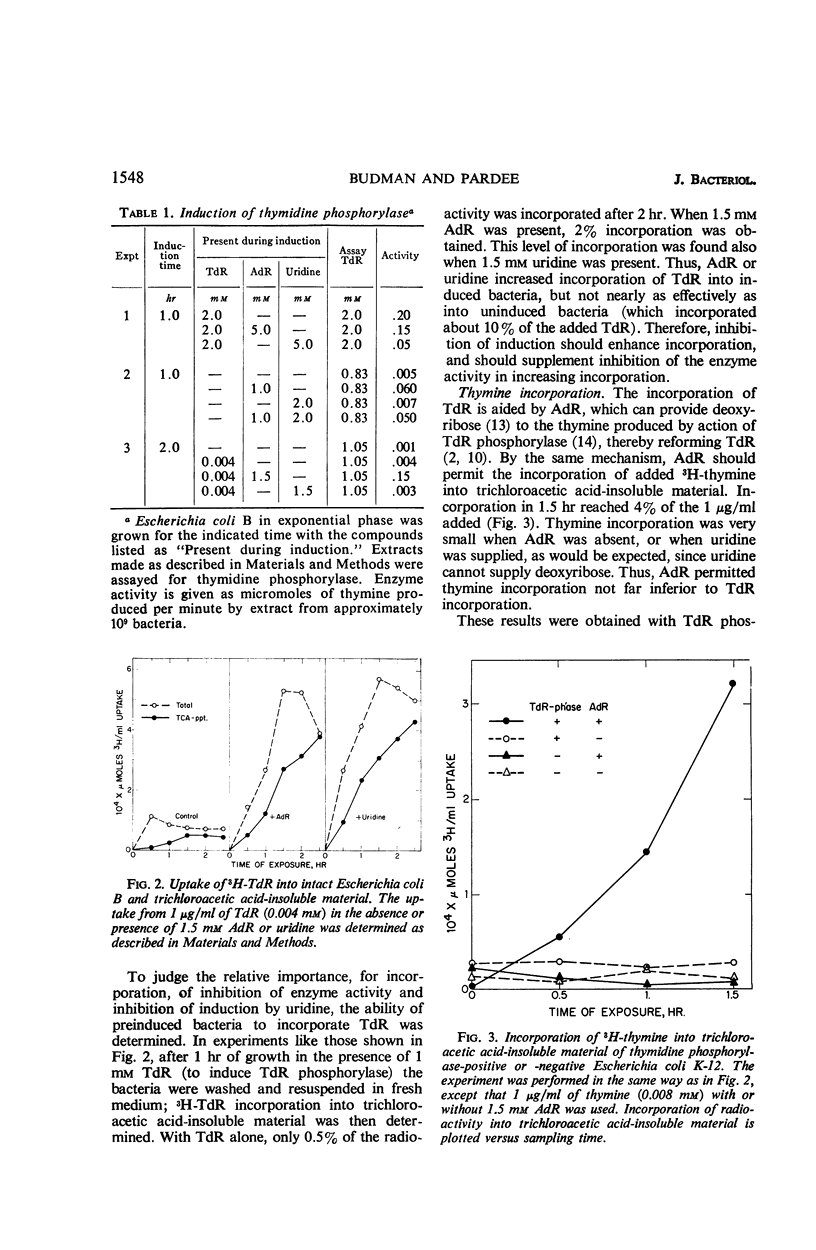
Thymidine is poorly incorporated into deoxyribonucleic acid (DNA) of Escherichia coli. Its incorporation is greatly increased by uridine, which acts in two ways. Primarily, uridine competitively inhibits thymidine phosphorylase (E.C.2.4.4), and thereby prevents the degradation of thymidine to thymine which is not incorporated into normally growing E. coli. Uridine also inhibits induction of the enzyme by thymidine. It prevents the actual inducer, probably a deoxyribose phosphate, from being formed rather than competing for a site on the repressor. The inhibition of thymidine phosphorylase by uridine also accounts for inhibition by uracil compounds of thymine incorporation into thymine-requiring mutants. Deoxyadenosine also increases the incorporation of thymidine, by competitively inhibiting thymidine phosphorylase. Deoxyadenosine induces the enzyme, in contrast to uridine. But this is offset by a transfer of deoxyribose from deoxyadenosine to thymine. Thus, deoxyadenosine permits incorporation of thymine into DNA, even in cells induced for thymidine phosphorylase. This incorporation of thymine in the presence of deoxyadenosine did not occur in a thymidine phosphorylase-negative mutant; thus, the utilization of thymine seems to proceed by way of thymidine phosphorylase, followed by thymidine kinase. These results are consistent with the data of others in suggesting that wild-type E. coli cells fail to utilize thymine because they lack a pool of deoxyribose phosphates, the latter being necessary for conversion of thymine to thymidine by thymidine phosphorylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GRETHER S. UPTAKE AND INCORPORATION OF THYMINE, THYMIDINE, URACIL, URIDINE, AND 5-FLUOROURACIL INTO THE NUCLEIC ACIDS OF BACILLUS SUBTILIS. J Bacteriol. 1965 Apr;89:1011–1014. doi: 10.1128/jb.89.4.1011-1014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Bradford R. M. Metabolism of thymineless mutants of Escherichia coli. I. Absence of thymidylate synthetase activity and growth characteristics of two sequential thymineless mutants. J Bacteriol. 1967 Mar;93(3):845–852. doi: 10.1128/jb.93.3.845-852.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., BARNER H. D. Studies on the induction of thymine deficiency and on the effects of thymine and thymidine analogues in Escherichia coli. J Bacteriol. 1956 May;71(5):588–597. doi: 10.1128/jb.71.5.588-597.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958 Nov;30(2):428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Mutant bacteria showing efficient utilization of thymidine. J Bacteriol. 1966 Jun;91(6):2390–2391. doi: 10.1128/jb.91.6.2390-2391.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Technique for starvation of Escherichia coli of thymine. J Bacteriol. 1965 Oct;90(4):1153–1154. doi: 10.1128/jb.90.4.1153-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Igarashi K., Yura T. A deoxythymidine kinase-deficient mutant of Escherichia coli. I. Isolation and some properties. Biochim Biophys Acta. 1967 Aug 22;145(1):41–51. doi: 10.1016/0005-2787(67)90652-1. [DOI] [PubMed] [Google Scholar]
- Jayaraman K., Müller-Hill B., Rickenberg H. V. Inhibition of the synthesis of beta-galactosidase in Escherichia coli by 2-nitrophenyl-beta-D-fucoside. J Mol Biol. 1966 Jul;18(2):339–343. doi: 10.1016/s0022-2836(66)80251-6. [DOI] [PubMed] [Google Scholar]
- MANSON L. A., LAMPEN J. O. The metabolism of desoxyribose nucleosides in Escherichia coli. J Biol Chem. 1951 Dec;193(2):539–547. [PubMed] [Google Scholar]
- MANS R. J., KOCH A. L. Metabolism of adenosine and deoxyadenosine by growing cultures of Escherichia coli. J Biol Chem. 1960 Feb;235:450–456. [PubMed] [Google Scholar]
- MANTSAVINOS R., ZAMENHOF S. Pathways for the biosynthesis of thymidylic acid in bacterial mutants. J Biol Chem. 1961 Mar;236:876–882. [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. 3. On the regulation of the deoxyadenosine triphosphate and deoxycytidine triphosphate pools of Escherichia coli. Biochim Biophys Acta. 1966 Oct 24;129(1):104–115. doi: 10.1016/0005-2787(66)90012-8. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., CASSHYAP P. SUBSTRATE SPECIFICITY AND INDUCTION OF THYMIDINE PHOSPHORYLASE IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1789–1793. [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN M. DEOXYRIBOSYL TRANSFER. II. NUCLEOSIDE:PYRIMIDINE DEOXYRIBOSYLTRANSFERASE ACTIVITY OF THREE PARTIALLY PURIFIED THYMIDINE PHOSPHORYLASES. J Biol Chem. 1964 Aug;239:2622–2627. [PubMed] [Google Scholar]


