Abstract
Affective neuroscience has been strongly influenced by the view that a ‘feeling’ is the perception of somatic changes and has consequently often neglected the neural mechanisms that underlie the integration of somatic and other information in affective experience. Here, we investigate affective processing by means of functional magnetic resonance imaging in nine cortically blind patients. In these patients, unilateral postgeniculate lesions prevent primary cortical visual processing in part of the visual field which, as a result, becomes subjectively blind. Residual subcortical processing of visual information, however, is assumed to occur in the entire visual field. As we have reported earlier, these patients show significant startle reflex potentiation when a threat-related visual stimulus is shown in their blind visual field. Critically, this was associated with an increase of brain activity in somatosensory-related areas, and an increase in experienced negative affect. Here, we investigated the patients’ response when the visual stimulus was shown in the sighted visual field, that is, when it was visible and cortically processed. Despite the fact that startle reflex potentiation was similar in the blind and sighted visual field, patients reported significantly less negative affect during stimulation of the sighted visual field. In other words, when the visual stimulus was visible and received full cortical processing, the patients’ phenomenal experience of affect did not closely reflect somatic changes. This decoupling of phenomenal affective experience and somatic changes was associated with an increase of activity in the left ventrolateral prefrontal cortex and a decrease of affect-related somatosensory activity. Moreover, patients who showed stronger left ventrolateral prefrontal cortex activity tended to show a stronger decrease of affect-related somatosensory activity. Our findings show that similar affective somatic changes can be associated with different phenomenal experiences of affect, depending on the depth of cortical processing. They are in line with a model in which the left ventrolateral prefrontal cortex is a relay station that integrates information about subcortically triggered somatic responses and information resulting from in-depth cortical stimulus processing. Tentatively, we suggest that the observed decoupling of somatic responses and experienced affect, and the reduction of negative phenomenal experience, can be explained by a left ventrolateral prefrontal cortex-mediated inhibition of affect-related somatosensory activity.
Keywords: emotion, phenomenal experience, affective feeling, startle reflex potentiation, ventrolateral prefrontal cortex
Introduction
Human emotions are complex phenomena. According to most theorists, emotions comprise multiple somatic and behavioural responses that are associated with a phenomenal experience of affect (for review, see Kleinginna and Kleinginna, 1981). These responses require different levels of cortical stimulus processing. For example, when cortical processing of emotional visual stimuli is reduced by backward masking (Macknik and Livingstone, 1998), these stimuli can still elicit affective somatic responses. Subjects show increased skin conductance responses (SCRs) to masked fear-conditioned visual stimuli (Esteves et al., 1994) and covert facial mimicry to masked fearful faces (Dimberg et al., 2000). At the behavioural level, drinking behaviour can be modulated by masked angry or happy faces (Winkielman et al., 2005). Invisible visual stimuli can also affect judgements of visible stimuli. For example, masked fearful or happy faces shown in one visual hemifield can influence reaction times to visible congruent or incongruent faces shown simultaneously in the other visual hemifield (Tamietto and de Gelder, 2008). Similarly, masked fearful or happy faces can influence affective judgements of subsequently presented meaningless ideographs (Murphy and Zajonc, 1993; Murphy et al., 1995).
Evidence for affective responses to visual stimuli in the complete absence of early cortical stimulus processing comes from studies in cortically blind patients. In these patients, cortical processing of visual stimuli is altered due to unilateral postgeniculate disconnection or destruction of primary visual cortex, while the retina and retino-tectal projections remain largely intact. Although these patients deny any visual sensation in the affected part of their visual field, they respond affectively to emotional stimuli shown in their blind field. These responses include somatic response such as startle reflex potentiation (Hamm et al., 2003; Anders et al., 2004a), behavioural responses such as above chance discrimination of emotional facial expressions or gestures in forced choice paradigms (de Gelder et al., 1999; Pegna et al., 2005; de Gelder and Hadjikhani, 2006), and judgements of simultaneously presented visible stimuli (de Gelder et al., 2001, 2005).
Currently, it is unclear how such somatic and behavioural affective responses, without in-depth cortical stimulus processing, relate to phenomenal affective experience. One of the most influential theories in affective neuroscience postulates that changes (or in fact any emotional response that can be assumed to be associated with somatic changes) should always be associated with an experience of affect, independent of the context. More recent theories of emotion assume that phenomenal emotional experiences are a result of integration of information from different internal and external sources (e.g. Russell, 2003; Scherer, 2005). Thus, similar somatic changes could be associated with different emotional experiences, depending on the context.
Empirical evidence concerning the dependence between somatic changes and affective experience is ambiguous. In an early study, Schachter and Singer (1962) showed that sympathetic arousal can be perceived as either positive or negative affect; depending on the information subjects were given. On the other hand, Robles et al. (1987) found that masked threat- or joy-related stimuli inserted into a neutral movie influenced the subjects’ self-reported affect, suggesting that invisible stimuli can modify phenomenal affective experience even in the presence of competing information. Masking other studies, however, report a dissociation of behavioural responses and self-reported affect. For example, in the study by Winkielman (2005), participants reported no changes of affect when they were exposed to masked happy or angry faces although these stimuli influenced their consumption behaviour. Finally, there is evidence that the degree to which information resulting from subcortical processing modulates emotional responses, and reaches awareness, depends on the level of concurrent cortical processing. Jolij and Lamme (2005) exposed subjects to very brief presentations of happy or sad faces. The depth of cortical processing of these stimuli was modulated by transcranial magnetic stimulation (TMS) over primary visual cortex at variable delays. They found that participants could report the valence of the affective face only if TMS abolished cortical processing.
Here, we report a case of decoupling of somatic responses and affective experience in cortically blind patients. Because of their lesions to the visual cortical pathway, these patients provide a rare model to study the relation between somatic responses and phenomenal experience of affect under two conditions: in the absence versus presence of stimulus information resulting from in-depth cortical processing. Affective somatic responses to visual stimuli presented in the blind visual field (BVF) of these patients are assumed to be mediated by a subcortical retino-tecto-thalamic route to the amygdala (Rosen et al., 1992; Morris et al., 1997; Linke et al., 1999; Morris et al., 1999, 2001; Pegna et al., 2005), a pathway that has also been termed the ‘quick-and-dirty pathway’. Visual stimuli presented in the sighted visual field (SVF) of these patients are also assumed to be processed via the subcortical pathway, but at the same time, they are processed via the retino-geniculo-cortical pathway that subserves and in-depth cortical visual processing phenomenal visual experience.
We investigated affective processing in a group of nine cortically blind patients. In the first part of this study we showed that, in line with the presumption that somatic responses to visual stimuli can be mediated via the subcortical pathway, these patients show significant startle reflex potentiation when a visual stimulus that had previously been paired with an unpleasant scream is shown in their BVF. Critically, during blind field stimulation, this affective somatic response was associated with increased brain activity in somatosensory-related cortex, and an increase of self-reported negative affect (Anders et al., 2004a). Here we investigated the relation between somatic responses and phenomenal experience of affect when the stimulus was presented in the sighted field, that is, when it was visible and received full cortical processing. To anticipate our findings, we found a decoupling of somatic responses and phenomenal experience of affect during stimulation of the SVF. This was associated with an increase of brain activity in the ventrolateral prefrontal cortex (VLPFC) and a decrease of brain activity in somatosensory-related cortex. As the VLPFC has previously been associated with information integration (Prabhakaran et al., 2000) and control of affect (Ochsner and Gross, 2005), we further investigated the relation between somatic responses, VLPFC activity and affect-related somatosensory activity. On the basis of these findings we suggest a model of VLPFC-mediated passive suppression of affect.
Materials and methods
Subjects
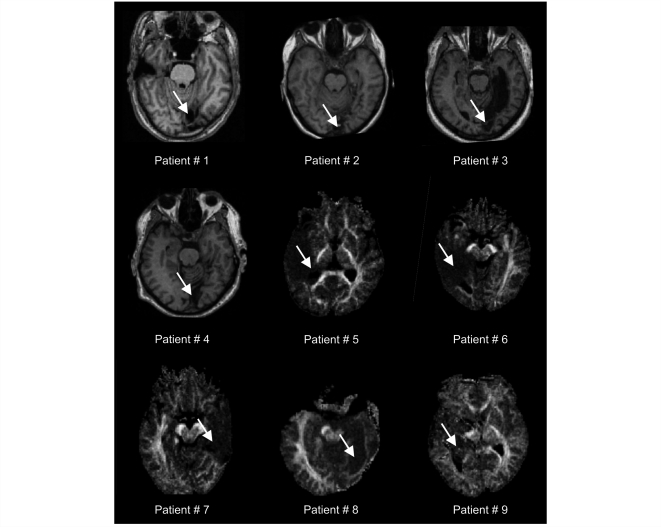
Nine patients with postgeniculate lesions resulting in partial deafferentiation or destruction of left (n = 3) or right (n = 6) striate cortex participated in the study. High resolution T1 and diffusion-weighted magnetic resonance images confirmed lesions corresponding to visual field defects identified by automated Tübinger perimetry (TAP) or manual perimetry in all patients. Details of lesions are given in Table 1 and Fig. 1. Patients were informed that they would be shown a face in their BVF and SVF and that they should pay attention to their emotional feelings even if they could not see anything. All patients gave written informed consent before participation, and the study was approved by the local ethics committee.
Table 1.
Clinical data
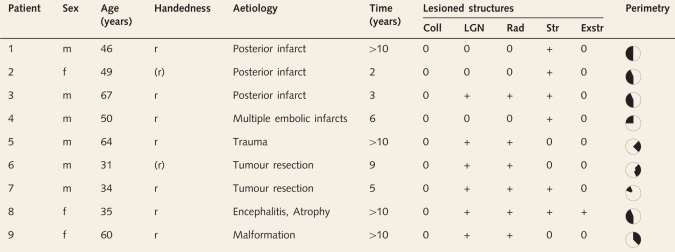 |
r = right handed; (r) = relearned right handed; Time = time since lesion; Coll = superior colliculus; LGN = lateral geniculate nucleus of the thalamus; Rad = optic radiation; Str = striate cortex; Exstr = extrastriate visual cortex, 0 intact, + lesioned (as assessed by neuroradiological examination of T1-weighted and diffusion-weighted MR images). Visual field defects were assessed with TAP except in Patient 5 for whom manual perimetry was used (left is left).
Figure 1.
Anatomical sections (Patients 1–4 with infarction of the primary visual cortex) or fractional diffusion anisotropy maps (Patients 5–9 with lesions affecting the optic radiation) showing the lesion of each patient. Arrows indicate lesions. Left is left.
Experimental design
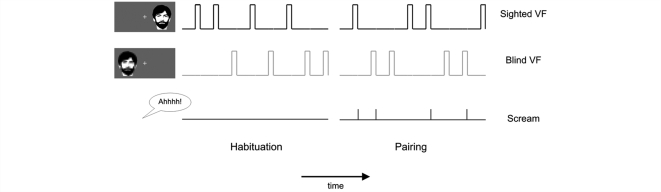
Patients underwent six functional magnetic resonance imaging (fMRI) runs during which they lay supine on the scanner couch and fixated a crosshair through a head-coil-mounted mirror. Stimuli were back-projected on a translucent screen mounted at the end of the scanner couch, and luminance during blank screen baseline and visual stimulation was matched. Additionally, the inside of the scanner and head coil were covered with black cardboard to prevent light scatter. The visual stimulus was a high-contrast, low-frequency greyscale image of a bearded male face adapted to satisfy the residual capacities within cortical visual field defects (e.g. Sahraie et al., 2008). During a 12 s stimulus presentation time the face expanded, from 0° visual angle to a size that was individually adjusted to the size of the absolute visual field defect of each patient as revealed by perimetry (approximately 6° × 8.5°). A single visual stimulus was used for habituation and pairing because we did not want to make any a priori assumptions about visual discrimination in the blind field.
The face appeared pseudorandomly 11° left or right to the fixation cross, with an intertrial interval of 24, 32, or 40 s and an additional jittering of 1s relative to scan onset (Fig. 2). Scanning was divided into two habituation runs and four pairing runs. During each run, the face was shown four times in the subject's cortically BVF, and four times in the SVF). In pairing runs, half of the face presentations in either visual field terminated with an unpleasant human scream, which was applied through fMRI compatible headphones (Baumgart et al., 1998) and individually adjusted in loudness to be unpleasant but not painful. Habituation trials served as baseline to control for unspecific effects due to face presentation. White noise startle probes were delivered during half of the blank screen baseline intervals (9 or 10 s after stimulus offset) and half of the face presentations (6 or 7 s after stimulus onset). Previous research showed that this design leads to a significant increase of brain activity, startle reflex potentiation, SCRs, and self-reported negative affect and arousal in healthy controls during pairing trials compared to habituation trials (Anders et al., 2005).
Figure 2.
Experimental design. The visual stimulus (a grey-scale male face) was presented in the SVF and BVF of nine cortically blind patients (first/second row). In habituation runs, which served as a baseline, the visual stimulus was always shown alone. In pairing runs, half of the visual stimulus presentations ended with an unpleasant human scream (third row). Each patient participated in two habituation runs and four pairing runs (only one of each is shown).
Data acquisition
Sixty-four T2*-weighted echoplanar images (1.5 Tesla Magnetom Vision, Siemens, Germany, 44 coronal slices, slice thickness 3 + 1.5 mm gap, 56 × 64 voxels, in plane resolution 3 × 3 mm2, TE 33 ms, TR 4 s) were acquired during each run. Each run was preceded by five scans which were not included in the analysis to allow for T1 saturation.
Startle eyeblink amplitudes were recorded with infrared oculography (Kimmig et al., 1999; Anders et al., 2004b). Skin conductance was recorded with commercial MRI compatible equipment (Vitaport II, Becker Meditec, Karlsruhe, Germany) using standard Ag/AgCl electrodes affixed to the skin surface underneath the M. abductor hallucis of the left foot halfway between phalanx and calcaneus.
After each run, subjects rated their feelings during two additional face presentations in each hemifield and to two randomly intermixed blank screen presentations. Nine-point self-assessment manikins for valence and arousal (SAM; Bradley and Lang, 1994) were shown after each trial, and subjects chose the number underneath the scale that corresponded best to their affective experience during that trial (valence, 1 = very unpleasant to 9 = very pleasant; arousal, 1 = very calm to 9 = very excited).
Eye gaze fixation was controlled throughout the experiment with infrared oculography. Functional MRI data and physiological recordings of one patient, of four trials of a second patient and of seven trials of a third patient were excluded because saccades during blind field stimulation led to an overlap of the foveal sparing of the patient and the visual stimulus (Table 2).
Table 2.
Number of trials included in each analysis
| Patient | fMRI data | Startle data face/baseline | SCR data face/baseline | Self-report face/baseline |
|---|---|---|---|---|
| n = 32a | n = 24/n = 24b | n = 48/n = 24c | n = 24/n = 12d | |
| 1 | 32 | 8/9 | 48/24 | 24/12 |
| 2 | 32 | – | – | 24/12 |
| 3 | 28 | 17/19 | 40/20 | 20/10 |
| 4 | 32 | 22/22 | 48/24 | 24/12 |
| 5e | 25 | – | 41/24 | 24/12 |
| 6f | 28 | 14/12 | 44/24 | 24/12 |
| 7 | 32 | 10/9 | 48/24 | 24/12 |
| 8g | – | – | – | 24/12 |
| 9 | 32 | – | – | 24/12 |
The whole experiment included 48 trials (2 x 8 habituation trials and 4 × 8 pairing trials, see Fig. 2).
a Total number of trials available for fMRI data analysis (4 × 4 pairing trials that were followed by the scream were not included in the fMRI data analysis).
b Total number of trials available for startle analysis (startle probes were delivered in half of all trials, and half of all baseline intervals).
c Total number of trials available for SCR analysis (all trials and half of the preceding baselines intervals were included in the SCR analysis).
d Total number of trials available for analysis of self-report (each of the six runs was followed by four face presentations and two blank screen presentations).
f fMRI and physiological data of seven blind field trials were excluded because of instable eye gaze fixation.
e fMRI and physiological data of four blind field trials were excluded because of instable eye gaze fixation.
g All fMRI and physiological data were excluded because of instable eye gaze fixation.
See ‘Materials and methods’ for details.
All analyses used the same data processing as in Anders et al. (2004a).
Analysis of somatic responses and self-reported affect
Startle eye blink data were digitized at 1000 Hz, temporally smoothed [Gaussian kernel 10 ms full width at half maximum (FWHM)] and visually inspected for artefacts. In addition to the subjects/trials that were excluded because of unstable fixation (see above), three subjects did not tolerate startle probe intensities that reliably elicited startle reflexes, and 37% of the trials of the remaining subjects had to be discarded because time series did not show an approximately Gaussian shape (Table 2). For the remaining trials, eye blink amplitudes were determined as the maximal differential voltage between 21 and 150 ms after startle probe onset, relative to the mean of a 20 ms baseline beginning with startle probe onset (Anders et al., 2004b). Eye blink amplitudes of each run were scaled to the average eye blink amplitude during blank screen baseline during that run to allow for sensor drifts and non-specific effects due to scream delivery and habituation across runs. Finally, to derive a measure of startle reflex potentiation due to pairing, startle eye blink amplitudes during pairing runs were contrasted with startle eye blink amplitudes during habituation runs (Anders et al., 2005).
Skin conductance data were temporally smoothed (1 s FWHM). SCR amplitudes were determined as the log transformed {ln[SCR (µS) + 1]} largest change in conductance between 9 s before and 1 s after picture onset (baseline) and between 1 s and 10 s after picture onset (Lang et al., 1993). Because of recording errors, no skin conductance data were obtained from two subjects (Table 2). Trials during which no SCR was observed (amplitudes < 0.05 µS) were not included in the final analysis (roughly 50% of trials). SCR amplitudes of each run were subtracted with SCR amplitudes during blank screen baseline of that run to remove non-specific effects due to scream delivery and habituation effects across runs. As for eyeblink amplitudes, differential SCR during pairing runs were contrasted with differential SCR during habituation runs to derive a measure of SCR increase during pairing (Anders et al., 2005).
Finally, affect rating ratings during face presentations obtained after each run were subtracted with affect ratings during blank screen baseline after that run, and differential ratings during pairing runs were contrasted with differential ratings during habituation runs.
Paired t-tests were used to compare somatic responses during face presentations in the SVF and BVF. Because of the larger variability of self-reports during SVF than during BVF stimulation we used the non-parametric McNemar test of significance of change to compare self-reported affect during stimulus presentation in the SVF and BVF. To test for correlations between somatic responses and brain activity, startle eye blink amplitudes were rank-transformed.
Analysis of fMRI data
Functional MRI data were analysed with SPM99 (Wellcome Trust Centre of Imaging Neuroscience, London, UK). Preprocessing included 3D motion correction, slice acquisition time correction, normalization into MNI space (Montreal Neurological Institute), spatial smoothing (FWHM 12 mm), and temporal filtering (high pass cut off period 78 s, low pass kernel FWHM 4 s). A linear model was used to estimate BOLD (blood oxygen level dependent) signal amplitudes during face presentations for each subject. These models included one regressor to model face presentations that were not followed by the scream, one regressor to model face presentations that were followed by the scream (pairing runs only), and two additional regressors that modelled startle probe delivery and scream presentations (pairing runs only) per run. All BOLD signals were modelled as box car functions representing stimulus onset and duration convolved with a synthetic haemodynamic response function as implemented in SPM99. Additionally, estimated head movements were included as regressors in each model. Linear combinations of parameter estimates representing average BOLD signals during face presentations were calculated separately for habituation runs and pairing runs, and for the BVF and SVF of each subject (BVF contrasts are identical to those reported in Anders et al., 2004a). Only face presentations that were not followed by the scream were included in these contrasts. To obtain effects due to pairing for each visual field, average parameter estimates during pairing and habituation runs were subtracted.
These contrasts were then entered into a paired t-test to test for differences between SVF and BVF stimulation. The resulting statistical parametric map (SPM) was thresholded at T = 7.1 (corresponding to an uncorrected voxel-wise probability of error of P = 0.0001). To be classified as significant, clusters had to comprise at least five contiguous voxels [corresponding to P = 0.05, corrected for multiple comparisons (Worsley et al., 1996)]. A subsequent region of interest (ROI) analysis assessed whether brain activity in the parietal somatosensory region, that showed a significant increase of activity during BVF stimulation (Anders et al., 2004a), would show a significantly smaller increase during stimulation of the SVF. For this analysis, the parameter estimates described above were extracted from the most significantly activated voxel during BVF stimulation, and differences between SVF and BVF stimulation were assessed with a paired t-test. Correlation analyses were based on parameter estimates extracted from the most significantly activated voxel in the VLPFC.
Results
Data yield
An overview of neuroimaging, somatic and self-report data obtained from each patient is given in Table 2.
Somatic responses and self-reported affect
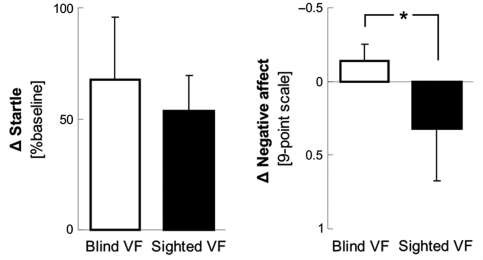
Startle potentiation did not differ during stimulation of the SVF and the BVF (T = 0.7, df = 4, P > 0.50, Fig. 3). Post hoc analysis revealed that startle eyeblink amplitudes were significantly increased during pairing compared to habituation runs during both BVF (T = 2.4, df = 4, P < 0.05) and SVF (T = 3.3, df = 4, P < 0.05) stimulation.
Figure 3.
Startle reflex potentiation and self-reported negative affect during stimulation of the BVF and SVF. Negative affect values indicate more negative affect. Responses during pairing runs are subtracted with responses during habituation runs. Error bars represent standard errors of the mean. The asterisk indicates a significant difference between stimulation of the SVF and the BVF.
However, patients reported significantly less negative affect during stimulation of the SVF than during stimulation of the BVF (McNemar χ2 = 4, df = 1, P < 0.05, Fig. 3). Furthermore, post hoc analysis revealed that during stimulation of the SVF self-reported negative affect did not increase from habituation to pairing, not even at trend level (T = −0.9, df = 8). Thus, despite similar somatic responses, patients showed a reduction of negative affective experience during stimulation of the SVF compared to the BVF.
No significant effects were observed in SCR amplitudes and self-reported arousal.
Brain activity
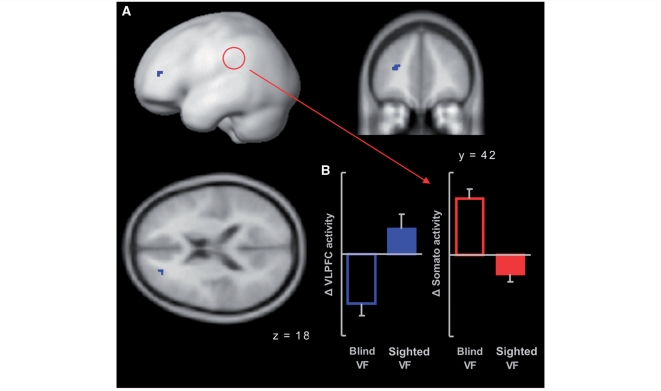
In the whole brain analysis, significantly stronger pairing-induced brain activity during stimulation of the SVF than during stimulation of the BVF was observed in the left VLPFC [cluster size = 9 voxels, peak T = 13.6, peak MNI coordinates x = −27, y = 42, z = 18, corresponding to the middle frontal gyrus and BA 46 (Eickhoff et al., 2005), Fig. 4]. Pairing resulted in an increase of VLPFC activity during stimulation of the SVF and a decrease of VLPFC activity during stimulation of the BVF. In the whole brain analysis, we detected no region that showed less activity during stimulation of the SVF than during stimulation of the BVF.
Figure 4.
(A) Random effects statistical parametric map (SPM) showing a significantly stronger increase of BOLD activity during stimulation of the SVF than during stimulation of the BVF in the left VLPFC. The SPM is thresholded at a voxel-wise probability of false positives of P = 0.0001 and an extent threshold of five contiguous voxels (corresponding to P < 0.05 corrected for multiple comparison across the whole volume), and shown as surface projection and superimposed on coronal and axial sections of a standard brain (left is left). (B) Bar charts showing VLPFC and affect-related somatosensory activity during stimulation of the SVF and BVF. All responses during pairing runs are subtracted with responses during habituation runs. Affect-related somatosensory activity was extracted from the most significantly activated voxel in a region that showed significant pairing-induced activity during blind field stimulation (indicated by the red circle in A, MNI coordinates x = –42, y = –42, z = 45; Anders et al., 2004a). Error bars represent standard errors of the mean.
Subsequent region of interest (ROI) analysis of brain activity in the parietal somatosensory-related region that showed significant pairing-induced activity during blind field stimulation (MNI coordinates x = −42, y = −42, z = 45, Anders et al., 2004a) revealed an effect opposite to that observed in the VLPFC. In this region, pairing-induced activity was significantly weaker during stimulation of the SVF than during stimulation of the BVF (T = 6.7, df = 7, P < 0.001, uncorrected, Fig. 4).
Correlation analyses
VLPFC and startle reflex potentiation
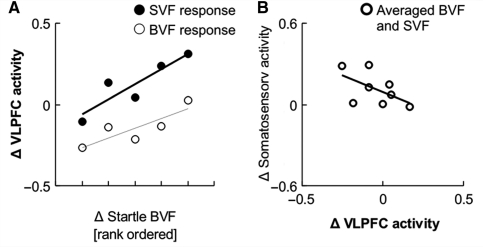
The first question concerning the role of VLPFC in integration of somatic information and information resulting from in-depth cortical stimulus processing was whether this region would show a sensitivity to startle reflex potentiation, and whether this sensitivity would be increased during stimulation of the SVF, which would indicate a role of the VLPFC in monitoring and weighting somatic information. To test this, we regressed VLPFC activity during stimulation of the SVF and BVF onto startle reflex potentiation and tested for an interaction between visual field and startle reflex potentiation. Startle reflex potentiation during BVF stimulation was used as regressor in both cases because we reasoned that this would be a better measure of subcortically triggered responses than startle reflex potentiation during stimulation of the SVF (which might have been modulated by cortical afferents). There was a significant positive correlation between VLPFC activity and startle reflex potentiation across subjects in both visual fields (SVF, r = 0.90, T = 3.7, df = 3, P = 0.02; BVF, r = 0.84, T = 2.7, df = 3, P = 0.04, Fig. 5) that tended to be stronger during stimulation of the SVF than during stimulation of the BVF (slope SVF versus slope BVF T = 1.8, df = 3, P = 0.08). Thus, the VLPFC was sensitive to subcortically triggered startle potentiation, and this sensitivity tended to be enhanced during face presentations in the SVF.
Figure 5.
(A) Relation between subcortically triggered startle potentiation and VLPFC activity during stimulation of the BVF (r = 0.84, P = 0.04) and SVF (r = 0.90, P = 0.02). (B) Relation between VLPFC activity and affect-related somatosensory activity (r = –0.54, P = 0.08). All responses during pairing are subtracted with responses during habituation runs.
VLPFC and somatosensory activity
The second question concerning the VLPFC was whether the observed negative relation between VLPFC activity and affect-related somatosensory activity across conditions would also be observed across subjects. This would further support an inhibitory influence between the VLPFC and affect-related somatosensory activity. Because correlations between VLPFC and affect-related somatosensory activity did not differ between visual fields [z(rSVF − rBVF) = 1.06, df = 6, P = 0.28, two-tailed], we merged data across both visual fields. This revealed a trend for a negative correlation between VLPFC and affect-related somatosensory activity across subjects (r = −0.52, T = 1.5, df = 6, P = 0.09, Fig. 5).
Discussion
The current study investigated the relation between somatic responses and phenomenal experience of affect in nine cortically blind patients. Although these patients showed similar somatic responses (startle potentiation) when a threat-related face was shown in their BVF or SVF, they reported significantly less negative affect when the stimulus was shown in their SVF. In other words, when the stimulus was visible and received full cortical processing the patients’ reported phenomenal experience of affect did not closely reflect somatic changes. This is reminiscent of a study in healthy subjects in which the subjects’ ability to discriminate between happy and sad faces under conditions of poor stimulus visibility depended on the level of cortical processing: only if cortical processing was reduced, subjects could discriminate positive and negative affect (Jolij and Lamme, 2005).
In the current study, the decoupling of phenomenal experience and somatic changes was associated with an increase of activity in left VLPFC and a decrease of affect-related somatosensory activity. VLPFC activity was sensitive to subcortically triggered startle reflex potentiation, with a trend for an increased sensitivity during stimulation of the SVF. In addition, increasing VLPFC activity tended to be associated with decreasing affect-related somatosensory activity across subjects. Together, these findings show that similar somatic responses can be associated with different phenomenal experiences of affect, depending on the level of cortical stimulus processing. Furthermore, they provide evidence for a role of the VLPFC in this process.
The role of the VLPFC
The VLPFC (here we use the term VLPFC to refer to the part of the lateral prefrontal cortex above z = 0 and below z = 30, see Ochsner and Gross, 2005) has been implicated in maintenance of goals, integration of relevant information, and response selection both in pure cognitive tasks (e.g. Konishi et al., 1999; Prabhakaran et al., 2000; Andersson et al., 2004) and in behaviour involving affect (for reviews see Spence et al., 2004; Ochsner and Gross, 2005).
In the current study, VLPFC activity was increased when the patients’ phenomenal experience of affect was decoupled from somatic changes. Moreover, the stronger the somatic changes were, the stronger was the increase in VLPFC activity. This is in line with a role of the VLPFC in monitoring and integrating information about somatic changes and contextual information.
In addition to its role in monitoring and integrating information, neuroimaging studies also suggest a specific role of the VLPFC in control of affect. A growing body of literature provides evidence for a modulatory role of the VLPFC in voluntary regulation of emotion (for review, see Ochsner and Gross, 2005). However, VLPFC activity is also observed during unintended, ‘passive’ suppression of affect as in placebo effects (Wager et al., 2004; Petrovic et al., 2005; Kong et al., 2006), when cognitive processing is required against an affective background (Yamasaki et al., 2002; Northoff et al., 2004; Blair et al., 2007), or in ambiguous social situations that require response inhibition (Cunningham et al., 2004; Greene et al., 2004). Behavioural studies have shown that voluntary emotion regulation can effectively modulate startle reflex potentiation (Jackson et al., 2000; Dillon and Labar, 2005; Eippert et al., 2007), and imaging studies on voluntary emotion inhibition often report a modulation of activity in the amygdala (Beauregard et al., 2001; Ochsner et al., 2002, 2004; Ohira et al., 2006; Eippert et al., 2007), a structure that mediates startle reflex potentiation (Rosen and Davis, 1988; Angrilli et al., 1996). In the current study, increased VLPFC activity did not result in a significant inhibition of startle reflex potentiation. Instead, we found a significant reduction of affect-related somatosensory activity. Inhibition of affect-related cortical activity in medial prefrontal, insular and parietal regions has commonly been reported in both studies on voluntary inhibition of emotion (Ochsner et al., 2002, 2004; Kalisch et al., 2005, 2006) and studies in which affective information was passively suppressed (Gorno-Tempini et al., 2001; Northoff et al., 2004; Wager et al., 2004). Thus, while voluntary inhibition of affect might effectively inhibit the amygdala and its efferents, involuntary ‘passive’ suppression of affect might mainly attenuate activity in cortical regions representing affective somatic responses. This might lead to a decoupling of somatic changes and phenomenal experience of affect and, in the case of threat-related stimuli, result in a significant reduction of negative affective experience.
Arousal-responses and arousal-related brain areas
Increased VLPFC activity often concurs with increased activity in the anterior cingulate cortex (ACC) (Beauregard et al., 2001; Yamasaki et al., 2002; Greene et al., 2004; Wager et al., 2004; Petrovic et al., 2005; Phan et al., 2005; Kong et al., 2006; Eippert et al. 2007), a region that has been shown to play a specific role in monitoring and signalling of conflict (Botvinick et al., 1999; Carter et al., 2000, Bishop et al., 2004) and bodily states of arousal (Critchley et al., 2003). It is interesting to note that neither anterior cingulate cortex activity nor SCR (an indicator of sympathetic arousal) was increased in the current study. Thus, one might speculate that conflict-related anterior cingulate cortex activity and arousal might occur only if conflicting information results from cortical processing, while subcortical affective information might reach the VLPFC cortex directly via orbitofrontal projections from the amygdala (McDonald, 1998; Cavada et al., 2000).
Clinical implications
The current study shows that phenomenal experience of affect and somatic changes can be decoupled in neurological patients. Altered phenomenal experience of affect is a central aspect of many neurological and psychiatric syndromes; and it is perhaps the most disturbing factor from the patient's perspective. The current study provides evidence that inappropriate affective experience may be the result of disturbed processing at different levels. Inflated or shallow affective experiences might be a direct consequence of inflated or shallow subcortically mediated somatic responses, but they might also result from disturbed cortical control over somatic responses and/or cortical areas that represent these responses. For example, a study by Carlsson et al. (2004) found that patients with small animal phobia and healthy subjects showed similar amygdala responses to phobia-related stimuli when backward masking prevented cortical processing. Only when stimulus presentation times were long enough to allow full cortical processing did patients show prolonged amygdala activity, and this was associated with a reduction of VLPFC activity. This suggests that in phobia it is disturbed cortical processing and regulation that leads to the disproportionate fear response. In a different study, Rauch et al. (2000) found abnormally increased amygdala activity in response to fearful facial expressions in post-traumatic stress disorder patients even when backward masking prevented cortical processing, possibly indicating altered subcortical processing in patients with post-traumatic stress disorder.
Limitations and open questions
The current study shows that in a rare neurological sample, processing of the same visual stimulus can be associated with different phenomenal experiences of affect despite similar somatic responses, and thereby provides important insights into human emotion. At the same time, it leaves some challenging questions unresolved.
First, it is not completely clear why the patients did not report negative affect during stimulation of the SVF, particularly as healthy controls did report negative affect when they underwent the same experimental procedure as the patients (Anders et al., 2005). One possibility is that in the patients, subcortical and cortical representations of the visual stimulus acquired a different meaning. In healthy controls the unpleasant scream was always preceded by in-depth cortical visual processing of the face. In contrast, in the patients, only half of the screams were preceded by in-depth cortical visual processing. The other half of screams was preceded by subcortical processing only. Thus the cortical representation of the face might not have become a reliable predictor of threat.
Secondly, because we did not want to make any a priori assumptions regarding visual discrimination in the blind field, we did not include a second visual stimulus that was not followed by the scream. Therefore, we cannot exclude that affective somatic information influenced affective experience to a certain degree even when the face was visible, and that the patients would have experienced a visual stimulus that was completely unrelated to the scream as even more positive.
Thirdly, the correlation analyses in the current study rely on between-subject analyses of a limited number of subjects. This makes it impossible to fully investigate different relations between somatic responses, brain activity and phenomenal experience. It would be highly interesting to see whether the observed reduction of negative affective experience, when the stimulus was visible, could be explained more comprehensively when more variables were included in the model.
Finally, the current study does not reveal if, and if so how, cortical processing might have altered concurrent subcortical processing. While previous studies have provided evidence that the nodes of the putative subcortical colliculo-amygdalar pathway are also activated when full cortical processing takes place, they also suggest that functional connectivity within this pathway can be modulated by concurrent cortical processing (Morris et al., 1999, 2001; Williams et al., 2006). In this study, startle potentiation, a response component that is assumed to be mediated by the colliculo-amygdalar pathway, did not differ between the SVF and BVF. It will be a challenging task for future studies to investigate in more detail how cortical processing modulates subcortical processing.
Conclusion
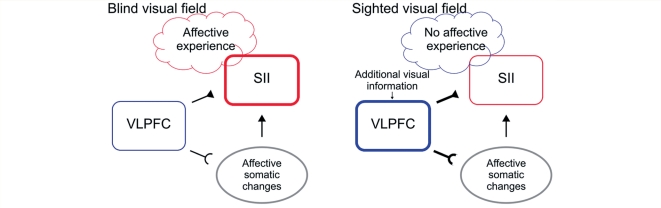
The current study shows that somatic responses and phenomenal experience of affect can be decoupled depending on how much cortical processing a stimulus receives. Taken together, our data provide evidence for a model in which the VLPFC integrates information about subcortically triggered affective somatic responses and information resulting from in-depth cortical stimulus processing. In this model, increased VLPFC activity leads to an inhibition of affect-related somatosensory activity (Fig. 6). Tentatively, we suggest that it is this inhibition of affect-related somatosensory activity that can lead to a decoupling of somatic changes and experienced affect, and to a reduction of negative phenomenal experience, as observed in the current study.
Figure 6.
A model incorporating the observed relations between affective somatic changes (startle reflex potentiation), VLPFC activity, and affect-related somatosensory activity in SII during stimulation of the BVF and SVF. During stimulation of the BVF, affect-related somatic responses are associated with an increase of activity in SII and reports of negative affective experience (left). During stimulation of the SVF, additional stimulus information resulting from in-depth cortical processing is available, and VLPFC sensitivity to somatic responses increases. Increased VLPFC activity, in turn, attenuates somatosensory-related activity. We suggest that this reduction of affect-related somatosensory activity leads to a decoupling of somatic changes and experienced affect and a reduction of negative phenomenal experience (right).
Funding
Junior Science Program (WIN) of the Heidelberger Academy of Sciences and Humanities.
Acknowledgement
The authors thank John-Dylan Haynes for comments on an earlier version of the manuscript.
Glossary
Abbreviations
- BVF
blind visual field
- fMRI
functional magnetic resonance imaging
- SCR
skin conductance response
- SVF
sighted visual field
- VLPFC
ventrolateral prefrontal cortex
References
- Anders S, Birbaumer N, Sadowski B, Erb M, Mader I, Grodd W, et al. Parietal somatosensory association cortex mediates affective blindsight. Nat Neurosci. 2004a;7:339–40. doi: 10.1038/nn1213. [DOI] [PubMed] [Google Scholar]
- Anders S, Lotze M, Wildgruber D, Erb M, Grodd W, Birbaumer N. Processing of a simple aversive conditioned stimulus in a divided visual field paradigm: an fMRI study. Exp Brain Res. 2005;162:213–9. doi: 10.1007/s00221-004-2145-1. [DOI] [PubMed] [Google Scholar]
- Anders S, Weiskopf N, Lule D, Birbaumer N. Infrared oculography-validation of a new method to monitor startle eyeblink amplitudes during fMRI. Neuroimage. 2004b;22:767–70. doi: 10.1016/j.neuroimage.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Ochsner KN, Cooper J, Robertson E, Gabrieli SW, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119(Pt 6):1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Baumgart F, Kaulisch T, Tempelmann C, Gaschler-Markefski B, Tegeler C, Schindler F, et al. Electrodynamic headphones and woofers for application in magnetic resonance imaging scanners. Med Phys. 1998;25:2068–70. doi: 10.1118/1.598368. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Ohman A. Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4:340–53. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15:806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Hadjikhani N. Non-conscious recognition of emotional body language. Neuroreport. 2006;17:583–6. doi: 10.1097/00001756-200604240-00006. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Morris JS, Dolan RJ. Unconscious fear influences emotional awareness of faces and voices. Proc Natl Acad Sci USA. 2005;102:18682–7. doi: 10.1073/pnas.0509179102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Pourtois G, van Raamsdonk M, Vroomen J, Weiskrantz L. Unseen stimuli modulate conscious visual experience: evidence from inter-hemispheric summation. Neuroreport. 2001;12:385–91. doi: 10.1097/00001756-200102120-00040. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Pourtois G, Weiskrantz L. Non-conscious recognition of affect in the absence of striate cortex. Neuroreport. 1999;10:3759–63. doi: 10.1097/00001756-199912160-00007. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Labar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behav Neurosci. 2005;119:1118–24. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychol Sci. 2000;11:86–9. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves F, Dimberg U, Öhman A. Automatically elicited fear: Conditioned skin conductance responses to masked facial expressions. Cognition & Emotion. 1994;8:393–413. [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI, Schupp HT, Treig T, Dressel A, Kessler C. Affective blindsight: intact fear conditioning to a visual cue in a cortically blind patient. Brain. 2003;126:267–75. doi: 10.1093/brain/awg037. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–22. [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jolij J, Lamme VA. Repression of unconscious information by conscious processing: evidence from affective blindsight induced by transcranial magnetic stimulation. Proc Natl Acad Sci USA. 2005;102:10747–51. doi: 10.1073/pnas.0500834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30:1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. J Cogn Neurosci. 2005;17:874–83. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Greenlee MW, Huethe F, Mergner T. MR-eyetracker: a new method for eye movement recording in functional magnetic resonance imaging. Exp Brain Res. 1999;126:443–9. doi: 10.1007/s002210050751. [DOI] [PubMed] [Google Scholar]
- Kleinginna PR, Kleinginna AM. A Categorized List of Emotion Definitions, with Suggestions for a Consensual Definition. Motivation and Emotion. 1981;5:354–79. [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–91. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Linke R, De Lima AD, Schwegler H, Pape HC. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J Comp Neurol. 1999;403:158–70. [PubMed] [Google Scholar]
- Macknik SL, Livingstone MS. Neuronal correlates of visibility and invisibility in the primate visual system. Nat Neurosci. 1998;1:144–9. doi: 10.1038/393. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–52. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Neural responses to salient visual stimuli. Proc R Soc Lond B Biol Sci. 1997;264:769–75. doi: 10.1098/rspb.1997.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96:1680–5. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: affective priming with optimal and suboptimal stimulus exposures. J Pers Soc Psychol. 1993;64:723–39. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Murphy ST, Monahan JL, Zajonc RB. Additivity of nonconscious affect: combined effects of priming and exposure. J Pers Soc Psychol. 1995;69:589–602. doi: 10.1037//0022-3514.69.4.589. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21:202–12. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–33. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Khateb A, Lazeyras F, Seghier ML. Discriminating emotional faces without primary visual cortices involves the right amygdala. Nat Neurosci. 2005;8:24–5. doi: 10.1038/nn1364. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, et al. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–6. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing – induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–69. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nat Neurosci. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Robles R, Smith R, Carver CS. Influence of subliminal visual images on the experience of anxiety. Personality and Social Psychology Bulletin. 1987;13:399–410. [Google Scholar]
- Rosen JB, Davis M. Enhancement of acoustic startle by electrical stimulation of the amygdala. Behav Neurosci. 1988;102:195–202. doi: 10.1037//0735-7044.102.2.195. 324. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–33. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110:145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ. Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia. 2008;46:879–85. doi: 10.1016/j.neuropsychologia.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Scherer KR. What are emotions? And how can they be measured? Social Science Information. 2005;44:695–729. [Google Scholar]
- Spence SA. The deceptive brain. J R Soc Med. 2004;97:6–9. doi: 10.1258/jrsm.97.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B. Affective blindsight in the intact brain: neural interhemispheric summation for unseen fearful expressions. Neuropsychologia. 2008;46:820–8. doi: 10.1016/j.neuropsychologia.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–71. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull. 2005;31:121–35. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in mages of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA. 2002;99:11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]