Abstract
The behavioural variant of frontotemporal dementia is a progressive neurodegenerative syndrome characterized by changes in personality and behaviour. It is typically associated with frontal lobe atrophy, although patterns of atrophy are heterogeneous. The objective of this study was to examine case-by-case variability in patterns of grey matter atrophy in subjects with the behavioural variant of frontotemporal dementia and to investigate whether behavioural variant of frontotemporal dementia can be divided into distinct anatomical subtypes. Sixty-six subjects that fulfilled clinical criteria for a diagnosis of the behavioural variant of frontotemporal dementia with a volumetric magnetic resonance imaging scan were identified. Grey matter volumes were obtained for 26 regions of interest, covering frontal, temporal and parietal lobes, striatum, insula and supplemental motor area, using the automated anatomical labelling atlas. Regional volumes were divided by total grey matter volume. A hierarchical agglomerative cluster analysis using Ward's clustering linkage method was performed to cluster the behavioural variant of frontotemporal dementia subjects into different anatomical clusters. Voxel-based morphometry was used to assess patterns of grey matter loss in each identified cluster of subjects compared to an age and gender-matched control group at P < 0.05 (family-wise error corrected). We identified four potentially useful clusters with distinct patterns of grey matter loss, which we posit represent anatomical subtypes of the behavioural variant of frontotemporal dementia. Two of these subtypes were associated with temporal lobe volume loss, with one subtype showing loss restricted to temporal lobe regions (temporal-dominant subtype) and the other showing grey matter loss in the temporal lobes as well as frontal and parietal lobes (temporofrontoparietal subtype). Another two subtypes were characterized by a large amount of frontal lobe volume loss, with one subtype showing grey matter loss in the frontal lobes as well as loss of the temporal lobes (frontotemporal subtype) and the other subtype showing loss relatively restricted to the frontal lobes (frontal-dominant subtype). These four subtypes differed on clinical measures of executive function, episodic memory and confrontation naming. There were also associations between the four subtypes and genetic or pathological diagnoses which were obtained in 48% of the cohort. The clusters did not differ in behavioural severity as measured by the Neuropsychiatric Inventory; supporting the original classification of the behavioural variant of frontotemporal dementia in these subjects. Our findings suggest behavioural variant of frontotemporal dementia can therefore be subdivided into four different anatomical subtypes.
Keywords: behavioural variant frontotemporal dementia, atrophy, cluster analysis, voxel-based morphometry
Introduction
The behavioural variant of frontotemporal dementia (bvFTD) is a progressive neurodegenerative syndrome in which subjects show early changes in personality and behaviour (Neary et al., 1998; Josephs, 2008). While bvFTD is considered a single syndromic entity, there is considerable variability in its clinical presentation. For example, subjects have been sub-classified into those showing apathetic behaviour and those showing disinhibited behaviour (Hodges, 2001; Snowden et al., 2002; Le Ber et al., 2006). As the disease progresses, patients can exhibit changes in language (Grossman et al., 2004; Blair et al., 2007; Wicklund et al., 2007), although the extent of these language abnormalities can also vary between subjects (Wicklund et al., 2007). Clinicopathological studies have shown that bvFTD is highly variable in terms of underlying pathology (Hodges et al., 2004; Kertesz et al., 2005; Forman et al., 2006; Josephs et al., 2006a), with ∼60% of subjects showing underlying deposition of the TAR DNA binding protein 43 (TDP-43) and the other 40% showing deposition of the microtubule associated protein tau (MAPT) (Kertesz et al., 2005; Josephs et al., 2006a). In fact, of all the clinical variants of frontotemporal dementia, bvFTD is the most difficult in which to predict the underlying pathology; although there is now some suggestion that behavioural features may differ between different pathologic forms (Hu et al., 2007a, b).
The behavioral variant of FTD is traditionally considered to be associated with atrophy of the frontal lobes, usually symmetrical, although asymmetric right-sided atrophy has been reported (Fukui and Kertesz, 2000; Rosen et al., 2002; Seeley et al., 2008). These changes are consistent with the role of the frontal lobes in behavioural control (Cummings, 1993; Rosen et al., 2005; Williams et al., 2005; Peters et al., 2006). However, recent studies using advanced imaging techniques have shown that bvFTD is also associated with atrophy in a network of other limbic areas that are likely to be involved in the fine tuning of behaviour, including the insula, striatum, anterior cingulate and amygdala (Rosen et al., 2002; Boccardi et al., 2005; Whitwell et al., 2005b; Barnes et al., 2006). Atrophy of temporal lobe structures, such as the hippocampus, and parahippocampal gyrus, has also been reported in bvFTD subjects (Galton et al., 2001a; Grossman et al., 2004; Barnes et al., 2006; Whitwell et al., 2009). The patterns of anatomic destruction in bvFTD therefore spread outside the frontal lobes and also vary to a large degree across different cohorts. It has also been demonstrated that subjects with a diagnosis of bvFTD can show differing patterns of atrophy according to behavioural profile and pathology (Cummings, 1993; Hodges, 2001; Snowden et al., 2001; Liu et al., 2004; Josephs et al., 2006b; Le Ber et al., 2006; Whitwell et al., 2006; Massimo et al., 2009). Specifically, studies have found that patients with an apathetic syndrome show dorsolateral and medial frontal changes, while patients with a disinhibited syndrome show orbitofrontal and temporal lobe changes (Le Ber et al., 2006; Massimo et al., 2009). In addition, many behavioural features have been associated with the right temporal lobe (Miller et al., 1993; Edwards-Lee et al., 1997; Thompson et al., 2003; Zamboni et al., 2008; Chan et al., 2009). These results therefore suggest that variability in behavioural presentation may mirror anatomic variability.
The behavioral variant of FTD is therefore a clinically, pathologically and anatomically heterogeneous disorder. The aim of this study was to assess the variability in patterns of atrophy present in bvFTD and investigate whether bvFTD consists of different anatomical subtypes that may help explain the clinical and pathological heterogeneity. Previous studies in bvFTD have utilized group level analysis techniques, such as voxel-based morphometry (Rosen et al., 2002; Boccardi et al., 2005), which provide useful information about patterns of atrophy across the whole brain in cohorts of subjects. However, these techniques do not allow the assessment of atrophy at the individual level, which is essential to assess variability across subjects and identify specific anatomical variants. It is also important in assessing variability to be able to sample throughout the entire brain. Traditional manual measurements, while providing data at the individual level, are time-consuming to perform and therefore performing multiple measurements throughout the brain in large samples of subjects is not feasible. The brain has, however, been carefully parcellated into many different regions of interests in a number of different brain atlases. One commonly used atlas is the automated anatomical labelling atlas in which regions of interest have been defined on a single subject scan (Tzourio-Mazoyer et al., 2002). This template can be warped onto a subject's scan and used to measure regional volumes (Jack et al., 2008b). This technique is automated and allows the measurement of multiple regions of interest across the brain of subjects and therefore will be utilized in this study.
Methods
Subjects
We identified all subjects from the Mayo Clinic Alzheimer's Disease Research Centre (ADRC) or Alzheimer's Disease Patient Registry (ADPR) that had a clinical diagnosis of bvFTD (Neary et al., 1998) and a volumetric MRI scan. The ADRC and ADPR are longitudinal prospective studies in which subjects undergo annual clinical and neuropsychological assessments and MRI scans. All subjects had been seen by a Behavioural Neurologist in the Department of Neurology, Mayo Clinic. The Mini-Mental State Examination (MMSE) (Folstein et al., 1975), Clinical Dementia Rating Scale (CDR) (Hughes et al., 1982), Dementia Rating Scale (DRS) (Mattis, 1988), and the brief questionnaire form of the Neuropsychiatric Inventory (NPI-Q) (Cummings, 1994; Kaufer et al., 2000) were administered. The results of the NPI-Q were not used in the clinical diagnosis of the subjects. All MRI were reviewed and scans were rejected for poor quality (such as motion or susceptibility artefacts) or the presence of other pathologies (such as large cortical infarcts, tumour or other structural abnormalities) that may influence the structural analysis. The first available MRI was used in all cases. A total of 79 subjects were identified, of which 13 were excluded from the study (eight because the scans were performed before 1994 using an older MRI protocol, three due to poor quality and one due to a large cortical infarct which would interfere with regional quantification), resulting in a total of 66 subjects available for this study. The subject demographics for the 66 bvFTD subjects are shown in Table 1. A behavioural neurologist (K.A.J.) who was not involved in the initial diagnosis reviewed the medical records of all cases to abstract data, ensure that the diagnostic codes in the database were indeed correct and to ensure that cases evaluated before 1999 fulfilled current clinical criteria for bvFTD (Neary et al., 1998). A total of seven cases had been evaluated and diagnosed before 1999 using older clinical criteria (Lund-Manchester, 1994), and hence in these cases the recent clinical criteria (Neary et al., 1998) were retrofitted.
Table 1.
Subject demographics
| Controls | bvFTD | P-value† | bvFTD subtypes | P-value‡ | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 30) | (n = 66) | Temporal dominant (n = 6) | Temporofronto parietal (n = 27) | Frontotemporal (n = 12) | Frontal dominant (n = 21) | |||
| No. females (%) | 19 (63) | 39 (59) | 0.69 | 4 (67) | 13 (48) | 8 (67) | 14 (67) | 0.53 |
| Age at MRI, year (IQR) | 63 (57–71) | 59 (53–67) | 0.36 | 49 (42–51) | 66 (56–71) | 59 (56–62) | 59 (51-66) | 0.004 |
| Age at onset, year (IQR) | – | 56 (50–63) | NA | 44 (39–50) | 62 (53–67) | 55 (51–58) | 56 (50-64) | 0.004 |
| Time from onset-MRI, year (IQR) | – | 3.0 (1.5–5.9) | NA | 4.5 (3.0–6.0) | 4.0 (1.9–7.0) | 2.8 (1.4–6.0) | 2.0 (1.5-3.3) | 0.31 |
| Education, year (IQR) | 15 (12–16) | 15 (12–16) | 0.92 | 14 (12–15) | 16 (12–17) | 16 (12–18) | 14 (12-16) | 0.73 |
| No. of APOE e4 carrier (%) | 7 (23) | 12 (25) | 0.87 | 0 | 5 (26) | 2 (18) | 5 (36) | 0.63 |
| Pathological/genetic diagnosis | ||||||||
| FTLD-TDP type 1(PGRN+,PGRN−) | 0 | 8 (26%)3,4 | NA | 0 | 3 (30%)1,1 | 2 (28%)1,1 | 3 (30%)1,2 | NA |
| FTLD-TDP type 2(PGRN+,PGRN−) | 0 | 1 (3%)0,1 | NA | 0 | 1 (10%)0,1 | 0 | 0 | NA |
| FTLD-TDP type 3(PGRN+,PGRN−) | 0 | 5 (16%)0,4 | NA | 0 | 2 (20%)0,2 | 1 (14%) | 2 (20%)0,2 | NA |
| FTLD-UPS(PGRN+,PGRN-) | 0 | 1 (3%)0,1 | NA | 0 | 0 | 1 (14%)0,1 | 1 (10%) | |
| FTLD-Tau (Pick's disease) | 0 | 1 (3%) | NA | 0 | 0 | 1 (14%) | 0 | NA |
| FTLD-Tau (Corticobasal degeneration) | 0 | 2 (7%) | NA | 0 | 1 (10%) | 0 | 1 (10%) | NA |
| FTLD-Tau (Progressive supranuclear palsy) | 0 | 1 (3%) | NA | 0 | 0 | 0 | 1 (10%) | NA |
| FTLD-Tau (FTDP-17) | 0 | 9 (29%)a | NA | 5 (100%) | 1 (10%) | 2 (29%) | 1 (10%) | NA |
| Alzheimer's disease | 0 | 3 (10%) | NA | 0 | 2 (20%) | 0 | 1 (11%) | NA |
†P-value for the comparison of the bvFTD and control groups.
‡P-value for the comparison across the four bvFTD subtypes.
a Two of the subjects with mutations in the MAPT gene also have pathological confirmation.
Data are shown as median [inter-quartile range (IQR)]. NA = not applicable; APOE = apolipoprotein E; FTLD = frontotemporal lobar degeneration; FTLD-Tau = FTLD with tau immunoreactive inclusions; FTDP-17 = FTLD with mutations in the microtubule associated protein tau gene MAPT; FTLD-UPS = FTLD with ubiquitin-only-immunoreactive inclusions; APOE = apolipoprotein E; PGRN+ = number of subjects that have screened positive for mutations in the PGRN gene; PGRN− = number of subjects that have screened negative for mutations in the PGRN gene.
Significant P-values (< 0.05) are shown in bold.
The bvFTD subjects were matched by age and gender to a group of 30 healthy controls. All control subjects were prospectively recruited into the ADRC or the ADPR and were identified from the ADRC/ADPR database. Controls were identified as individuals who (i) were independently functioning community dwellers; (ii) did not have active neurologic or psychiatric conditions; (iii) had no cognitive complaints; (iv) had a normal neurological and neurocognitive examination; and (v) were not taking any psychoactive medications in doses that would affect cognition.
Neuropsychology
Neuropsychological data performed within 4 months of the scan date were collected and analysed. We selected two neuropsychological tests to represent each of the domains of memory, language, executive and visuospatial function. The memory tests included the Wechsler Memory Scale–Revised (WMS-R) Logical Memory (LM) delayed recall and Visual Reproduction (VR) delayed recall subtests (Wechsler, 1987). The Boston Naming Test (BNT) (Kaplan et al., 1983) and Category Fluency test (animals, vegetables and fruits) (Lucas et al., 1998) were used to test language functioning. The executive tests included Trail Making Test B (War Department, 1944) and the Controlled Oral Word Association Test (COWAT) (Benton and Hamsher, 1989). The visuospatial tests included the Picture Completion and Block Design subtests of the Wechsler Adult Intelligence Scale (WAIS)–Revised or Third Edition (WAIS-R/WAIS-III) (Wechsler, 1981, 1997). All tests were administered by experienced psychometrists and supervised by clinical neuropsychologists. The age-corrected scaled scores from the WAIS subtests were used for comparison while the raw scores were used for all other tests. WAIS-R and WAIS-III age-corrected scaled scores were used because each test's raw scores have been converted to a common metric (i.e. scaled score) that allows meaningful combinations. The raw scores from these tasks have different metrics, precluding statistical combination. The neuropsychological results are shown in Table 2. Some neuropsychological tests could not be administered to some subjects due to the advanced state of their dementia, but were included in this analysis to encompass as broad a spectrum of bvFTD as possible. Some controls were missing neuropsychological data; this is primarily due to changes in the battery administered to control participants over time.
Table 2.
Cognitive data at time of scan
| Domain | Test | Na | Controls | bvFTD | bvFTD subtypes |
P-value‡ | |||
|---|---|---|---|---|---|---|---|---|---|
| (n = 30) | (n = 66)† | Temporal dominant (n = 6) | Temporofronto parietal (n = 27) | Frontotemporal (n = 12) | Frontal dominant (n = 21) | ||||
| General Cognitive function | MMSE (IQR)b | 30:62 | 29 (29–30) | 26 (21–28) | 28 (25–29) | 26 (22–28) | 27 (23–27) | 25 (15–26) | 0.073 |
| CDR-SB (IQR) | 30 : 65 | 0 | 5.0 (3.0–8.0) | 4.0 (2.5–5.5) | 4.5 (2.5–7.0) | 6.0 (4.0–8.5) | 5.5 (4.5–9.0) | 0.13 | |
| DRS (IQR)b | 29 : 49 | 142 (139–143) | 121 (105–131) | 133 (115–136) | 126 (105–132) | 119 (102–125) | 119 (78–123) | 0.34 | |
| Memory | WMS-LM (IQR)b | 22 : 42 | 22 (18–28) | 5 (1–13) | 1 (0–2) | 5 (1–19) | 5 (1–12) | 11 (5–16) | 0.002 |
| WMS-VR (IQR)b | 22 : 42 | 29 (25–33) | 7 (0–14) | 2 (0–7) | 7 (2–14) | 6 (0–9) | 9 (0–18) | 0.13 | |
| Language | BNT (IQR)b | 22 : 54 | 57 (55–58) | 49 (36–54) | 30 (26–37) | 49 (37–54) | 49 (45–56) | 51 (39–56) | 0.006 |
| Cat. Fluency (IQR)b | 22 : 51 | 48 (44–55) | 21 (17–30) | 27 (21–30) | 22 (16–31) | 24 (18–26) | 19 (17–26) | 0.66 | |
| Executive | Trails B (IQR) | 17 : 54 | 55 (47–69) | 209 (107–300) | 81 (70–121) | 199 (142–300) | 259 (151–300) | 300 (77–300) | 0.24 |
| COWAT (IQR)b | 22 : 50 | 44 (38–49) | 23 (16–32) | 33 (17–34) | 28 (18–33) | 23 (14–29) | 18 (7–20) | 0.069 | |
| Visuospatial | WAIS-PC (IQR) | 22 : 48 | 11 (9–12) | 5 (4–6) | 6 (5–7) | 6 (4–7) | 5 (4–6) | 5 (4–6) | 0.37 |
| WAIS-BD (IQR) | 22 : 47 | 10 (8–12) | 6 (4–7) | 6 (6–7) | 6 (5–7) | 6 (4–7) | 6 (4–8) | 0.86 | |
†Significant difference was observed between bvFTD and control group on all variables.
‡P-value for the comparison across the four bvFTD subtypes.
a Number of control subjects : bvFTD subjects with available test scores.
b Low score on test represents impaired performance.
Data are shown as median (inter-quartile range). All data are raw scores, except for the WAIS in which age-corrected scaled scores are shown. CDR-SB = CDR sum of boxes (scored out of 18); VR = Visual Reproduction delayed recall; BNT = Boston Naming Test; Trails B = Trail Making Test B; Cat. Fluency = Category fluency; BD = Block Design; PC = Picture Completion; MMSE = mini-mental state examination (scored out of 30); DRS = Dementia Rating Scale (scored out of 144).
P-values at trend level (P < 0.1) are shown in bold.
Pathological assessment
Autopsy data were available in 25 of the bvFTD subjects. Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for Alzheimer's Disease (Mirra et al., 1991). In all cases, pathological assessment and diagnosis was conducted by one of two expert neuropathologists (D.W.D. or J.E.P.) as described earlier (Knopman et al., 2003). All cases were reclassified based on the recent consensus recommendation for the nomenclature for neuropathological subtypes of FTD (Mackenzie et al., 2009).
Frontotemporal lobar degeneration with TAR DNA binding protein 43 (FTLD-TDP) was diagnosed if there was neuronal loss and gliosis in frontal and temporal cortices, as well as ubiquitin and TDP-43 immunoreactive neuronal inclusions. FTLD-TDP type 1 was diagnosed if in frontotemporal cortices and hippocampal dentate granule cells of the hippocampus there is a mixture of TDP-43 immunoreactive neuronal cytoplasmic inclusions and dystrophic neurites; FTLD-TDP type 2 if there is a predominance of dystrophic neurites, and FTLD-TDP type 3 if there is a predominance of cytoplasmic inclusions (Mackenzie et al., 2006).
FTLD-Tau was diagnosed if there were tau positive immunoreactive neuronal or glial inclusions or genetic determination of a mutation in the MAPT gene. FTLD-Tau subjects were further sub-classified into their major pathological subtypes. Subjects were diagnosed as having progressive supranuclear palsy if there was neuronal loss and gliosis, as well as characteristic tau-positive lesions including tufted astrocytes, coiled bodies and globose neurofibrillary tangles in cardinal nuclei (subthalamic nucleus, thalamic and brainstem nuclei, etc.) that met diagnostic criteria for progressive supranuclear palsy (Hauw et al., 1994). Subjects were given a pathological diagnosis of corticobasal degeneration if there was cortical neuronal loss and gliosis with balloon neurons and tau-positive lesions including astrocytic plaques, corticobasal bodies and abundant neuropil threads that were located in cardinal regions that met diagnostic criteria for corticobasal degeneration (Dickson et al., 2002). A diagnosis of Pick's disease was made if there were neurofilament positive balloon neurons and silver and tau-positive rounded Pick bodies in the cerebral cortex, and subcortical grey structures (Dickson, 2001).
Cases with ubiquitin-only-immunoreactive inclusions, i.e. the inclusions were not immunoreactive to TDP-43 (Josephs et al., 2008a; Mackenzie et al., 2008) were designated FTLD-UPS.
A diagnosis of Alzheimer's disease was made if the Braak neurofibrillary tangle stage (Braak and Braak, 1991) was V or VI and pathological findings met the National Institute of Aging and Reagan Institute Working Group (NIA-Reagan) diagnostic criteria for high probability Alzheimer's disease (Hyman and Trojanowski, 1997).
Genetic assessments
Analysis of MAPT exons 1, 7 and 9–13 was performed using primers and conditions that were described earlier (Hutton et al., 1998). In addition, exons 0–13 and the 3′-untranslated region of the progranulin (PGRN) gene were amplified by polymerase chain reaction (PCR) using our previously published primers and protocol (Baker et al., 2006; Gass et al., 2006). PCR amplicons were purified using the Multiscreen system (Millipore, Billerica, MA, USA) and then sequenced in both directions using Big Dye chemistry following manufacturer's protocol (Applied Biosystems, Foster City, CA, USA). Sequence products were purified using the Montage system (Millipore) prior to being run on an ABI 3730 DNA Analyser. Sequence data were analysed using either SeqScape or Sequencher software.
MRI acquisition
All MRI studies were performed with a standardized imaging protocol (Jack et al., 2008b). Scans were performed on different scanners but all were GE Signa with body resonance module gradient sets and transmit–receive single channel head coils. All scanners underwent a standardized quality control calibration procedure every morning, which monitored geometric fidelity over a 200 mm volume along all three cardinal axes, signal-to-noise ratio and transmit gain. All images underwent pre-processing correction for gradient non-linearity (Jovicich et al., 2006) and intensity non-uniformity (Sled et al., 1998) as described earlier (Jack et al., 2008a).
Atlas-based parcellation
An atlas-based parcellation technique was employed using Statistical Parametric Mapping release 2005 (SPM5) and the automated anatomical labelling atlas (Tzourio-Mazoyer et al., 2002), in order to generate grey matter volumes for different regions of interest across the brain of each subject. The following 26 mutually exclusive regions of interest were analysed: medial frontal, orbitofrontal, lateral inferior frontal, lateral superior frontal, medial temporal, lateral temporal, temporal pole, medial parietal, lateral parietal, insula, caudate nucleus, lentiform nucleus and supplemental motor area (left and right hemispheres were assessed separately for each region).
The high-resolution T1-weighted single-patient brain image (Tzourio-Mazoyer et al., 2002) with atlas labels was normalized to a customized template using the unified segmentation method in SPM-5 (Ashburner and Friston, 2005), giving a discrete cosine transformation which normalizes the atlas brain to custom template space. Each region of interest in the atlas was edited in template space by an experienced image analyst (M.M.S.), in order to improve the accuracy of the atlas. Each patient MRI scan was then spatially normalized to the custom template, giving a discrete cosine transformation, which normalizes the MRI of each patient to the custom template. Then for each patient, the inverse transformation was applied to the atlas in order to warp the atlas to the patient's native anatomical space. Each patient scan was segmented into grey matter, white matter and cerebrospinal fluid (CSF) in native space. The segmented grey matter probability map generated from the unified segmentation routine was thresholded at a value of 0 to create a binary grey matter mask, and multiplied by the patient-specific warped atlas, to generate a custom grey matter atlas for each patient, parcellated into the aforementioned regions of interest. Grey matter volumes were calculated for each region of interest for each patient by multiplying the mean grey matter probability by the total number of voxels within a region of interest and by the voxel volume. In addition, total grey matter volume was calculated for each patient from the grey matter segmentation. All regional grey matter volumes were divided by total grey matter volume to correct for differences in global atrophy between subjects. This step was performed because we were interested in clustering subjects by the relative involvement of each region rather than just by global severity.
Cluster analysis
We performed a hierarchical agglomerative cluster analysis which begins with each patient in his or her own cluster and at each step combines the two most ‘similar’ clusters until the last two clusters are combined into a single cluster with all patients (Johnson and Wichern, 1982). Such a hierarchical method gives a sequence of nested clusters and allows the analyst to choose the number of clusters to work with. This cluster analysis was performed using the 26 grey matter corrected region of interest volumes for each of the 66 subjects with bvFTD. We used Ward's clustering linkage method of combining clusters. At each step, Ward's method chooses which pair of clusters to combine next by merging the pair of clusters that minimizes the sum of square errors, or sum of squared deviations from the cluster mean, across all clusters. Note that unlike in regression modelling, cluster analysis does not involve parameter estimation and therefore the analyst need not be concerned with overfitting resulting from considering too many variables given the sample size.
While clusters were formed using the method described earlier, principal components analysis was used to provide some validation of the clustering as well as an insight into the underlying dimensionality of the data. Principal components analysis is a technique by which a high-dimensional data set (with possible correlation between the dimensions) is projected onto to a lower dimensional (uncorrelated) space by retaining the maximum amount of data variability. Computationally, this is performed using singular value decomposition via matrix arithmetic. From a conceptual standpoint, the first component finds the coefficients for each region of interest that can be combined to account for the maximum amount of variability in the data and the coefficients of each of the succeeding components account for as much as the remaining variability as possible. The first two principal components can be interpreted as the best two dimensional representation of the full data set and informative of the highest variability in the data.
Voxel-based morphometry
Voxel-based morphometry (Ashburner and Friston, 2000) using SPM5 was used to assess patterns of grey matter loss in each cluster of subjects that was identified from the cluster analysis compared to controls. This comparison assesses overall patterns of grey matter loss in each subtype without correcting for grey matter volume. A number of pre-processing steps were performed as described earlier (Jack et al., 2008b). We first created sample-specific (customized) templates and tissue probability maps by normalizing and segmenting all scans with the standard Montreal Neurological Institute template and tissue probability maps and averaging the normalized tissue probability maps. All images were normalized to the customized template and segmented using the customized tissue probability maps into grey matter, white matter and CSF, followed by the hidden Markov random field clean-up step (Zhang et al., 2001). All grey matter images were modulated and smoothed with a Gaussian kernel of 8 mm full-width at half maximum.
Grey matter differences between each cluster-defined bvFTD subtype and controls were assessed using t-tests within the general linear model framework of SPM (corrected for multiple comparisons using the family-wise error correction at P < 0.05). In addition, two one-sided t-tests were performed between each pair of subtypes (say A and B), one looking for regions that showed greater loss in A than B, and the other looking for regions that showed greater loss in B than A. These analyses were assessed at a more lenient statistical threshold of P < 0.005 using the false discovery rate correction for multiple comparisons.
Statistical analysis
We tested for group differences in demographic variables between controls and bvFTD subtypes using Wilcoxon rank-sum/Mann–Whitney U-tests when analysing numeric variables and chi-squared test when analysing categorical variables. Differences among the bvFTD subtypes on numeric variables were assessed using the Kruskal–Wallis test, differences in gender; proportion of apolipoprotein E e4 carriers and proportion of subjects with each NPI-Q behaviour were assessed with the Fisher exact test due to the low expected counts in the corresponding two-by-four table.
Neuropsychological variables were not normally distributed due to floor and ceiling effects and skewness. We therefore used the proportional odds logistic regression (POLR) model to accommodate non-normal distributions and allow us to adjust for differences due to age. The POLR model is an ordinal logistic regression model that can be thought of as generalizing the Wilcoxon rank-sum/Mann–Whitney U-test to a regression context (Harrell, 2001). This model can be interpreted as treating the neuropsychological score as a coarsened version of an underlying continuum representing constructs such as ‘memory ability’ or ‘language facility’. The neuropsychological test score can therefore be used to rank order subjects along the continuum with those reaching the floor or ceiling assigned to the highest or lowest categories, respectively. For example, subjects naming 10 animals in the category fluency test are treated as more impaired than those naming 20, but cannot be assumed to have half the language facility.
In our proportional odds logistic regression models, the response or dependent variable was the neuropsychological score, patient group was the predictor of primary interest, and age was an adjustment covariate. For each neuropsychological variable, we fitted two models. The first tested for differences between the control and bvFTD subtypes and the second tested for differences among the cluster-defined subtypes. Significance was assessed using a likelihood ratio test, comparing a model with age and group versus a model with age only.
Results
Cluster analysis
In our cluster analysis, each of the 66 bvFTD subjects started in a cluster on his/her own and was progressively clustered with others. The results of the cluster analysis are illustrated by the dendrogram in Fig. 1. The horizontal axis of the dendrogram is a measure of dissimilarity. A distinct grouping of all subjects can be obtained by drawing a vertical line at any point along the dendrogram. This vertical line can be thought of as cluster cut-points. At the penultimate stage of the clustering, i.e. just prior to all bvFTD subjects being merged into a single non-informative group, the two remaining clusters demarcated by the vertical blue line, divide the sample into two clusters of equal size. At one stage prior to this point, there are four clusters as demarcated by the red line. These clusters are quite different as indicated by the horizontal distance that must be traversed before the clusters are merged. If we were to move the vertical line further to the left then we end up with a two-patient subgroup. Because of the limited generalizability of a two-patient cluster, we drew our cluster cut-point at the red four-cluster mark and next report differences between these four potential bvFTD subtypings.
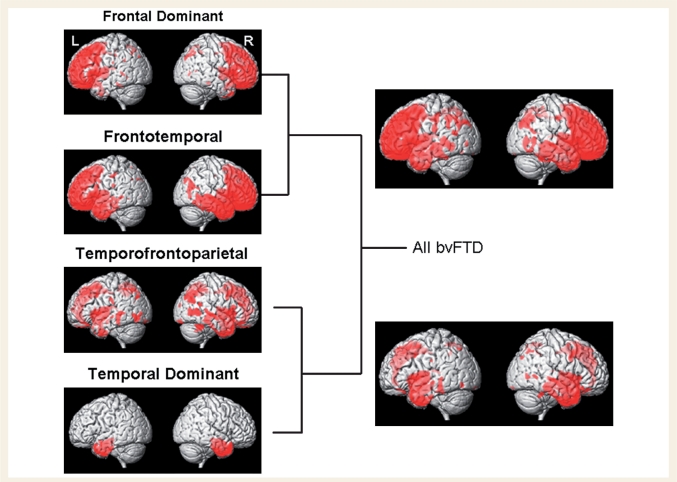
Figure 1.
The dendrogram created by the cluster analysis. Each of the 66 bvFTD subjects started in a cluster on his/her own and were then progressively clustered together. Individual patients are indicated by cluster and given an arbitrary number within cluster. The distance along the x-axis represents a measure of similarity between subjects, such that the closer the distance the greater the similarity between the subjects. We then applied voxel-based morphometry to the final two levels of clustering (represented by blue and red lines). TD = temporal dominant subtype, TFP = temporofrontoparietal subtype, FT = frontotemporal subtype, FD = frontal dominant subtype.
Voxel-based morphometry analysis
Comparisons to controls
We performed two levels of voxel-based morphometry analysis, assessing patterns of grey matter loss in subjects in the four subtypes of bvFTD relative to controls, as well as at the penultimate stage of the dendrogram when these four subtypes are collapsed into two groups (Fig. 1).
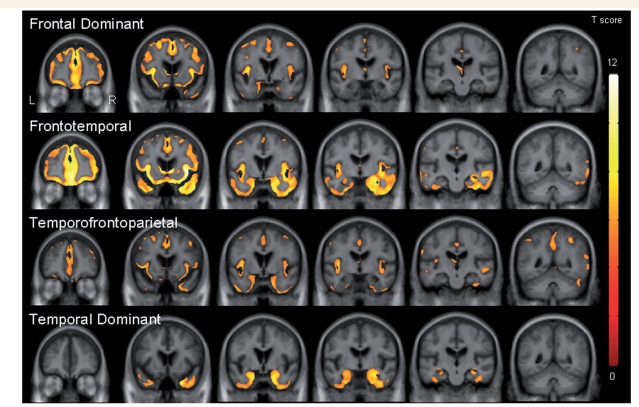
It is clear from Fig. 2 that the subjects in the bottom branch of the dendrogram were characterized by grey matter loss predominantly located in temporal lobes, with less severe involvement of frontal lobes, when compared to controls. This half of the dendrogram consisted of two bvFTD subtypes. The first of these subtypes showed grey matter loss only in temporal lobes, involving temporal pole, medial temporal lobe (hippocampus, amygdala and fusiform gyrus) and inferior temporal gyri, compared to controls (Figs 2 and 3). Grey matter loss was slightly more severe in the right hemisphere. We therefore refer to this subtype as ‘temporal dominant’. The second of the subtypes showed grey matter loss in anterior temporal lobes, medial frontal lobe, and medial and lateral parietal lobes, compared to controls (Figs 2 and 3). The temporal lobe involvement included temporal pole, anterior medial temporal lobe and inferior temporal lobes. There was also some involvement of insula, orbitofrontal cortex and dorsolateral frontal lobes. To reflect the fact that the temporal, frontal and parietal lobes are all similarly affected we will refer to this subtype as ‘temporofrontoparietal’.
Figure 2.
Three dimensional surface renderings showing patterns of grey matter loss in each of the four bvFTD subtypes that were identified from the cluster analysis compared to controls (corrected for multiple comparisons using family wise error at P < 0.05). The results are shown on a schematic version of the dendrogram in order to illustrate the relationship between patterns of atrophy and cluster position. Patterns of grey matter loss are also shown at the level of the dendrogram when the bvFTD subjects are divided into two groups.
Figure 3.
Coronal slices through the template showing patterns of grey matter loss in each of the four subtypes compared to controls (corrected for multiple comparisons using family-wise error at P < 0.05).
In contrast, the subjects in the top branch of the dendrogram were characterized by more severe loss in frontal lobes, with less severe involvement of temporal and parietal lobes, when compared to controls (Fig. 2). Once again, this side of the dendrogram consisted of two subtypes. The first of these subtypes showed grey matter loss throughout frontal lobes but also with involvement of temporal lobes, compared to controls (Figs 2 and 3). Frontal lobe grey matter loss was observed throughout orbitofrontal, dorsolateral and medial frontal lobes. The temporal lobe loss involved temporal pole, medial temporal lobe and inferior and middle temporal gyri, and was most severe in the right temporal lobe. Grey matter loss was also observed in insula, anterior cingulate, caudate nucleus and right putamen. There was also minor involvement of right parietal lobe. We will refer to this subtype as ‘frontotemporal’ to reflect the fact that they showed predominantly frontal and temporal loss. The second of these subtypes showed grey matter loss relatively isolated to frontal lobes, involving orbitofrontal, dorsolateral and medial frontal lobes, as well as anterior cingulate, insula and caudate nucleus (Figs 2 and 3). Only small scattered regions of loss were identified in temporal and parietal lobes. Therefore, this subtype will be referred to as ‘frontal dominant’. These patterns have been summarized in Table 3.
Table 3.
Relative schematic of the degree of atrophy, cognitive and behavioural deficits across the four subtypes
| Feature | bvFTD subtypes |
|||
|---|---|---|---|---|
| Temporal dominant | Temporofrontoparietal | Frontotemporal | Frontal dominant | |
| Temporal lobe atrophy | ↓↓↓ | ↓↓ | ↓↓ | – |
| Frontal lobe atrophy | – | ↓↓ | ↓↓↓ | ↓↓↓ |
| Parietal lobe atrophy | – | ↓↓ | ↓ | ↓ |
| Memory deficit | ↓↓↓ | ↓↓ | ↓↓ | ↓ |
| Language deficit | ↓↓↓ | ↓↓ | ↓↓ | ↓ |
| Executive function deficit | ↓ | ↓↓ | ↓↓↓ | ↓↓↓ |
| Visuospatial deficit | ↓ | ↓ | ↓ | ↓ |
| Behaviour abnormalities | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
Comparisons between subtypes
Figure 4 shows the results of direct comparisons between the four different bvFTD subtypes. The temporal-dominant subtype showed greater involvement of temporal lobe, particularly right temporal pole, medial and inferior temporal lobe, than the frontal-dominant subtype.
Figure 4.
Coronal slices through the template showing how patterns of grey matter loss differ between the four subtypes on direct comparison (corrected for multiple comparisons using false discovery rate at P < 0.005). Patterns of grey matter loss were assessed in each subtype compared to each other subtype. The TD subtype showed greater loss in the temporal lobes than the FD subtype, the TFP subtype showed greater loss in the parietal lobes than the TD subtype, the FT subtype showed greater loss in the frontal lobes than the TD and TFP subtypes, the FT subtype showed greater loss in the temporal lobe than the FD subtype and the FD subtype showed greater loss in the frontal lobes than the TD subtype. TD = temporal dominant subtype, TFP = temporofrontoparietal subtype, FT = frontotemporal subtype, FD = frontal dominant subtype.
The temporofrontoparietal subtype showed greater grey matter loss in parietal lobes compared to the temporal-dominant subtype.
The frontotemporal subtype showed greater involvement of frontal lobes, anterior insula and caudate nuclei than the temporal-dominant subtype, and greater involvement of the frontal lobes, anterior insula, caudate, right putamen and right anterior temporal lobe compared to the temporofrontoparietal subtype. The frontotemporal subtype also showed greater involvement of right temporal lobe, right anterior insula and caudate nuclei than the frontal-dominant subtype.
The frontal-dominant subtype showed greater involvement throughout frontal lobes and anterior insula, with some greater involvement of parietal lobe, than the temporal-dominant subtype.
Principle component analysis
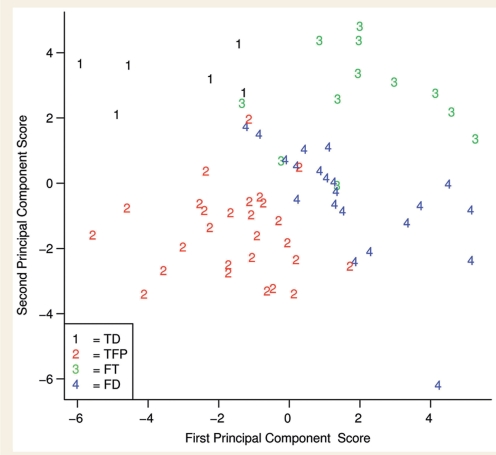
Figure 5 represents a scatter plot of the first two principal components of the 26 region of interest data set. Even in just two dimensions, rather than the original 26, the plot shows good separation between the four subtypes of bvFTD, further validating the separation of these four bvFTD subtypes. The scatter of different subjects along the axes is as expected and the scores conform to patterns seen in the voxel-based morphometry maps: the temporal-dominant subjects have negative first principal component scores and positive second principal component scores indicating large temporal lobe volume loss; the temporofrontoparietal subjects have typically negative first principal component scores and negative second principal component scores suggesting large temporoparietal volume loss with relatively less frontal loss than in the frontal-dominant and frontotemporal subtypes; the frontotemporal subjects have mostly positive first principal component scores and positive second principal component scores suggesting frontal and temporal volume loss; and the frontal-dominant subjects have positive first principal component scores and on an average close to zero second principal component scores reflecting the severe frontal volume loss but less temporal and parietal loss.
Figure 5.
Scatter-plot showing separation of the four bvFTD subtypes using the first two principal components in a principal components analysis. Based on the weights of the principal component analysis the first principal component (horizontal axis) captures the variability of the grey matter differences in the temporal and frontal lobes with positive projection of the first principal component indicating large frontal lobe atrophy and negative projection of the first principal component indicating large temporal lobe atrophy. The weights of the second principal component (vertical axis) capture the rest of the variability of the grey matter differences in the temporal lobe and mainly parietal lobes with positive projection indicating large temporal lobe atrophy and negative projection indicating large parietal lobe atrophy. TD = temporal dominant subtype, TFP = temporofrontoparietal subtype, FT = frontotemporal subtype, FD = frontal dominant subtype.
Subject demographics
Demographics for the subjects in each of the four bvFTD subtypes are shown in Table 1. There was a significant difference across subtypes in age at MRI (P = 0.004) and age at onset (P = 0.004), with youngest age observed in the temporal-dominant subtype. There was no difference across subtypes in years of education, time from onset to scan and gender ratio. A total of 48% of the cohort had either a pathologically or genetically confirmed diagnosis. Of the five subjects in the temporal-dominant subtype with a confirmed diagnosis, 100% had a mutation in the MAPT gene [two (c.1920 + 3G>A; IVS10 + 3G>A), two (c.1842T>G; p.Asn279Lys) and one (c.1920 + 16C>T; IVS10 + 16C>T)]. Lower proportions of subjects with mutations in MAPT were observed in the other subtypes [(c.1842T>G; p.Asn279Lys), (c.1920 + 16C>T; IVS10 + 16C>T), (c.1919G>A; p.Ser305Asn) and (c.1907C>T; p.Pro301Leu)]. Subjects with the pathological diagnosis of FTLD-TDP were found in the temporofrontoparietal, frontotemporal and frontal-dominant subtypes; with type 1 and 3 cases observed across all three subtypes and the only type 2 case observed in the temporofrontoparietal subtype. Pathological evidence of motor neuron degeneration was identified in three of the frontal-dominant subjects (two type 3 and one type 1), all of which also had clinical features of motor neuron disease. Only three of the FTLD-TDP subjects, all type 1, screened positive for PGRN. These three subjects were in the temporofrontoparietal, frontotemporal and frontal-dominant subtypes. Subjects with Pick's disease or progressive supranuclear palsy were found in the frontotemporal and frontal-dominant subtypes. All subjects with a pathological diagnosis of Alzheimer's disease were found in the temporofrontoparietal subtype and the frontal-dominant subtype.
Cognitive and behavioural testing
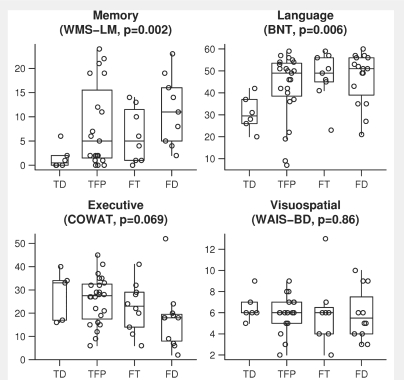
Cognitive results for the subjects in each of the four subtypes are shown in Table 2. As expected, the bvFTD group as a whole performed worse on all measures compared to the control group (each P < 0.001). No significant differences were observed across the four subtypes in tests of general cognitive function, although the frontal-dominant subtype tended to perform slightly poorer than the other subtypes for MMSE (P = 0.07). There was a significant difference across the four subtypes in performance on WMS-R Logical Memory delayed recall (P = 0.002) (Fig. 6), with subjects in the temporal-dominant subtype performing the worst and subjects in the frontal-dominant subtype performing the best. Similar trends were observed in WMS-R Visual reproduction delayed recall although they did not reach significance. There was also a significant difference across the four subtypes in the Boston Naming Test (P = 0.006) (Fig. 6), with worst performance in the temporal-dominant subtype and best performance in the frontal-dominant subtype. There was no significant difference observed with the category fluency test. There was a trend for the COWAT (P = 0.069) (Fig. 6), with poor performance observed in the frontal-dominant subtype and best performance in the temporal-dominant subtype. These same tendencies were observed with the Trail Making Test B. There were no significant differences across the four subtypes in tests of visuospatial functioning (Fig. 6). These results are illustrated in Table 3.
Figure 6.
Box-plots with individual data points superimposed showing neuropsychological test scores for the domains of memory (WMS-R logical memory delayed recall; WMS-LM), language (Boston Naming test; BNT), executive function (Controlled Oral Word Association Test; COWAT) and visuospatial function (WAIS block design; WAIS-BD) for each sub cluster. The y-axis represents raw scores for each test, except for the WAIS in which age-corrected scaled scores are shown. In all cases, a low score represents impaired performance. TD = temporal-dominant subtype, TFP = temporofrontoparietal subtype, FT = frontotemporal subtype, FD = frontal-dominant subtype.
The results of the NPI-Q are shown in Table 4. There was no significant difference observed across the subtypes on the total NPI-Q score or in the proportion of subjects with each type of behaviour. The most common behaviour in the temporofrontoparietal, frontotemporal and frontal-dominant subtypes was apathy. However, the most common behaviour in the temporal-dominant subtype was changes in appetite and eating behaviour.
Table 4.
Behavioural and psychiatric data from the NPI-Q at time of scan
| NPI feature | bvFTD | bvFTD subtypes |
P-value‡ | |||
|---|---|---|---|---|---|---|
| (n = 66) | Temporal dominant (n = 6) | Temporofronto parietal (n = 27) | Frontotemporal (n = 12) | Frontal dominant (n = 21) | ||
| Total NPI-Q severity score | 8 (4–12) | 8 (3–19) | 8 (3–11) | 12 (8–15) | 8 (4–12) | 0.22 |
| Agitation | 24 (45) | 3 (60) | 9 (43) | 7 (64) | 5 (31) | 0.35 |
| Anxiety | 23 (43) | 2 (40) | 8 (38) | 8 (73) | 5 (31) | 0.18 |
| Apathy | 43 (81) | 3 (60) | 16 (76) | 10 (91) | 14 (88) | 0.43 |
| Appetite and eating behaviours | 35 (66) | 4 (80) | 11 (52) | 8 (73) | 12 (75) | 0.46 |
| Night-time behaviour | 19 (37) | 2 (40) | 9 (45) | 5 (50) | 3 (19) | 0.31 |
| Delusions | 13 (25) | 2 (40) | 5 (24) | 4 (36) | 2 (13) | 0.39 |
| Depression | 26 (49) | 1 (20) | 12 (57) | 4 (36) | 9 (56) | 0.40 |
| Disinhibition | 29 (55) | 3 (60) | 11 (52) | 8 (73) | 7 (44) | 0.53 |
| Euphoria | 19 (36) | 0 | 8 (38) | 6 (55) | 5 (31) | 0.24 |
| Hallucinations | 4 (8) | 0 | 1 (5) | 2 (18) | 1 (6) | 0.62 |
| Irritability | 22 (42) | 3 (60) | 9 (43) | 6 (55) | 4 (25) | 0.36 |
| Aberrant motor behaviour | 22 (42) | 3 (60) | 6 (29) | 6 (55) | 7 (44) | 0.40 |
‡P-value for the comparison across the four bvFTD subtypes.
NPI-Q data were available for 53 bvFTD subjects. The total NPI-Q severity score is shown as median (inter-quartile range) and the data for each behaviour are shown as number of subjects (%). The control subjects showed no behaviours on the NPI-Q which were available in 25 subjects.
Discussion
This study demonstrates that bvFTD is not an anatomically homogeneous syndrome. Our cluster analysis approach showed that subjects with bvFTD can be divided into four anatomically different subtypes, two of which are defined by prominent frontal atrophy and two of which are defined by prominent temporal lobe atrophy. In fact, 50% of our cohort did not show a frontal-dominant pattern of loss, but instead showed a more temporal lobe predominant pattern, which is not traditionally associated with a diagnosis of bvFTD.
Of the frontal prominent subtypes, one showed grey matter loss relatively restricted to the frontal lobes and hence was named the ‘frontal-dominant’ subtype. The other showed severe grey matter loss in the frontal lobes but also with loss in the anterior temporal lobes, and hence was named the ‘frontotemporal’ subtype. The frontotemporal subtype showed significantly greater temporal lobe loss than the frontal-dominant subtype when they were compared directly. Similarly, of the temporal prominent subtypes, one showed grey matter loss restricted to the inferior and medial temporal lobes, particularly in the right temporal lobe, and was named the ‘temporal-dominant’ subtype. The other showed a more temporoparietal pattern of grey matter loss with additional involvement of the medial frontal lobes, and was hence named the ‘temporofrontoparietal’ subtype. In fact, the temporofrontoparietal subtype showed significantly greater grey matter loss in the parietal lobe than the temporal-dominant subtype. Both the frontotemporal and frontal-dominant subtypes showed greater grey matter loss in the frontal lobes than the temporal-dominant subtype, and conversely the temporal-dominant subtype showed greater temporal lobe atrophy than the frontal-dominant subtype. The four subtypes therefore differ in which regions show the most striking grey matter loss, thus validating the clustering algorithm and suggesting that these are different anatomical subtypes of bvFTD. We do not mean to conclude that these are the only regions involved in these subtypes, but rather that they are the most dominant. One could hypothesize that the dominant regions in each subtype may reflect the earliest sites of the disease, suggesting differing patterns of progression in each subtype.
Although not available for every subject, cognitive data collected on these subjects demonstrated differences between these four different subtypes which concur with the anatomical differences. The frontal dominant and frontotemporal subtypes tended to show poorer performance on the tests of executive function than the other subtypes, which supports the results of previous studies that have demonstrated a relationship between executive dysfunction and frontal lobe damage and dysfunction (Fine et al., 2008; Broome et al., 2009). Conversely, the temporal-dominant subtype performed significantly worse on tests of naming and memory than the other three subtypes. Previous studies have demonstrated correlations between atrophy of the temporal lobes, particularly the anterior temporal lobe, and confrontation naming (Mummery et al., 2000; Grossman et al., 2004; Williams et al., 2005), and there is a well-established relationship between atrophy of the medial temporal lobe, particularly the hippocampus, and memory performance (Trenerry et al., 1993; Petersen et al., 2000). The frontal-dominant subtype performed the best on tests of memory and naming reflecting the fact that the temporal lobes were relatively spared in this subtype. The temporofrontoparietal and frontotemporal subtypes performed similarly on the tests of memory and naming reflecting the fact that temporal lobe loss was a feature of both subtypes, although the frontotemporal group performed worse on tests of executive function. Performance on the tests of visuospatial function were impaired in all subtypes compared to the controls, but did not differ across subtypes. This runs counter to expectations given the relatively small amount of parietal lobe atrophy observed, particularly in the frontal, frontotemporal and temporal-dominant subtypes. It is possible however that while the Picture Completion and Block Design tests load most strongly on visuospatial function, they may also be affected by executive function resulting in poorer performance in our subtypes that show executive dysfunction.
The total NPI-Q score did not differ between the four subtypes. This is expected since all subjects in this study fulfilled criteria for bvFTD and therefore would have had behaviour or personality changes; providing validation of the clinical diagnosis in these subtypes. The proportion of subjects with each of the NPI-Q behaviours also did not differ across subtypes suggesting that the anatomical subtypes do not map neatly onto different behavioural subtypes (Snowden et al., 2001; Le Ber et al., 2006). The most common behaviour in all the subtypes that showed involvement of the frontal lobes was apathy, occurring in over 76% of subjects. Previous studies have similarly noted a high frequency of apathy in cohorts of bvFTD subjects (Levy et al., 1996; Perri et al., 2005; Rosen et al., 2005). A number of previous studies have also found correlations using both MRI and single photon emission computed tomography (SPECT) with apathy and change in the frontal lobes (Rosen et al., 2005; Le Ber et al., 2006; McMurtray et al., 2006; Peters et al., 2006; Zamboni et al., 2008; Massimo et al., 2009). Consistent with these findings was the fact that apathy was not the most common behaviour in the temporal-dominant subtype. Instead, the temporal-dominant group showed a high proportion of subjects with changes in appetite and eating behaviour. Studies have reported frequent changes in eating behaviour, such as food fads, in subjects with temporal lobe atrophy (Thompson et al., 2003), although others have shown that binge eating and the presence of a sweet tooth localize to the orbitofrontal cortex (Whitwell et al., 2007c; Woolley et al., 2007). Some previous studies have demonstrated associations between disinhibition and the temporal lobes (Liu et al., 2004; Le Ber et al., 2006; McMurtray et al., 2006; Zamboni et al., 2008). While disinhibition was relatively common in both the temporal dominant and frontotemporal subtypes in our study, we failed to observe any significant differences across subtypes suggesting that the anatomical correlate is unclear, at least based on the present study.
The pattern of atrophy identified in the temporal-dominant subtype could be considered unusual for subjects with a diagnosis of bvFTD. The striking anterior temporal lobe pattern could actually be considered more typical of a diagnosis of semantic dementia, in which subjects show early deficits in naming (Warrington, 1975; Mummery et al., 2000; Chan et al., 2001; Galton et al., 2001b; Rosen et al., 2002; Josephs et al., 2008d). The subjects in this bvFTD subtype did indeed perform poorly on tests of naming reflecting the involvement of the left anterior temporal lobes (Mummery et al., 2000; Grossman et al., 2004; Williams et al., 2005); however, all six patients in this subtype showed severely abnormal behaviours at onset hence fulfilling criteria for bvFTD, and not semantic dementia (Table 5). However, these subjects showed greater right than left temporal lobe loss. Abnormal behaviours, including social awkwardness, disinhibition, changes in eating behaviour and aggression, have previously been observed in FTD subjects with right > left temporal lobe atrophy (Miller et al., 1993; Edwards-Lee et al., 1997; Thompson et al., 2003; Chan et al., 2009). An important feature to note about this group is that all six of the temporal-dominant subjects had a positive family history with five genetically confirmed to have a mutation in MAPT. We have previously demonstrated that subjects with mutations in MAPT show severe grey matter loss in the anterior temporal lobe (Whitwell et al., 2009). This group of subjects was also younger than the other bvFTD subtypes as is typically found in MAPT mutation carriers (Pickering-Brown et al., 2008; Whitwell et al., 2009). These results therefore confirm that a right dominant temporal pattern of loss can occur in the context of bvFTD and not just in the context of semantic dementia. More detailed prospective behavioural and cognitive assessments may be able to determine whether these subjects can be identified on clinical measures alone.
Table 5.
Description of subjects in the temporal dominant group
| Gender | Age at onset | Clinical features at presentation | Family history | MAPT mutation | |
|---|---|---|---|---|---|
| 1 | F | 53 | Inappropriate behaviours, for example inappropriate touching and talking, apathy, anxiety with a tendency to worry, paranoia, forgetfulness and difficulty naming and recognizing familiar faces | Y | Unknown |
| 2 | F | 51 | Mental rigidity and inflexibility, such as difficulty in adjusting to changes in her environment, lack of initiative, difficulty recalling names of people and forgetfulness | Y | Y |
| 3 | M | 41 | Poor planning and organization, difficulty with directions, apathy, inappropriate comments, sweet cravings, increased appetite, weight gain and forgetfulness | Y | Y |
| 4 | F | 48 | Poor judgement, obsessive behaviours, tendency to pace around, tendency to purchase and cook the same foods, sweet cravings, tendency to eat more ‘junk foods’, anxiety and forgetfulness | Y | Y |
| 5 | M | 21 | Poor judgement, inappropriate behaviours, tendency to wonder, physical aggressiveness, obsessive behaviours, urinary incontinence, weight gain, and poor personal hygiene | Y | Y |
| 6 | F | 39 | Poor judgement, difficulty with problem solving and multi-tasking, voracious appetite and will eat foods quickly and with both hands, forgetfulness and difficulty recalling names of people | Y | Y |
The temporofrontoparietal subtype also showed an unusual pattern of loss for subjects with bvFTD, with involvement of the temporal and parietal lobes, posterior cingulate gyrus, as well as the medial frontal lobes. Three of the subjects in this subtype had pathological diagnoses of either Alzheimer's disease or corticobasal degeneration which are both associated with parietal lobe damage (Whitwell et al., 2007b; Josephs et al., 2008b). There have been previous reports of both Alzheimer's disease and corticobasal degeneration presenting with bvFTD syndrome (Johnson et al., 1999; Josephs et al., 2006a). Similarly, we have previously shown more parietal lobe atrophy in subjects with a clinical presentation of aphasia and Alzheimer's disease pathology, compared to subjects with aphasia without Alzheimer's disease pathology (Josephs et al., 2008c). A high proportion of subjects in the temporofrontoparietal subtype also had a pathological diagnosis of FTLD-TDP, which is typically associated with a temporal-dominant pattern of atrophy as well as frontal and parietal involvement (Whitwell et al., 2005a, 2006, 2007a). Parietal lobe atrophy is particularly severe in FTLD-TDP subjects with mutations in PGRN (Whitwell et al., 2007a). Mutations in PGRN were not associated with one particular subtype in this study suggesting heterogeneity in the dominant regions of loss, although we only had three PGRN positive subjects. It is notable however that PGRN was not associated with the temporal-dominant subtype confirming our previous suggestion that patterns of atrophy can be useful in distinguishing MAPT and PGRN mutations (Whitwell et al., 2009). A pattern of atrophy involving the temporal and parietal lobes, in the context of bvFTD, could therefore be suggestive of underlying Alzheimer's disease, corticobasal degeneration or even FTLD-TDP pathology.
The frontal dominant and frontotemporal subtypes show what is probably considered the most typical patterns of atrophy for subjects with bvFTD, with prominent involvement of the frontal lobes. In both subtypes grey matter loss was observed throughout the frontal lobe, including orbitofrontal cortex, medial and dorsolateral frontal regions. They were also both associated with volume loss in the caudate nuclei. While the pathological diagnoses found in these patients were varied, it is notable that motor neuron degeneration, Pick's disease and progressive supranuclear palsy were only found in these frontal subtypes. All three of these pathological diagnoses have been associated with frontal atrophy (Brenneis et al., 2004; Whitwell et al., 2005a; Boxer et al., 2006; Whitwell et al., 2006; Josephs et al., 2008b). In particular, FTLD-TDP with motor neuron degeneration has been found to have atrophy restricted to the frontal lobes (Whitwell et al., 2006) which concurs with the focal patterns of atrophy identified in the frontal-dominant subtype. While it is clear that these two subtypes are anatomically different, with one subtype associated with temporal atrophy and the other not, it is possible that the pattern of atrophy in the frontotemporal subtype may reflect a later stage of the frontal-dominant subtype, where the temporal lobe has become progressively involved over time. The subjects in the frontotemporal subtype have had the disease slightly longer than those in the frontal-dominant subtype (2.8 versus 2.0 years) although these differences were not significant. There was a trend however for worse performance on the MMSE in the frontal-dominant subtype which would argue against this subtype being an earlier version of the frontotemporal subtype. Longitudinal follow-up of subjects in the frontal-dominant subtype will be needed to properly investigate this issue. Similarly, one could suggest that the involvement of the parietal lobe in the temporofrontoparietal subtype represents a more advanced version of the others. However, the degree of frontal involvement in this subtype was less than that observed in the frontotemporal and frontal-dominant subtypes, and the degree of temporal involvement was less than that observed in the temporal-dominant subtype, suggesting that the temporofrontoparietal subtype is not simply a more progressed version of these other subtypes.
The study has some limitations that should be discussed. First, the number of subjects, while large for the entire cohort, was relatively small for each of the different subtypes. This may have influenced our power to detect cognitive and behavioural differences across groups. Second, while the clinical and neuropsychological data were collected prospectively from within the ADRC or ADPR, this study was retrospective in nature which was associated with difficulties such as missing behavioural and neuropsychological data. In addition, seven subjects were evaluated by a neurologist before the most recent clinical criteria were published (Neary et al., 1998) and so in these cases the recent clinical criteria had to be fitted retrospectively.
In summary, we have demonstrated the existence of four different anatomical subtypes of bvFTD: (i) the temporal-dominant subtype showing grey matter loss predominantly involving the temporal lobes; (ii) the temporofrontoparietal subtype showing involvement of the temporal, frontal and parietal lobes; (iii) the frontotemporal subtype showing frontal and temporal grey matter loss; and (iv) the frontal-dominant subtype showing grey matter loss predominantly involving the frontal lobes. These subtypes tend to differ on cognitive tests and importantly may prove to have different pathologic and genetic underpinnings. We hypothesize that the pattern of progression of atrophy over time will differ in each of these subtypes, although this will need to be assessed using longitudinal data. These findings are important as they confirm that bvFTD is not homogeneous. This heterogeneity could affect the outcome of clinical trials that use atrophy patterns as biomarkers of disease progression in bvFTD.
Funding
National Institutes of Health Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078; National Institute on Aging, Bethesda, MD (grants P50 AG16574, U01 AG06786 and R01 AG11378); Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation, USA; the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; National Institutes of Health Construction Grant (NIH C06 RR018898).
Acknowledgements
We would like to acknowledge Dr Mary Machulda for providing neuropsychological advice, Tina Schriever for neuropsychological data collection, and Dr Rosa Rademakers and Matthew Baker for performing genetic analyses.
Glossary
Abbreviations
- bvFTD
the behavioural variant of frontotemporal dementia
- FTLD-TDP
frontotemporal lobar degeneration with TAR DNA binding protein 43 immunoreactive inclusions
- MAPT
microtubule associated protein tau
- NPI-Q
the brief questionnaire form of the Neuropsychiatric Inventory
- PGRN
progranulin gene
- WAIS
Wechsler Adult Intelligence Scale
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol. 2006;63:1434–19. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Manual. Iowa City: University of Iowa; 1989. Multilingual Aphasia Examination. [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 2007;13:237–45. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:246–9. [PMC free article] [PubMed] [Google Scholar]
- Broome MR, Matthiasson P, Fusar-Poli P, Woolley JB, Johns LC, Tabraham P, et al. Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287–98. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease.[see comment] Ann Neurol. 2001;49:433–42. [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of Pick's disease. Neurology. 2001;56:S16–20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Fine EM, Delis DC, Dean D, Beckman V, Miller BL, Rosen HJ, et al. Left frontal lobe contributions to concept formation: a quantitative MRI study of performance on the Delis-Kaplan Executive Function System Sorting Test. J Clin Exp Neuropsychol. 2008:1–8. doi: 10.1080/13803390802419017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Kertesz A. Volumetric study of lobar atrophy in Pick complex and Alzheimer's disease. J Neurol Sci. 2000;174:111–21. doi: 10.1016/s0022-510x(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Gomez-Anson B, Antoun N, Scheltens P, Patterson K, Graves M, et al. Temporal lobe rating scale: application to Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatr. 2001a;70:165–73. doi: 10.1136/jnnp.70.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001b;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Harrell FEJ. Regression modeling strategies. New York: Springer-Verlag; 2001. [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–19. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology. 2001;56:S6–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hu WT, Mandrekar JN, Parisi JE, Knopman DS, Boeve BF, Petersen RC, et al. Clinical features of pathologic subtypes of behavioural—variant frontotemporal dementia. Arch Neurol. 2007a;64:1611–16. doi: 10.1001/archneur.64.11.1611. [DOI] [PubMed] [Google Scholar]
- Hu WT, Parisi JE, Knopman DS, Boeve BF, Dickson DW, Ahlskog JE, et al. Clinical features and survival of 3R and 4R tauopathies presenting as behavioural variant frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007b;21:S39–43. doi: 10.1097/WAD.0b013e31815bf5e5. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008a;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008b;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied multivariate statistical analysis. Upper Saddle River, NJ: Prentice Hall; 1982. [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol. 2008a;116:159–67. doi: 10.1007/s00401-008-0397-8. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006a;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008b;29:280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008c;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Jack CR, Parisi JE, Dickson DW. Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol. 2006b;63:1632–8. doi: 10.1001/archneur.63.11.1632. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Vemuri P, Senjem ML, Boeve BF, Knopman DS, et al. The anatomic correlate of prosopagnosia in semantic dementia. Neurology. 2008d;71:1628–33. doi: 10.1212/01.wnl.0000334756.18558.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. 2nd. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, et al. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–65. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioural distinctions. Arch Neurol. 1996;53:687–90. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioural disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–8. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo's older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- Lund-Manchester Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–18. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–93. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- McMurtray AM, Chen AK, Shapira JS, Chow TW, Mishkin F, Miller BL, et al. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66:517–22. doi: 10.1212/01.wnl.0000197983.39436.e7. [DOI] [PubMed] [Google Scholar]
- Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–13. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, et al. Alzheimer's disease and frontal variant of frontotemporal dementia—a very brief battery for cognitive and behavioural distinction. J Neurol. 2005;252:1238–44. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–9. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–7. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioural variant frontotemporal dementia. Arch Neurol. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140–3. doi: 10.1192/bjp.180.2.140. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioural -cognitive implications. Neurology. 2003;61:1196–203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- War Department. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. Army Individual Test Battery. [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. New York: Psychological Corporation; 1997. [Google Scholar]
- Whitwell JL, Jack CR, Jr, Baker M, Rademakers R, Adamson J, Boeve BF, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol. 2007a;64:371–6. doi: 10.1001/archneur.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Senjem ML, Baker M, Rademakers R, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009;72:813. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Senjem ML, Josephs KA. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology. 2006;66:102–4. doi: 10.1212/01.wnl.0000191395.69438.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005a;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007b;130:1777–86. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007c;35:207–13. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Watt HC, Harvey RJ, Rossor MN, Fox NC. A volumetric magnetic resonance imaging study of the amygdala in frontotemporal lobar degeneration and Alzheimer's disease. Dement Geriatr Cogn Disord. 2005b;20:238–44. doi: 10.1159/000087343. [DOI] [PubMed] [Google Scholar]
- Wicklund AH, Rademaker A, Johnson N, Weitner BB, Weintraub S. Rate of cognitive change measured by neuropsychologic test performance in 3 distinct dementia syndromes. Alzheimer Dis Assoc Disord. 2007;21:S70–8. doi: 10.1097/WAD.0b013e31815bf8a5. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71:736–42. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]