Abstract
The mammalian brain is a remarkably complex organ comprising millions of neurons, glia and various other cell types. Its impressive cytoarchitecture led to the long standing belief that it is a structurally static organ and thus very sensitive to injury. However, an area of striking structural flexibility has been recently described at the centre of the brain. It is the subependymal zone of the lateral wall of the lateral ventricles. The subependymal zone—like a beating heart—continuously sends new cells to different areas of the brain: neurons to the olfactory bulbs and glial cells to the cortex and the corpus callosum. Interestingly, the generation and flow of cells changes in response to signals from anatomically remote areas of the brain or even from the external environment of the organism, therefore indicating that subependymal neurogenesis—as a system—is integrated in the overall homeostatic function of the brain. In this review, it will be attempted to describe the fundamental structural and functional characteristics of the subependymal neurogenic niche and to summarize the available evidence regarding its plasticity. Special focus is given on issues such as whether adult neural stem cells are activated after neurodegeneration, whether defects in neurogenesis contribute to neuropathological conditions and whether monitoring changes in neurogenic activity can have a diagnostic value.
Keywords: adult neural stem cells, neurodegeneration, neurogenesis, subependymal zone, subventricular zone
Introduction
The mammalian brain is a remarkably complex organ comprising millions of neurons and glia; cells that communicate with each other via billions of cellular processes by forming various types of cell–cell interactions (from tight junctions to synapses). This impressive cytoarchitecture led to the long standing belief that the adult brain is a structurally static organ and thus very sensitive to any kind of injury. Over the last decades, it became apparent that this wealth of cellular interconnections is not as static as it was initially believed. For example, the synaptic organization of the adult brain is very plastic, constantly dependent on the electrical activity of neuronal networks and facilitated by the sprouting of new processes and the recession of existent ones. This structural plasticity does not alter the size and the gross anatomy of the brain but is very important in terms of its function.
Recently an area of striking structural flexibility has been discovered at the centre of the brain. It is the subependymal zone (SEZ) of the lateral wall of the lateral ventricles (frequently also referred to as the subventricular zone, in line with the embryonic proliferative areas). The SEZ—like a beating heart—continuously sends new cells to different areas of the brain: neurons to the olfactory bulbs and glial cells to the cortex and the corpus callosum. Interestingly, the generation and flow of cells changes in response to signals from the periphery of the brain or even from the external environment of the organism, therefore indicating that neurogenesis, as a system, is integrated in the overall homeostatic function of the brain.
This review will attempt to summarize the available evidence regarding the structural and functional flexibility of the SEZ. Which cell types of the niche respond to external signals and through which molecular mechanisms? What are the implications of the functional flexibility of the SEZ? To what degree might the plasticity of the SEZ cause pathologies in the brain, and could the detection of alterations in the function of the SEZ be used to diagnose neuropathologies of the brain? Finally, what are the open questions in this field of research?
It must be noted that the vast majority of the data available so far on this subject derive from studies performed on rodents; therefore this review will focus on the SEZ neurogenic system of the rodent adult brain, with the exception of a few references on the human SEZ or data from other model organisms, when this is deemed appropriate.
Neurogenesis in the adult brain
During embryonic development the central nervous system (CNS) is formed by the consecutive divisions of different populations of neuronal stem and progenitor cells that are located around the ventricles of the brain and the central canal of the spinal cord (Caviness et al., 2003). Post-natally the majority of the remaining neuronal precursors exit the cell cycle and terminally differentiate, mostly into astrocytes and oligodendrocytes, resulting in the gradual reduction of the peri-ventricular zones to the ependymal single-cell layer that lines the ventricles and the spinal canal. Notably, two specialized areas where neurogenesis persists into adulthood have been described: the SEZ of the lateral walls of the lateral ventricles and the subgranular zone of the dentate gyrus in the hippocampal formation. These are small and compact areas that, in rodents, are not separated from the differentiated brain tissue by any obvious anatomical barrier (in contrast, the human SEZ neurogenic niche is bordered by a myelin-rich zone) (Quinones-Hinojosa and Chaichana, 2007) and that continuously contribute new cells to the olfactory bulbs (Luskin, 1993; Lois and Alvarez-Buylla, 1994) and the corpus callosum (Menn et al., 2006) or to the granule cell layer of the dentate gyrus (Seri et al., 2001, 2004). The current hypothesis is that these adult neural stem cells (NSCs) are the direct progeny of embryonic NSCs (Alvarez-Buylla et al., 2001) and even though these two types of NSCs differ in their morphology, their position within the tissue and their function (embryonic NSCs build the CNS, while adult NSCs serve a more homeostatic function), they both retain similar properties (Ahlenius et al., 2009) and are regulated by similar signalling pathways (Kazanis et al., 2008).
The SEZ niche: cell types, architecture and the microenvironment
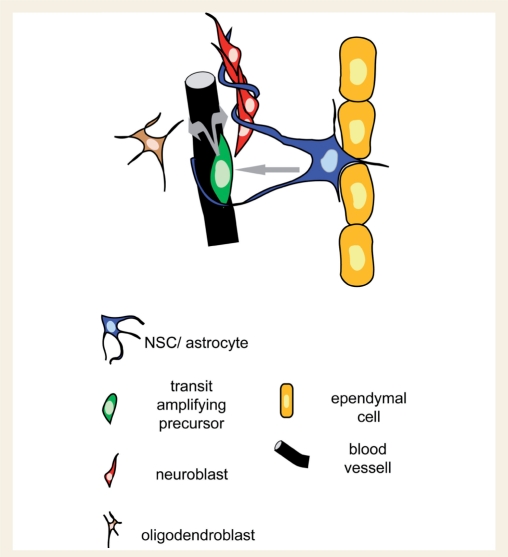
The SEZ is a thin area, beneath the ependymal cell layer, that contains three cell types of the NSC lineage: NSCs that generate transit amplifying precursors (TaPs) that after few self-renewing divisions produce more committed progenitors, either neuroblasts or oligodendrocyte precursors (OPCs) (Fig. 1) (Morshead et al., 1994; Doetsch et al., 1999).
Figure 1.
Graphic illustration of the basic cellular elements of the SEZ neurogenic niche. NSCs with astroglial morphology, located adjacent to ependymal cells, give rise to TaPs which are divided proximal to blood vessels in order to generate neuroblasts or OPCs (follow the grey arrows). Neuroblasts migrate out of the niche in chains, surrounded by astroglial processes.
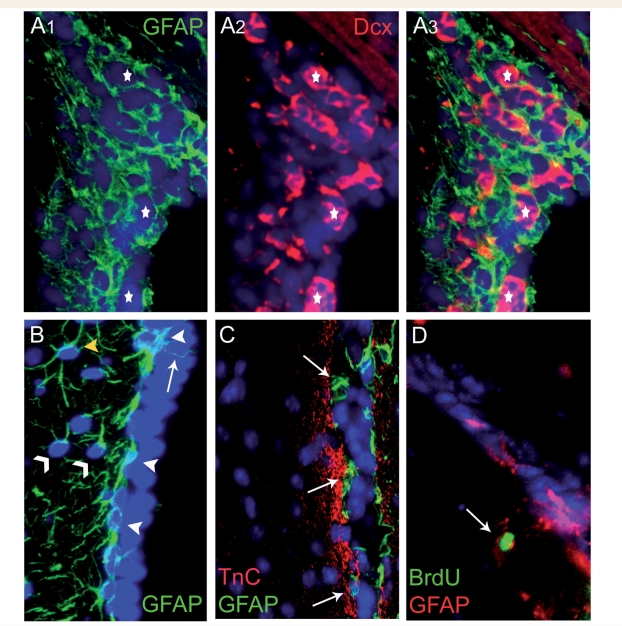
The cytoarchitecture of the SEZ is tightly controlled, with each one of the neuronal progenitor types occupying spatially distinct positions. NSCs have astroglial morphology, are located near ependymal cells and along with the non-neurogenic astrocytes of the SEZ are altogether known as type-B cells. Type-B cells can be separated in different sub-types depending on: (i) their exact position towards the ependymal cell layer (Shen et al., 2008); (ii) their morphology and their proliferative behaviour (astrocytes called ‘type-B1’ proliferate less than the smaller and more basally located ‘B2’ astrocytes) (Doetsch et al., 1997); or (iii) whether they are in contact with the ventricle, through a monociliated process emerging between the ependymal cells, exposing them to the content of the cerebrospinal fluid (CSF) (Alvarez-Buylla et al., 2001; Doetsch et al., 2002a) (Fig. 2). In addition, distinct pools of type-B astrocytes have been described, based on the expression of various markers (e.g. platelet-derived growth factor receptor α, LeX) (Capela and Temple, 2002; Jackson et al., 2006) and on their possible role: as NSCs, astrocytes located at the border of the SEZ creating an extracelluar matrix-rich area, or to form the tubes in which neuroblasts migrate (Doetsch et al., 1997; Kazanis et al., 2007) (Fig. 2). Regarding the neurogenic astrocytes, no definite marker to identify them has been reported so far. NSCs are relatively quiescent (Doetsch et al., 1999) therefore, the use of bromodeoxyuridine (BrdU)-retention has been employed as a method allowing the specific labelling of NSCs (Ramirez-Castillejo et al., 2006; Kazanis et al., 2007) (Fig. 2D), although the accuracy of this protocol has invited some criticism (Kiel et al., 2007).
Figure 2.
Different types of type-B cells. (A) Type-B astrocytes form the tubes through which neuroblasts migrate towards the olfactory bulbs. Stars indicate clusters of neuroblasts ensheathed by astrocytes. (B) Type-B astrocytes can be distinguished according to their position and morphology. Some type-B cells are located next to ependymal cells (white arrowheads) and occasionally extend a process towards the ventricle (arrow). Other type-B cells (with one or two processes) are located tangentially to the ependymal cell layer (thin arrowheads) and few of them reside further away from the ventricle and have a more typical astroglial morphology (yellow arrowhead). (C) Type-B astrocytes (arrows) are also found at the borders of the SEZ, in contact with the tenascin-C (TnC) rich extracellular matrix zone. (D) The majority of bromodeoxyuridine (BrdU)-retaining cells in the SEZ are type-B astrocytes (an example indicated by the arrow). BrdU retention is examined by administering BrdU cumulatively for a few days (3 days in the specific example shown) and then sacrificing the animals after a 40-day chase period. BrdU retention is considered as a sign of slow cell cycle, compatible with the behaviour of potential NSCs, because actively dividing cells dilute BrdU and become immuno-negative. (The lateral ventricle is at the right side of each image). GFAP = glial fibrillary acidic protein.
TaPs are the most mitotically active pool of progenitors in the SEZ. They tend to form small clusters and are situated nearer to blood vessels compared to all other progenitor types (Shen et al., 2008; Tavazoie et al., 2008). Distinct subpopulations of TaPs exist, defined by the differential expression of neurogenic or gliogenic transcription factors, indicating that lineage commitment is determined at this progenitor stage. TaPs expressing Pax6 are committed to neuronal fate while those expressing Olig2 to glial fate (Hack et al., 2005).
Neuroblasts constitute the most motile progenitor population, since they have been shown to migrate out of the SEZ via tubes formed by astroglial processes and located near blood vessels (Doetsch et al., 1997; Tavazoie et al., 2008). They express the polysialiated form of neural cell adhesion molecule (PSA-NCAM) and doublecortin and are cells mainly committed towards the neuronal fate (Hack et al., 2005) although experimental data indicate that they can be respecified towards oligodendroglial phenotype in areas of demyelination (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002).
Niche-derived oligodendrocyte precursors are the less studied precursor population of the SEZ. There is augmenting evidence indicating that OPCs are generated in the niche and then migrate within the corpus callosum (Menn et al., 2006; Magalon et al., 2007; Nait-Oumesmar et al., 2008; Gonzalez-Perez et al., 2009). However, their generation within the niche has not been definitely demonstrated and studied and is likely to be confounded by the existence of Olig2 and NG2 positive oligodendrocyte precursors, of parenchymal origin, in very close proximity to the SEZ (Komitova et al., 2009).
How specialized is the neurogenic microenvironment of the SEZ? The niche assumes its final structure during early post-natal life as a result of an active transformation process which has not been fully explored (Alvarez-Buylla et al., 2001; Alves et al., 2002; Merkle et al., 2004; Peretto et al., 2005) and the mechanisms that lead to the creation of the neurogenic niche in this specific anatomic location are still unknown. Regarding the origin of the neuronal progenitors contained within the SEZ, it has been established that they are not only derived from progenitors already situated at the respective area during embryonic life, but are also derived from precursors that have migrated there from distant (e.g. dorsal cortex) progenitor pools (Willaime-Morawek et al., 2006). This diversity in the origin of NSCs located in the SEZ may underline the recent observation that NSCs positioned in distinct parts of the niche generate distinct types of differentiated progeny (Merkle et al., 2007).
Recent research has shown that the ependymal cell layer is not morphologically similar all around the ventricles of the brain; within the neurogenic area ependymal cells and NSCs create specific pinwheel structures (Mirzadeh et al., 2008). In contrast, other characteristics of the niche microenvironment, like the protrusions of the blood vessel basal lamina towards ependymal cells (termed fractones) and the existence of a reduced barrier between the blood vessels and the extracellular space, although only observed in perivetricular areas, they are not restrained in the neurogenic part of the SEZ of the lateral ventricles (Mercier et al., 2002; Tavazoie et al., 2008).
The extracellular matrix composition of the niche is significantly different from that of the surrounding mature tissue. Apart from the laminin-β and collagen I-rich fractones that branch around NSCs and progenitors (Mercier et al., 2002; Kerever et al., 2007), other extracellular matrix molecules present in the SEZ are matrix metalloproteinases, brevican (Jaworski and Fager, 2000), tenascin-C (Jaworski and Fager, 2000; de Chevigny et al., 2006; Kazanis et al., 2007), chondroitin/dermatan sulphate proteoglycans (von Holst et al., 2006; Akita et al., 2008), as well as the trisaccharide LeX/SSEA-1/CD15 that is expressed on NSCs and TaPs and is shed in the microenvironment (Capela and Temple, 2002). These molecules are highly expressed during embryonic development and are subsequently downregulated in early post-natal life and replaced by proteoglycans like brevican, neurocan and versicans that form the mature brain parenchymal extracellular matrix (Bandtlow and Zimmermann, 2000; Novak and Kaye, 2000; Ruoslahti, 1996; Rauch, 1997).
Box 1 The structure of the subependymal zone: open questions.
Identification of definite markers for neural stem cells.
Are neuroblasts and oligodendrocyte precursors two distinct pools of progenitors?
Why does neurogenesis persist only in this specific periventricular area (the subependymal zone of the lateral wall of the lateral ventricles of the brain)?
Plasticity of the SEZ
Cell dynamics
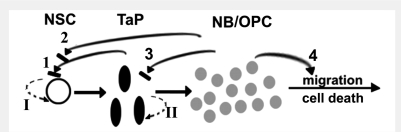
The SEZ is an open system, in which cells are continuously generated and then either die or flow away. The cell dynamics of the neurogenic niche are tightly buffered in such a way that although new cells are constantly generated, the numbers of TaPs and neuroblasts within the niche remain stable at any developmental stage. However, a significant decline in the overall neurogenic activity of the ageing niche has been demonstrated (Enwere et al., 2004; Luo et al., 2006; Tang et al., 2009), as a result of decreased numbers of all types of progenitors (NSCs, TaPs and neuroblasts) (Ahlenius et al., 2009). The hierarchy of the neuronal lineage within the SEZ was determined from analysis of the regeneration of the niche, after depletion of actively dividing cells (TaPs and neuroblasts) achieved by infusion of the anti-mitotic drug cytosine arabinoside (AraC) (Doetsch et al., 1999), or by cell-specific irradiation (Morshead et al., 1994). NSCs remain relatively quiescent and divide occasionally to generate TaPs that, after few rounds of cell cycles, produce neuroblasts and OPCs, the cells that are moving away from the system (Figs 1 and 3). The number of TaPs and neuroblasts is controlled in such a way that they do not exceed normal levels even during regeneration of the depleted niche, indicating the existence of multiple intrinsic and extrinsic signals regulating cell dynamics. Many growth factors and morphogens (e.g. brain-derived neurotrophic factor, fibroblast growth factor, vascular endothelial growth factor, nitric oxide as well as different components of the Wnt signalling pathways) have been shown to regulate the numbers of proliferating cells within the SEZ (Chen et al., 2005a, b, 2007; Adachi et al., 2007; Young et al., 2007). However, in many of the studies, these factors are supplied in excess (i.e. in non-physiological ranges) and it has been difficult to dissect the specific progenitor population(s) that they affect.
Figure 3.
Extrinsic and intrinsic mechanisms regulating the numbers of NSCs/progenitor cells in the SEZ. NSCs remain relatively quiescent; their random (oscillation-like) activation is indicative of the operation of intrinsic clock mechanisms (I). Their massive activation after the depletion of their progeny suggests the existence of progenitor-dependent feedback, extrinsic inhibition (1, 2). TaPs are mitotically active cells; their numbers are controlled via intrinsic mechanisms (II), as indicated by the observation that they start generating neuroblasts after certain numbers of divisions during regeneration of the niche, but they might be also receiving feedback inhibition similarly to NSC (3). Neuroblasts or OPC numbers in the niche are regulated by their controlled outward migration and cell death (4). NB = neuroblast.
Notably, investigation of the SEZ in many different experimental animal models of neuropathology as well as in conditions of environmental enrichment and of exercise has revealed that the neurogenic niche retains a significant level of plasticity, something that is also observed in tissue from patients with neurological diseases. Plasticity in this case is defined as any alteration in the numbers or the phenotype of the cells that reside in the niche and as any alteration in the structure of the niche microenvironment. Degenerative lesions in the brain result in increases in the size of the SEZ which is able to respond to different types of degeneration by producing the appropriate committed progenitors (neuroblasts in neurodegeneration, OPCs after demyelination) (Nait-Oumesmar et al., 1999). In the majority of published studies the number of proliferating cells in the SEZ has been measured, rather than the total number of cells or the size in terms of volume. In many reports, changes in the numbers of dividing cells have been interpreted as changes in the size of the SEZ, however these conclusions need to be treated with caution because of the absence of direct measurements of the size of the SEZ, partially due to the lack of anatomical or molecular markers to define the niche, apart from the expression of Tenascin C at its borders with the laterally located striatum (Fig. 2) (Kazanis et al., 2007). Increased proliferative activity of the SEZ has been reported in experimental models of traumatic brain injury (Ramaswamy et al., 2005) and of ischaemia in rodents (Zhang et al., 2004, 2008; Komitova et al., 2005b), as well as in human cases of stroke (Curtis et al., 2007). Enhanced proliferation in the SEZ has also been observed in patients suffering from epileptic seizures (Grote and Hannan, 2007) and multiple sclerosis (Nait-Oumesmar et al., 2007), although findings from animal experimental models showing recruitment of SEZ-generated oligodendrocytes in demyelination lesions (Picard-Riera et al., 2002; Nait-Oumesmar et al., 2007) have not yet been confirmed in human tissue. Neurogenesis is increased in human cases and animal models of Huntington's disease while it is decreased in patients and animal models of Alzheimer's and Parkinson's disease (Hoglinger et al., 2004; Elder et al., 2006; Curtis et al., 2007). The normal SEZ neurogenic activity is not altered by exercise or environmental enrichment, but these external conditions influence its activity after ischaemia (Komitova et al., 2005a, b).
How is the plasticity of the SEZ regulated and how is it translated at the level of specific cell types? Any exogenous induction of symmetric self-renewing divisions of a progenitor pool, over the asymmetric divisions that generate more committed progeny, results in decreased numbers of downstream populations (Doetsch et al., 2002a; Bauer and Patterson, 2006; Jackson et al., 2006). However, effects in the opposite direction (e.g. any effects of an increase in the size of progenitor populations to upstream cell-populations), that would indicate the existence of feedback signalling, have not been properly studied. Bulbectomy i.e. ablation of the area where neuroblasts are destined to migrate to, results in slower migration and accumulation of neuroblasts in the SEZ, and leads to significant decrease in overall cell proliferation in the niche. This has, however, not been extensively investigated and might be confounded by other non-specific effects (Kirschenbaum et al., 1999; Keilhoff et al., 2006). An interesting finding regarding the control of SEZ neurogenesis has emerged from animal models of Huntington's disease. Increased neurogenesis was observed only in animal models of the disease based on the use of cytotoxic substances, inducing inflammatory reactions, while no change was observed in transgenic models, in which cell loss is minimal. Therefore, it emerges that the presence of inflammatory signals in the SEZ might be an important factor in the regulation of neurogenesis (Phillips et al., 2005) a hypothesis also supported by in vitro and in vivo data showing that activation of microglia can induce neurogenesis (Walton et al., 2006; Thored et al., 2009). Nevertheless, the possible role of inflammation in adult neurogenesis remains controversial, since recent experimental work in an animal model of demyelination indicated impaired proliferation caused by the inflammatory microenvironment (Pluchino et al., 2008), while examination of tissue from patients suffering from multiple sclerosis suggested sustained activation of the SEZ (Nait-Oumesmar et al., 2007).
Whether the post-injury increased production of cells in the SEZ and their subsequent migration towards sites of degeneration is eventually followed by functional integration in the tissue is an issue of paramount importance in the field of regenerative medicine, however only limited experimental work has addressed it. Morphological studies have revealed that SEZ-derived oligodendrocytes form mature myelin sheaths around axons (Menn et al., 2006) and SEZ-derived new neurons survive and maturate within areas of stroke (Arvidsson et al., 2002; Yamashita et al., 2006), but more direct functional studies are still lacking.
Box 2 Cell dynamics in the subependymal zone: open questions.
Identification of the existence of feedback signalling in the regulation of distinct neuronal progenitor pools.
The role of inflammation in the activity of the subependymal zone.
Plasticity of the NSC population
Within the normal SEZ, NSCs divide very slowly as evidenced by the fact that those that are dividing during a period of administration of BrdU retain the labelling for longer times and by the observation that long infusions of AraC do not deplete the NSC pool (Doetsch et al., 1999). When and how are NSCs becoming mitotically active? The mechanism that controls the random activation of NSCs in vivo remains unexplored and it could depend on intrinsic clock-like regulators (or oscillators) or on external cues from the local microenvironment. Recently it has been shown that clock genes are expressed in the SEZ (Kimiwada et al., 2009) and that neurogenic transcription factors, such as Mash1 and Neurogenin 1, control the expression of clock genes (Zhang et al., 2007). The question of whether neurodegeneration can stimulate the proliferation of NSCs situated in the SEZ also remains open. Indirect evidence has indicated that NSCs are activated after ischaemic injury in mice (Zhang et al., 2004) and data from human cases of multiple sclerosis suggest that chronic demyelination results in increased numbers of cells with NSC characteristics (Nait-Oumesmar et al., 2007). Moreover, in the hippocampal neurogenic niche, neurogenesis after traumatic brain injury is driven by the activation of quiescent neuronal progenitors (Yu et al., 2008). However, Doetsch and colleagues (2002a) clearly showed that the numbers of TaPs can quickly be doubled without any increased division of NSCs. This issue could be of significance in regenerative medicine because it is necessary to assess if the neurogenic response of endogenous niches can be increased after neurodegeneration. Notably, there is one experimental condition in which NSCs are massively induced to divide and this is the infusion of the anti-mitotic AraC that results in the ablation of actively dividing neuronal progenitors of the niche (Doetsch et al., 1999). The elimination of TaPs and neuroblasts transiently activates NSCs, until at least the pool of their immediate progeny (the TaPs) is reconstituted, indicating the operation of a progenitor-dependent NSC inactivation signal (Fig. 3) (Kazanis et al., 2007). However, elimination of NSC progeny is not sufficient to drive the mitotic activation of NSCs on its own, because under prolonged infusions of AraC (continued even after TaPs and neuroblasts are totally ablated), NSCs remain quiescent and start dividing only after the end of the infusion of the drug (Doetsch et al., 1999 and unpublished data). This observation suggests that additional controlling mechanisms (e.g. feed-forward signals) must regulate the activation of NSCs.
Few molecules have experimentally been shown to specifically affect the behaviour of NSCs. Leukaemia inhibitory factor (Bauer and Patterson, 2006) is a mitogen shown to induce the expansion of the NSC population by promoting symmetric self-renewing divisions against lineage progression. Pigment epithelium-derived factor (PEDF) and platelet-derived growth factor both induce asymmetric divisions of NSCs, leading to increased numbers of downstream progenitor populations, although favouring different lineage commitments (PEDF neuronal and PDGF oligodendroglial) (Jackson et al., 2006; Ramirez-Castillejo et al., 2006). Short infusions of epidermal growth factor result in increased numbers of type-B cells contacting the CSF via a monociliated process, an observation interpreted as a sign of activation of NSCs (Doetsch et al., 2002a). Finally, although interfering with bone morphogenetic protein signalling via the deletion of Smad4 in NSCs does not affect their proliferation, it does lead to the generation of more oligodendrocyte committed progenitors (Colak et al., 2008).
Stem cell behaviour is generally thought to be dependent on the contact with adjacent supporting cells. This derives from extensive experimental work in the Drosophila melanogaster (fruit fly) germ cell niches (Chen and McKearin, 2005; Fuller and Spradling, 2007) in which germ cells retain their function as long as they remain anchored to the adjacent hub cells. Recent experimental work has also indicated that in some cases the stem cell-extracellular matrix interaction can be equally important, as in the case of follicle stem cells in the Drosophila ovary, where laminin/integrin interactions on the membrane of stem cells regulate their self-renewal (O’Reilly et al., 2008). At present there is no direct evidence to suggest the unique importance of any single interaction between NSCs and any cellular or parenchymal component of the SEZ. The physical positioning of NSCs adjacent to ependymal cells implies an interaction similar to the hub cell/stem cell interaction in the Drosophila germ cell niches. This is supported by the finding that ependymal cells are a source of NSC-activating factors, like PEDF (Ramirez-Castillejo et al., 2006) and the pro-neurogenic bone morphogenetic protein signalling modulator noggin (Lim et al., 2000; Peretto et al., 2004). In vitro and in vivo data indicate that the interaction between NSCs and blood vessel endothelial cells might be important in neurogenesis (Shen et al., 2004) and blood vessel endothelial cells are a source of PEDF and leukemia inhibitory factor (Riquelme et al., 2008). Finally, it is important to highlight the potentially important role of the NSC monociliated process that is contacting the CSF (Alvarez-Buylla et al., 2001; Mirzadeh et al., 2008). Primary cilia have been shown to affect neurogenesis in the subgranular zone (Han et al., 2008) and are involved in Shh signalling (Rohatgi et al., 2007), a molecule to which adult NSCs respond (Palma et al., 2005). Nevertheless, no evidence currently supports any link between pertrubations on the above-described pathways and disease-induced plasticity of the SEZ.
Box 3 Plasticity of the neural stem cell pool: open questions.
At what developmental stage do neural stem cells become quiescent?
Is the occasional activation of neural stem cells dependent on intrinsic or extrinsic regulation?
Do neural stem cells of the subependymal zone become activated after neurodegeneration?
How are neural stem cells prevented from becoming mitotically active in toxic conditions (e.g. in the presence of anti-mitotics)?
Is lineage commitment determined at the level of the neural stem cell?
What is the contribution of cell–cell and cell–extracellular matrix interactions in the control of the quiescence of neural stem cells?
Plasticity of the TaP population
During the post-AraC regeneration of the niche, TaPs are generated from neural stem cells and their pool reaches normal levels quite quickly. Neuroblasts start being produced only after TaP numbers approximate the 40% of the normal niche (Kazanis et al., 2007), indicating the operation of an intrinsic mechanism that controls the number of self-renewing divisions and the time of differentiation to neuroblasts. The cell-cycle inhibitor p27Kip1 is one gene that has been directly shown to control the size of the TaP pool (Doetsch et al., 2002b) and therefore could be involved in this process.
Because TaPs are the most mitotically active cells in the niche, even a moderate increase in the number of self-renewing divisions can result in a quick and significant expansion of their pool and therefore lead to higher numbers of neuroblasts and OPCs; however, their contribution to disease or activity dependent plasticity of the SEZ has not been directly addressed. TaPs are responsive to growth factors and morphogens such as Shh (Rohatgi et al., 2007). Although not in direct contact with the CSF (Tavazoie et al., 2008) intracerebroventricular infusion of epidermal growth factor (Doetsch et al., 2002a; Komitova et al., 2005b) results in significantly increased self-renewing divisions of TaPs, in limited generation of neuroblasts and increased production of oligodendrocytes (Gonzalez-Perez et al., 2009). TaPs are situated next to blood vessels which they contact in areas devoid of pericytes and astrocytic endfeet (Tavazoie et al., 2008), and therefore might be exposed to peripheral signals. Two TaP sub-populations have been identified in the SEZ: those expressing the pro-neurogenic transcription factor Pax6 and those expressing the oligodendroglial commitment factor Olig2 (Hack et al., 2005). However, potential alterations in the size of distinct TaP subpopulations (neuronal or glial committed) in any model of neurodegeneration have not been examined so far.
Plasticity of the neuroblast and OPC population
Neuroblasts seem to be the least plastic pool of progenitors since they do not exhibit high self-renewing capacity (Doetsch et al., 1999; Nait-Oumesmar et al., 1999; Komitova et al., 2005b) and recent experimental work has revealed that it is very difficult to induce their conversion into glial progenitors after they have migrated out of the niche (Hack et al., 2005). Therefore, it seems that they carry lineage decisions that have been determined at the NSC or TaP level, a sensible hypothesis since neuroblasts/OPCs are exposed to divert environmental signals during their long migration process. However, some experimental results have indicated that neuroblasts recruited to lesions of demyelination can downregulate markers of neuronal commitment and be respecified towards a glial fate, although direct evidence is still lacking (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002). Regulation of the numbers of neuroblasts is probably achieved via cell death and migration (Morshead and van der Kooy, 1992) (Fig. 3). In any case, alterations in the size of the neuroblast and OPC pools can be the result of changes in any upstream progenitor pool and therefore the plasticity potential of neuroblasts/OPCs cannot be investigated without the parallel investigation of the other progenitor populations.
The niche microenvironment as a regulator of neurogenesis
Although the plasticity of the SEZ, in terms of numbers of proliferating progenitors, has been vigorously investigated in different conditions, very little is known regarding the structural plasticity of the niche microenvironment. In general, stem cell niches are considered to be stable milieus that retain their supportive properties even after the depletion of stem cells and their progeny (Kai and Spradling, 2003). In the case of the SEZ it is extremely difficult to study a neuronal progenitor void niche because surgical removal of NSCs and progenitors is not feasible (in contrast to the mammary gland for example) and because the quiescence of NSCs does not enable their total depletion by pharmacological means or irradiation.
Only limited data exist about the extracellular matrix profile or other structural characteristics of the SEZ (i.e. the blood vessel network), in different models of disease and activity or after growth factor-induced increase in proliferation. The density of the vessel network has been reported to be transiently decreased after ischaemic injury (Thored et al., 2007), but angiogenesis and blood vessel permeability were found to increase after cortical injury (Gotts and Chesselet, 2005a, b). A comprehensive RNA microarray study revealed upregulation of numerous extracellular matrix components and growth factors after stroke, but didn’t include any anatomical investigation of the changes (Liu et al., 2007). Conclusively, the structural plasticity of the SEZ prior or in parallel to an increase or decrease in the number of progenitors has not yet been assessed.
Neurogenesis in the SEZ is regulated by stimuli originating in remote areas
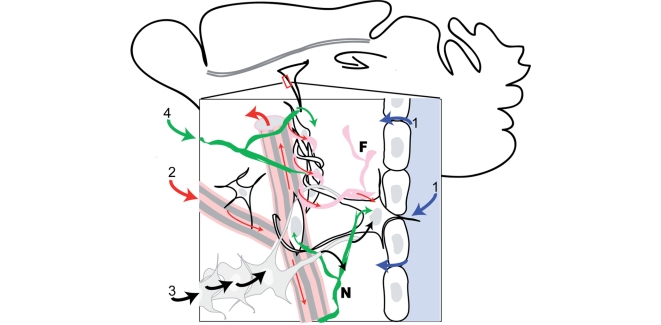
How do events occurring in anatomically remote areas of the brain induce responses of the SEZ? (Fig. 4). Cues might be provided by the contact of the niche with the CSF that allows cells of the SEZ to sense information on the state of the organism reflected in the concentration of hormones, growth factors and metabolites (Maurer, 2008). A second pathway could be provided by the extensive meninges-vessels-fractones basal lamina continuum that connects the surface of the brain with the centrally located SEZ (Mercier et al., 2002). In addition to these pathways, the niche extracellular space shows a high degree of communication with the blood vessel content (Tavazoie et al., 2008) which has been shown to be enhanced after cortical injury (Gotts and Chesselet, 2005b). The structure of the blood vessel network of the niche changes after ischaemic brain injury (Thored et al., 2007), a rearrangement that might trigger responses from neuronal progenitors on its own. Another pathway through which information might be conveyed from the periphery to the SEZ, is the astrocytic network formed by the gap junctions that couple neighbouring astroytes (Giaume and Venance, 1998; Houades et al., 2006). Finally, the activity of the SEZ is regulated by neurotransmitters and, therefore, can be directly influenced by the activity of neuronal networks. For example, the decreased proliferation observed in Parkinson's disease might be due to the loss of dopaminergic innervation in the SEZ (Curtis et al., 2007; Grote and Hannan, 2007). In addition, serotoninergic innervation is also observed in the SEZ area (Diaz et al., 2009) and serotonin has been documented to increase neurogenesis in the SEZ, especially by targeting proliferation of neuroblasts (Banasr et al., 2004; Encinas et al., 2006; Qiu et al., 2007). Serotonin-dependent modulation of neurogenesis in the subgranular zone of the hippocampus (e.g. by the use of selective serotonin reuptake inhibitors) has significant potential anti-depressive effects (Santarelli et al., 2003); however, the effects of serotonin in neurogenesis in the SEZ and the subgranular zone seem to be mediated by different receptors (Banasr et al., 2004). A few studies have provided evidence for regulatory activity of γ-amino-butyric acid (GABA) in the SEZ. GABA is the principle inhibitory neurotransmitter in the adult CNS but has an excitatory effect (as during development) in the SEZ (Wang et al., 2003; Ge et al., 2007). Isolated rat neuroblasts express the GABA-A receptor and GABA has been found to decrease neuroblast migration (Bolteus and Bordey, 2004). Therefore, it has been suggested that it could participate in a feedback loop between NSCs and neuroblasts, controlling the number of neuroblasts produced at a given time (Liu et al., 2005).
Figure 4.
Pathways of signal integration in the SEZ. The neurogenic niche of the SEZ is located at the centre of the brain and receives information via several distinct pathways: (1) Molecules contained in the cerebrospinal fluid can enter the niche microenvironment via ependymal cell controlled transport, diffusion or uptake from the astrocytic processes (blue arrows). (2) Information can be transferred via the content of the blood vessel network (big red arrows) or the fractones (F)-vessel-meninges basal lamina continuum (thin red arrows). (3) Signals can be transported through the syncytia formed by astrocytes (black arrows). (4) Regulation can also be achieved via neuronal activity, mediated by the release of neurotransmitters from neurites (N) located in the SEZ (green arrows).
Plasticity of the SEZ as a cause of brain pathologies
Brain malformations that develop during embryonic life (such as microcephaly) are directly attributed to aberrant neurogenesis, since the size of the brain is dependent on the divisions of embryonic neuronal progenitors. In contrast, it is very difficult to directly assess whether defects in adult neurogenesis can also cause pathologies, mostly because samples from patients are taken only after a disease has been diagnosed, thus making it difficult to distinguish cause from effect. In addition, it has proved difficult to experimentally address the role of specific molecules in adult neurogenesis (using transgenics technology) because most of the regulatory mechanisms are common in the embryo and the adult (Kazanis et al., 2008) therefore resulting in perturbations of early brain development. Experiments where neurogenesis is conditionally disturbed specifically in the post-natal brain are lacking and exogenously induced perturbations (irradiation, growth factor injections) have been studied only for short-term effects and not in a time-frame suitable for the development of diseases. Therefore, the hypothesis that impaired adult neurogenesis may lead to brain pathology is based predominantly on the indications of correlation between the decline in neurogenesis in the ageing brain (Luo et al., 2006; Tang et al., 2009) and the development of dementia. The study of neurogenesis in the other adult niche, the subgranular zone, has revealed that the abolishment of hippocampal neurogenesis leads to impaired formation of trace memories (Shors et al., 2001) and computational approaches suggest that incorporation of new neurons to hippocampal circuits might contribute to memory formation (Aimone et al., 2009), thus supporting the hypothesis of aberrant neurogenesis as a cause of neuropathology. However, these data remain highly correlative and need to be investigated further (Bruel-Jungerman et al., 2007).
Box 4 Neurogenesis in the subependymal zone and neuropathology: open questions.
Does adult neurogenesis contribute significantly to the normal function of the brain and to regeneration after injury?
Would the permanent depletion of adult neurogenesis lead to neuropathologies and would it affect repair after degeneration?
Would abnormally increased neurogenesis lead to neuropathologies?
Studying the plasticity of the SEZ: implications to treatment
Modelling the neurogenic microenvironment
As discussed earlier, the spatio-temporal characteristics of SEZ plasticity have not been extensively examined until now. Data from experimental studies indicate that the disease-induced activation of the SEZ is transient (Nait-Oumesmar et al., 1999; Thored et al., 2007). However, observations from human material taken from patients with long-term neurodegenerative diseases suggest that the size of the SEZ can change over extensive periods (Curtis et al., 2007). It remains unclear, however, whether these changes in the size of the niche are caused by long-term alterations in the size of the NSC pool or by the continuously altered activity of downstream progenitor pools, or by both. To answer this question a detailed analysis of the cell cycle characteristics and of the ratios of distinct progenitor populations in the SEZ has to be undertaken in the diseased brain. Most relevant to the issue of the temporal characteristics of the plasticity of the SEZ and maybe more important in terms of regenerative medicine, is the issue of the spatial and structural parameters of this plasticity. Is any change in the number of progenitors followed by a similar change in the size of the niche, or in other words, do progenitors survive and function only within very specific microenviroments? It will be of great importance to identify the structural parameters that change prior and in parallel to changes in the numbers and the activity of neuronal progenitors. Recently, it was revealed that NSCs are not evenly distributed along the lateral walls of the lateral ventricles but that there are hot-spots of increased abundance of stem cells (Mirzadeh et al., 2008), implying that the neurogenic area might have the potential to host augmented numbers of NSCs (in the less densely populated spots). Further to this observation, a detailed investigation of the interactions between neuronal progenitors and blood vessels showed that dividing cells contact the vessels in specific spots, characterized by the absence of pericytes and asrocytic endfeet (Tavazoie et al., 2008). What happens when the blood vessel network needs to support the proliferation of increased numbers of progenitors? Existing data suggest that the niche blood vessel network is more static than in other areas (Shen et al., 2008) although stroke leads to a transient reduction of vessel density (Thored et al., 2007) and injury promotes angiogenesis in the SEZ (Gotts and Chesselet, 2005b). Evidence from a recent microarray study showed increased expression of growth factors, morphogens and extracellular matrix molecules after stroke (Liu et al., 2007), but it is not known whether this was due only to an increase in the expression of these factors or whether it also involved a parallel spread of the area of expression. The elucidation of the temporal and structural parameters of the plasticty of the SEZ will allow the extraction of important information on the ability to recapitulate the NSC microenvironment in vitro and to construct ex vivo neurogenic niches. It will also allow the assessment of the expected efficiency of neuronal progenitor transplantations in areas of lesions, i.e. in microenvironments distinctly different from that of the neurogenic niche.
Box 5 Modelling the subependymal zone microenironment: open questions.
What is the capacity of the subependymal zone to host expanded numbers of neural stem and progenitor cells?
What changes of the microenvironment (extracellular matrix composition, blood vessel network) accompany any alteration in the numbers of neural stem and progenitor cells?
Do neuronal progenitors create their microenvironment or are they dependent on heterologous, supporting cells?
SEZ: a beating heart that senses pathologies
Although the only known contribution of the SEZ neurogensesis is the provision of neurons to the olfactory bulbs and perhaps oligodendrocytes to the corpus callosum, the above described evidence clearly demonstrates that it is significantly altered almost in all cases of neuropathology (as a side effect or to enhance regeneration or for unknown specific reasons). Recently a magnetic resonance spectroscopy (MRS) method allowing the imaging of neuronal progenitors in the live human brain was developed (Manganas et al., 2007). It is therefore possible that in the near future the size of the SEZ (reflecting its activation/deactivation status) could be used as an additional diagnostic and prognostic criterion, especially since the evidence thus far indicates that SEZ neurogenesis is not altered by life-style changes (exercise or environmental enrichment) (Komitova et al., 2005b). For example, if a baseline reflecting the size of the SEZ in the healthy brain could be estimated and the time course of activation of the SEZ after stroke or in cases of dementias could be established, it would facilitate accurate assessment of the recovery phase of a post-stroke patient and of the stage and grade of the disease in a demented patient. Another example can be multiple sclerosis, where the presence of neuronal progenitors at a site of lesion has been correlated with the stage of demyelination (active, chronic etc) (Nait-Oumesmar et al., 2007). Therefore, MRS or any other similar emerging methods (Adach and Kawabata, 2005) could be used for the non-invasive assessment of the progress of the disease. In other words, measuring the beat of the ‘heart of the brain’ and the flow of cells migrating away from the niche might prove to be as efficient in assessing the well being of the brain as measuring the pulses of the heart has been in assessing the general condition of the organism for hundreds of years.
Funding
National Institutes of Health—National Institute of Biomedical Imaging and Bioengineering Quantum Grant Project (1P20EB00706).
Acknowledgements
The author kindly acknowledges the critical contribution of Dr. Ifigeneia Mavranezouli (University College London), Prof. Charles ffrench-Constant (MRC Centre for Regenerative Medicine and Centre for Multiple Sclerosis Research, University of Edinburgh) and of Dr Veronique Marthiens (University of Cambridge) through many interesting and provocative discussions.
Glossary
Abbreviations
- OPCs
oligodendroglial precursors
- NSCs
neural stem cells
- PEDF
Pigment epithelium-derived factor
- SEZ
subependymal zone
- TaPs
transit amplifying precursors
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–36. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Kawai J, Uehara G, Ogata H, Miyamoto M, Kawabata S, et al. A SQUID biomagnetometer system for measurement of a human cervical spinal cord evoked field. Superconductor Science And Technology. 2005;18:S303–7. [Google Scholar]
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–19. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita K, von Holst A, Furukawa Y, Mikami T, Sugahara K, Faissner A. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells. 2008;26:798–809. doi: 10.1634/stemcells.2007-0448. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alves JA, Barone P, Engelender S, Froes MM, Menezes JR. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol. 2002;52:251–65. doi: 10.1002/neu.10087. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–60. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–90. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26:12089–99. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–31. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–75. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–8. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Gene circuitry controlling a stem cell niche. Curr Biol. 2005;15:179–84. doi: 10.1016/j.cub.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005a;25:2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005b;25:281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: the role of BDNF and FGF2. Mol Cell Neurosci. 2007;36:108–20. doi: 10.1016/j.mcn.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–46. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Eriksson PS, Faull RL. Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol. 2007;34:528–32. doi: 10.1111/j.1440-1681.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- de Chevigny A, Lemasson M, Saghatelyan A, Sibbe M, Schachner M, Lledo PM. Delayed onset of odor detection in neonatal mice lacking tenascin-C. Mol Cell Neurosci. 2006;32:174–86. doi: 10.1016/j.mcn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Diaz D, Valero J, Airado C, Baltanas FC, Weruaga E, Alonso JR. Sexual dimorphic stages affect both proliferation and serotonergic innervation in the adult rostral migratory stream. Exp Neurol. 2009;216:357–64. doi: 10.1016/j.expneurol.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002a;36:1021–34. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Verdugo JM, Caille I, Alvarez-Buylla A, Chao MV, Casaccia-Bonnefil P. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002b;22:2255–64. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, De Gasperi R, Gama Sosa MA. Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73:931–40. [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–65. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–4. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. EGF induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–43. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Mechanisms of subventricular zone expansion after focal cortical ischemic injury. J Comp Neurol. 2005a;488:201–14. doi: 10.1002/cne.20609. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005b;194:139–50. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin Exp Pharmacol Physiol. 2007;34:533–45. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–72. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Houades V, Rouach N, Ezan P, Kirchhoff F, Koulakoff A, Giaume C. Shapes of astrocyte networks in the juvenile brain. Neuron Glia Biol. 2006;2:3–14. doi: 10.1017/S1740925X06000081. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–99. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Fager N. Regulation of tissue inhibitor of metalloproteinase-3 (Timp-3) mRNA expression during rat CNS development. J Neurosci Res. 2000;61:396–408. doi: 10.1002/1097-4547(20000815)61:4<396::AID-JNR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA. 2003;100:4633–8. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Belhadi A, Faissner A, Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. J Neurosci. 2007;27:13991–6. doi: 10.1523/JNEUROSCI.3279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Lathia J, Moss L, ffrench-Constant C. StemBook eTSCRC editor. StemBook. Vol stembook.1.15.1; 2008. The neural stem cell microenvironment. http://www.stembook.org/node/490. [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology. 2006;31:1165–76. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–42. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimiwada T, Sakurai M, Ohashi H, Aoki S, Tominaga T, Wada K. Clock genes regulate neurogenic transcription factors, including NeuroD1, and the neuronal differentiation of adult neural stem/progenitor cells. Neurochem Int. 2009;54:277–85. doi: 10.1016/j.neuint.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–80. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005a;36:1278–82. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhao LR, Gido G, Johansson BB, Eriksson P. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur J Neurosci. 2005b;21:2397–405. doi: 10.1111/j.1460-9568.2005.04072.x. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–16. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Meng H, Chopp M. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007;146:1053–61. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–52. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci. 2007;25:761–71. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MH. Proteomics of brain extracellular fluid (ECF) and cerebrospinal fluid (CSF) Mass Spectrom Rev. 2008 doi: 10.1002/mas.20213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–88. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–4. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–82. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992;12:249–56. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–66. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci USA. 2007;104:4694–9. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U, Kaye AH. Extracellular matrix and the brain: components and function. J Clin Neurosci. 2000;7:280–90. doi: 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- O'Reilly AM, Lee HH, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008;182:801–15. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–44. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Dati C, De Marchis S, Kim HH, Ukhanova M, Fasolo A, et al. Expression of the secreted factors noggin and bone morphogenetic proteins in the subependymal layer and olfactory bulb of the adult mouse brain. Neuroscience. 2004;128:685–96. doi: 10.1016/j.neuroscience.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J Comp Neurol. 2005;487:407–27. doi: 10.1002/cne.20576. [DOI] [PubMed] [Google Scholar]
- Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington's disease are attributable to the in vivo microenvironment. J Neurosci. 2005;25:11564–76. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci USA. 2002;99:13211–16. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–78. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G, Helmeste DM, Samaranayake AN, Lau WM, Lee TM, Tang SW, et al. Modulation of the suppressive effect of corticosterone on adult rat hippocampal cell proliferation by paroxetine. Neurosci Bull. 2007;23:131–6. doi: 10.1007/s12264-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–24. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron SR, Aroca-Aguilar JD, Sanchez P, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–9. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- Rauch U. Modeling an extracellular environment for axonal pathfinding and fasciculation in the central nervous system. Cell Tissue Res. 1997;290:349–56. doi: 10.1007/s004410050940. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–37. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–92. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Tang H, Wang Y, Xie L, Mao X, Won SJ, Galvan V, et al. Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia. Neurobiol Aging. 2009;30:299–308. doi: 10.1016/j.neurobiolaging.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–94. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wang DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaime-Morawek S, Seaberg RM, Batista C, Labbe E, Attisano L, Gorski JA, et al. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–68. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–55. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ng KL, Li JD, He F, Anderson DJ, Sun YE, et al. Prokineticin 2 is a target gene of proneural basic helix-loop-helix factors for olfactory bulb neurogenesis. J Biol Chem. 2007;282:6917–21. doi: 10.1074/jbc.C600290200. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–8. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Roberts C, LeTourneau Y, Lu M, Zhang L, et al. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab. 2008;28:602–11. doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]