Abstract
Multiple sclerosis (MS) remains without an effective treatment in spite of intense research efforts. Interferon-beta (IFN-β) reduces duration and severity of symptoms in many relapsing-remitting MS patients, but its mechanism of action is still not well understood. Moreover, IFN-β and other available treatments must be given parenterally and have a variety of adverse effects. Certain naturally occurring flavonoids, such as luteolin, have anti-oxidant and anti-inflammatory effects, including inhibition of activated peripheral blood leukocytes from MS patients. Luteolin also inhibits mast cells, as well as mast cell-dependent T cell activation, recently implicated in MS pathogenesis. Moreover, luteolin and structurally similar flavonoids can inhibit experimental allergic allergic encephalomyelitis (EAE), an animal model of MS in rodents. An appropriate luteolin formulation that permits sufficient absorption and reduces its metabolism could be a useful adjuvant to IFN-β for MS therapy.
Introduction
This issue includes an interesting article by Sternberg et al. showing that the flavonoid luteolin inhibits IL-1, TNF and metalloproteinase-9 (MMP-9) release from activated peripheral blood mononuclear cells (PBMCs) from multiple sclerosis (MS) patients, and that the effect of luteolin is augmented by concurrent administration of interferon-beta (IFN-β). This paper extends previous similar results with quercetin that required higher concentrations of the flavonoid [1].
Discussion
Luteolin with or without IFN-β, could be helpful in MS by not only inhibiting PBMC release of cytokines, but also by inhibiting T cells, which we recently showed can be superstimulated by mast cells, an action also inhibited by luteolin [2]. In addition to T cells, recent evidence implicates also TH2 processes typically associated with allergic reactions [3-5], which involve mast cells (Fig. 1). In fact, mast cells have been considered as the next target for MS therapy [6-8].
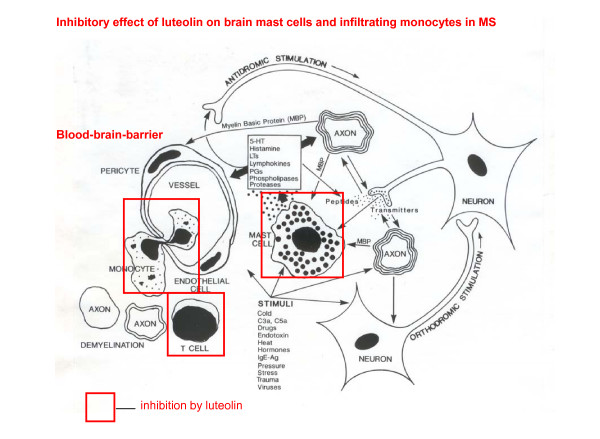
Figure 1.
Diagrammatic representation of the inhibitory effect of luteolin on brain mast cells and infiltrating monocytes in the pathogenesis of multiple sclerosis.
Brain MS plaques also contain activated mast cells [9,10], which have been associated with brain demyelination [11-13]. Gene array analysis also showed that MS plaques had increased gene expression for the IgE receptor (FcεRI), the histamine-1 receptor and the protease tryptase, all of which are associated with mast cells [14-16]. Mast cell tryptase is elevated in the CSF of MS patients [17], can activate peripheral mononuclear cells to secrete TNF and IL-6 [18], as well as stimulate protease-activated receptors (PAR) to induce widespread inflammation [19]. Brain mast cells can secrete TNF [20], which is involved in both brain inflammation [21] and blood-brain-barrier (BBB) permeability [22]. In fact, BBB disruption precedes any pathologic signs of MS [23] and mast cells can disrupt the BBB [24,25].
Flavonoids such as quercetin have potent anti-oxidant and anti-inflammatory activity [26]. Quercetin and luteolin also inhibit human cultured mast cell release of histamine, leukotrienes and prostaglandin D2 [27], as well as IL-6, IL-8, TNF-α and tryptase [28,29]. Moreover, quercetin and luteolin inhibit mast cell activation stimulated by IL-1 [30] leading to selective release of IL-6. Luteolin also inhibits IL-6 release from microglia cells [31], and from astrocytes [32]. Flavonoids can also inhibit myelin phagocytosis by macrophages [33], as well as inhibit EAE [34-36].
Conclusion
Quercetin and its structurally related luteolin are safe [37]. The fact remains that less than 10% of flavonoids are absorbed orally [37]. Novel ways of delivering select flavonoid combinations would be required to assure sufficient plasma levels, especially if luteolin were to also inhibit brain inflammation. Such a test nutraceutical formulation has already been tried on a number of relapsing-remitting MS patients treated with INF-β with encouraging positive results.
Competing interests
TCT has been awarded US patents No 6,689,748; 6,984,667 and EPO No 1365777 that cover the use of flavonoids in inflammatory diseases; he has also filed (3/30/04) US patent applications No. 10/811,826; 11/214,831; 11/999,991; 12/151,268 specifically covering combinations of flavonoids, including luteolin with INF-β, for the treatment of MS.
References
- Sternberg Z, Chadha K, Lieberman A, Hojnacki D, Drake A, Zamboni P, Rocco P, Grazioli E, Weinstock-Guttman B, Munschauer F. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol. 2008;205:142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolfberg A, Theoharides TC. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- Pedotti R, De Voss JJ, Steinman L, Galli SJ. Involvement of both 'allergic' and 'autoimmune' mechanisms in EAE, MS and other autoimmune diseases. Trends Immunol. 2003;24:479–484. doi: 10.1016/S1471-4906(03)00233-3. [DOI] [PubMed] [Google Scholar]
- Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, Sobel RA, Steinman L. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-F. [DOI] [PubMed] [Google Scholar]
- Zappulla JP, Arock M, Mars LT, Liblau RS. Mast cells: new targets for multiple sclerosis therapy? J Neuroimmunol. 2002;131:5–20. doi: 10.1016/S0165-5728(02)00250-3. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Mast cells in plaques of multiple sclerosis. Acta Neurol Scand. 1974;50:611–618. doi: 10.1111/j.1600-0404.1974.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Krüger PG, Bø L, Myhr KM, Karlsen AE, Taule A, Nyland HI, Mørk S. Mast cells and multiple sclerosis: a light and electron microscopic study of mast cells in multiple sclerosis emphasizing staining procedures. Acta Neurol Scand. 1990;81:31–36. doi: 10.1111/j.1600-0404.1990.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Dietsch GN, Hinrichs DJ. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988;444:195–198. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Dimitriadou V, Letourneau RJ, Rozniecki JJ, Vliagoftis H, Boucher WS. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain demyelination: changes resembling early stages of demyelination. Neuroscience. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-J. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Bomprezzi R, Ringnér M, Kim S, Bittner ML, Khan J, Chen Y, Elkahloun A, Yu A, Bielekova B, Meltzer PS, Martin R, McFarland HF, Trent JM. Gene expression profile in multiple sclerosis patients and healthy controls: identifying pathways relevant to disease. Hum Mol Genet. 2003;12:2191–2199. doi: 10.1093/hmg/ddg221. [DOI] [PubMed] [Google Scholar]
- De Jager PL, Hafler DA. Gene expression profiling in MS: what is the clinical relevance? Lancet Neurol. 2004;3:269. doi: 10.1016/S1474-4422(04)00731-8. [DOI] [PubMed] [Google Scholar]
- Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- Malamud V, Vaaknin A, Abramsky O, Mor M, Burgess LE, Ben-Yehudah A, Lorberboum-Galski H. Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-alpha, IL-6 and IL-1 beta: possible relevance to multiple sclerosis. J Neuoimmunol. 2003;138:115–122. doi: 10.1016/S0165-5728(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Evidence that brain mast cells can modulate neuroinflammatory responses by tumor necrosis factor-α production. Neuroreport. 1998;9:95–98. doi: 10.1097/00001756-199801050-00019. [DOI] [PubMed] [Google Scholar]
- Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-α transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137–141. doi: 10.1016/S0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- Kim KS, Wass CA, Cross AS, Opal SM. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/S0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo CL, Theoharides TC. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–944. doi: 10.1038/sj.bjp.0706246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, Hyun MC, Kwon TK, Kim YH, Kim SH. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008;31:1303–1311. doi: 10.1007/s12272-001-2110-5. [DOI] [PubMed] [Google Scholar]
- Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br J Pharmacol. 2006;148:208–215. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, de Vries HE, Pol SM van der, Berg TK van den, van Tol EA, Dijkstra CD. Flavonoids inhibit myelin phagocytosis by macrophages; a structure-activity relationship study. Biochem Pharmacol. 2003;65:877–885. doi: 10.1016/S0006-2952(02)01609-X. [DOI] [PubMed] [Google Scholar]
- Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S, Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24:542–552. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, Alblas J, Pol SM van der, van Tol EA, Dijkstra CD, de Vries HE. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]