Abstract
The occurrence of a chloramphenicol-acetylating enzyme, similar to that found in Escherichia coli, carrying an R factor was investigated in various gram-negative bacilli. The acetylated products of chloramphenicol were identified by chromatography and quantitatively assayed after benzene extraction. The investigated strains were of the Salmonella-Arizona group, the Klebsiella-Aerobacter group, Serratia marcescens, the Proteus group, and Pseudomonas aeruginosa, most of which were isolated from 1947 to 1957. Both chloramphenicol-sensitive and -resistant strains were included, but none of them was able to transfer chloramphenicol resistance by conjugation. In the Proteus group, a significant level of a chloramphenicol-acetylating enzyme was found in most strains, whether they were sensitive or resistant to chloramphenicol; the resistant strains showed higher levels of the enzyme. Some chloramphenicol-sensitive strains lacked this enzyme. Only the sensitive strains containing the enzyme could easily produce chloramphenicol-resistant mutants with higher enzyme activity. Thus, the chloramphenicol resistance of this group can be reasonably explained on the basis of the chloramphenicol-acetylating enzyme. All of the Pseudomonas aeruginosa strains were resistant to chloramphenicol, and most strains showed low levels of the enzyme (which, however, did not appear sufficient to explain their resistance). All of the strains of the other groups (except one strain of Enterobacter cloacae) lacked the enzyme, although most strains of the Klebsiella-Aerobacter group and of S. marcescens were resistant to chloramphenicol. With respect to the origin of the resistance gene of the R factor, it is noteworthy that the strains of Proteus mirabilis isolated in 1947 possessed this enzyme before the discovery of chloramphenicol.
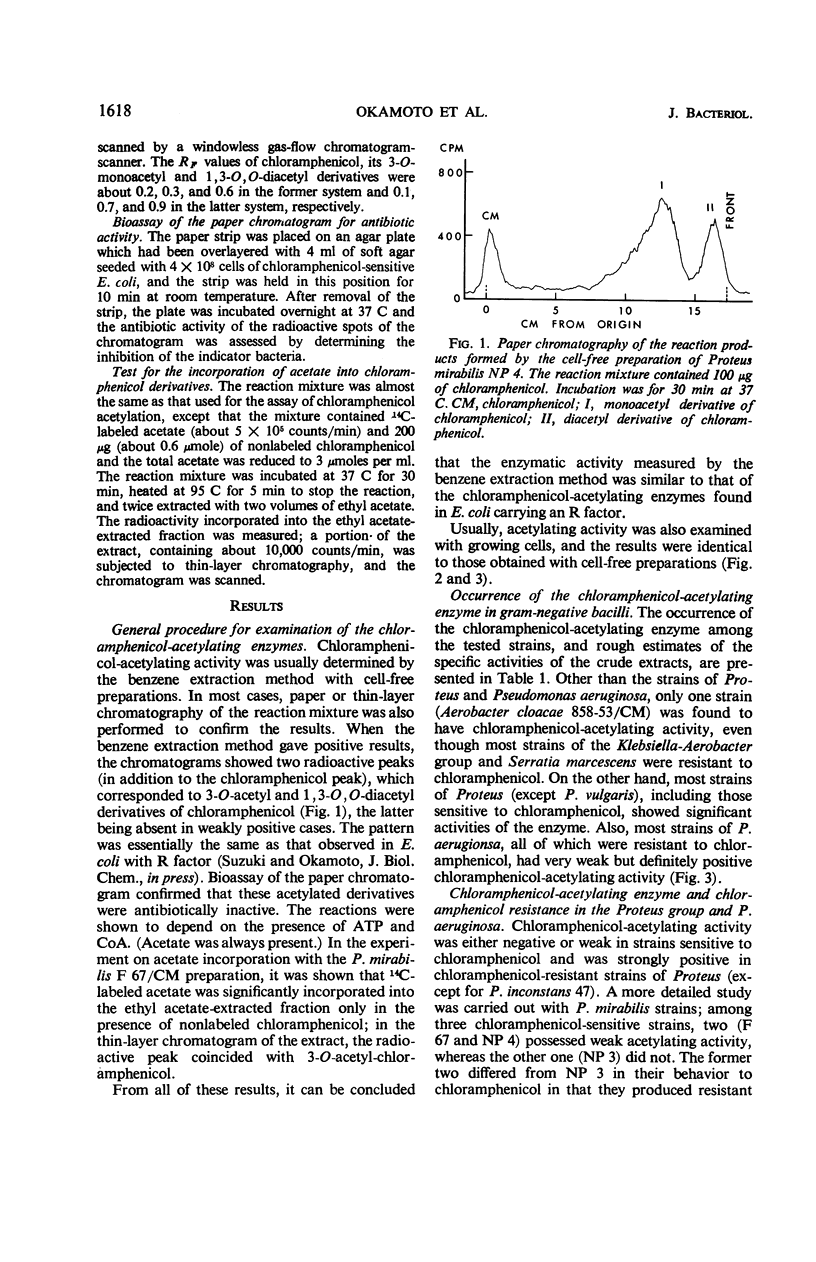
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EWING W. H., TANNER K. E., DENNARD D. A. The Providence group: an intermediate group of enteric bacteria. J Infect Dis. 1954 Mar-Apr;94(2):134–140. doi: 10.1093/infdis/94.2.134. [DOI] [PubMed] [Google Scholar]
- MIYAMURA S., OKETANI S. [Studies on chloramphenicol inactivation by microorganisms. 2. Relation between chloramphenicol inactivation and chloramphenicol resistance in various microorganisms]. Nihon Saikingaku Zasshi. 1962 Apr;17:294–296. [PubMed] [Google Scholar]
- NAMIOKA S., SAKAZAKI R. Etude sur les Rettgerella. Ann Inst Pasteur (Paris) 1958 Apr;94(4):485–499. [PubMed] [Google Scholar]
- OKAMOTO S., MIZUNO D. MECHANISM OF CHLORAMPHENICOL AND TETRACYCLINE RESISTANCE IN ESCHERICHIA COLI. J Gen Microbiol. 1964 Apr;35:125–133. doi: 10.1099/00221287-35-1-125. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- SAKAZAKI R., NAMIOKA S. Biochemical studies on Voges-Proskauer positive enteric bacteria. Jpn J Exp Med. 1957 Oct;27(5):273–282. [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S., Kono M. Basis of chloramphenicol resistance in naturally isolated resistant staphylococci. J Bacteriol. 1966 Sep;92(3):798–799. doi: 10.1128/jb.92.3.798-799.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


