Abstract
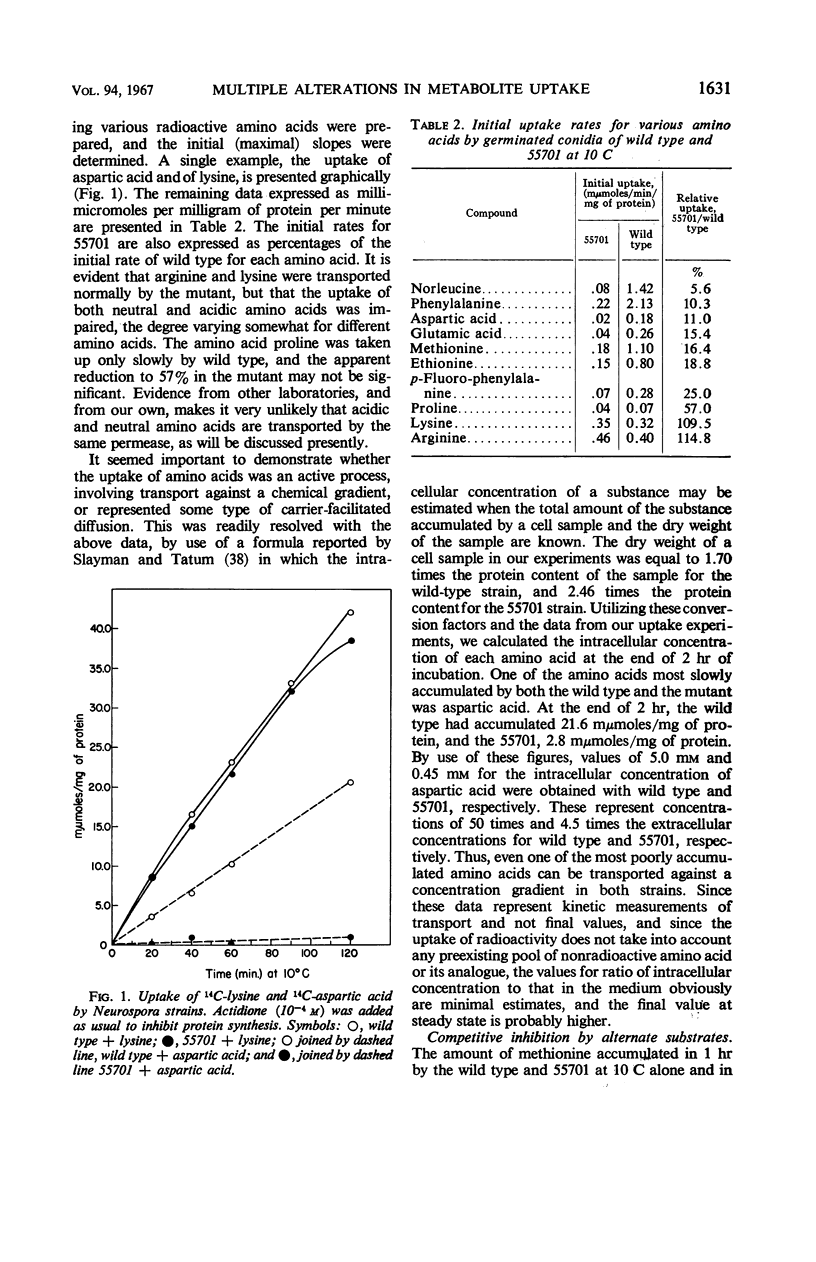
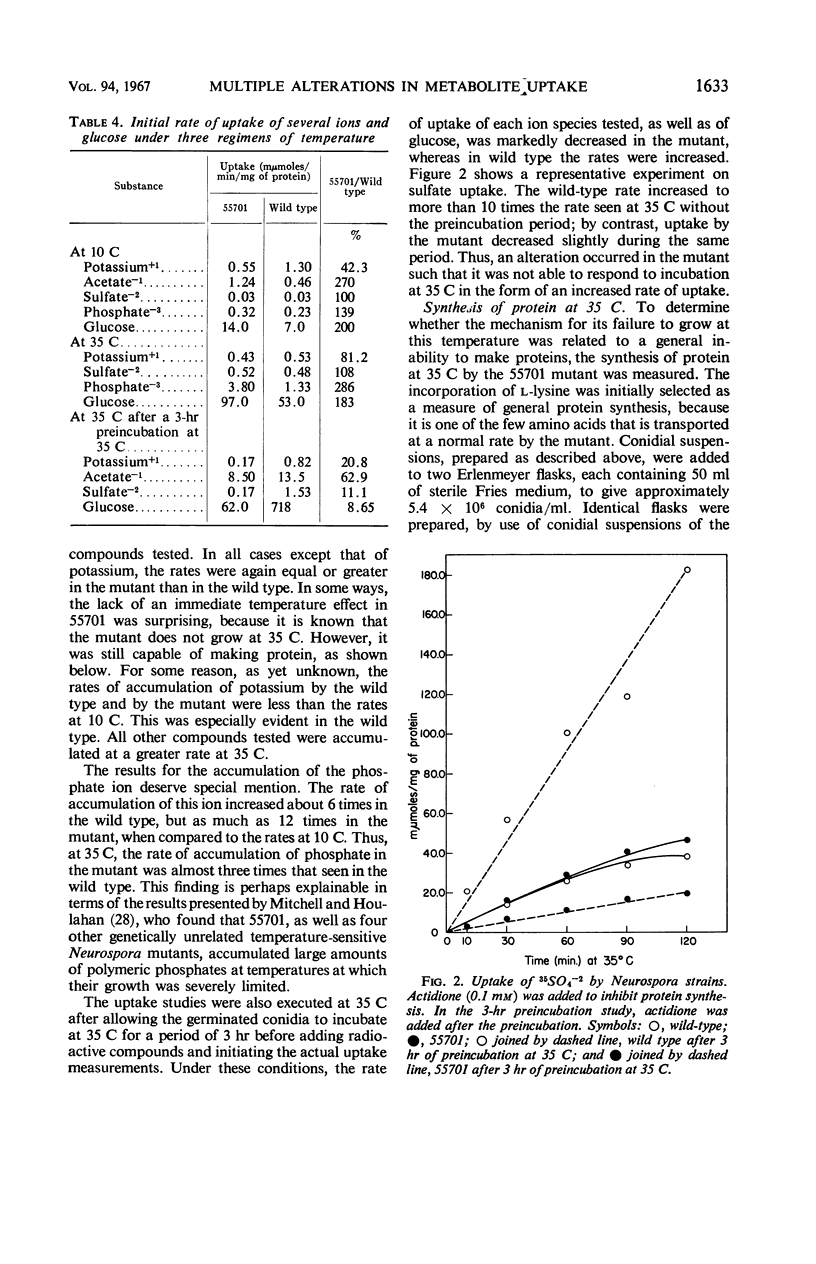
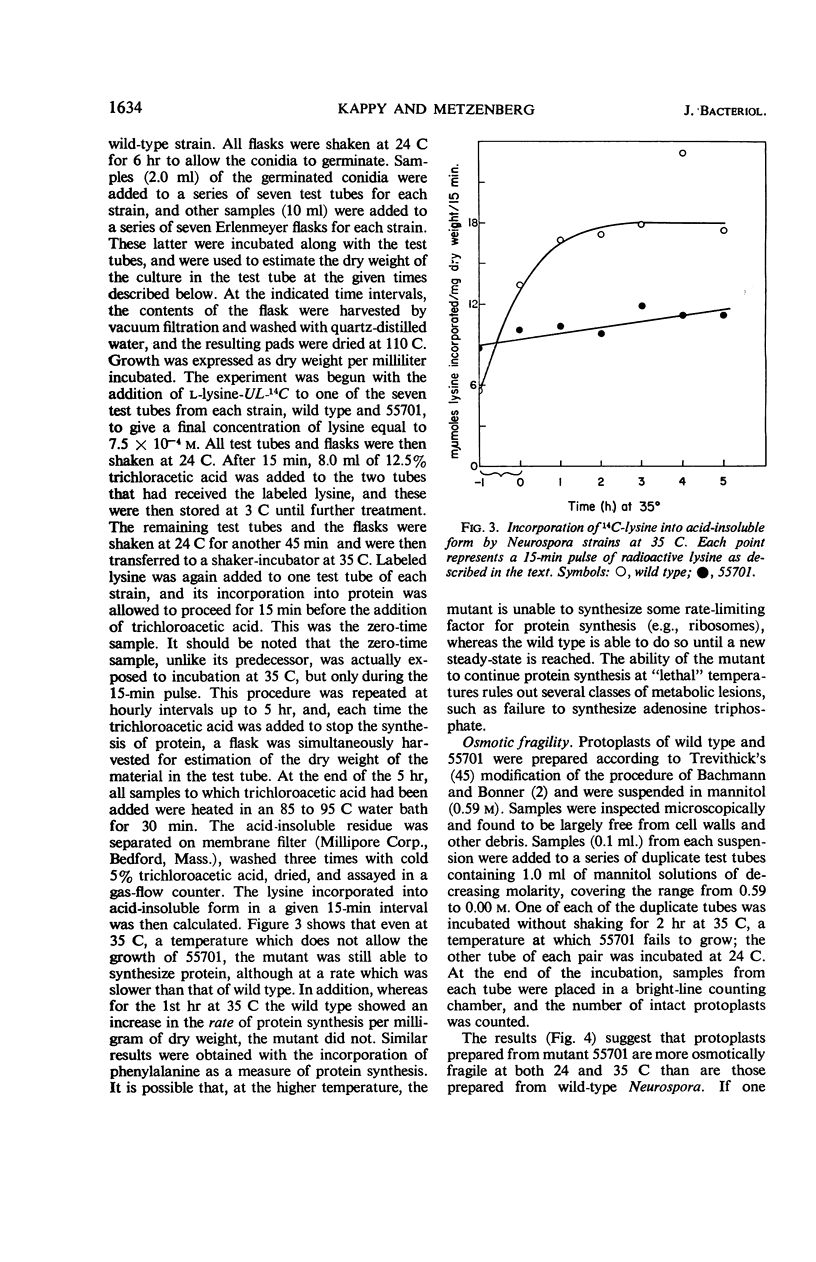
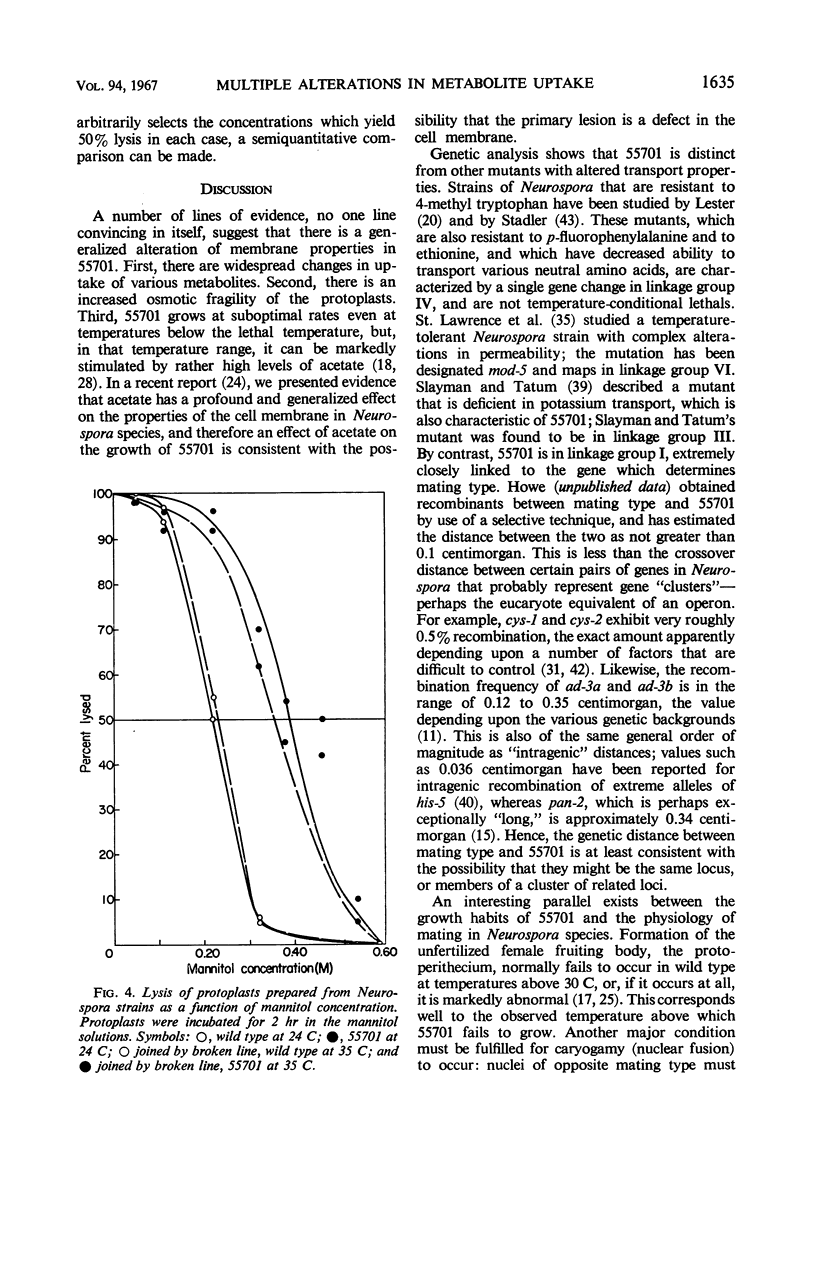
A temperature-conditional lethal mutant of Neurospora crassa, un-t (55701), was resistant to neutral amino acid analogues by virtue of a decreased ability to transport these analogues and their natural congeners across the cell membrane. The uptake of acidic, but not basic, amino acids was also impaired, as was the uptake of potassium ions. After preincubation above the tolerated temperature, the ability to take up a still wider variety of metabolites was greatly reduced. Protoplasts of the mutant were more osmotically fragile than those of wild type. The possibility that the mutant has a generalized membrane defect is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- BACHMANN B. J., BONNER D. M. Protoplasts from Neurospora crassa. J Bacteriol. 1959 Oct;78:550–556. doi: 10.1128/jb.78.4.550-556.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUERLE R. H., GARNER H. R. THE ASSIMILATION OF ARGININE AND LYSINE IN CANAVANINE RESISTANT AND SENSITIVE STRAINS OF NEUROSPORA CRASSA. Biochim Biophys Acta. 1964 Nov 8;93:316–322. doi: 10.1016/0304-4165(64)90381-2. [DOI] [PubMed] [Google Scholar]
- BROCKMAN H. E. EFFECTS OF AMINO ACIDS ON THE UTILIZATION OF TRYPTOPHAN AND INDOLE FOR GROWTH BY A MUTANT OF NEUROSPORA CRASSA. J Gen Microbiol. 1964 Jan;34:31–41. doi: 10.1099/00221287-34-1-31. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle M. B., Pittenger T. H. Mitotic recombination in pseudo-wild types of Neurospora. Genetics. 1965 Sep;52(3):609–625. doi: 10.1093/genetics/52.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE SERRES F. J. Recombination and interference in the ad-3 region of Neurospora crassa. Cold Spring Harb Symp Quant Biol. 1958;23:111–118. doi: 10.1101/sqb.1958.023.01.015. [DOI] [PubMed] [Google Scholar]
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- Dreyfuss J., Pardee A. B. Regulation of sulfate transport in Salmonella typhimurium. J Bacteriol. 1966 Jun;91(6):2275–2280. doi: 10.1128/jb.91.6.2275-2280.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. 3. Studies of the transport process. Biochim Biophys Acta. 1966 Jan 4;112(1):63–73. doi: 10.1016/s0926-6585(96)90009-6. [DOI] [PubMed] [Google Scholar]
- HOWE H. B., Jr Determining mating type in Neurospora without crossing tests. Nature. 1961 Jun 10;190:1036–1036. doi: 10.1038/1901036a0. [DOI] [PubMed] [Google Scholar]
- Kappy M. S., Metzenberg R. L. Studies on the basis of ethionine-resistance in Neurospora. Biochim Biophys Acta. 1965 Oct 18;107(3):425–433. doi: 10.1016/0304-4165(65)90186-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBIN M., KESSEL D. H., BUDREAU A., GROSS J. D. The isolation of bacterial mutants defective in amino acid transport. Biochim Biophys Acta. 1960 Aug 26;42:535–538. doi: 10.1016/0006-3002(60)90836-2. [DOI] [PubMed] [Google Scholar]
- Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966 Feb;91(2):677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNELLY-INGLE C. A., FROST L. C. THE EFFECT OF TEMPERATURE ON THE PRODUCTION OF PERITHECIA BY NEUROSPORA CRASSA. J Gen Microbiol. 1965 Apr;39:33–42. doi: 10.1099/00221287-39-1-33. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L., KAPPY M. S., PARSON J. W. IRREPARABLE MUTATIONS AND ETHIONINE RESISTANCE IN NEUROSPORA. Science. 1964 Sep 25;145(3639):1434–1435. doi: 10.1126/science.145.3639.1434. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. BIOCHEMICAL MECHANISMS OF DRUG RESISTANCE. Annu Rev Microbiol. 1964;18:347–366. doi: 10.1146/annurev.mi.18.100164.002023. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Parson J. W. Altered repression of some enzymes of sulfur utilization in a temperature-conditional lethal mutant of Neurospora. Proc Natl Acad Sci U S A. 1966 Mar;55(3):629–635. doi: 10.1073/pnas.55.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkres K. D., Woodward D. O. On the genetics of enzyme locational specificity. Proc Natl Acad Sci U S A. 1966 May;55(5):1217–1224. doi: 10.1073/pnas.55.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E. Cysteine mutant strains of Neurospora. Genetics. 1965 Oct;52(4):801–808. doi: 10.1093/genetics/52.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Résistance a l'éthionine chez Saccharomyces cerevisiae. II. Etude physiologique. Genetics. 1966 Oct;54(4):993–1006. doi: 10.1093/genetics/54.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K., SIMON E. J. An impaired concentrating mechanism for amino acids in mutants of Escherichia coli resistant to L-canavanine and D-serine. Biochim Biophys Acta. 1959 Apr;32:582–583. doi: 10.1016/0006-3002(59)90650-x. [DOI] [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- SMITH B. R. INTERALLELIC RECOMBINATION AT THE HIS-5 LOCUS IN NEUROSPORA CRASSA. Heredity (Edinb) 1965 May;20:257–276. doi: 10.1038/hdy.1965.33. [DOI] [PubMed] [Google Scholar]
- SORSOLI W. A., SPENCE K. D., PARKS L. W. AMINO ACID ACCUMULATION IN ETHIONINE-RESISTANT SACCHAROMYCES CEREVISIAE. J Bacteriol. 1964 Jul;88:20–24. doi: 10.1128/jb.88.1.20-24.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STLAWRENCE P., MALING B. D., ALTWERGER L., RACHMELER M. MUTATIONAL ALTERATION OF PERMEABILITY IN NEUROSPORA: EFFECTS ON GROWTH AND THE UPTAKE OF CERTAIN AMINO ACIDS AND RELATED COMPOUNDS. Genetics. 1964 Dec;50:1383–1402. doi: 10.1093/genetics/50.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin S., Ames B. N., FerroLuzzi-Ames G. Effect of the alpha-hydrazino analogue of histidine on histidine transport and arginine biosynthesis. J Biol Chem. 1966 Jul 25;241(14):3424–3429. [PubMed] [Google Scholar]
- Slayman C. W., Tatum E. L. Potassium transport in Neurospora. 3. Isolation of a transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):184–193. doi: 10.1016/0926-6585(65)90102-0. [DOI] [PubMed] [Google Scholar]
- Stadler D R. A Map of Linkage Group VI of Neurospora Crassa. Genetics. 1956 Jul;41(4):528–543. doi: 10.1093/genetics/41.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler D. R. Genetic control of the uptake of amino acids in Neurospora. Genetics. 1966 Aug;54(2):677–685. doi: 10.1093/genetics/54.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Trevithick J. R., Metznberg R. L. The invertase isozyme formed by Neurospora protoplasts. Biochem Biophys Res Commun. 1964 Jul 1;16(4):319–325. doi: 10.1016/0006-291x(64)90033-6. [DOI] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. I. Specificity and kinetics. J Bacteriol. 1966 Dec;92(6):1698–1705. doi: 10.1128/jb.92.6.1698-1705.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


