Abstract
Photodynamic therapy (PDT) can cause lethal photodamage by both direct and indirect mechanisms. Direct modes of cell death relate to nonspecific necrosis and the initiation of signaling pathways that elicit apoptosis, autophagy or both. In this report, effects of low-dose and high-dose PDT are explored, comparing sensitizers that localize in the endoplasmic reticulum (the porphycene termed CPO) or mitochondria (mesochlorin). To explore the role of autophagy, two cell lines were examined—the murine L1210 leukemia and an Atg7 knockdown derivative of L1210. The Atg7 gene is central to the process of autophagy. High-dose PDT with either sensitizer resulted in a substantial loss of the Bcl-2 protein. As Bcl-2 regulates both apoptosis and autophagy, loss of this protein can lead to initiation of either or both processes. Low-dose PDT with either sensitizer resulted in the initiation of apoptosis in the L1210/Atg7− cell line and a 20% loss of viability. In contrast, the same PDT dose led to the rapid appearance of autophagic cells in the L1210 line, less apoptosis and only a 5% loss of viability. These results are consistent with autophagy serving as a pro-survival response via the recycling of damaged organelles. At a higher PDT dose more apoptosis was again seen in the L1210/Atg7− line, but both cell lines exhibited comparable cytotoxicity in colony formation assays. We conclude that autophagy offers protection from the phototoxic effects of low-dose PDT, but can serve as an alternate death mode when the PDT dose is increased.
INTRODUCTION
Photodynamic therapy (PDT) involves the treatment of neoplastic cells and tissues with photosensitizing agents that generate reactive oxygen species upon irradiation. PDT can eradicate malignant cells by direct tumor cell kill, vascular shutdown and immunologic effects (1). Oleinick’s group was the first to describe an apoptotic response to PDT following sensitization with Pc 4, a phthalocyanine that evoked mitochondrial and endoplasmic reticulum (ER) photodamage (2). This group later proposed that apoptosis was not a necessary component of post-PDT cell death, but could serve as a means for disposing of dead cells (3,4). Indeed, recent studies have shown that PDT is cytotoxic to sensitized cells that have defects in their apoptotic program (5,6).
Macroautophagy (hereafter referred to as autophagy) is a physiologic process by which cytosol and entire organelles become enclosed within a double-membrane vacuole termed the autophagosome. Upon fusion of a lysosome with an autophagosome, the contents of the latter undergo degradation. Although autophagy was originally described as a survival response to nutrient deprivation, it may also provide other pro-survival functions (7,8). Indeed, Lemasters has proposed that the pro-apoptotic consequences of damaged mitochondria can be attenuated by the selective autophagy of the damaged organelles (9). On the other hand, several recent studies suggest that autophagy can also serve as a cell death pathway (10–12).
We (13) and another group (14) recently reported that autophagy occurs during PDT protocols involving sensitizers that localize to the ER. We (15,16) and others (17) also demonstrated that mitochondrial- and ER-associated Bcl-2 is among the targets damaged/inactivated in PDT protocols involving sensitizers that target these organelles. These effects on Bcl-2 are important within the context of both apoptosis and autophagy. The former effect is well known, but it has been shown that loss of Bcl-2 protein or function can also initiate the development of autophagy (18,19).
At issue in PDT studies, as well as in other protocols in which Bcl-2 may be a target, is whether autophagy constitutes a survival or pro-death pathway. In the present report we examined the relationships among PDT dose, inductions of autophagy and apoptosis, and the functioning of autophagy as either a pro-survival or death pathway. These studies utilized cell cultures sensitized with agents that localize to the mitochondria or the ER and catalyze Bcl-2 photodamage upon irradiation. Analyses were performed in L1210 cultures, and a derivative of the L1210 line in which autophagy could not occur because of silencing of the Atg7 gene (20). Our studies suggest that autophagy can function as either a prosurvival or death pathway in PDT protocols involving ER and mitochondrial sensitizers. The former function is obvious at low-dose PDT conditions, whereas the latter function contributes to the killing of those cells having a phenotype that precludes the development of an apoptotic response (13), or those cells that survive the initial wave of apoptosis after high-dose PDT.
MATERIALS AND METHODS
Chemicals and biologicals
The porphycene CPO (21) was synthesized by Dr. Gracça Vicente, Department of Chemistry, Louisiana State University. Mesochlorin (MC), the reduced analog of mesoporphyrin, was provided by Porphyrin Products (Logan, UT). Stock solutions of these agents were prepared in DMSO. Amino acids and tissue culture media were purchased from Sigma-Aldrich (St. Louis, MO). Sterile horse serum was purchased from Gibco-BRL (Grand Island, NY). HO33342, TMRM, MitoTracker Green and DEVD-rhodamine 110 were from Molecular Probes (Eugene, OR).
Cell lines
Murine leukemia L1210 cells and an Atg7 knockdown derivative of L1210 were used in all experiments reported here. Cells were grown in sealed flasks using Fisher’s Medium (Sigma-Aldrich) containing 10% horse serum and supplemented with 1 mM glutamine, 1 mM mercaptoethanol and gentamicin. As Fisher’s medium is no longer commercially available, an approximation of the formula was prepared by supplementing the α-MEM formulation (Gibco-BRL) with MgCl2 (45 mg L−1), methionine (75 mg L−1), phenylalanine (30 mg L−1), valine (30 mg L−1) and folic acid (9 mg L−1).
Gene-silencing methodology
A retroviral vector that encoded a short hairpin RNA construct directed against murine Atg7 was obtained from Open Biosystems (Huntsville, AL). The protocol for the construction and selection of an L1210 derivative cell line that stably expresses shRNAs to Atg7 has been published (20). L1210/Atg7− cells were periodically monitored by Western blotting to insure continued silencing of Atg7.
Western blots
Cells were lysed in SDS-PAGE buffer, and the lysate heated to 100°C for 5 min. Aliquots containing 40 µg of protein per well were used for Western blot analysis (20). A rabbit antibody to Bcl-2 was obtained from BD-Pharmingen (San Jose, CA). An antibody to the murine LC3 protein was provided by Proteintech Group, Inc. (Chicago, IL). A rabbit polyclonal antibody to a peptide mapping to the carboxy terminus of human Atg7 was purchased from Prosci Inc. (Poway, CA). To inhibit hydrolysis of LC3-II by lysosomal proteases, incubation buffers were supplemented with the protease inhibitors E64d and acetylpepstatin A (22).
Viability
Clonogenic assays were used to determine loss of viability. Serial dilutions of cell suspensions were plated on soft agar. After 7- to 9-day growth in a humidified chamber under 5% CO2, colonies were counted and compared with untreated control values. All such experiments were carried out in triplicate. The plating efficiency of control L1210 cell cultures was approximately 70%.
Cell culture and PDT protocols
Cells (7 mg mL−1, 3.5 × 106 cells mL−1) were incubated in growth medium with 25 mM HEPES buffer (pH 7.4) replacing NaHCO3, to promote maintenance of a near-neutral pH at a high cell density. PDT studies involved incubations for 30 min at 37°C with 2 µM CPO or MC. After the loading incubation, the cells were washed and resuspended in fresh medium at 10°C and irradiated. The light source was a 600-W quartz-halogen lamp with IR radiation attenuated by a 10-cm layer water and an 850-nm low-pass filter. Irradiation was carried out at 600–650 nm as defined by interference filters (Oriel, Stratford, CT). The cell suspensions were then incubated for additional times at 37°C as specified in the text, or used for clonogenic assays.
Sensitizer transport
To assess the possible effects of Atg7 silencing on uptake of CPO or MC, cells were incubated with 2 µM levels of these agents for 30 min at 37°C, then washed and homogenized in 10 mM Triton X-100 buffer. The fluorescence in the cell pellets vs supernatant fluid was then determined using 400 ± 20 nm excitation. Fluorescence emission from these samples was assessed with a multichannel analyzer and the area under the curve integrated to provide an indication of drug levels in cells and medium. The distribution ratio was calculated in terms of µg per mg cells (wet weight)/µg per mL medium.
Microscopy
Fluorescence images were acquired using a Nikon Eclipse E600 microscope and a CoolSnap HQ CCD camera (Photo-metrics). Images were processed using MetaMorph software (Universal Imaging). A Uniblitz shutter was used to control exposure of the stage to the excitation source. This lamp shutter was configured to open and close with the camera shutter, thereby minimizing photo-bleaching of samples.
For electron microscopy, L1210 cells were fixed with glutaraldehyde and osmium tetroxide, treated with uranyl acetate and lead citrate for enhanced protein and lipid staining, and then dehydrated in ethanol. The cell pellets were embedded in epon resin and cut with an ultramicrotome to a 70-nm thickness before viewing.
Fluorescent probes and procedures
Fluorescent probes used were HO33342 (chromatin) and Mito Tracker Green (a probe for mitochondria). After labeling for 5 min at 37°C, cells were washed once with fresh medium and examined by fluorescence microscopy using appropriate excitation and emission wavelengths (16,23). For MC localization studies, cultures were incubated for 5 min at 37°C, collected by centrifugation and washed once with fresh medium before microscopic examination. This involved excitation of fluorescence at 400 ± 20 nm, with emission detected at 600–700 nm.
DEVDase assays
Cells were collected 60 min after irradiation, washed, and lysed in 200 µL of buffer containing 50 mM Tris (pH 7.2), 0.03% Nonidet P-40 and 1 mM DTT. The lysate was briefly sonicated and the debris removed by centrifugation at 10 000 g for 1 min. The supernatant fluid (100 µL) was mixed with 40 µM DEVD-R110, 10 mM HEPES (pH 7.5), 50 mM NaCl, and 2.5 mM DTT in a total volume of 200 µL. The rate of increase in fluorescence emission, resulting from the release of rhodamine-110 from the fluorogenic substrate, was measured over 30 min at room temperature using a fluorescence plate reader. Control determinations were made on untreated cells. Each assay was performed in triplicate.
RESULTS
Sub-cellular localization of CPO and MC
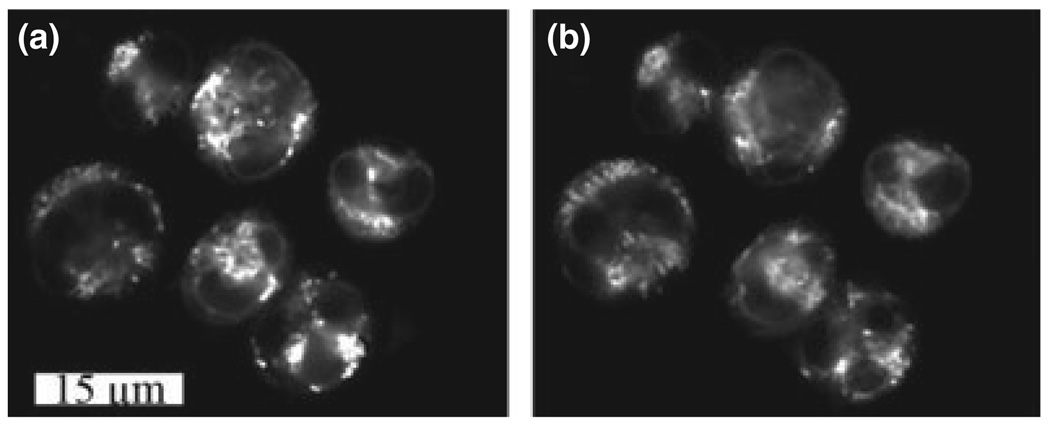
Localization of CPO in the ER has already been reported (24). MC labeling patterns in L1210 cells duplicated the mitochondrial fluorescence of MitoTracker Green (Fig. 1). These fluorescence localization results with both sensitizers were not affected by Atg7 silencing (data not shown).
Figure 1.
Fluorescence localization patterns of MC (a) and MTG (b) in L1210 cells.
Expression of Atg7 and protein photosensitivity
The protein product of the Atg7 gene is required for development of the autophagosome (25,26). Expression of Atg7 shRNA in the L1210/Atg7− cell line effectively reduced Atg7 protein content (Fig. 2, compare a with d). PDT (LD90 conditions) with either sensitizer did not affect Atg7 protein content in wild-type L1210 cells (Fig. 2a–c).
Figure 2.
Western blot analyses of Atg7 expression in L1210 cells before (a) and directly after an LD90 PDT dose with CPO (b) or MC (c). Lane (d) depicts Atg7 expression in the L1210/Atg7− cell line. In this and all other Western blots, the total protein loading was 40 µg per well.
Sensitizer transport studies
A 30-min incubation of L1210 cells with a 2 µM concentration of CPO led to a drug-distribution ratio of 3.9 ± 0.3. With MC, the corresponding values were 7.9 ± 0.4. When the Atg7 gene was silenced, the results were quite similar: 3.6 ± 0.4 and 8.1 ± 0.4, respectively.
Effects of PDT on viability and DEVDase activation
With CPO as the photosensitizing agent, a light dose of 135 mJ cm−2 caused a 90% loss of viability in both the L1210 and L1210/Atg7− lines, as monitored by colony formation assays (Table 1). MC yielded an LD90 effect, in both cell lines, with a 120 mJ cm−2 light dose (Table 1). A corresponding examination of lower light doses revealed that the L1210 cell line was more resistant to photodamage with either sensitizer. Light doses that caused only a 5% loss of viability in L1210 cells represented LD20 doses in the L1210/Atg7− line (Table 1). DEVDase-specific activities, an indicator of procaspases-3 and -7 activation, were elevated following irradiation of cells loaded with either sensitizer (Table 1). DEVDase activities following low-dose PDT with either sensitizer were greater in the L1210/Atg7− line. The same was true under LD90 conditions (Table 1).
Table 1.
DEVDase activation and viability studies after low vs high dose using CPO or MC as the photosensitizer.
| Cell line | |||||
|---|---|---|---|---|---|
| Light dose (mJ cm−2) |
L1210 | L1210/Atg7− | |||
| Sensitizer | Viability* | DEVDase† | Viability* | DEVDase† | |
| None | N/A | 100 ± 3 | 0.43 ± 0.14 | 100 ± 4 | 0.56 ± 0.18 |
| CPO | 35 | 94 ± 2 | 1.1 ± 0.2 | 80 ± 1 | 2.4 ± 0.3 |
| 135 | 11 ± 3 | 9.3 ± 0.9 | 13 ± 4 | 13.6 ± 1.1 | |
| MC | 30 | 95 ± 2 | 1.4 ± 0.3 | 78 ± 3 | 2.9 ± 0.3 |
| 120 | 13 ± 4 | 10.8 ± 1.1 | 11 ± 2 | 14.7 ± 0.8 | |
CPO = porphycene; MC = mesochlorin.
Viability was estimated by clonogenic assays.
DEVDase activity is expressed in terms of nmol product min−1 mg−1 protein measured 60 min after irradiation.
Apoptosis and autophagy after low-dose PDT
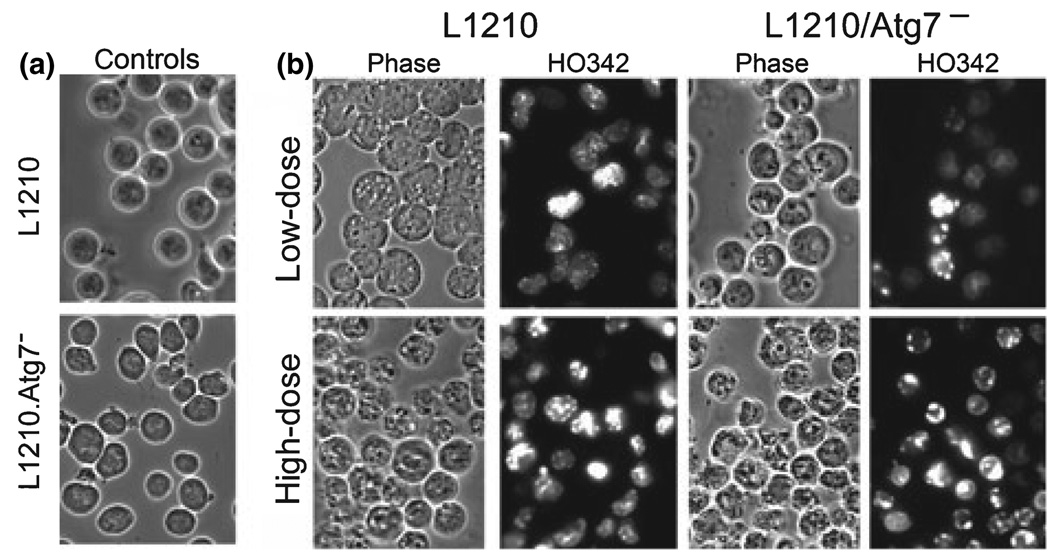
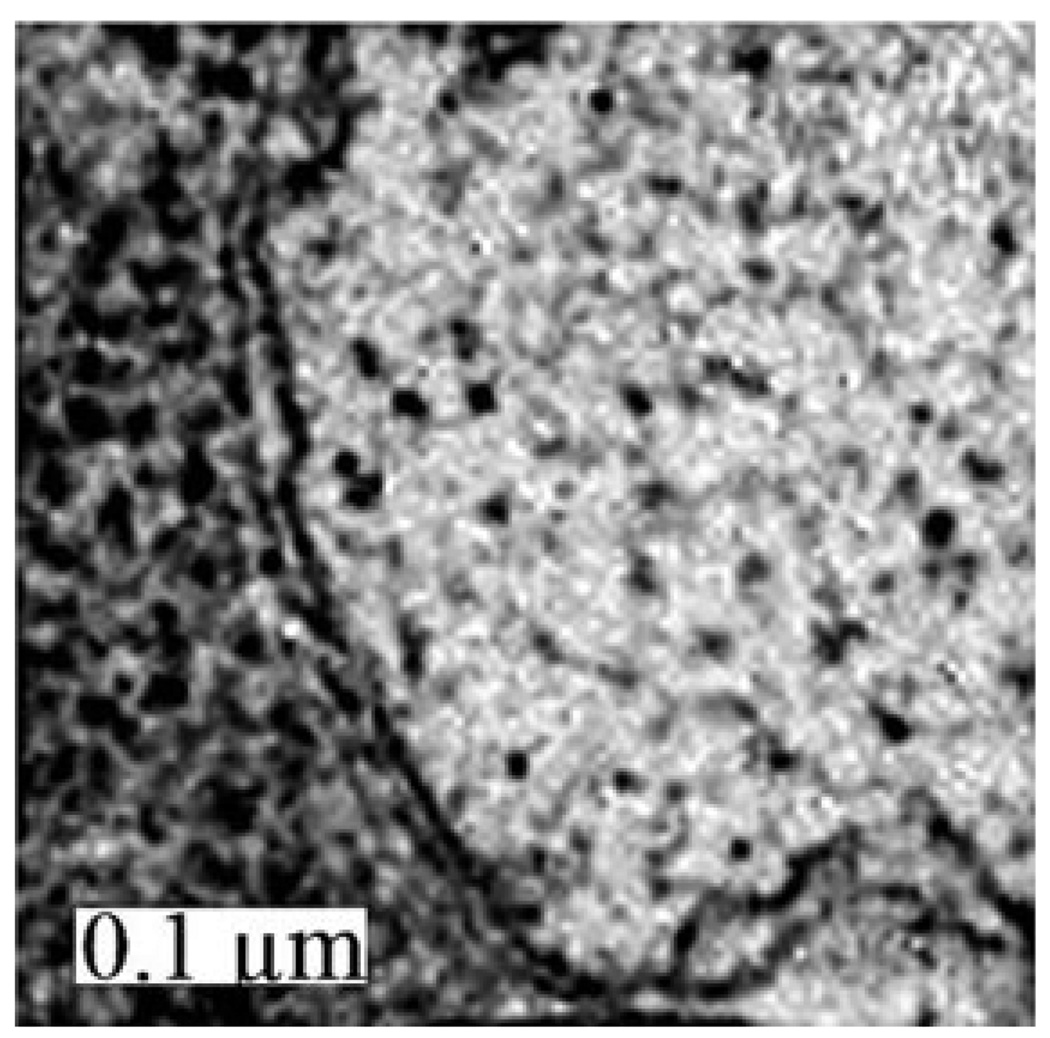
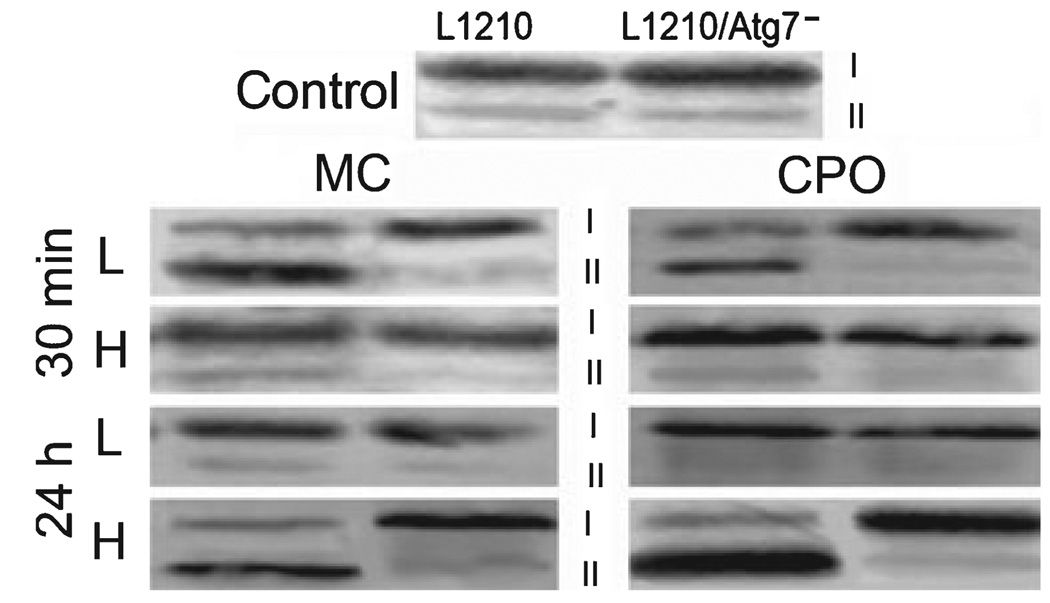
Many cytosolic vacuoles were observed in MC-sensitized L1210 cells within 30 min of administration of an LD5 PDT dose (Fig. 3, top row). These vacuoles exhibited the double membrane structure characteristic of autophagosomes (Fig. 4). In addition, a conversion of LC3-I to LC3-II occurred during the same time frame in low-dose irradiated, MC- and CPO-sensitized L1210 cultures (Fig. 5). In contrast, neither vacuolization nor LC3-I to LC3-II conversion occurred in L1210 cells within 30 min of administration of an LD90 dose (Fig. 3 bottom row, Fig. 5). Instead, cultures exhibited a significant percentage of cells with condensed chromatin (Fig. 3 bottom row) and markedly elevated DEVDase activities (Table 1). However, not all cells were initially killed in this initial wave of apoptosis. Highly vacuolated cells were observed 24 h after irradiation in MC- (data not shown) and CPO-sensitized cultures (13), as were substantial conversions of LC3-I to LC3-II (Fig. 5).
Figure 3.
(a) Phase-contrast images (1000×) of control L1210 and L1210/Atg7− cells. (b) Phase-contrast and HO342 stained images of L1210 and L1210/Atg7− cells after low-dose or high-dose PDT (light doses are defined in Table 1). MC-sensitized cells were photographed 30 min after irradiation.
Figure 4.
Electron microscopic examination of vacuoles in L1210 cells 30 min after exposure to low-dose PDT using MC as the photosensitizing agent.
Figure 5.
Western blots showing processing of the LC3-I protein to the faster-migrating LC3-II species in L1210 vs L1210/Atg7− cells. Analyses are of control cultures (top), and cultures harvested 30 min or 24 h after administration of low- or high-dose PDT with MC (left) or CPO (right).
We observed no significant levels of vacuolization or conversion of LC3-I to LC3-II in MC-sensitized L1210/Atg7− cells following exposure to either low- or high-dose PDT conditions (Fig. 3 and Fig 5). Instead, chromatin condensation and DEVDase activation occurred in a dose-dependent fashion.
Bcl-2 photodamage
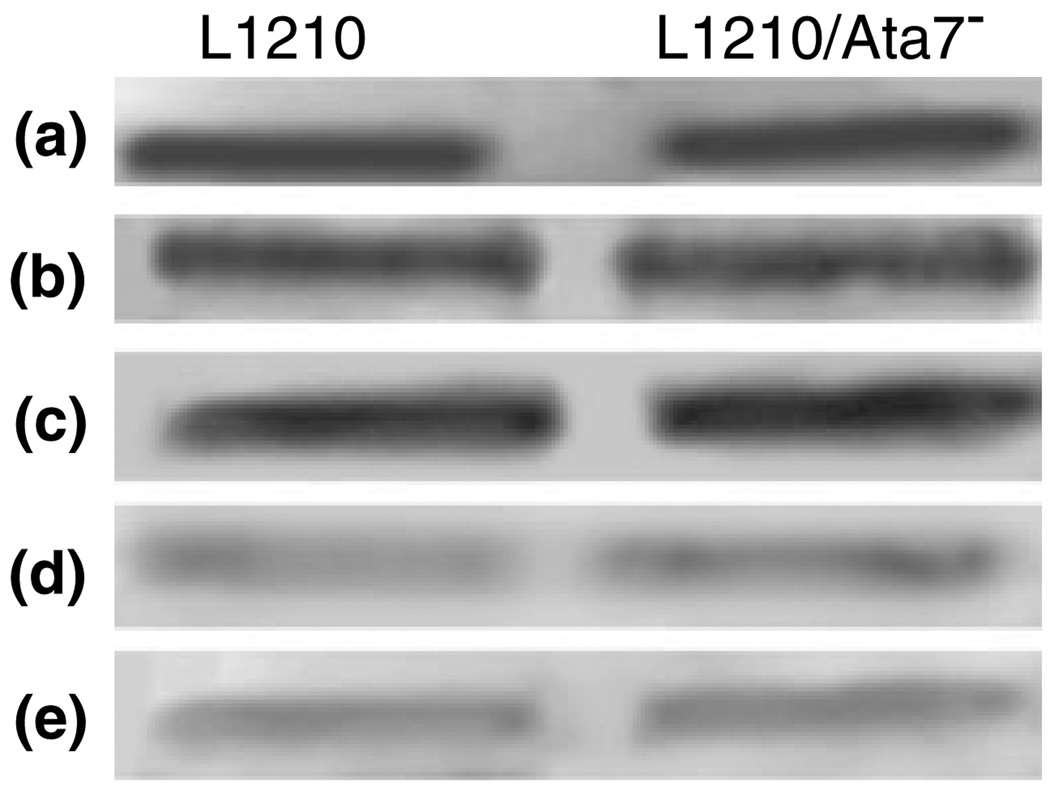
Exposure of MC-sensitized L1210 or L1210/Atg7− cells to low-dose PDT had negligible effects on Bcl-2 levels, as analyzed by Western blotting. However, at the higher PDT dose level, a substantial loss of Bcl-2 protein was detected in both cell lines (Fig. 6). The samples for these analyses were acquired immediately after irradiation, before any additional incubation had occurred. As MC targets mitochondria and CPO the ER, these data suggest that there are approximately equal levels of Bcl-2 associated with each site.
Figure 6.
Western blot analyses of Bcl-2 expressions in L1210 and L1210/Atg7− cells. Cultures were sensitized with MC or CPO and irradiated at low vs high PDT doses (see Table 1 for details). Cultures were harvested immediately after irradiation for analyses of Bcl-2. a = controls, b = MC low dose, c = CPO low dose, d = MC high dose, e = CPO high dose.
DISCUSSION
Cells undergoing autophagy generally swell, develop autophagosomes, and convert LC3-I to LC3-II in a caspase-independent manner (27). We recently reported that L1210 cells undergo both autophagy and apoptosis following PDT with the ER sensitizer CPO (13). An examination of the resulting vacuoles by electron microscopy clearly revealed the double-membrane structure of these vacuoles (28). In the current study, we demonstrate that similar processes occur in L1210 cultures following PDT with the mitochondrial sensitizer MC. We expect that close examination of these vacuoles, perhaps aided by appropriate staining techniques, will reveal evidence that mitochondria are being recycled after photodamage by MC, and ER after CPO, but this has not yet been established.
Molecular (18,19) and pharmacological (23) approaches for reducing Bcl-2 expression, or modulating its activity, have been shown to induce autophagy in several cell types, including L1210. Low-dose PDT conditions had little effect upon the Bcl-2 content of MC-sensitized L1210 cultures, whereas dramatic reductions were observed following treatment with PDT doses in the LD90 range. Hence, it was surprising to observe the rapid induction (within 30 min of irradiation) of autophagy in cultures treated with low- but not high-dose PDT. Although speculative, it is conceivable that in addition to Bcl-2, high-dose PDT initially damages proteins needed for the development of autophagy. Cells that survive the rapid apoptotic death caused by high PDT doses may subsequently recover sufficiently to mount an autophagic response at a latter time. As for the autophagic phenotype occurring shortly after low-dose PDT, presumably other signals over-ride suppressive effects that Bcl-2 normally has on the initiation of autophagy.
The question of whether autophagy functions as a pro-survival or death pathway following stressor insult is a ‘‘hot topic’’ (11,29,30). Our comparative analyses of L1210 and L1210/Atg7− survival suggest that the induction of autophagy clearly plays a pro-survival role following low dose irradiation in PDT protocols using either CPO or MC as the sensitizer. Specifically, PDT conditions causing 5% killing in the L1210 line promoted 20% killing, and more apoptosis in the L1210/Atg7− line. After high-dose (LD90) PDT, both cell lines initiated a strong and rapid apoptotic response, with the level of DEVDase activation greater in the L1210/Atg7− line. In high-dose L1210 treatment groups, the preponderance of cells observed 24 h after irradiation had autophagic characteristics. Presumably, autophagic death of these cells ultimately occurs. In this regard, we also have implicated autophagy in the death of L1210 cultures by two chemotherapeutic anti-tumor agents (20).
Levels of cytosolic calcium ion have been suggested as one determinant of the initiation of autophagy (31), although some questions regarding this interpretation have been raised (32). As the ER is known to be a major site of Ca2+ sequestration, ER photodamage has the potential for promoting both processes via calcium translocation. In a previous report (24), we demonstrated that the level of Ca2+ release after ER photodamage was insufficient to initiate apoptosis. The situation with regard to autophagy is unknown at this time. Another potential factor in the post-PDT death mode is crosstalk between the ER and mitochondria. The relationship of this phenomenon as a determinant of the death mode has recently been reviewed (33). ER-to-mitochondrial signals appear to play a role in the initiation of apoptosis after ER photodamage: loss of Bcl-2 associated with the ER leads to association of Bax and/or Bak with the mitochondrial membrane and a subsequent loss of the membrane potential (16). The role of mitochondrial vs ER Bcl-2 in the initiation of autophagy is also unknown. Is autophagy after PDT mainly a consequence of organelle photodamage, or does the loss of Bcl-2 protein also play a role?
In summary, our studies suggest that autophagy can function as either a pro-survival or death pathway in PDT protocols. At sub-optimal PDT doses autophagy plays a protective role, perhaps by promoting the rapid removal of photodamaged mitochondria or ER, and thereby preventing the initiation of those apoptotic pathways triggered by damage to these organelles. At optimal PDT doses, both apoptosis and autophagy may contribute to cell death. Autophagic cell death is likely germane to the processes whereby PDT is cytotoxic to cells defective in apoptosis. Two studies have demonstrated a PDT-induced autophagic phenotype in apoptosis-resistant cells, and their death, following treatment with ER sensitizers and irradiation (13,14). Lastly, it should be noted that the induction of autophagy in PDT protocols may have an additional therapeutic benefit. Autophagic cells exhibit enhanced presentation of processed cytosolic and membrane peptides associated with the major histocompatibility complex class II complex (34). This has the potential for leading to enhanced immunologic recognition and elimination of tumor cells.
Acknowledgements
These studies were supported by grant CA 23378 from the NIH. Ann Marie Santiago and Nakaiya Okan-Mensah provided excellent technical assistance during the course of this work. We thank Dr. James Hatfield, Department of Pathology at the John Dingell VA Hospital, for electron microscopy image acquisition.
Footnotes
This invited paper is part of the Symposium-in-Print: Photodynamic Therapy.
REFERENCES
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR, Oleinick NL. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991;51:5993–5996. [PubMed] [Google Scholar]
- 3.Whitacre CM, Satoh TH, Xue L, Gordon NH, Oleinick NL. Photodynamic therapy of human breast cancer xenografts lacking caspase-3. Cancer Lett. 2002;179:43–49. doi: 10.1016/s0304-3835(01)00853-9. [DOI] [PubMed] [Google Scholar]
- 4.Chiu SM, Xue LY, Usuda J, Azizuddin K. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br. J. Cancer. 2003;89:1590–1597. doi: 10.1038/sj.bjc.6601298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu SM, Xue LY, Azizuddin K, Oleinick NL. Photodynamic therapy-induced death of HCT 116 cells: Apoptosis with or without Bax expression. Apoptosis. 2005;10:1357–1368. doi: 10.1007/s10495-005-2217-0. [DOI] [PubMed] [Google Scholar]
- 6.Xue LY, Chiu SM, Oleinick NL. Photodynamic therapy-induced death of MCF-7 human breast cancer cells: A role for caspase-3 in the late steps of apoptosis but not for the critical lethal event. Exp. Cell Res. 2001;263:145–155. doi: 10.1006/excr.2000.5108. [DOI] [PubMed] [Google Scholar]
- 7.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky DJ. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005;118(Pt 1):7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 11.Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Lindsten T, Thompson CB. Cell death in the absence of bax and bak. Cell Death Differ. 2000;13:1272–1276. doi: 10.1038/sj.cdd.4401953. [DOI] [PubMed] [Google Scholar]
- 13.Kessel D, Vicente MG, Reiners JJ., Jr Initiation of apoptosis and autophagy by photodynamic therapy. Lasers Surg. Med. 2006;38:482–488. doi: 10.1002/lsm.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I, Grooten J, Agostinis P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006;20:756–758. doi: 10.1096/fj.05-4305fje. [DOI] [PubMed] [Google Scholar]
- 15.Kim HR, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999;59:3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 16.Kessel D, Castelli M. Evidence that bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochem. Photobiol. 2001;74:318–322. doi: 10.1562/0031-8655(2001)074<0318:etbitt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20:3420–3427. doi: 10.1038/sj.onc.1204441. [DOI] [PubMed] [Google Scholar]
- 18.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Saeki K, Yuo A, Okuma E, Yazaki Y, Susin SA, Kroemer G, Takaku F. Bcl-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ. 2000;7:1263–1269. doi: 10.1038/sj.cdd.4400759. [DOI] [PubMed] [Google Scholar]
- 20.Kessel D, Reiners JJ, Jr, Hazeldine ST, Horwitz JP. The role of autophagy in the death of L1210 leukemia cells initiated by the new anti-tumor agents XK469 and SH80. Mol. Cancer Ther. 2007;6:370–379. doi: 10.1158/1535-7163.MCT-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toledano H, Edrai R, Kimel S. Photodynamic damage by liposome-bound porphycenes: Comparison between in vitro and in vivo models. J. Photochem. Photobiol. B, Biol. Sci. 1998;42:20–27. doi: 10.1016/S1011-1344(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 22.Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM. The dynamics of autophagy visualized in live cells: From autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. doi: 10.4161/auto.1.1.1495. [DOI] [PubMed] [Google Scholar]
- 23.Kessel D, Reiners JJ., Jr Initiation of apoptosis and autophagy by the Bcl-2 antagonist HA14-1. Cancer Lett. 2007 doi: 10.1016/j.canlet.2006.09.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessel D, Castelli M, Reiners JJ., Jr Ruthenium red-mediated suppression of Bcl-2 loss and Ca2+ release initiated by photodamage to the endoplasmic reticulum: Scavenging of reactive oxygen species. Cell Death Differ. 2005;12:502–511. doi: 10.1038/sj.cdd.4401579. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 2005;280:31582–31586. doi: 10.1074/jbc.M506736200. [DOI] [PubMed] [Google Scholar]
- 27.Misushima N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Kessel D, Vicente MGH, Reiners JJ., Jr Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy. 2006;2:289–290. doi: 10.4161/auto.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B, Yuan J. Autophagy in cell death: An innocent convict? J. Clin. Invest. 2005;115:2679–2788. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskelinen E-L. Doctor Jeckyll and Mister Hyde: Autophagy can promote both cell survival and cell death. Cell Death Differ. 2005;12:1468–1372. doi: 10.1038/sj.cdd.4401721. [DOI] [PubMed] [Google Scholar]
- 31.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Swerdlow S, Distelhorst CW. Bcl-2-regulated calcium signals as common mediators of both apoptosis and autophagy. Dev. Cell. 2007;12:178–179. doi: 10.1016/j.devcel.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Walter L, HajnÓczky G. Mitochondria and endo-plasmic reticulum: The lethal interorganelle cross-talk. J. Bioenerg. Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- 34.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Mulle M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl Acad. Sci. USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]