Abstract
Purpose
To undertake mutation screening in the connexin 46 (GJA3) gene in seven congenital cataract families of Indian origin.
Methods
Seven Indian families with congenital cataract were analyzed by detailed family history and clinical evaluation. Each family had two to five affected members. Mutation screening was carried out in the candidate gene, connexin 46 (GJA3), using bidirectional sequencing of amplified products. Segregation of the observed change with the disease phenotype was further tested by restriction fragment length polymorphism (RFLP).
Results
Sequencing of the coding region of GJA3 showed the presence of a novel, heterozygous C260T change in one family (CC-472) who had two affected members. The cataract phenotype gave the appearance like a "pearl box" in these two affected individuals of this family. The observed C260T substitution created a novel restriction enzyme site for NlaIII and resulted in substitution of highly conserved threonine at position 87 by methionine (T87M). NlaIII restriction digestion analysis revealed this nucleotide change was not in unaffected members of this family or in 100 unrelated control subjects (200 chromosomes) with the same ethnic background.
Conclusions
This is a novel mutation identified in the second transmembrane domain of the connexin 46. These findings thus expand the mutation spectrum of the GJA3 in association with congenital cataract.
Introduction
Congenital cataract is one cause of childhood blindness worldwide. It is clinically and genetically a highly heterogeneous lens disorder, with autosomal dominant inheritance being most common. Congenital cataract can occur either as an isolated anomaly or in association with other ocular anomalies or as a component of multisystemic disorders. Its incidence is estimated to be between 2.2 and 2.49 per 10,000 infants [1,2]. In one-third of the cases worldwide, congenital cataract has been reported as familial [3,4]. At least 35 loci and mutations in 15 genes have been identified as being involved in the pathogenesis of various forms of congenital and developmental cataracts [5].
The eye lens, an avascular organ, is highly dependent on intercellular communication for volume regulation and metabolic homeostasis [6]. This is achieved by cell-to-cell communication via gap junctions, which are encoded by the connexin genes. These gap junctions facilitate the exchange of ions, metabolites, signaling molecules, and other molecules that have a molecular weight up to 1 kDa between adjacent cells [7]. In humans, more than 20 genes coding connexins of varying molecular mass ranging between 25-62 kDa have been identified. Three of these, connexin 43, connexin 46, and connexin 50, are expressed in the lens [8]. Mutations in either connexin 46 or in connexin 50 have so far been linked with congenital cataract [9,10].
The aim of present study was to identify the mutations in the connexin 46 (GJA3) gene in seven congenital cataract families of Indian origin. Upon sequence analysis of the GJA3, we identified a heterozygous C260T change resulting in the substitution of a highly conserved threonine by methionine at codon 87 (T87M) in the affected individuals of one family (CC-472). The change cosegregated completely with the disease phenotype. This is a novel mutation and has not been reported previously with congenital cataract.
Methods
Clinical evaluation and collection of genetic material
The present study was undertaken in collaboration with Dr. Daljit Singh Eye Hospital, Amritsar, Punjab (India). The study protocols adhered to the tenets of the Declaration of Helsinki and were approved by the institutional review board of Guru Nanak Dev University, Amritsar. A pediatric ophthalmologist performed a detailed clinical examination on each proband and selected relatives. The exam included Snellen visual acuity, A-scan ultrasonography, and slit-lamp examination with pupil dilation. The phenotypes were documented using slit-lamp photography. A detailed family history and pedigree were recorded for each case. In the present study we analyzed seven congenital cataract families, each of whom had two to five affected members. Four families had the autosomal dominant mode of inheritance and three presented with the autosomal recessive inheritance pattern. After affected and unaffected family members gave informed consent, 5-10 ml venous blood was drawn, and DNA was extracted for subsequent molecular genetic analysis.
PCR and DNA sequencing
GJA3 (GenBank NM_021954), located at 13q11 and consisting of a single coding exon encoding 435 amino acids, was sequenced using previously published primer sequences [11]. Genomic DNA from two affected and one unaffected individual from each family were amplified. PCR and sequencing reactions were performed following conditions detailed elsewhere [12,13]. Electrophoresis of purified sequencing reaction products was performed on 5% urea-polyacrylamide gel on ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA), and data was analyzed using sequence analysis software version 3.4.1 (Applied Biosystems).
Restriction endonuclease analysis
The DNA fragment harboring the mutation was amplified for both affected and unaffected family members, and PCR products were digested with NlaIII restriction enzyme following directions given by the manufacturer (New England Biolabs, Beverly, MA). Restriction digestion products were analyzed on a 2.5% agarose gel. In addition, 100 unrelated controls from similar ethnic background were also similarly tested.
Results
In the present study, four families with autosomal-dominant congenital cataract and three families with autosomal-recessive congenital cataract were investigated. Each family had 2-5 affected members with different clinical morphologies (one family each with pearl box, posterior cortical, granular, absorbed membranous, and round ball cataract and two autosomal-recessive families with posterior subcapsular type cataract). Mutation analyses of the connexin 46 (GJA3) gene in these seven families revealed a novel heterozygous mutation in only one family (CC-472). In this family, a son and his mother were affected with bilateral congenital cataract (Figure 1A). The opacities involved both the embryonal and fetal nuclei separated by a clear space. Fine white spots made up the opacity of the fetal nucleus (Figure 1B). The embryonal nucleus was composed of coarse white spots of various sizes that were round when located toward the center and linear-shaped in the periphery. There was an optically empty space between the embryonal nuclear and fetal nuclear opacities. The peripheral zone outside the fetal nucleus was clear. The cataract phenotype gave the appearance of a box produced by the fetal nuclear opacities and pearls by the embryonal nuclear opacities (Figure 1C). Hence, this phenotype was termed "pearl box" cataract.
Figure 1.
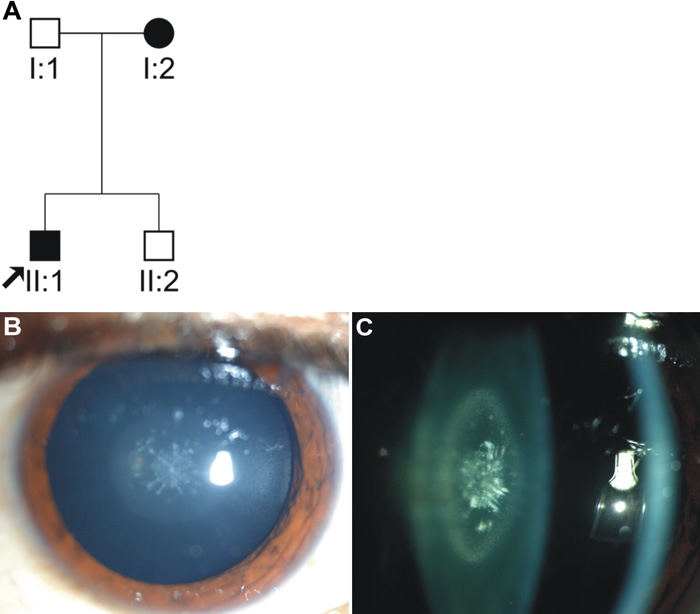
Pedigree of family CC-472 and lens photograph of proband. A: Pedigree of family CC-472 with two affected individuals: mother (I:2) and son (II:1). Circle represents females while squares indicate males. Shaded shapes indicate affected individuals. B: Oblique illumination of phenotype (II:1) showing white spots of different sizes and shapes in the fetal nucleus. C: Optical section showing wide surface opacity of the fetal nucleus composed of fine white spots. The space between the surface opacity and central white spots is optically empty.
Direct sequencing of GJA3 revelaed a novel heterozygous C>T transition (Figure 2A) at position 260 (c. 260C>T) in the affected individuals of CC-472 family. It is this transition that led to the replacement of highly conserved threonine with methionine at codon 87 (Thr87Met). This substitution created a novel NlaIII restriction site that segregated completely with the disease phenotype in this family (Figure 2C). This nucleotide change was not observed in the 100 control subjects from similar ethnic background tested by restriction digestion analysis (data not shown). Testing of six additional families revealed no disease-linked change or any polymorphic change in GJA3.
Figure 2.

Mutation analysis of family CC-472. A: DNA sequence of a portion of GJA3, showing the heterozygous 260C>T transition that changes threonine to methionine at codon 87 in affected individual (II:1). B: DNA sequence electropherogram of unaffected individual (I:1). C: NlaIII restriction digestion analysis of amplified DNA at the mutation site. The 308 bp PCR product is cleaved into four fragments (306 bp, 261 bp, 45 bp, and 2 bp) in affected individuals (I:2 and II:1) and into two fragments (306 bp and 2 bp) in unaffected individuals (I:1 and II:2).
Discussion
We report a novel change, T87M in the connexin 46 gene, in one family (CC-472) who has what we term "pearl box" cataract. The T87M substitution is likely to be disease causative as it segregated among the affected members only and was not detected either in unaffected family members or in 100 unrelated controls. The 260C>T substitution observed in the present study resulted in the replacement of polar amino acid threonine (T) with nonpolar amino acid methionine (M) at codon 87 (T87M) localized in the second transmembrane domain (M2) of the connexin 46 polypeptide (Figure 3). This is the first identified mutation that lies within in the second transmembrane domain (M2) of the connexin 46 polypeptide associated with novel "pearl box" cataract.
Figure 3.
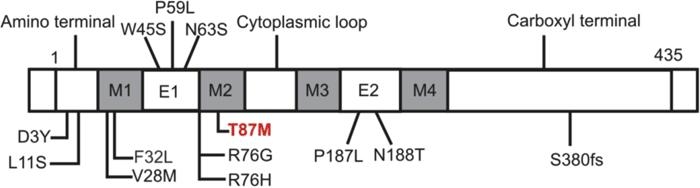
Schematic diagram of the connexin 46 polypeptide and locations of identified mutations. Connexin 46 polypeptide (435 amino acids) has nine structural domains including a cytoplasmic NH2-terminus, four transmembrane domains (M1-M4), two extracellular loops (E1-E2), a cytoplasmic loop, and a cytoplasmic COOH-terminus (modified figure from Bennett et al. [31]). The relative location of the T87M mutation (indicated in red) and other reported mutations associated with congenital cataracts in humans are marked.
A multiple sequence alignment of the amino acid sequences of connexin 46 showed that threonine at position 87 is phylogenetically conserved in the second transmembrane domain of connexin 46 in different species (Figure 4A) and also in different human α-connexins (Figure 4B). This indicates that the threonine at position 87 in connexin 46 is likely to be functionally important and the T87M mutation may therefore has a detrimental physiological effect. In the connexin 50 (GJA8) gene localized at 1q21, corresponding mutations (P88S and P88Q) in the second transmembrane domain (M2) resulting in zonular pulverulent [14] and lamellar pulverulent cataract [15], respectively, have been reported. Pal et al. [16] further reported that P88S mutant connexin 50 abolishes the function of the gap junction channel and acts in a dominant negative manner. In humans, P87S and P87A mutations located within M2 of the connexin 32 (GJB1) gene have been reported in association with Schwann cell dysfunction and peripheral nerve degeneration in X-linked Charcot-Marie-Tooth disease [17-19]. The authors hypothesized that these mutations (P87S and P87A) in M2 of the connexin 32 polypeptide may impair voltage-dependent opening and closing of gap junctions in electrically excitable tissues. Suchyna et al. [20] have also demonstrated that in the connexin 26 polypeptide proline at codon 87 has a function in voltage-gating of gap junctions. Although, the effect of T87M mutation observed in the present study on the activity of the connexin 46 has not been directly tested, we speculate that, like P88S in the GJA8 and other dominantly inherited mutations reported in different connexins, this mutation also results in inappropriate association of connexins and alters the function of endogenous wild-type connexins in the affected individuals in a dominant negative way.
Figure 4.
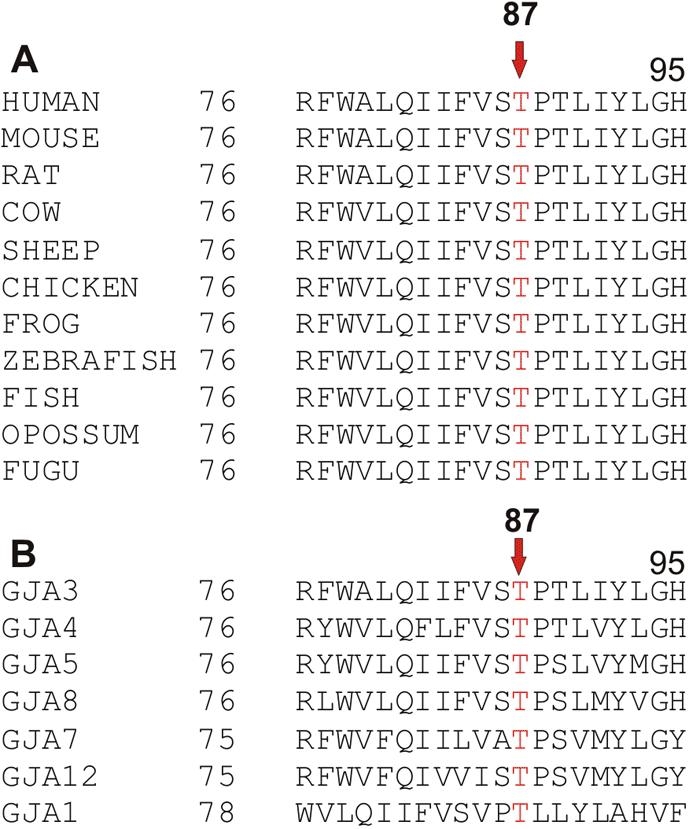
A multiple sequence alignment of amino acid sequences of connexin 46 in different species and in different human alpha-connexins. Alignment data indicate that threonine is highly conserved in different species (A) and in different human alpha-connexins (B). Arrow points to the conserved threonine at position 87, which is marked in red.
Defects in the connexin 46 and connexin 50 genes have been reported to cause cataract in mice. Point mutations A47C and V64A in the connexin 50 gene have been reported to result, respectively, in nuclear opacities (No2) and nuclear with posterior suture opacities (Aey5) in mice [21,22]. Gong et al. [23] reported that mice homozygous for disrupted connexin 46 developed nuclear cataracts due to failure in maintenance of differentiation and of crystallins solubility, while connexin 50 knockout mice had reduced ocular growth along with nuclear cataract [24,25].Targeted replacement of connexin 50 with connexin 46 in mice has revealed the role of connexin 50 in lens and eye growth and that of connexin 46 in maintaining differentiation by nonspecific restoration of intercellular communication [26].
The cataract phenotypes that are reported to be linked with the GJA3 mutations share genotype-phenotype similarities to some extent, but they also exhibit some differences with respect to the appearance and location of opacities within the lens. At this point, 12 mutations in GJA3 have been reported to be associated with autosomal-dominant congenital cataract in humans involving different domains of connexin 46 polypeptide (Table 1). Most of the cataract phenotypes linked with mutations in the GJA3 are of nuclear or zonular pulverulent types. The phenotype observed in present study (CC-472 family) is different in its appearance from the earlier reported types (Table 1) as it appears like pearls in a box (Figure 1B,C). The differences in the morphologies of cataract phenotypes associated with mutations in the GJA3 in different families may be attributed to the action of modifier genes or environmental factors that could affect the expression of the connexin 46 gene and hence resulting cataract types.
Table 1. Reported mutations in GJA3 associated with different congenital cataract phenotypes in different families.
|
Amino acid change |
Location |
Cataract type |
Phenotype description |
Family origin |
Reference |
| D3Y |
-NH2 terminal cytoplasmic loop |
Zonular pulverulent |
Progressive zonular pulverulent cataract |
Flispanic |
[27] |
| L11S |
-NH2 terminal cytoplasmic loop |
Ant-egg |
Lamellar cataract with dense ant-egg like structures imbedded in the lens, primarily confined to the perinuclear layers and to lesser degree in the fetal nucleus |
Danish |
[9] |
| V28M |
First transmembrane domain |
Variable |
Variable cataract types like total, anterior capsular cataract with posterior cortical opacities in different individuals |
Indian |
[28] |
| F32L |
First transmembrane domain |
Nuclear pulverulent |
Punctate opacities in the central zone of the lens limited to the embryonal nucleus |
Chinese |
[29] |
| W45S |
First extracellular loop |
Nuclear |
Progressive nuclear cataract |
Chinese |
[30] |
| P59L |
First extracellular loop |
Nuclear punctate |
Coarse punctate opacities located in the central or nuclear region of the lens |
American |
[31] |
| N63S |
First extracellular loop |
Zonular pulverulent |
Coarse and granular opacities in the central zone of the lens. Fine dust-like opacities predominated in die peripheral zone of the lens. |
Caucasian |
[11] |
| R76G |
Boundary of first extracellular loop and second transmembrane domain |
Total |
Total leas opacification |
Indian |
[28] |
| R76H |
Boundary of first extracellular loop and second transmembrane domain |
Nuclear pulverulent |
Faint lamellar nuclear opacity surrounding pulverulent nuclear opacities, some with fine gold dots or haze and some with needle-like peripheral riders |
Australian |
[32] |
| T87M |
Second transmembrane domain |
Pearl box |
A bunch of white spots seen in the embryonal nucleus. The central white spots distributed in a radial manner. The space between the surface opacity and central white spots is optically empty. Surface opacity gives the appearance of a box while central white spots as of pearls in it. |
Indian |
Present study |
| P187L |
Second extracellular loop |
Zonular pulverulent |
Central dust-like opacity affecting the embryonal, fetal and infantile nucleus of the lens surrounded by snowflake-like opacities in the anterior and posterior cortical region of the lens |
Caucasian |
[33] |
| N188T |
Second extracellular loop |
Nuclear pulverulent |
Progressive, central pulverulent opacity affecting the embryonal, fetal and infantile nucleus of the lens |
Chinese |
[34] |
| S380fs | -COOH terminal cytoplasmic loop | Zonular pulverulent | Coarse and granular opacities in the central zone of the lens. Fine dust-like opacities predominated in die peripheral zone of the lens. | Caucasian | [11] |
Mutation spectrum of the connexin 46 (GJA3) gene and cataract phenotypes in different congenital cataract families.
In summary, we describe a novel heterozygous T87M mutation in the connexin 46 polypeptide associated with "pearl box" cataract. On the basis of observed phenotypic as well as genotypic variability as compared to previously published reports, the present study further expands the genetic and phenotypic heterogeneity of congenital cataract.
Acknowledgements
We thank the patients and their family members for taking part in this study. This work was supported by the CSIR grant 5/258/20/2003-NMITLI and DBT/BT/IN/FRG/JRS/2003-04 sanctioned to J.R.S.
References
- 1.Rahi JS, Dezateux C, British Congenital Cataract Interest Group. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42:1444–8. [PubMed] [Google Scholar]
- 2.Wirth MG, Russell-Eggitt IM, Craig JE, Elder JE, Mackey DA. Aetiology of congenital and paediatric cataract in an Australian population. Br J Ophthalmol. 2002;86:782–6. doi: 10.1136/bjo.86.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund AM, Eiberg H, Rosenberg T, Warburg M. Autosomal dominant congenital cataract; linkage relations; clinical and genetic heterogeneity. Clin Genet. 1992;41:65–9. doi: 10.1111/j.1399-0004.1992.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 4.Vanita, Singh JR, Singh D. Genetic and segregation analysis of congenital cataract in the Indian population. Clin Genet. 1999;56:389–93. doi: 10.1034/j.1399-0004.1999.560507.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Guo X, Xiao X, Yi J, Jia X, Hejtmancik JF. Clinical description and genome wide linkage study of Y-sutural cataract and myopia in a Chinese family. Mol Vis. 2004;10:890–900. http://www.molvis.org/molvis/v10/a107/ [PubMed] [Google Scholar]
- 6.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 8.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 9.Hansen L, Yao W, Eiberg H, Funding M, Riise R, Kjaer KW, Hejtmancik JF, Rosenberg T. The congenital "ant-egg" cataract phenotype is caused by a missense mutation in connexin46. Mol Vis. 2006;12:1033–9. http://www.molvis.org/molvis/v12/a116/ [PubMed] [Google Scholar]
- 10.Vanita V, Hennies HC, Singh D, Nurnberg P, Sperling K, Singh JR. A novel mutation in GJA8 associated with autosomal dominant congenital cataract in a family of Indian origin. Mol Vis. 2006;12:1217–22. http://www.molvis.org/molvis/v12/a138/ [PubMed] [Google Scholar]
- 11.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–64. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanita V, Singh D, Robinson PN, Sperling K, Singh JR. A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant "cerulean cataract" in an Indian family. Am J Med Genet A. 2006;140:558–66. doi: 10.1002/ajmg.a.31126. [DOI] [PubMed] [Google Scholar]
- 13.Vanita V, Hejtmancik JF, Hennies HC, Guleria K, Nurnberg P, Singh D, Sperling K, Singh JR. Sutural cataract associated with a mutation in the ferritin light chain gene (FTL) in a family of Indian origin. Mol Vis. 2006;12:93–9. http://www.molvis.org/molvis/v12/a10/ [PubMed] [Google Scholar]
- 14.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant "zonular pulverulent" cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–32. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol. 1999;276:C1443–6. doi: 10.1152/ajpcell.1999.276.6.C1443. Erratum in: Am J Physiol 1999; 277:section C. [DOI] [PubMed] [Google Scholar]
- 17.Bort S, Nelis E, Timmerman V, Sevilla T, Cruz-Martinez A, Martinez F, Millan JM, Arpa J, Vilchez JJ, Prieto F, Van Broeckhoven C, Palau F. Mutational analysis of the MPZ, PMP22 and Cx32 genes in patients of Spanish ancestry with Charcot-Marie-Tooth disease and hereditary neuropathy with liability to pressure palsies. Hum Genet. 1997;99:746–54. doi: 10.1007/s004390050442. [DOI] [PubMed] [Google Scholar]
- 18.Janssen EA, Kemp S, Hensels GW, Sie OG, de Die-Smulders CE, Hoogendijk JE, de Visser M, Bolhuis PA. Connexin32 gene mutations in X-linked dominant Charcot-Marie-Tooth disease (CMTX1). Hum Genet. 1997;99:501–5. doi: 10.1007/s004390050396. [DOI] [PubMed] [Google Scholar]
- 19.Nelis E, Simokovic S, Timmerman V, Lofgren A, Backhovens H, De Jonghe P, Martin JJ, Van Broeckhoven C. Mutation analysis of the connexin 32 (Cx32) gene in Charcot-Marie-Tooth neuropathy type 1: identification of five new mutations. Hum Mutat. 1997;9:47–52. doi: 10.1002/(SICI)1098-1004(1997)9:1<47::AID-HUMU8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Suchyna TM, Xu LX, Gao F, Fourtner CR, Nicholson BJ. Identification of a proline residue as a transduction element involved in voltage gating of gap junctions. Nature. 1993;365:847–9. doi: 10.1038/365847a0. [DOI] [PubMed] [Google Scholar]
- 21.Steele EC, Jr, Lyon MF, Favor J, Guillot PV, Boyd Y, Church RL. A mutation in the connexin 50 (Cx50) gene is a candidate for the No2 mouse cataract. Curr Eye Res. 1998;17:883–9. doi: 10.1076/ceyr.17.9.883.5144. [DOI] [PubMed] [Google Scholar]
- 22.Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, Hrabe de Angelis M. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res. 2001;73:867–76. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- 23.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–43. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 24.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–25. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–74. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 26.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 27.Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis. 2006;12:791–5. http://www.molvis.org/molvis/v12/a88/ [PubMed] [Google Scholar]
- 28.Devi RR, Reena C, Vijayalakshmi P. Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol Vis. 2005;11:846–52. http://www.molvis.org/molvis/v11/a100/ [PubMed] [Google Scholar]
- 29.Jiang H, Jin Y, Bu L, Zhang W, Liu J, Cui B, Kong X, Hu L. A novel mutation in GJA3 (connexin46) for autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2003;9:579–83. http://www.molvis.org/molvis/v9/a70/ [PubMed] [Google Scholar]
- 30.Ma ZW, Ma Z, Zheng JQ, Zheng J, Yang F, Li J, Ji J, Li XR, Li X, Tang X, Yuan XY, Yuan X, Zhang XM, Zhang X, Sun HM, Sun H. Two novel mutations of connexin genes in Chinese families with autosomal dominant congenital nuclear cataract. Br J Ophthalmol. 2005;89:1535–7. doi: 10.1136/bjo.2005.075184. Erratum in: Br J Ophthalmol. 2006; 90:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett TM, Mackay DS, Knopf HL, Shiels A. A novel missense mutation in the gene for gap-junction protein alpha3 (GJA3) associated with autosomal dominant "nuclear punctate" cataracts linked to chromosome 13q. Mol Vis. 2004;10:376–82. http://www.molvis.org/molvis/v10/a47/ [PubMed] [Google Scholar]
- 32.Burdon KP, Wirth MG, Mackey DA, Russell-Eggitt IM, Craig JE, Elder JE, Dickinson JL, Sale MM. A novel mutation in the Connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J Med Genet. 2004;41:e106. doi: 10.1136/jmg.2004.018333. Erratum in: J Med Genet. 2005; 42:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees MI, Watts P, Fenton I, Clarke A, Snell RG, Owen MJ, Gray J. Further evidence of autosomal dominant congenital zonular pulverulent cataracts linked to 13q11 (CZP3) and a novel mutation in connexin 46 (GJA3). Hum Genet. 2000;106:206–9. doi: 10.1007/s004390051029. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Wang J, Dong B, Man H. A novel connexin46 (GJA3) mutation in autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2004;10:668–71. http://www.molvis.org/molvis/v10/a80/ [PubMed] [Google Scholar]


