Abstract
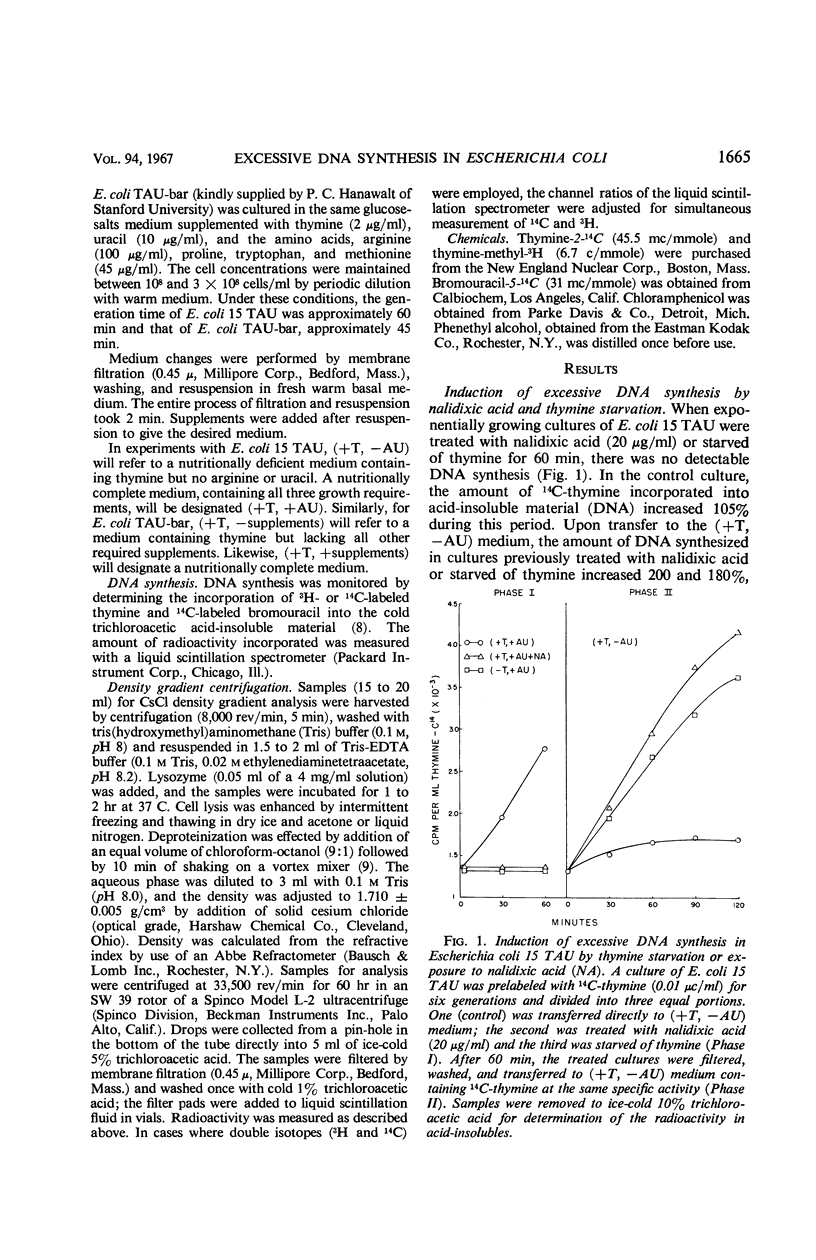
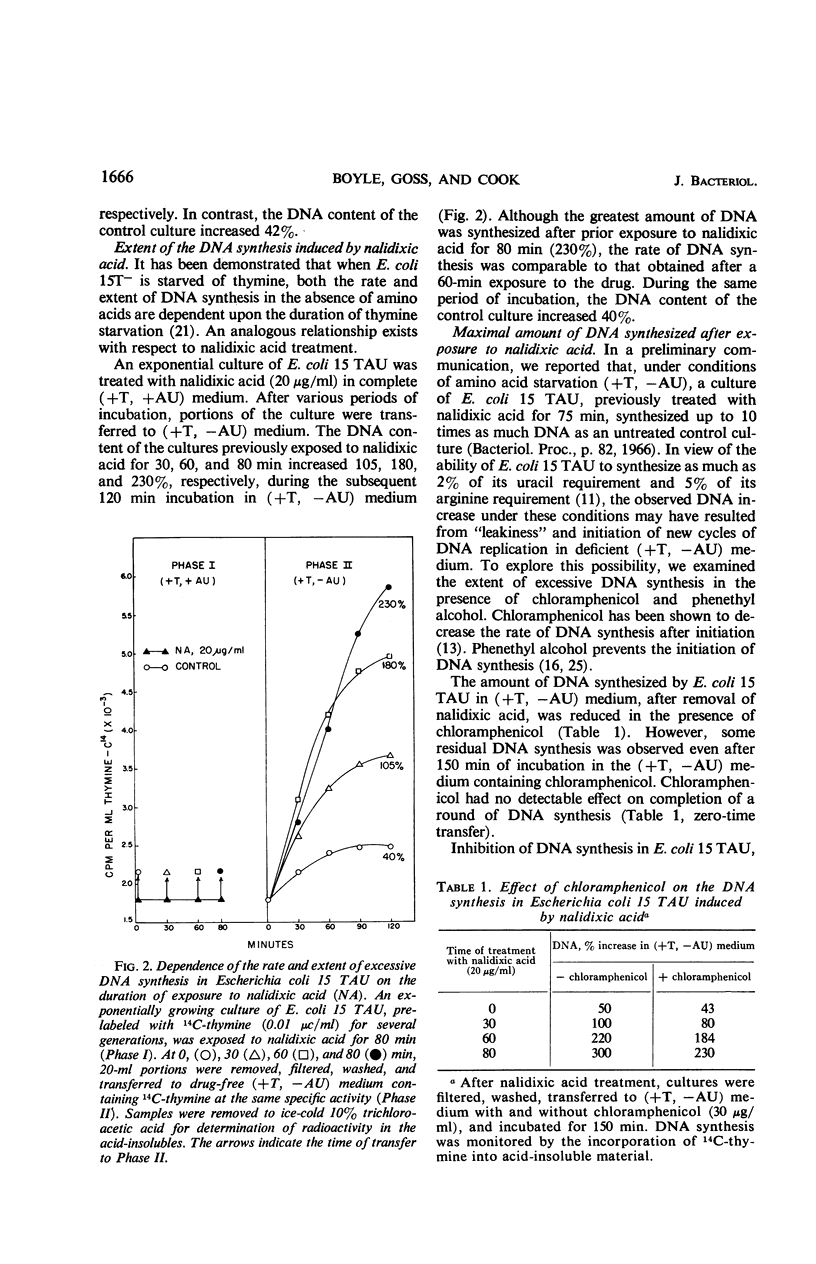
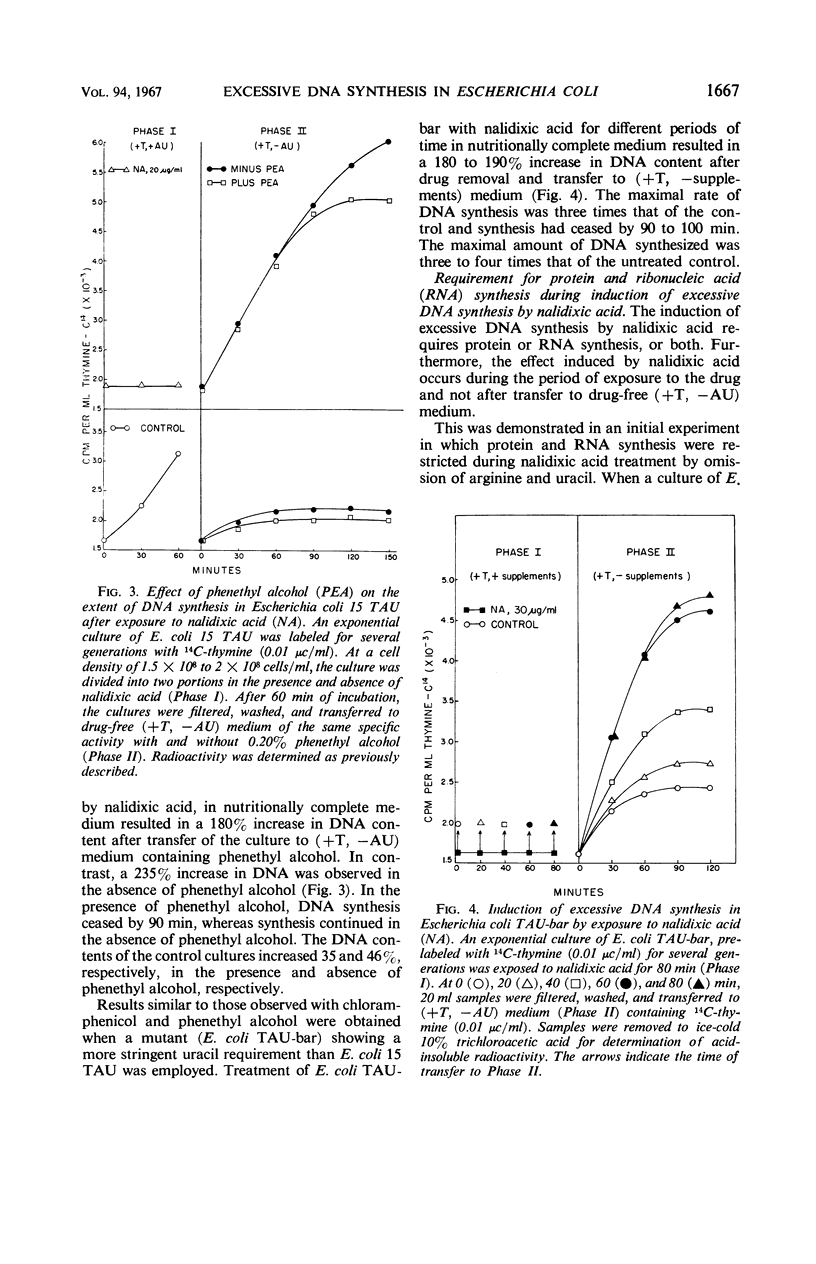
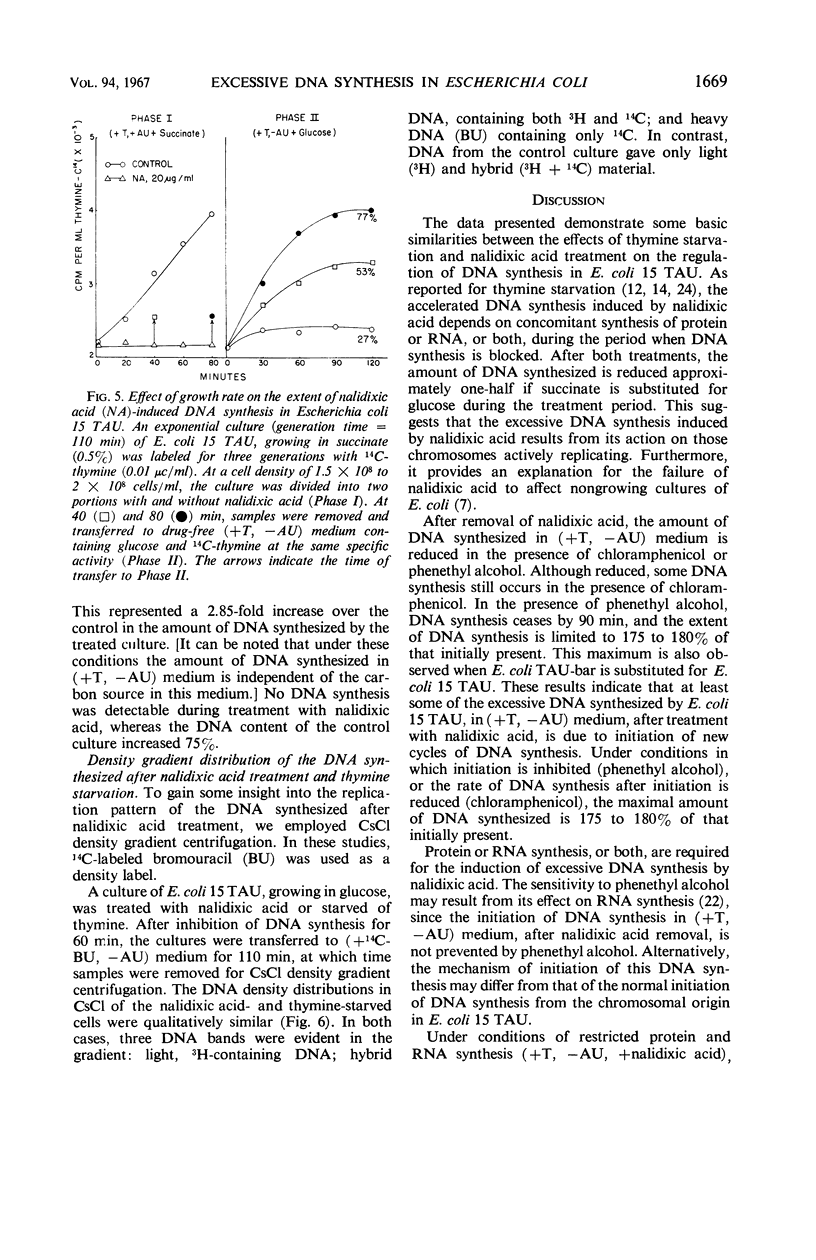
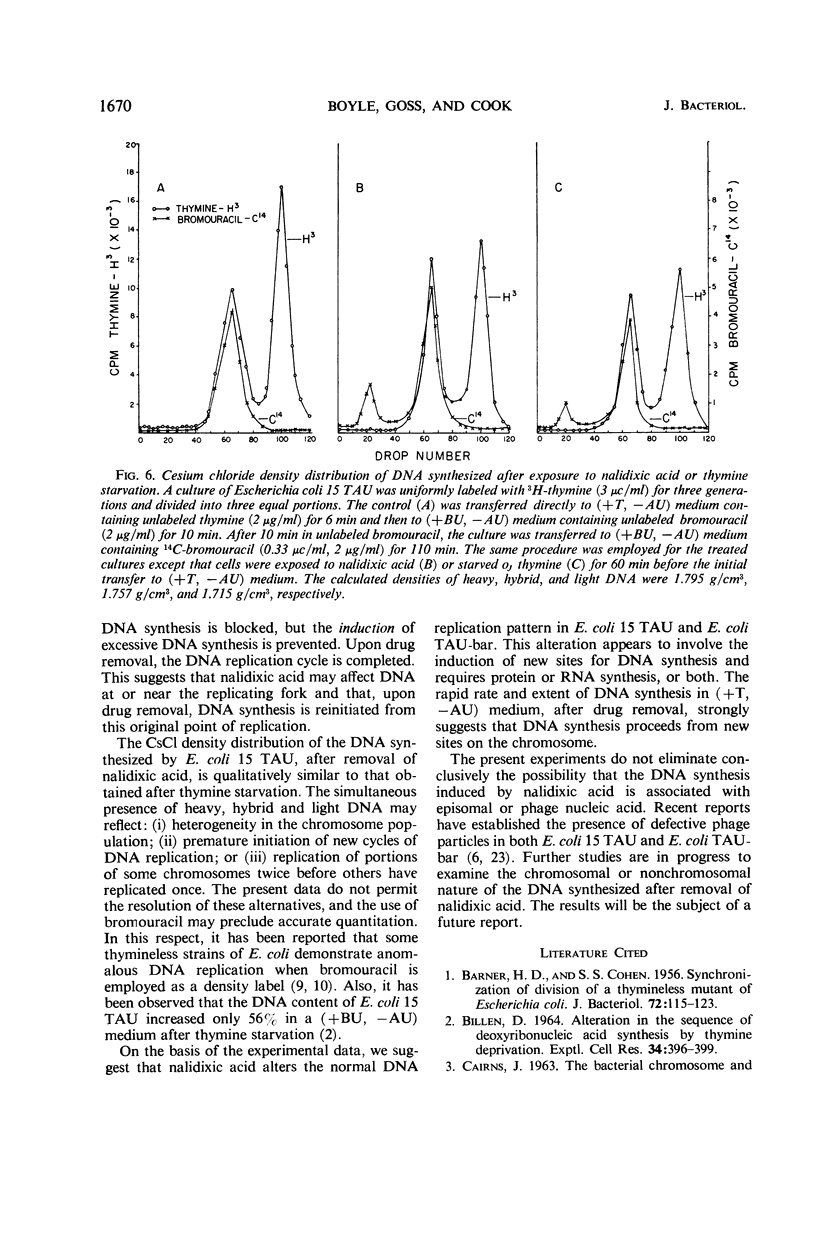
Prior treatment of Escherichia coli with nalidixic acid in nutritionally complete medium altered the subsequent pattern of deoxyribonucleic acid (DNA) synthesis normally observed in nutritionally deficient medium. Transfer of E. coli 15 TAU to an amino acid- and pyrimidine-deficient medium usually resulted in a 40 to 50% increase in DNA content. Previous treatment with nalidixic acid caused a 200 to 300% increase in DNA content under these conditions. The extent of this DNA synthesis depended on the duration of prior exposure to nalidixic acid. The maximal rate of synthesis was obtained after a 40- to 60-min exposure to nalidixic acid and was two to three times that of the control. The induction of this excessive DNA synthesis was prevented by chloramphenicol or phenethyl alcohol, but the synthesis of this DNA was only partially sensitive to these agents. With E. coli TAU-bar, the rate of DNA synthesis, after removal of nalidixic acid, was similar to that of E. coli 15 TAU, but the maximal amount of DNA synthesized was 180 to 185% of that initially present. Cesium chloride density gradient analysis demonstrated that DNA synthesis after removal of nalidixic acid occurs by a semiconservative mode of replication. The density distribution of this DNA was similar to that obtained after thymine starvation. These results suggest that nalidixic acid treatment may induce additional sites for DNA synthesis in E.coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNER H. D., COHEN S. S. Synchronization of division of a thymineless mutant of Escherichia coli. J Bacteriol. 1956 Jul;72(1):115–123. doi: 10.1128/jb.72.1.115-123.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLEN D. ALTERATION IN THE SEQUENCE OF DEOXYRIBONUCLEIC ACID SYNTHESIS BY THYMINE DEPRIVATION. Exp Cell Res. 1964 Apr;34:396–397. doi: 10.1016/0014-4827(64)90374-x. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Cook T. M., Deitz W. H., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. IV. Effects on the stability of cellular constituents. J Bacteriol. 1966 Feb;91(2):774–779. doi: 10.1128/jb.91.2.774-779.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAMPTON E. W., BRINKLEY B. R. EVIDENCE OF LYSOGENY IN DERIVATIVES OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:446–452. doi: 10.1128/jb.90.2.446-452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P., Jr, Hanawalt P. Selectivity for thymine over 5-bromouracil by a thymine-requiring bacterium. Biochim Biophys Acta. 1966 Aug 17;123(2):356–363. doi: 10.1016/0005-2787(66)90288-7. [DOI] [PubMed] [Google Scholar]
- Hewitt R., Suit J. C., Billen D. Utilization of 5-bromouracil by thymineless bacteria. J Bacteriol. 1967 Jan;93(1):86–89. doi: 10.1128/jb.93.1.86-89.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAZIR D., BARNER H. D., FLAKS J. G., COHEN S. S. Some physiological and genetic properties of a strain of Escherichia coli requiring thymine, arginine and uracil. Biochim Biophys Acta. 1959 Aug;34:341–353. doi: 10.1016/0006-3002(59)90287-2. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- LARK K. G. Studies on the mechanism regulating periodic DNA synthesis in synchronized cultures of Alcaligenes fecalis. Biochim Biophys Acta. 1960 Dec 4;45:121–132. doi: 10.1016/0006-3002(60)91432-3. [DOI] [PubMed] [Google Scholar]
- Lark C. Regulation of deoxyribonucleic acid synthesis in Escherichia coli: dependence on growth rates. Biochim Biophys Acta. 1966 Jun 22;119(3):517–525. doi: 10.1016/0005-2787(66)90128-6. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: a comparison of the effects of phenethyl alcohol treatment with those of amino acid starvation. J Mol Biol. 1966 Sep;20(1):9–19. doi: 10.1016/0022-2836(66)90113-6. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: alternate replication of two chromosomes at slow growth rates. J Mol Biol. 1965 Aug;13(1):105–126. doi: 10.1016/s0022-2836(65)80083-3. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA T. The molecular synchrony and sequential replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1963 Apr;49:551–559. doi: 10.1073/pnas.49.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ H. S., CARR H. S., ROSE H. M. PHENETHYL ALCOHOL. I. EFFECT ON MACROMOLECULAR SYNTHESIS OF ESCHERICHIA COLI. J Bacteriol. 1965 May;89:1354–1369. doi: 10.1128/jb.89.5.1354-1369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. J. Some effects of bromouracil on the kinetics of thymineless death in Escherichia coli. J Mol Biol. 1966 Sep;20(1):21–28. doi: 10.1016/0022-2836(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Soska J., Lark K. G. Regulation of nucleic acid synthesis in Lactobacillus acidophilus R-26. Biochim Biophys Acta. 1966 Jun 22;119(3):526–539. doi: 10.1016/0005-2787(66)90129-8. [DOI] [PubMed] [Google Scholar]
- TREICK R. W., KONETZKA W. A. PHYSIOLOGICAL STATE OF ESCHERICHIA COLI AND THE INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS BY PHENETHYL ALCOHOL. J Bacteriol. 1964 Dec;88:1580–1584. doi: 10.1128/jb.88.6.1580-1584.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



