Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Despite significantly improved diagnosis and treatment in recent years, the long-term therapeutic effect is compromised by the frequent recurrence and metastasis, of which the molecular mechanisms are not fully understood. Our initial studies in established HCC cell lines with different metastatic capabilities indicated a correlation of metastasis with the resistance to apoptosis and therefore the ability to survive in stressed conditions. Subsequent investigation revealed that increased expression of X-linked inhibitor-of-apoptosis protein (XIAP) was correlated with the resistance to apoptosis and enhanced invasiveness in vitro, which could contribute to increased metastatic foci in vivo. Furthermore, we found that nearly 90% of clinical samples from advanced HCC patients expressed high levels of XIAP. Patients with XIAP-positive tumors had a significantly increased risk to relapse, which was resulted from metastasis, following total liver resection and orthotopic liver transplantation. Indeed, XIAP expression could be an independent prognostic factor for predicting disease-free survival rate and overall survival rate of these patients. XIAP expression was also highly correlated with advanced cases that exceeded the Milan criteria and could be a prognostic factor for disease-free survival in these patients as well. Conclusion: our studies have revealed an important molecule in controlling HCC metastasis, defined a biomarker that can be utilized to predict HCC recurrence and patient survival following treatment, and suggest that XIAP can be a molecular target subject to intervention to reduce metastasis and recurrence.
Keywords: Apoptosis, liver cancer
Introduction
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide (1). The prognosis of HCC has been significantly improved in recent years due to earlier diagnosis and more effective treatments. However, tumor recurrence and metastasis are still the major obstacles for long term survival (2, 3). Although liver resection followed by liver transplantation is considered as curative treatment for HCC, the overall recurrence rate due to distant metastasis or intra-hepatic reappearance could be as high as 65% and 43% after the procedure, respectively (4). Therefore, elucidating the molecular mechanism of HCC recurrence is important for the development of more effective treatment modalities.
The process of metastasis can be summarized as a series of sequential events that include intravasation, transport via the circulatory system, trapping at a secondary site, extravasation, and growth in the secondary site (5). Metastasis is actually an inefficient process by which only a small percentage of tumor cells that leave the primary site could survive to form metastatic foci at new sites (6). It is likely that metastasis can fail at any given stage of the process due to cell destruction by the immune system, blood flow turbulence, hypoxia, nutrition deprivation, or unsuitable tissue microenvironment. The susceptibility of tumor cells to death during metastasis can directly affect the efficiency of metastasis (7). Tumor cells with higher metastatic potentials are likely more resistant to cell death. Thus it is critical to explore the molecular relationship between cell survival and metastasis.
It's well known that deregulated apoptosis contribute to carcinogenesis, abnormal cell differentiation, and resistance to chemotherapy. Multiple mechanisms could contribute to enhanced survival and reduced apoptosis in tumor cells, among which the control of caspase activation would be the key. Depending on how caspases are activated through the intrinsic or extrinsic pathway, cancer cells may develop different means to suppress the activation. The inhibitor-of-apoptosis proteins (IAP) represent a large family of endogenous caspases inhibitors, which include X-linked IAP (XIAP), c-IAP1, c-IAP2 and Survivin (8). Among them, XIAP is perhaps the most relevant and most potent caspases inhibitor (9). XIAP could suppress the activation of an initiator caspase, caspase-9 and the effector caspases (caspase-3, and -7) via its interaction through the BIR3 and BIR2 domains (10). Since the effector caspases are the common downstream caspases for both the extrinsic pathway (death receptor pathway) and the intrinsic pathway (mitochondria pathway) XIAP could block both pathways potently.
Increased XIAP has been reported in a variety of human tumors including esophageal carcinoma (11), clear cell renal carcinoma (12), ovarian carcinoma (13), lymphoma (14) and HCC (15). In some cases, this expression was found to be associated with reduced survival (12). Although over-expression of XIAP in cancer cells is linked to increased resistance to apoptosis, exactly how this survival advantage benefits tumors at various stage of tumor development and progression is less clear. Nevertheless, it seems that suppression of XIAP could be beneficial in cancer therapy (16). Indeed, anti-sense oligonucleotides against XIAP have been developed and found to possess therapeutic effects in both in vivo and vitro studies (17, 18). Currently, a novel second generation anti-sense oligonucleotide against XIAP, AEG35156, is under assessment in multiple phase I trials in cancer patients (19).
Here we investigated the expression of XIAP in established HCC cell lines and in HCC patients, and demonstrated the role of this anti-apoptosis molecule in metastasis, tumor recurrence and therapeutic outcome of HCC patients.
Experimental Procedures
Animals, cell lines and reagents
Male BALB/c nude mice (nu/nu) (Shanghai Institute of Material Medica, Chinese Academy of Science, Shanghai, China) at the age of 4 to 6 weeks were housed in laminar flow cabinets under specific pathogen free conditions. The mice were cared for and handled according to the recommendations of the National Institute of Health Guidelines for Care and Use of Laboratory Animals. Experimental protocol was approved by Shanghai Medical Experimental Animal Care Committee.
The metastasis-capable human HCC cell lines MHCC-97L and HCCLM3 were established through consecutive in vivo selection based on lung metastasis and in vitro clonal expansion (20). The human HCC cell line SMMC7721 has a very low metastatic potential (21). All three cell lines were cultured in DMEM supplemented with 10% fetal bovine serum and standard supplements. HCCLM3 cells stably expressing a shRNA against XIAP was established via retroviral infection and puromycin selection.
All chemical reagents were obtained from Sigma (St. Louis, MO). The following antibodies were used: anti-XIAP (for immunoblot: BD Biosciences, San Diego, CA; for immunohistochemistry: R&D system, Minneapolis, MN), anti- Bcl-XL (Cell Signaling, Boston, MA), anti-Bcl-2 (clone 6A8, BD Biosciences), anti-Mcl-1 (BD Biosciences), anti-c-IAP1, anti-c-IAP2, anti-NF-κB p65RelA, anti-Bak, anti-Bax, and anti-p53 (Santa Cruz Biotech, Santa Cruz, CA), anti-Survivin (R&D Systems), anti-Bid (22) and anti-β-actin (Sigma).
Recombinant adenovirus expressing human XIAP (Ad-Y5-XIAP) or the control viral vector (Ad-Y5) were prepared in the University of Pittsburgh Biotechnology core facility as described previously (23).
RNAi-mediated downregulation of XIAP
For cell culture study, a siRNA hairpin duplex against human XIAP (NM_001167, base 241-259, GTGGTAGTCCTGTTTCAGC) (Cell Signaling) was used to knock down XIAP in cultured cell lines. A random scrambled siRNA (GGAUAACCUCAAUUCGGUU) (Invitrogen) was used as a negative control. To establish stable cell lines expressing shRNA against XIAP, the same oligonucleotides were cloned into the RNAi-Ready pSIREN-RetroQ retroviral vector system (Clontech, Mountain View, CA). For retroviral infection, PA317 packaging cells were transfected with 10 μg of the appropriate retroviral constructs using Polyfect (Qiagen). The culture supernatants containing the viral particles were collected 24 h after transfection and filtered for subsequent use.
Analysis of apoptosis, invasiveness and metastasis
Cells undergoing apoptosis were determined by the typical nuclear morphology of condensation and fragmentation, following the staining with Hoechst 33342 (5 μg/ml). Caspase-3 activities were determined as previously described (24). The membrane invasion assay was performed in Matrigel-coated invasion chambers (Becton Dickinson Labware, Franklin Lakes, NJ) as previously described (25). DMEM with 10% fetal bovine serum was added to the lower chamber as a chemoattractant. 2.5 × 104 cells were plated in the upper chamber. Following incubation at 37°C in a humidified 5% CO2 atmosphere for 48 hours, the cells in the upper chamber and on the Matrigel were mechanically removed with a cotton swab. The cells on the outer surface of the membrane were stained with Diff-Quick stain (Dade Behring Inc., Newark, DE) according to the manufacturer's instructions. The invading cells were examined, counted, and photographed by digital microscopy (Nikon Eclipse TE 200, Melville, NY). Six fields were counted per filter in each group, and the experiment was repeated three times.
For in vivo metastasis analysis, HCC cells (5×106) in 0.2 ml PBS were injected subcutaneously into the left upper flank region of the nude mice. The mice were sacrificed 42 days later and the lungs were removed for histopathological examinations. Tumor volume at the injection site was calculated by the formula v = (a × b2)/2 (where a= the larger diameter b = the perpendicular diameter). In addition, each lung was fixed in 10% formalin buffer and paraffin-embedded. Five serial sections were made for each lung block and the total number of microscopic lung metastatic foci was then determined as previously described (20).
Patient samples
Patient samples were obtained following informed consent according to an established protocol approved by the Ethic Committee of Fudan University. Data do not contain any information that may lead to the identification of the patients.
Matched pairs of primary HCC samples and adjacent liver tissues were used for the construction of a tissue microarray (Shanghai Biochip Co., Ltd. Shanghai, China) as previously described (26). Briefly, duplicates of one-millimeter diameter cylinders from two different areas, the center of the tumor tissue and the liver tissue adjacent to the tumor, a total of four punches, were included in each case, along with different controls, to ensure reproducibility and homogenous staining of the slides. Sections of 4-μm thickness were placed on 3-aminopropyltriethoxysilane (APES)-coated slides for subsequent staining with an anti-XIAP antibody using a manufacturer-recommended two-step Envision method (DAKO, Carpinteria, CA).
Statistical Analysis
Comparisons of quantitative data were analyzed by Student's t test between two groups, or by one-way ANOVA for multiple groups. Categorical data were analyzed by Fisher's exact test. The Kaplan-Meier method was used to determine survival probability and the differences were assessed by the log-rank test. Cox multivariate regression analysis was used to determine independent prognostic factors. All analyses were performed using the SPSS software (v. 13.0, Chicago, IL).
Results
Differential response of HCC cell lines to apoptosis is correlated with their metastatic capabilities
In order to understand whether the metastasis capability of HCC cells could be correlated with the ability to survive, we compared the apoptotic response of three human HCC cell lines, which had previously characterized for their different metastatic capability (20, 21, 27). A number of apoptotic stimuli were applied, which include a death receptor ligand, TNF-α; a DNA-damaging agent, etopside; a general protein kinas inhibitor, staurosporine (STS); a proteasome inhibitor, MG132 and an oxidant, H2O2. These agents are capable of inducing apoptosis through the extrinsic death receptor pathway and/or the intrinsic mitochondria pathway.
We found that the most metastatic HCCLM3 cells were also the most resistant while the least metastatic SMMC7721 cells the most sensitive (Fig. 1A). The extent of apoptotic response of MHCC97L cell, which had an intermediate level of metastatic capability, was between those of SMMC7721 and HCCLM3 cells. The dose-response pattern of the three cell lines as illustrated in the case of H2O2 treatment clearly indicated their differential sensitivities in correlation with their metastatic capability (Fig. 1B). Thus while SMMC7721 were sensitive to H2O2 even at the low dose of 250 mM, MHCC97L cells became sensitive only at the high dose of 600 μM. On the other hand, HCCLM3 cells were resistant to H2O2 even at the high dose. This dose-dependent response pattern could be also observed in other treatments (data now shown). Furthermore, the time-response patterns were also clearly correlated well with the metastatic capability with SMMC7721 cells dying more rapidly than the other two cell lines (Fig. 1C).
Figure 1. Diverse sensitivity to apoptotic stimuli in HCC cell lines.
(A). HCCLM3 (white column), MHCC97L (gray column) and SMMC7721 (black column) were treated with TNFα (10 ng/ml) plus actinomycin D (0.2 μg/ml), etopside (0.6 mM), MG132 (5 μM), STS (0.5 μM) or H2O2 (600 μM) for 48 hours (TNFα, etopside, MG132) or 24 hours (STS, H2O2). Percentages of apoptotic cells were determined by nuclear staining with Hoechst 33342. (B). Cells were treated with H2O2 at different concentrations as indicated for 24 hours. Apoptosis was determined as in A. (C). Cells were treated with etopside (0.6 mM) or MG132 (5 μM) for different times as indicated. Apoptosis was determined as in A. (D). Cells were treated as in A for 24 hours. Cytosol caspase-3-like activity was determined using Ac-DEVD-AFC as the substrate. All data shown are mean±SD. For panels A, C and D, comparisons were made between SMMC7721 cells and HCCLM3 as indicated. For panel B, comparisons were made between SMMC-7721 and HCCLM3 cells, and between MHCC97L and HCCLM3 cells at three different H2O2 concentrations as indicated. (**: p < 0.01, one way ANOVA).
Activation of caspases is a hall marker of apoptosis. We found that caspase-3 activities in the treated cells were correlated with the nuclear staining assay with the highest detected in HCCLM3 cells (Fig. 1D). Caspase-8 and capase-9 activities were similar to those of caspase-3 (data not shown). The apoptosis of these cells could be suppressed by z-VAD-fmk, a general caspase inhibitor (data not shown). Taken together, it seems that cells that are more metastatic are also more apoptosis-resistant.
Differential expression of XIAP may account for the variable resistance of HCC cells to apoptosis
We examined whether there could be differential expression of key apoptotic regulatory molecules that would correlate with the observed variations in apoptosis sensitivity in these HCC cells. We found that there were no differences in the expression of several anti-apoptosis molecules, such as Bcl-XL, Bcl-2, c-IAP1, c-IAP2, Survivin and a NF-κB subunit, p65RelA (Fig. 2A), and pro-death molecules, such as Bax and Bid (Fig. 2B).
Figure 2. Expression of major apoptosis regulating molecules in HCC cell lines.
Lysates of HCCLM3, MHCC97L and SMMC7721 cells were subjected to SDS-PAGE followed by immunoblot assays with antibodies against molecules that protect cells from apoptosis (A) or molecules that promote apoptosis (B).
Paradoxically, the most sensitive cell line, SMMC7721, expressed a higher level of Mcl-1, an anti-death Bcl-2 family protein, and a lower level of pro-death molecule, p53, suggesting that these molecules might not be responsible for the differential apoptosis sensitivity. Although SMMC7721 cells expressed a higher level of a pro-death Bcl-2 family protein, Bak, the level of Bak did not show a noticeable difference between HCCLM3 and MHCC97L cells (Fig. 2B), which rendered it harder to attribute the differential apoptotic response of these two cell lines to this factor, although we could not rule it out. However, the expression of XIAP, a pro-survival molecule of the IAP family, seemed to better correlate well with the apoptosis sensitivity with the highest expression in HCCLM3 cells and lowest expression in the SMMC7721 cells (Fig. 2A). Because of this close correlation, we reasoned that XIAP would likely be a major factor determining the apoptosis behavior of these cells among the various contributing molecules. We thus decided to focus our study on XIAP.
To confirm the role of XIAP, we introduced a specific siRNA into HCCLM3 cells, which effectively knocked down the expression XIAP (>80%) in this cell line without affecting the expression of other IAP molecules, such as c-IAP1 and c-IAP2 (Fig. 3A). Correspondingly, this led to a significant increase in the apoptotic response (Fig. 3B). Conversely, over-expression of XIAP in SMMC7721 cells (Fig. 3C) significantly raised the resistant level to multiple apoptotic stimuli (Fig. 3D). These findings thus suggest that the different levels of XIAP in these cell lines could at least in part explain their differential sensitivity to apoptotic stimulation.
Figure 3. Alteration of XIAP levels in SMMC7721 and HCCLM3 cells changes their susceptibility to apoptosis.
(A). HCCLM3 cells were transfected with a negative siRNA (200 nM)(lane 2, Neg) or a siRNA against XIAP (100-200 nM, lanes 3 and 4) for 48 hours. The levels of XIAP and other molecules were compared to the parental cells (lane 1). (B). HCCLM3 cells transfected with control siRNA (white column) or siRNA against XIAP (black column) for 48 hours were treated with etopside (Etop. 600 μM), STS (1 μM), or TNFα (10 ng/ml) plus actinomycin D (0.2 μg/ml) for 24 hours. Apoptosis was determined by nuclear staining. (C). SMMC7721 were infected with an adenovirus expressing the vector only (lane 2, Ad-C) or human XIAP (lane 3, Ad-XIAP) for 48 hours. The levels of XIAP and other molecules were compared to the non-infected parental SMMC7721 cells (lane 1, Pa), or HCCLM3 (lane 4). (D). SMMC7721 cells infected with control adenovirus (white column) or XIAP adenovirus (black column) for 48 hours were treated with etopside (Etop. 250 μM), H2O2 (200 μM) or MG132 (5 μM) for 24 hours. Apoptosis was determined by nuclear staining. For panels B and D, data shown are mean±SD and asterisks indicate significant differences between the control groups and the over-expressed or knocked-down group (*: p < 0.05; **: p < 0.01, Student's t test).
Modulation of the expression of XIAP can alter the metastatic outcome of HCC cells
Since the level of XIAP expression in the three established HCC lines correlated with their metastatic capability, we investigated whether modulating the XIAP level could affect the metastasis in vivo. Toward this end we established HCCLM3 cells stably expressing a shRNA construct against XIAP. The same short hairpin sequence as used in siRNA-mediated knockdown experiment was cloned into an RNAi-ready pSIREN vector. Transient transfection of the vector into HCCLM3 demonstrated that this vector worked well in knocking down XIAP, resulting in more than 80% of inhibition in 120 hours (Fig. 4A). This sequence was then cloned into the RNAi-ready pSIREN-retroviral vector, from which a specific retrovirus was made for infection into HCCLM3 cells. Stable clones were selected that consistently expressed a much lower level of XIAP, compared to the cells expressing the control shRNA (Fig. 4B).
Figure 4. Stable suppression of XIAP expression affects the metastatic outcome of HCCLM3 cells.
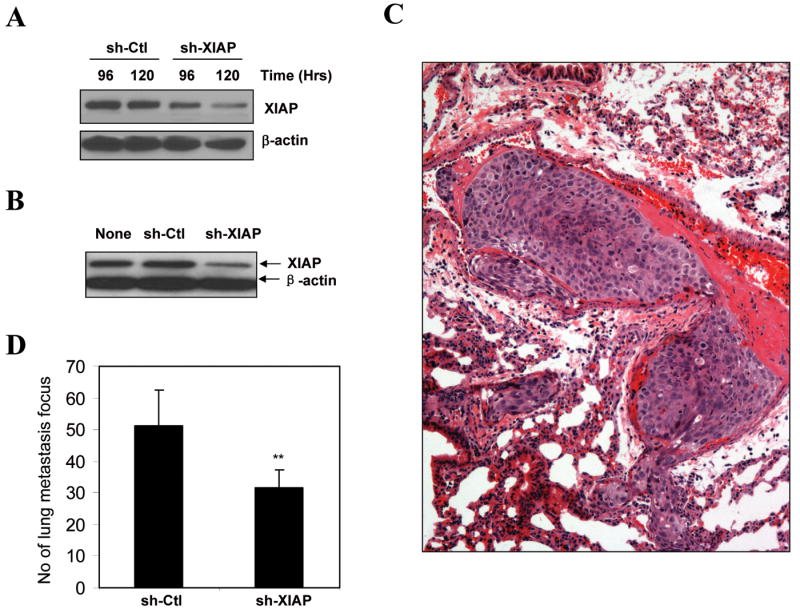
(A). HCCLM3 cells were transiently transfected with pSIREN-shRNA vector (sh-Ctl) or pSIREN-shRNA against XIAP (sh-XIAP) for 96 or 120 hours. Lysates were prepared and the level of XIAP was determined by immunoblot assay. (B). HCCLM3 cells were transfected with as in panel A, stable clones were selected and analyzed for XIAP expression by immunoblot assay. (C-D). The stable clones (5×106) were subcutaneously injected into nude mice (n = 5 per group). Mice were sacrificed 42 days later and lungs were histologically examined for metastatic foci. A typical metastatic colony formed by HCCLM3 cells stably expressing the pSIREN vector was shown in C. The total number (mean±SD) of lung metastatic foci was determined from 5 serial sections per lung (D). There was a significant difference in the metastatic capability between HCCLM3 cells stably expressing pSIREN-shRNA vector and HCCLM3 cells stably expressing pSIREN-shRNA against XIAP (**: p < 0.01, Student's t test).
A total of 5×106 XIAP-knocked-down HCCLM3 cells or control cells were subcutaneously injected into the flank of nude mice, respectively. Necropsy at the 42nd day indicated that the average tumor volume at the injection sites was not statistically different between the control and XIAP-knocked-down group (0.69±0.15 cm3 vs 0.66±0.26 cm3, p = 0.865, one-way ANOVA). While pulmonary metastasis could be detected in both groups at 100% rate (5/5 in each group), which were confirmed by histological examination (Fig. 4C), the number of metastatic foci revealed a significant reduced ability of HCCLM3 cells to establish individual metastatic foci (about 40% reduction) (Fig. 4D). These observations thus indicated that increased expression of XIAP did not affect significantly the growth of tumor cells at the primary site, but rather affect the outcome of the metastasis as revealed by the difference in the metastatic foci in the lung.
It is likely that XIAP could do so by providing survival advantage to the metastatic cells via its anti-apoptosis effect (Figs. 1 and 3), which would be important during the transit of these cells from the primary site to the secondary site and during the establishment of the colony at the secondary site (5-7). XIAP could alter other biological behavior of HCC cells, which might be also important for metastasis. Thus we investigated the impact of XIAP on the invasiveness of HCC cells in vitro. SMMC7721 cells that were treated with recombinant adenovirus expressing XIAP and HCCLM3 cells that were treated with siRNA against XIAP were analyzed for the invasiveness in Matrigel (Fig. 5). Compared to the control group, SMMC7721 cells over-expressing XIAP had a significantly increased ability to pass through the Matrigel, indicating an increased invasive behavior. As anticipated, HCCLM3 cells were more invasive than SMMC7721 cell. But knocking down XIAP in HCCLM3 resulted in a much reduced ability to pass through Matrigel. Taken together it is likely that XIAP could affect the metastatic outcome by several mechanisms.
Figure 5. Alteration of XIAP levels in SMMC7721 and HCCLM3 cells changes their invasiveness in vitro.
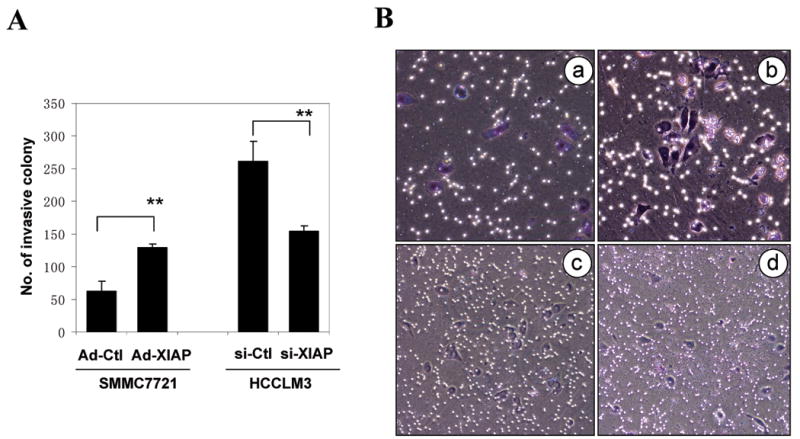
(A). SMMC7721 cells infected with control adenovirus (Ad-Ctl) or XIAP-expressing adenovirus (Ad-XIAP) and HCCLM3 cells transfected with control siRNA (si-Ctl) or siRNA against XIAP (si-XIAP) were subjected to in vitro invasion assay. About 2.5 × 104 cells were placed in each chamber at the beginning of the assay. The number (mean±SD) of invasive colony on each membrane was quantified for each group. Asterisks indicate significant differences between the control groups and the over-expressed or knocked-down groups (**: p < 0.01, Student's t test). (B). Representative images of SMMC7721 cells infected with control adenovirus (a) or XIAP-expressing adenovirus (b) and HCCLM3 cells transfected with control siRNA (c) or siRNA against XIAP (d) in the invasion assay as in A.
Expression of XIAP in HCC correlates with a poorer prognosis in patients
The above findings also suggest that XIAP could be an important factor in determining clinical outcomes of HCC patients. To determine whether this could be the case, we examined the expression of XIAP in 192 HCC samples in a tissue microarray by immunohistochemistry (Table 1). A majority of HCC samples (89.6%) were found to be highly positive for XIAP, which was observed primarily in the cytoplasm (Fig. 6A). In contrast, less than half of the adjacent liver tissues (45.8%) expressed XIAP (p <0.01, Fisher's exact test), suggesting that high XIAP expression was more of a characteristic of the HCC cells.
Table 1.
Upregulation of XIAP in HCC tissues.
| XIAP staining | Tumor (n=192) | Adjacent liver tissue (n=192) | p value |
|---|---|---|---|
| Negative | 20 | 104 | <0.001 |
| Positive | 172 | 88 |
HCC samples and the corresponding adjacent liver tissues (n = 192) in a tissue microarray were stained for XIAP expression, which was scored independently by two pathologists based on both staining intensity and the extent of XIAP expression across the section. Intensity of staining was scored as 0 (negative), 1 (weak), or 2 (strong). The extent of staining was based on the percentage of positive tumor cells: 0 (negative), 1 (1–25%), 2 (26–50%), 3(51–75%), and 4 (76–100%). The final score of each sample was assessed by summarizing the result of intensity and extent of staining. Therefore, each case was finally considered negative if the final score was 0–1 (-) or 2-3 (±), and positive if the final score was 4–5 (+) or 6–7 (++), respectively. The percentage of HCC samples showing positive staining of XIAP (172/192) is significantly higher than that of adjacent liver tissue (88/192) (p<0.001, Fisher's exact test).
Figure 6. High XIAP expression correlates with reduced survival in HCC patients receiving orthotopic liver transplantation.


(A). HCC samples in a tissue microarray were immunostained with an anti-XIAP antibody (a-d). Representative XIAP-positive sample (a-b) and XIAP-negative sample (c-d) were shown at 40× (a, c) and 100× (b, d) magnification. The corresponding H-E staining were shown in panels a′-d′. (B-G). The disease-free (B, D, F) and overall (C, E, G) survival rates of 192 HCC patients underwent OLT were between the XIAP-positive and XIAP-negative groups. B-C, all patients; D-E, patients who met Milan criteria; F-G, patients who exceeded Milan criteria. Significance was determined using the log-rank test.
These 192 paired samples were from advanced HCC patients who underwent total liver resection followed by OLT. They had been followed up for a median length of 58 months for the clinical outcome. Segregation of these patients into the XIAP-positive and XIAP-negative groups did not reveal significant correlations with any single clinopathological parameters (Table 2). However, these groups are significantly correlated with Milan criteria, which consider both the number and the size of the tumor nodules (28). Thus patients who exceeded the Milan criteria were more likely to have an increased XIAP expression. The Milan criteria are widely used to predict the clinical outcome of HCC patients post-OLT (28). In fact, when the 192 patients were grouped according to the Milan criteria, those exceeding the Milan criteria had a significantly worse overall survival and disease-free survival than those matching the criteria (p= 0.00, data not shown). Consistently, the 1-year, 2-year and 3-year overall survival and disease-free survival following OLT were much worse for the XIAP-positive group than the XIAP-negative group (Table 3)(Fig. 6B-C). Because of the correlation of XIAP expression and Milan criteria, the prognostic value of XIAP expression is to a large degree overlapped with that of Milan criteria. Thus for patients who matched the Milan criteria, further segregation based on XIAP expression did not lead to significant differences in overall and disease-free survival, even the trend remained the same (Fig. 6D-E). However, in patients who exceeded the Milan criteria, those with increased XIAP expression had significantly shorter disease-free survival (Fig. 6F-G), indicating that XIAP expression could provide additional prognostic values beyond the Milan criteria in advanced cases.
Table 2.
Profiles of patients with XIAP-positive or XIAP-negative HCC
| Clinopathological variables | Tumor XIAP expression | p value1 | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Age | ≤ 60 yr. | 18 | 26 | 0.744 |
| > 60 yr. | 2 | 146 | ||
| Sex | Male | 18 | 160 | 0.644 |
| Female | 2 | 12 | ||
| Tumor Number | Solitary | 10 | 72 | 0.486 |
| Multiple | 10 | 100 | ||
| Maximal Tumor Size | ≤ 5 cm | 16 | 109 | 0.214 |
| > 5 cm | 4 | 63 | ||
| Liver Cirrhosis | Absent | 3 | 41 | 0.574 |
| Present | 17 | 131 | ||
| AFP2 | Positive | 14 | 127 | 0.790 |
| Negative | 6 | 45 | ||
| Child-Pugh Score3 | Class A | 17 | 128 | 0.524 |
| Class B | 2 | 36 | ||
| Class C | 1 | 8 | ||
| Tumor Differentiation4 | I - II | 17 | 136 | 0.770 |
| III - IV | 3 | 36 | ||
| Microsatellite Lesions5 | Absent | 16 | 107 | 0.143 |
| Present | 4 | 65 | ||
| Tumor Encapsulation5 | Absent | 12 | 102 | 1.000 |
| Present | 8 | 70 | ||
| Venous Invasion6 | Absent | 14 | 98 | 0.340 |
| Present | 6 | 74 | ||
| pTNM stage7 | I | 6 | 46 | 0.695 |
| II | 8 | 58 | ||
| III | 6 | 68 | ||
| Milan Criteria8 | Yes | 14 | 62 | 0.0065 |
| No | 6 | 110 | ||
HCC patients receiving OLT were divided into XIAP-negative group and XIAP-positive group. The patient and disease profiles in each group were compared.
Statistical analyses were conducted with Fisher's exact test for all the parameters. P values less than 0.05 were considered statistically significant.
AFP positive: serum level >20ng/ml, AFP negative: serum level ≤ 20ng/ml.
Child-Pugh scoring system was used to stratifying the severeness of underlying end-stage liver disease, which was judged by five clinical measures: total bilirubin, serum albumin, International Normalized Ratio (INR), ascites and hepatic encephalopathy. Class A: score 5-6, Class B: score 7-9, Class C: score 10-15.
Grading of differentiation status was performed according to the method of Edmondson and Steiner (35). The tumors were classified into two groups: well differentiated (Grades I and II) and poorly differentiated (Grades III and IV).
Microsatellite lesions and tumor encapsulation were determined by macroscopic pathological examination.
HCC with microscopic portal vein tumor thrombosis or macroscopic portal vein thrombosis indicates tumor venous invasion.
The pTNM classification for HCC was based on The American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) staging system (6th edition, 2002).
The Milan criteria is matched if a single solitary tumor is ≤5cm in diameter, or if in multiple-tumor cases, there are ≤3 nodules, and each nodule is ≤3 cm in diameter (28).
Table 3.
Expression of XIAP in HCC correlates with poorer prognosis following orthotopic liver transplantation
| Survival Measurement | XIAP Expression | p value | |
|---|---|---|---|
| Negative | Positive | ||
| 1-year overall survival (%) | 100.0±0.0 | 76.1±3.3 | 0.021 |
| 2-year overall survival (%) | 93.3±6.4 | 61.8±3.9 | |
| 3-year overall survival (%) | 93.3±6.4 | 57.5±4.4 | |
| 1-year disease-free survival (%) | 100.0±0.0 | 69.4±3.6 | 0.003 |
| 2-year disease-free survival (%) | 100.0±0.0 | 65.6±3.8 | |
| 3-year disease-free survival (%) | 100.0±0.0 | 63.4±4.3 | |
One hundred ninety-two (192) HCC patients underwent OLT were followed up for a median length of 58 months (range, 2 to 109 months). The overall and disease-free survival rates were determined. Disease-free survival is defined as period without recurrence, the diagnosis of which is based on the typical features presented in CT/MRI scan and an elevated serum alpha-fetal protein. The differences in these rates between patients whose original tumors were XIAP-positive and those whose original tumors were XIAP-negative are significant (log-rank test).
Finally we performed Cox multivariate regression analysis to determine the independent prognostic factors among the thirteen important clinopathological factors (Table 2). We found that positive XIAP expression in HCC, the presence of microsatellite lesions and a larger tumor size could be independent prognostic factors for both overall survival and disease-free survival (Table 4). Overall, patients with XIAP-positive HCC had about four times higher risk than patients with XIAP-negative HCC for tumor recurrence and for a shorter survival rate. Taken together, these findings suggest that XIAP expression is an important predicting factor for metastatic recurrence in HCC patients, which in turn affect their survival.
Table 4.
XIAP expression in HCC is an independent prognostic factor for HCC patients undergoing OLT
| Overall Survival | Disease-free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wald | p value | 95% CI for RR | RR | Wald | p value | 95% CI for RR | RR | |||
| Lower | Upper | lower | Upper | |||||||
| XIAP Expression (positive vs negative) | 3.7248 | 0.050 | 0.979 | 16.412 | 4.008 | 4.1981 | 0.040 | 1.066 | 17.855 | 4.363 |
| Microsatellite Lesions (present vs absent) | 6.7969 | 0.009 | 1.172 | 3.073 | 1.898 | 9.0329 | 0.003 | 1.293 | 3.386 | 2.092 |
| Maximal Tumor Size (> 5 cm vs < 5cm) | 3.8698 | 0.049 | 1.002 | 2.635 | 1.625 | 6.8798 | 0.008 | 1.178 | 3.109 | 1.914 |
Clinopathological parameters shown in Table 2, except the Milan criteria, were subjected to multivariate Cox regression analysis. Among them, positive XIAP expression, presence of microsatellite lesions, and a larger tumor size were found to be independent prognostic factors for both overall survival and disease-free survival. Significance test for the Wald values was performed and the p values are shown (significance level is ≤0.05). The relative risks (RR) of earlier death, as measured by overall survival, and recurrence, as measured by disease-free survival, in patients with XIAP-positive tumor, with microsatellite lesions or with a larger tumor size were also calculated together with the 95% confidence interval (CI).
Discussion
Hepatocellular carcinoma is one of the most prevalent cancers worldwide. Despite recent advances in diagnosis and treatment, the outcome is still far from optimal. Total liver resection followed by liver transplantation has emerged as an effective treatment for HCC, particularly for patients with advanced cirrhosis (29). Excellent results can be achieved when this combination of regime is performed following a strict or slightly loosened set of Milan criteria (28, 30). Although the 5-year survival rate seemed to be extended with a reduced recurrence rate, compared to the treatment by partial liver resection (28, 30-32), once the recurrence is established the treatment is still difficult and the prognosis is dismal. Recurrence in this setting is due to metastasis from the original tumors, which occurs before the procedure. However, the mechanism of metastasis and the factors affecting the metastasis in HCC are not fully understood, which impedes the development of effective measurements.
Metastasis is a complicated process, involving multiple steps (5). Generally, metastasis is an inefficient process and only a small percentage of cancer cells can manage through these steps to establish a metastatic tumor (6). Studies have indicated that resistance to cell death is intrinsically associated with metastasis (7). Our initial studies on three HCC cell lines with different metastasis potentials indicated that they indeed possessed different sensitivity to a diverse array of intrinsic and extrinsic apoptosis stimulation. Notably, a lower sensitivity was associated with a higher metastatic potential. Thus the highly metastatic HCCLM3 cells were much more resistant to apoptosis than the weakly metastatic SMMC-7721 cells. MHCC97L cells fell in between in terms of its metastatic capability and apoptosis resistance.
A number of factors could account for the differences in apoptosis sensitivity. Earlier studies showed that ratio of the anti-death versus the pro-death members of the Bcl-2 family proteins was critical to the response of many types of cancer cells to therapeutic agent-induced apoptosis (33). Increased expression of Bcl-2, Bcl-XL or Mcl-1 has been associated with poor therapeutic response and poor prognosis. However, in the three HCC cells that we investigated, the expression of the anti-death and pro-death Bcl-2 family proteins neither seemed to differ significantly nor seemed to correlate with their apoptosis sensitivity (Fig. 2).
In contrast, we observed that the expression level of XIAP in these cell lines was closely correlated with their apoptosis sensitivity. The IAP family consists of multiple members (8, 16). But XIAP may be the only member that has the authentic caspase-suppressive effect under normal conditions (9). Expression of XIAP in tumors has been well studied (11-14). Our observation in the three HCC cell lines indicate that only XIAP level is functionally discriminative (Fig. 2). Indeed, increasing XIAP expression in the sensitive SMMC-7721 cells significantly enhanced their resistance to multiple apoptotic stimuli. Conversely, down-regulation of XIAP in the resistant HCCLM3 cells significantly sensitized them to apoptosis.
Would the differential expression of XIAP contribute to the different metastatic capacity observed in these cell lines, perhaps due to their differential sensitivity to apoptosis? Our studies reveal that XIAP level is correlated with enhanced lung metastasis in vivo. These effects are likely due to an increased resistance to apoptosis, although XIAP may have other effects affecting metastasis, such as increased invasiveness. To determine that this effect of XIAP was not an artifact of experimental manipulation, we examined clinical HCC samples for XIAP expression. Furthermore, we investigated the impact of XIAP expression on HCC recurrence and survival in patients undergoing total liver resection followed by orthotopic liver transplantation. This therapeutic regime provides a good model to study metastasis as any recurrent foci must be derived from metastatic cells of the original tumors before and/or during the resection of the affected liver.
In 192 such cases that were followed, we found that XIAP expression was elevated in 89.6% HCC samples. To determine whether XIAP expression could be in general increased in HCC samples, we had also examined 75 additional HCC samples from patients who presented minor conditions without the need of total live resection and OLT. These samples were also from consecutive cases in a randomly determined period of time. We found increased XIAP expression in 77.3% of the cases (58/75). A high rate of XIAP expression (about 70%) in liver cancer had also been reported in another independent work (15). Thus in general XIAP expression is increased in a majority of HCC samples.
Most importantly, patients with XIAP-positive tumors had an increasing risk of recurrence and thus a significantly reduced disease-free survival rate. This led to a reduction in overall post-surgery survival rate as well. XIAP expression is a powerful independent prognostic factor. This finding is consistent with the results from cell culture and animal studies and with the notion that XIAP is an important factor affecting tumor metastasis and recurrence. Interestingly, XIAP expression has the similar prognostic values as the Milan criteria, likely due to the fact that increased XIAP expression is significantly more likely to be seen in patients who exceed the Milan criteria, i.e., in patients with more tumors and/or larger tumors. However, XIAP expression could reveal further survival difference even in those advanced patients.
Early studies had shown that XIAP expression was also negatively correlated with the prognosis of clear cell renal carcinoma (12) and that Survivin expression was negatively correlated with the prognosis of patients with HCC (34). Our study is consistent with these studies indicating the pro-cancer role of IAP molecules and clearly supports the notion that metastasis can be a key step where suppression of apoptosis by XIAP can be contributive.
In conclusion, through a series of studies using HCC cell lines, animal models and HCC patient samples, we have established the importance of XIAP in resistance to apoptosis, invasiveness, metastasis, and tumor recurrence in HCC patients. These effects seem to be interconnected and the survival advantage conferred by XIAP may be the major mechanism. Thus XIAP can be an important biomarker for HCC recurrence and could be therapeutically targeted to reduce HCC metastasis and recurrence.
Acknowledgments
We would like to thank Prof. Xiao-Ling Xu (Shanghai Institute of Foreign Trade) for assistance in statistical analysis.
Financial Support: This work is supported in part by US NIH grants R01CA83817 and CA111456 (to XMY) and by the National Natural Science Foundation of China (N.30571801) and Shanghai National Natural Science Foundation (05ZR14027) (to SYH and JF).
Abbreviations
- HCC
hepatocellular carcinoma
- IAP
inhibitor-of-apoptosis protein
- OLT
orthotopic liver transplantation
- XIAP
X-linked IAP
References
- 1.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY. Hepatocellular carcinoma surgery--review of the past and prospects for the 21st century. J Surg Oncol. 2005;91:95–96. doi: 10.1002/jso.20291. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 4.Wong LL. Current status of liver transplantation for hepatocellular cancer. Am J Surg. 2002;183:309–316. doi: 10.1016/s0002-9610(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 5.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 6.Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- 7.Townson JL, Naumov GN, Chambers AF. The role of apoptosis in tumor progression and metastasis. Curr Mol Med. 2003;3:631–642. doi: 10.2174/1566524033479483. [DOI] [PubMed] [Google Scholar]
- 8.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 9.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren S, Liu Z, et al. XIAP is Highly Expressed in Esophageal Cancer and its Downregulation by RNAi Sensitizes Esophageal Carcinoma Cell Lines to Chemotherapeutics. Cancer Biol Ther. 2007;6:973–980. doi: 10.4161/cbt.6.6.4195. [DOI] [PubMed] [Google Scholar]
- 12.Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert HE, et al. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol. 2004;35:1022–1028. doi: 10.1016/j.humpath.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res. 2003;13:399–404. doi: 10.3727/096504003108748410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akyurek N, Ren Y, Rassidakis GZ, Schlette EJ, Medeiros LJ. Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer. 2006;107:1844–1851. doi: 10.1002/cncr.22219. [DOI] [PubMed] [Google Scholar]
- 15.Shiraki K, Sugimoto K, Yamanaka Y, Yamaguchi Y, Saitou Y, Ito K, Yamamoto N, et al. Overexpression of X-linked inhibitor of apoptosis in human hepatocellular carcinoma. Int J Mol Med. 2003;12:705–708. [PubMed] [Google Scholar]
- 16.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, Mayer LD, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 18.Shaw TJ, Lacasse EC, Durkin JP, Vanderhyden BC. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2007 doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 19.LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, et al. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL, Liu YK, et al. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol. 2004;130:460–468. doi: 10.1007/s00432-004-0564-9. [DOI] [PubMed] [Google Scholar]
- 21.Bao ZJ, Wang Y, Zhan RZ. Clonal analysis of a hepatocarcinoma cell line: an experimental model of tumor heterogeneity. Cancer Research on Prevention and Treatment. 1995;22:65–67. [Google Scholar]
- 22.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 23.Hui H, Khoury N, Zhao X, Balkir L, D'Amico E, Bullotta A, Nguyen ED, et al. Adenovirus-Mediated XIAP Gene Transfer Reverses the Negative Effects of Immunosuppressive Drugs on Insulin Secretion and Cell Viability of Isolated Human Islets. Diabetes. 2005;54:424–433. doi: 10.2337/diabetes.54.2.424. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Zhao Y, He X, Kim TH, Kuharsky DK, Rabinowich H, Chen J, et al. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277:26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- 25.Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 27.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 29.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998;129:643–653. doi: 10.7326/0003-4819-129-8-199810150-00013. [DOI] [PubMed] [Google Scholar]
- 30.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 31.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. discussion 799-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 33.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 34.Ikeguchi M, Ueda T, Sakatani T, Hirooka Y, Kaibara N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol. 2002;11:33–40. doi: 10.1097/00019606-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]






